Abstract
LPPs (lipid phosphate phosphatases) are members of a family of enzymes that catalyse the dephosphorylation of lipid phosphates. The only known form of regulation of this family of enzymes is via de novo expression of LPP isoforms in response to growth factors. In this respect, we evaluated the effect of moderate increases in the expression of recombinant LPP1 on signal transduction by both G-protein-coupled receptors and receptor tyrosine kinases. We present evidence for a novel role of LPP1 in reducing PDGF (platelet-derived growth factor)- and lysophosphatidic acid-induced migration of embryonic fibroblasts. We demonstrate that the overexpression of LPP1 inhibits cell migration by reducing the PDGF-induced activation of p42/p44 MAPK (mitogen-activated protein kinase). This appears to occur via a mechanism that involves the LPP1-induced down-regulation of typical PKC (protein kinase C) isoform(s), which are normally required for PDGF-induced activation of p42/p44 MAPK and migration. In this regard, DAG (diacylglycerol) levels are high and sustained in cells overexpressing LPP1, suggesting a dynamic interconversion of phosphatidic acid into DAG by LPP1. This may account for the effects of LPP1 on cell migration, as sustained DAG is known to down-regulate PKC isoforms in cells. Therefore the physiological changes in the expression levels of LPP1 might represent a heterologous desensitization mechanism for attenuating PKC-mediated signalling and regulation of cell migration.
Keywords: cell migration, lipid phosphate phosphatase, lysophosphatidic acid, p42/p44 mitogen-activated protein kinase (MAPK), phosphatidic acid, platelet-derived growth factor
Abbreviations: CHO, Chinese hamster ovary; DAG, diacylglycerol; DAPI, 4,6-diamidino-2-phenylindole; DMS, N,N-dimethylsphingosine; EGF, epidermal growth factor; FCS, foetal calf serum; GPCR, G-protein-coupled receptor; LPA, lysophosphatidic acid; LPP, lipid phosphate phosphatase; MAPK, mitogen-activated protein kinase; MEF, mouse embryonic fibroblast; PA, phosphatidic acid; PDGF, platelet-derived growth factor; PKC, protein kinase C; PLD, phospholipase D; RTK, receptor tyrosine kinase; S1P, sphingosine 1-phosphate; SK1, sphingosine kinase 1
INTRODUCTION
LPPs (lipid phosphate phosphatases) are integral membrane proteins that display broad substrate specificity in vitro, catalysing the dephosphorylation of lipid phosphates [e.g. S1P (sphingosine 1-phosphate), LPA (lysophosphatidic acid), PA (phosphatidic acid) and C1P (ceramide 1-phosphate)] in a Mg2+-independent and N-ethylmaleimide-insensitive manner [1]. Three mammalian LPP isoforms have been cloned, termed LPP1, LPP2 and LPP3 [2–6]. LPP3 corresponds to the endoplasmic reticulum protein, Dri42, which is up-regulated during differentiation of intestinal epithelial cells [7]. Recently, LPP has been suggested to belong to a family of lipid phosphatases/phosphotransferases that also includes plasticity-related genes 1–4 and sphingomyelin synthases [8]. LPP is predicted to have six transmembrane domains, with the active site facing the extracellular side of the plasma membrane or the luminal side of intracellular membranes [9]. The presence of LPP1, LPP2 and LPP3 at the plasma membrane has been detected with isoform-selective antibodies [2,10–13] and epitope-tag antibodies [13–16]. LPP1 and LPP3 have also been identified in caveolae [11,12], whereas LPP2 and LPP3 are present in cytoplasmic vesicles in CHO (Chinese hamster ovary) cells [13]. LPP2 and LPP3 are constitutively co-localized with SK1 (sphingosine kinase 1), and LPP3/SK1 is recruited to a perinuclear compartment upon induction of PLD1 (phospholipase D1) in CHO cells [13].
