Abstract
When induced to differentiate, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle and undergo mitotic clonal expansion (MCE) followed by expression of genes that produce the adipocyte phenotype. The preadipocytes traverse the G1/S checkpoint synchronously as evidenced by the expression/activation of cdk2-cyclin-E/A, turnover of p27/kip1, hyperphosphorylation of Rb, translocation of cyclin D1 from nuclei to cytoplasm and GSK-3β from cytoplasm to nuclei, and incorporation of [3H]thymidine into DNA. As the cells cross the G1/S checkpoint, C/EBPβ acquires DNA-binding activity, initiating a cascade of transcriptional activation that culminates in the expression of adipocyte proteins. The mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor PD98059 delays, but does not block, MCE and differentiation, the extent of the delay causing a comparable delay in the expression of cell-cycle markers, MCE, and adipogenesis. The more potent and specific MEK inhibitor UO126 and the cyclin-dependent kinase inhibitor roscovitine, which inhibit the cell cycle at different points, block MCE, expression of cell cycle and adipocyte markers, as well as adipogenesis. These results show that MCE is a prerequisite for differentiation of 3T3-L1 preadipocytes into adipocytes.
Keywords: cell cycle‖adipogenesis‖3T3-L1 preadipocyte‖C/EBPα‖PPARγ
Obesity leads to hyperplasia of adipocytes through the recruitment and proliferation of preadipose cells in adipose tissue (1, 2). This hyperplasia is mimicked by the adipogenic differentiation program of 3T3-L1 preadipocytes. Treatment of growth-arrested 3T3-L1 preadipocytes with differentiation inducers initiates mitotic clonal expansion (MCE) before expression of genes that give rise to the adipocyte phenotype (3–5). Whether MCE is a prerequisite for terminal differentiation remains controversial (6), because definitive proof is lacking. This issue is addressed in the present investigation.
Peroxisome proliferator-activated receptor γ (PPARγ) and several members of the C/EBP family of transcription factors participate in a signaling cascade (4, 5, 7–10) that culminates in the transcriptional activation of genes that produce the adipocyte phenotype. C/EBPβ is expressed immediately (within 2–4 h) after hormonal induction. At this point in the differentiation program, however, C/EBPβ is unable to bind DNA (11) and thus cannot function as a transcriptional activator. Acquisition of DNA-binding activity is delayed until later in the program (at ≈14 h) and reaches a maximum ≈24 h after induction (11). The time at which DNA-binding activity (measured in vitro) is acquired by C/EBPβ corresponds exactly to the time at which it associates with centromeres through binding sites on centromeric satellite DNA ex vivo (11). Having acquired DNA-binding function, C/EBPβ transcriptionally activates both the C/EBPα and PPARγ genes through C/EBP regulatory elements in their proximal promoters (12–15). The preadipocytes exit the cell cycle after they have undergone approximately two rounds of mitosis, i.e., MCE. Because C/EBPα (16–19) and PPARγ (20) are both antimitotic, they seem to function as terminators of MCE. Together, C/EBPα and PPARγ then coordinately activate transcription of many adipocyte genes whose expression produces the differentiated phenotype (12, 21–25).
The present studies were undertaken to understand the kinetic relationship between the cell-cycle events of MCE during adipogenesis and to determine whether MCE is required for adipogenesis.
Materials and Methods
Two-day postconfluent (designated day 0) growth-arrested 3T3-L1 preadipocytes were induced to differentiate by using our standard protocol (26). At the times indicated, cells were stained with Oil Red O to detect cytoplasmic triglyceride, extracted and immunoblotted, or subjected to immunofluorescence microscopy, as described (11). For immunoblotting, cells were lysed and extracted, and equal amounts of protein were separated by SDS/PAGE. Antibodies to the cyclins, cyclin-dependent kinases (cdks), and Rb were obtained from Santa Cruz Biotechnology; mitogen-activated protein kinase (MAPK) and phospho-MAPK (Thr-202/Tyr-204) were obtained from Upstate Biotechnology (Lake Placid, NY); C/EBPα or 422/aP2 were from our laboratory (11, 27); and PPARγ was provided by Mitchell Lazar (University of Pennsylvania, Philadelphia).
Immunoprecipitation and in Vitro Kinase Reaction.
Preadipocytes were induced to differentiate, after which nuclear extracts were prepared every 4 h for 28 h (11). Nuclear extract (50 μg of protein) was prepared, cdk2 was immunoprecipitated with mouse monoclonal anti-cdk2 IgG, and the immune complex was subjected to in vitro kinase assay with Histone H1 as substrate as described (28).
FACS Analysis and [3H]Thymidine Incorporation into DNA.
Postconfluent 3T3-L1 preadipocytes were subjected to the differentiation protocol as above. At the times indicated, cells were trypsinized, washed with PBS, fixed with 2% (wt/vol) paraformaldehyde in PBS, and treated with 0.5 mg/ml RNase A for 1 h at room temperature. After staining with 0.1 mg/ml propidium iodide, DNA content was determined by FACS analysis. [3H]Thymidine incorporation into DNA was performed as described (11).
Results
Synchronous Reentry of the Cell Cycle upon Induction of Differentiation.
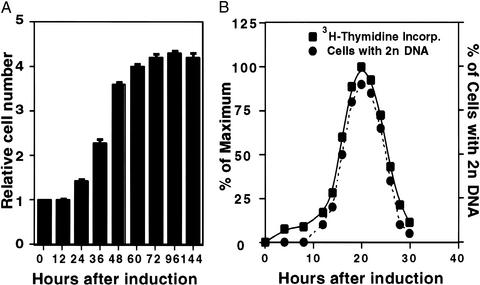
When postconfluent, growth-arrested 3T3-L1 preadipocytes