LPPs have the potential to influence physiological processes that are regulated by the GPCR (G-protein-coupled receptor) agonists S1P and LPA. This may involve dephosphorylation of extracellular S1P and LPA via an ecto-LPP activity, which may limit the bioavailability of these agonists at their respective GPCRs, S1P1–S1P5 and LPA1–LPA3 [4,14–16]. Recent studies have shown that the overexpression of LPP1 reduces both acute and chronic LPA-stimulated responses, including activation of p42/p44 MAPK (mitogen-activated protein kinase), PLD, DNA synthesis and cell division, while having no effect on LPA receptor function or expression [10,13,17]. Similarly, overexpression of LPP3 in ovarian cancer cells decreases colony formation and reduces tumour growth in vitro and in vivo [18]. In addition, overexpression of LPP2 or LPP3 reduces the S1P- and LPA-stimulated activation of p42/p44 MAPK in serum-deprived HEK-293 cells [10,13]. This effect is blocked by pre-treating HEK-293 cells with the caspase-3/7 inhibitor, Ac-DEVD-CHO (N-acetyl-Asp-Glu-Val-Asp-aldehyde) [13]. Therefore LPP2 and LPP3 appear to regulate the apoptotic status of serum-deprived HEK-293 cells. In this regard, LPP2 reduced basal intracellular PA levels, whereas LPP3 reduced intracellular S1P in serum-deprived HEK-293 cells [13]. These data are consistent with an important role for LPP2 and LPP3 in regulating an intracellular pool of PA and S1P respectively, which may govern apoptosis in response to cellular stress.
To date, there is no evidence that LPP isoforms are acutely regulated (e.g. phosphorylation) by extracellular stimuli. However, growth factors [e.g. EGF (epidermal growth factor)] and androgens have been shown to increase de novo expression of endogenous LPP3 protein and endogenous LPP1 mRNA transcript respectively [3,19]. In the present study, we have evaluated the functional consequences of increased LPP1 expression on signal transduction by both GPCRs and RTKs (receptor tyrosine kinases) in mammalian cells. This was achieved by comparing the ability of PDGF (platelet-derived growth factor), a growth factor that binds to a PDGFβ RTK, and a GPCR agonist, LPA, to stimulate migration of embryonic fibroblasts isolated from wild-type and LPP1 transgenic mice.
MATERIALS AND METHODS
Materials
All biochemicals including PDGF, LPA and FITC-conjugated secondary antibody were from Sigma Chemical Co. Cell culture supplies were from Invitrogen (Paisley, U.K.). Anti-phospho-p42/p44 MAPK (polyclonal) and anti-p42 MAPK antibodies were from New England Biolabs. Anti-PKC (protein kinase C) (recognizes α, β and γ) antibody was from Upstate Ltd. S1P was from Avanti. PDGFβ receptor plasmid construct was kindly given by Professor C.-H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden).
Cell culture
MEFs (mouse embryonic fibroblasts) derived from either wild-type or LPP1 transgenic mice [20] were maintained in Dulbecco's modified Eagle's medium containing 10% (v/v) FCS (foetal calf serum) and penicillin/streptomycin. HEK-293 cells that stably overexpress LPP1 [10] were maintained in minimum essential medium supplemented with FCS (10%, v/v) and penicillin/streptomycin. Cells were deprived of serum for 24 h before stimulation with agonist.
Transfection
Cells were transiently transfected with plasmid constructs as required. Cells at 75–95% confluence were placed in medium containing 1% FCS and transfected with 1 μg of plasmid construct following complex formation with LipofectAMINE™ 2000, according to the manufacturer's instructions. The cDNA-containing medium was removed after incubation for 24 h at 37 °C, and the cells were incubated for a further 18 h before agonist additions.
SDS/PAGE and Western blotting
Cell lysates were prepared using sample buffer containing 62 mM Tris/HCl, pH 6.7, 1.25% (w/v) SDS, 10% (v/v) glycerol, 3.75% (v/v) mercaptoethanol and 0.05% (w/v) Bromophenol Blue, and proteins were resolved by SDS/PAGE. Western blotting with specific antibodies was used to identify proteins of interest [10]. Immunoreactive proteins were visualized using enhanced chemiluminescence detection.
Immunofluorescence
Cells were grown on 12 mm glass coverslips to 60–90% confluence, and transfected as described above. Cells were fixed in 3.7% formaldehyde in PBS for 10 min, and then permeabilized in 0.1% (v/v) Triton X-100 in PBS for 1 min. Non-specific binding was reduced by pre-incubating cells in blocking solution containing 5% FCS/1% BSA in PBS for 1 h. Cells were incubated with primary antibodies (1:100 dilution of antibody in blocking solution) for 1 h at room temperature (or overnight at 4 °C), and then incubated with FITC-conjugated secondary antibody (1:100) for 1 h. Cells were mounted on glass slides using Vectashield mounting medium, and visualized using a Nikon E600 epifluorescence microscope.
LPP activity
A 100000 g membrane pellet was combined with a reaction mixture consisting of 50 μM [32P]PA (0.01 μCi/reaction), 8 mM Triton X-100, 100 mM Tris/maleate, pH 6.5, and 1 mM rac-glycerophosphate in 100 μl. After a 10 min incubation at 30 °C, 4 ml of 0.1 M phosphoric acid, 296 μl of water and 400 μl of 1 M HClO4 were added, and the unreacted substrate was extracted twice with water-saturated butan-1-ol. To a 500 μl aliquot of the reaction mixture, 50 μl of 125 mM ammonium molybdate was added, and the phosphomolybdate complex was extracted with 600 μl of 2-methyl-1-propanol/benzene (1:1, v/v). The radioactivity of the upper phase was measured by scintillation counting.
Cell migration
MEFs were grown to 90–95% confluence and were quiesced by serum starvation. The cell monolayer was wounded by a scratch using a 1 ml pipette tip. The wounded monolayer was then treated without or with agonist for 8 h, after which time the cells were stained with DAPI (4,6-diamidino-2-phenylindole) and the area of cell-free wound was digitally photographed. The wound healing effect is presented as the number of cells migrating into the wounded area within a standard field of view (×40 magnification).
RESULTS AND DISCUSSION
LPP1 functions to regulate p42/p44 MAPK activation in HEK-293 cells
We have used two cell model systems to evaluate the functional consequences of the overexpression of LPP1. First, we have used HEK-293 cells that have been stably transfected with a plasmid construct encoding LPP1. This results in an approx. 60–70-fold increase in LPP1 activity using dioleoyl-PA as a substrate [10]. We have previously shown that S1P and LPA stimulate activation of p42/p44 MAPK in HEK-293 cells [10]. This is mediated by lipid-phosphate GPCR, evident from data showing that the cellular responses to S1P or LPA are blocked by pertussis toxin, which functions to uncouple Gi from LPA and S1P receptors [10]. Overexpression of LPP1 in HEK-293 cells reduced the activation of p42/p44 MAPK by S1P, LPA and thrombin (Figure 1). However, a major focus here was to investigate whether LPP1 has an extended repertoire of functions in terms of being able to attenuate signalling by agents that use RTK. We therefore focused on PDGF, which binds to PDGF receptors to induce a cell response. In order to assess effects on PDGF signalling, HEK-293 cells were transfected with plasmid construct encoding the PDGFβ receptor. PDGF-induced responses in HEK-293 cells require ectopic expression of the PDGFβ receptor [21]. Our findings demonstrate that LPP1 overexpression markedly reduced PDGF-induced activation of p42/p44 MAPK (Figure 1). The effects of LPP1 are therefore heterologous in nature and are not restricted to one receptor type, and LPP1 can significantly modulate both GPCR- and RTK-mediated signalling. The activation of p42/p44 MAPK by PDGF in HEK-293 cells does not involve the synthesis of intracellular S1P that is subsequently released to act as an autocrine signal on S1P receptors [21]. This is based on data showing that the activation of this kinase pathway was not reduced by pre-treating cells with D,L-threo-dihydrosphingosine, an inhibitor of SK responsible for the synthesis of S1P [21]. Therefore an intracellular function for LPP1 is suggested by its ability to disrupt PDGFβ receptor signalling.
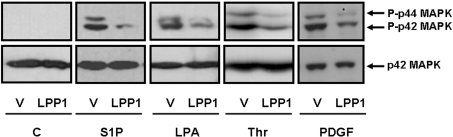
Figure 1. Functional role of LPP1 in regulating p42/p44 MAPK signalling in HEK-293 cells.
Stable vector- and LPP1-transfected HEK-293 cells were transiently transfected with or without a plasmid construct encoding the PDGFβ receptor. The cells were treated with or without 5 μM S1P, 5 μM LPA, 30 units/ml thrombin (Thr) or 10 ng/ml PDGF for 10 min. Lysates were Western-blotted using anti-phospho-p42/p44 MAPK antibody. The Western blot shows that the ligand-induced activation of p42/p44 MAPK is reduced in HEK-293 cells that stably overexpress LPP1. Blots were stripped and re-probed with anti-p42 MAPK antibody (to confirm equal protein loading). ‘V’ represents cells that are stably transfected with vector.
LPP1 functions to regulate p42/p44 MAPK activation in MEFs
The second approach to evaluate the functional consequence of increasing the expression of LPP1 on GPCR- and RTK-mediated signal transduction utilized MEFs from LPP1 transgenic mice. In this case, we have specifically addressed the question of the role of LPP1 in regulating endogenous PDGF receptor and LPA/S1P receptor signal transmission. MEFs derived from colonies of mice with different numbers of copies of the LPP1 gene {2, 8 and 20 times greater (2×, 8× and 20×) than that of wild-type mice; [20]} were used. The LPP activity increase was dependent on gene dosage, with an approx. 3.32-fold increase in LPP activity in the 20× compared with wild-type mice [LPP activity: control, 12.5±1.54 nmol/min per mg of protein; 8×, 30.48±3.9 nmol/min per mg of protein; 20×, 41.47±6.9 nmol/min per mg of protein; n=3–5 (means±S.E.M.) using 50 μM PA as substrate]. The increase in LPP activity is similar to the 3-fold stimulation of LPP activity as a consequence of de-novo synthesis of the enzyme in response to EGF [3], and is therefore comparable with physiological changes in LPP regulation in vivo.
The stimulation of p42/p44 MAPK by PDGF, S1P or LPA was reduced in the LPP1-overexpressing MEFs (Figures 2A–2C). Others have reported that PDGF stimulates the release of S1P from the MEFs [22], which acts in an autocrine manner to stimulate cell responses. We therefore considered the possibility that LPP1 might also exert its effects on PDGF receptor signalling by dephosphorylation of released S1P. However, we excluded the possible role for autocrine S1P in the PDGF-induced activation of p42/p44 MAPK and migration. This was evident from results showing that overexpression of Myc-tagged wild-type or dominant-negative SK1 (G81D) or SK2 (G213D) had no effect on the PDGF- or lipid phosphate-induced activation of p42/p44 MAPK in these cells (Figures 2D and 2E). In addition, the pre-treatment of cells with the SK inhibitor DMS (N,N-dimethylsphingosine) had no effect on the PDGF-, S1P- or LPA-induced activation of p42/p44 MAPK (Figure 2F). We have also used scratch injury of MEF monolayers to assess the effect of DMS on cell migration. Migration of wild-type MEFs in response to PDGF was evidenced by the appearance of cells in the scratch injury area. However, DMS was without effect on PDGF-induced cell migration (Figure 2F) at concentrations that inhibit SK [23].
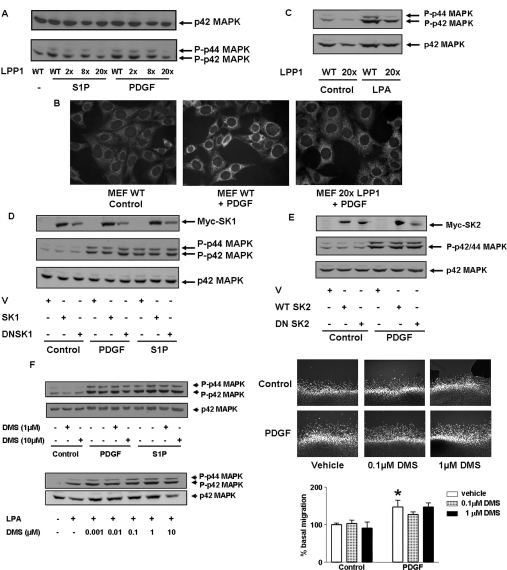
Figure 2. LPP1 reduces PDGF-induced activation of p42/p44 MAPK in LPP1 transgenic MEFs.
MEFs from wild-type (WT) and LPP1 transgenic mice were stimulated with or without PDGF (10 ng/ml) or S1P (5 μM) or LPA (5 μM) for 10 min. (A) The Western blot shows that the PDGF- and S1P-induced activation of p42/p44 MAPK is reduced in MEFs from LPP1 transgenic mice (with 2, 8 or 20 gene copies) compared with MEFs from wild-type mice; (B) immunofluorescent cell imaging with anti-phospho-p42/p44 MAPK antibody/FITC-conjugated secondary antibody and DAPI. (C) The Western blot shows that the LPA-induced activation of p42/p44 MAPK is reduced in MEFs from LPP1 transgenic mice compared with MEFs from wild-type mice. (D and E) Wild-type MEFs were transfected with plasmid constructs encoding (D) Myc-tagged wild-type SK1 or dominant-negative SK1 (DNSK1) or (E) Myc-tagged wild-type SK2 or dominant-negative SK2 (DNSK2). The Western blot shows that neither wild-type nor dominant-negative sphingosine kinase isoforms has an effect on p42/p44 MAPK activation. Also included in (D) and (E) (top panels) are Western blots probed with anti-Myc-tag antibody, showing overexpression of SK1/DNSK1 or SK2/DNSK2. (F) MEFs from wild-type mice were pre-treated with or without DMS (0.001–10 μM) for 10 min prior to stimulation with agonist. The Western blots (left) show the lack of effect of DMS on PDGF- or S1P- or LPA-induced p42/p44 MAPK activation. Also shown is the lack of effect of DMS on PDGF-induced cell migration in the scratch wound healing assay (upper right). The histogram (below) shows quantitative data for the cell migration assay performed in three or four determinations from a representative experiment (*P<0.01 compared with vehicle control).
LPP1 functions to regulate PDGF-stimulated cell migration
The ability of LPA or PDGF to induce migration was significantly reduced in MEFs from LPP1 transgenic mice (Figures 3A and 3B). S1P did not significantly stimulate cell migration of wild-type MEFs (Figure 3A), despite activating the p42/p44 MAPK pathway. A possible explanation for the ineffectiveness of S1P in wild-type MEFs is that this lysolipid can bind to both stimulatory (S1P1) and inhibitory (S1P2) receptors that mediate migration in these cells [22,24], leading to no net effect on the migration of MEFs. The migratory response to PDGF is dependent on the activation of p42/p44 MAPK, as evidenced by results showing that pre-treatment of cells with the inhibitor of MEK-1 (MAPK/extracellular-signal-regulated kinase kinase 1) activation, PD098059, markedly reduced PDGF-stimulated migration (Figure 3B). Therefore the action of LPP1 on PDGF responses can be explained by its ability to reduce the activation of p42/p44 MAPK in response to PDGF, which is required for PDGF-induced cell migration.
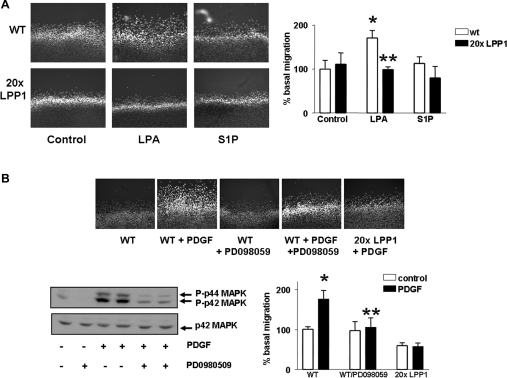
Figure 3. LPP1 reduces LPA- and PDGF-induced migration of MEFs.
In the scratch wound healing assay, MEFs from wild-type and LPP1 transgenic mice were stimulated with PDGF (10 ng/ml) or S1P (5 μM) or LPA (5 μM) for 8 h. The photographs show: (A) the inhibitory effect of LPP1 (MEFs from LPP1 transgenic mice) on LPA-induced cell migration [*P<0.01 compared with control; **P<0.01 compared with LPA response in wild-type (wt) MEFs]; and (B) the inhibitory effect of PD098059 (10 μM for 30 min) and the LPP1 transgene on PDGF-induced cell migration (*P<0.01 compared with the control; **P<0.01 compared with the PDGF response in the absence of PD098059). The Western blot confirms the inhibitory effect of PD098059 on the PDGF-induced activation of p42/p44 MAPK (10 min) in MEFs from wild-type mice. The histograms show quantitative data for the cell migration assay performed in three or four determinations from a representative experiment.
Role of PKC signalling
DAG (diacylglycerol) levels are high and sustained in HEK-293 cells stably expressing LPP1, and in MEFs from the LPP1 transgenic mice [10,20]. These findings support the notion that there is dynamic interconversion of PA into DAG in cells overexpressing LPP1. PA levels are not significantly altered. Thus the steady-state concentration of PA may be robustly ‘buffered’, by, for example, PLD and DAG kinase routes.
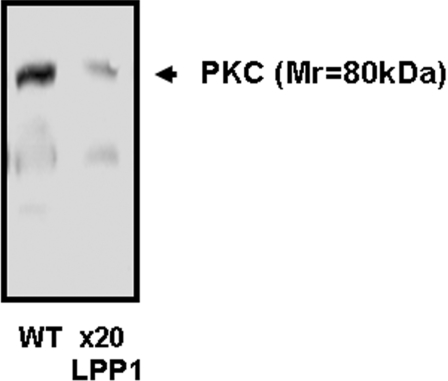
We hypothesized that the increase in DAG levels might affect signalling pathways that are specifically regulated by PKC, as sustained increases in DAG can induce down-regulation of PKC isoforms that use DAG as a co-activator with calcium. Therefore we investigated whether PKC might be involved in the PDGF-induced regulation of p42/p44 MAPK and cell migration, and whether the inhibitory effect of LPP1 on p42/p44 MAPK and migration is related to disruption of PKC signalling. This possibility is supported by recent studies by Campbell and Trimble [25]. These authors have shown that PKCβII is required for PDGF-induced p42/p44 MAPK activation and migration of vascular smooth muscle cells. Moreover, Yamamura et al. [26] have shown that down-regulation of PKCα inhibits the migration of endothelial cells. We first evaluated the effect of a PKC inhibitor, Ro318220, on p42/p44 MAPK activation and cell migration. The pre-treatment of wild-type MEFs with Ro318220 reduced PDGF- or S1P-induced activation of p42/p44 MAPK (Figure 4A) and inhibited PDGF-induced cell migration (Figure 4B). These findings suggest that PDGF-stimulated p42/p44 MAPK activation and cell migration are, indeed, PKC-dependent. Moreover, we observed that typical PKC (Mr=80000) isoform(s) expression levels were down-regulated in MEFs from LPP1 transgenic mice (Figure 5). Therefore the LPP1-dependent down-regulation of PKC may account for its ability to reduce both PDGF- and S1P-induced activation of p42/p44 MAPK, and PDGF-stimulated cell migration.
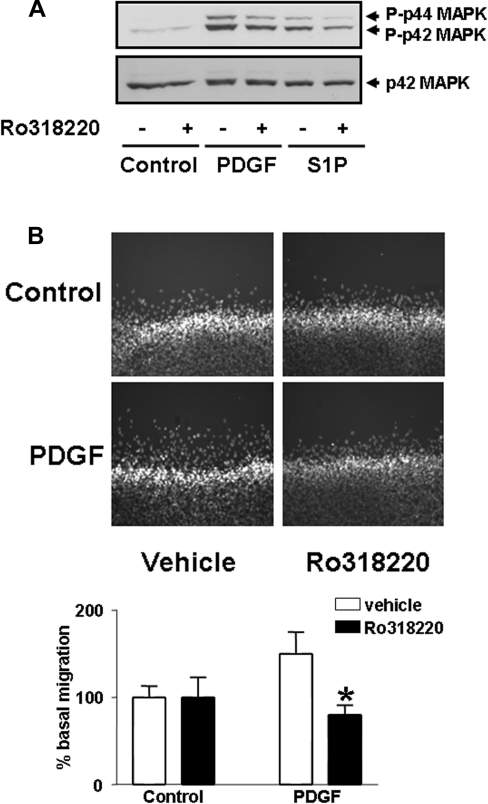
Figure 4. Role of PKC in PDGF-induced cell migration.
Wild-type MEFs were pre-treated with and without Ro318220 (10μM) for 10 min and then stimulated with (A) PDGF (10 ng/ml) or S1P (5 μM) for 10 min for p44/p42 MAPK assays, or (B) PDGF (10 ng/ml) for 8 h for the scratch wound healing assay. (A) The Western blot shows the inhibitory effect of Ro318220 on the PDGF- or S1P-induced activation of p42/p44 MAPK in MEFs from wild-type mice; (B) inhibitory effect of Ro318220 on PDGF-induced migration of wild-type MEFs in the scratch wound healing assay. The histogram shows quantitative data for the cell migration assay performed in three or four determinations from a representative experiment (*P<0.01 compared with the PDGF response).
Figure 5. Comparison of typical PKC isoform expression in wild-type compared with LPP1 transgenic MEFs.
Wild-type (WT) and LPP1 transgenic MEF lysates were subjected to Western blotting with anti-PKC antibody. The Western blot shows that typical PKC isoform(s) are markedly down-regulated in LPP1 transgenic MEFs.
Conclusion
We have shown here that LPP1 overexpression reduced the PDGF- and LPA-induced migration of MEFs derived from LPP1 transgenic mice. This is the first evidence to demonstrate that LPP1 overexpression reduces PDGFβ receptor signal transmission and response in mammalian cells. In addition, we suggest that typical PKC isoform(s) are markedly down-regulated in MEFs from LPP1 transgenic mice, and that this might account for the inhibitory effect of LPP1 on PDGF- or LPA-induced cell migration. The implication of these findings is that the reported increase in LPP expression evoked by growth factors might represent a heterologous desensitization mechanism that attenuates cell migratory responses that require a functional PKC signalling pathway.
Acknowledgments
This work was supported by BBSRC (Biotechnology and Biological Sciences Research Council) grants to S. P. and N. J. P. and an NIH grant (RO1 CA-92160) to G. T.
References
- 1.Brindley D. N., Waggoner D. W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 1998;273:24281–25284. doi: 10.1074/jbc.273.38.24281. [DOI] [PubMed] [Google Scholar]
- 2.Kai M., Wada I., Imai S., Sakane F., Kanoh H. Identification and cDNA cloning of 35-kDa phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J. Biol. Chem. 1996;271:18931–18938. doi: 10.1074/jbc.271.31.18931. [DOI] [PubMed] [Google Scholar]
- 3.Kai M., Wada I., Imai S., Sakane F., Kanoh H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J. Biol. Chem. 1997;272:24572–24578. doi: 10.1074/jbc.272.39.24572. [DOI] [PubMed] [Google Scholar]
- 4.Roberts R., Sciorra V. A., Morris A. J. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998;273:22059–22067. doi: 10.1074/jbc.273.34.22059. [DOI] [PubMed] [Google Scholar]
- 5.Tate R., Tolan D., Pyne S. Molecular cloning of magnesium-independent type 2 phosphatidic acid phosphatases from airway smooth muscle. Cell. Signalling. 1998;11:515–522. doi: 10.1016/s0898-6568(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 6.Leung D. W., Tompkins C. K., White T. Molecular cloning of two alternatively spliced forms of human phosphatidic acid phosphatase cDNAs that are differentially expressed in normal and tumor cells. DNA Cell Biol. 1998;17:377–385. doi: 10.1089/dna.1998.17.377. [DOI] [PubMed] [Google Scholar]
- 7.Barilá D., Plateroti M., Nobili F., Onetti Muda A., Xie T., Morimoto G., Perozzi G. The Dri 42 gene, whose expression is up-regulated during epithelial differentiation, encodes a novel endoplasmic reticulum resident transmembrane protein. J. Biol. Chem. 1996;271:29928–29936. doi: 10.1074/jbc.271.47.29928. [DOI] [PubMed] [Google Scholar]
- 8.Sigal Y. J., McDermott M. I., Morris A. J. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse function. Biochem. J. 2005;387:281–293. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q. X., Pilquil C. S., Dewald J., Berthiaume L. G., Brindley D. N. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem. J. 2000;345:181–184. [PMC free article] [PubMed] [Google Scholar]
- 10.Alderton F. A., Darroch P., Sambi B., McKie A., Ahmed I. S., Pyne N. J., Pyne S. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 2001;276:13452–13460. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- 11.Sciorra V. A., Morris A. J. Sequential actions of phospholipase D and phosphatidic acid phosphohydrolase 2b generate diglyceride in mammalian cells. Mol. Cell. Biol. 1999;10:3863–3876. doi: 10.1091/mbc.10.11.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanjundan M., Possmayer F. Pulmonary lipid phosphate phosphohydrolase in plasma membrane signaling platforms. Biochem. J. 2001;358:637–646. doi: 10.1042/0264-6021:3580637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long J., Darroch P. I., Wan K. F., Kong K. C., Ktistakis N. T., Pyne N. J., Pyne S. The regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem. J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasinska R., Zhang Q. X., Pilquil C. S., Singh I., Xu J., Dewald J., Dillon D. A., Bertiaume L. G., Carman G. M., Waggoner D. W., Brindley D. N. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 1999;340:677–686. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa T., Kai M., Wada I., Kanoh H. Cell surface activities of the human type 2b phosphatidic acid phosphatase. J. Biochem. (Tokyo) 2000;127:645–651. doi: 10.1093/oxfordjournals.jbchem.a022652. [DOI] [PubMed] [Google Scholar]
- 16.Jia Y.-J., Kai M., Wada I., Sakane F., Kanoh H. Differential localization of lipid phosphate phosphatases 1 and 3 to cell surface subdomains in polarized MDCK cells. FEBS Lett. 2003;552:240–246. doi: 10.1016/s0014-5793(03)00931-1. [DOI] [PubMed] [Google Scholar]
- 17.Hooks S. B., Santos W. L., Im D.-S., Heise C. E., Macdonald T. L., Lynch K. R. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 2001;276:4611–4621. doi: 10.1074/jbc.M007782200. [DOI] [PubMed] [Google Scholar]
- 18.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003;63:1073–1082. [PubMed] [Google Scholar]
- 19.Ulrix W., Swinnen J. V., Heynes W., Verhoeven G. Identification of phosphatidic acid phosphatase type 2a isozyme as an androgen-regulated gene in human prostatic adenocarcinoma cell line LNCaP. J. Biol. Chem. 1997;273:4660–4665. doi: 10.1074/jbc.273.8.4660. [DOI] [PubMed] [Google Scholar]
- 20.Yue J., Yokoyama K., Balazs L., Baker D. L., Smalley D., Pilquil C., Brindley D. N., Tigyi G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signalling. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., Pyne N. J. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- 22.Hobson J. P., Rosenfeldt H. M., Barak L. S., Olivera A., Poulton S., Caron M. G., Milstien S., Spiegel S. Role of sphingosine 1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 23.Waters C., Sambi B., Kong K. C., Thompson D., Pitson S. M., Pyne S., Pyne N. J. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J. Biol. Chem. 2003;278:6282–6290. doi: 10.1074/jbc.M208560200. [DOI] [PubMed] [Google Scholar]
- 24.Goparaju S. K., Jolly P. S., Watterson K. R., Bektas M., Alvarez S., Sarkar S., Mel L., Ishii I., Chun J., Milstien S., Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol. Cell. Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell M., Trimble E. R. Modification of PI3K- and MAPK-dependent chemotaxis in aortic vascular smooth muscle cells by protein kinase C beta II. Circ. Res. 2005;96:197–206. doi: 10.1161/01.RES.0000152966.88353.9d. [DOI] [PubMed] [Google Scholar]
- 26.Yamamura S., Nelson P. R., Kent K. C. Role of protein kinase C in attachment, spreading and migration of human endothelial cells. J. Surg. Res. 1996;63:349–354. doi: 10.1006/jsre.1996.0274. [DOI] [PubMed] [Google Scholar]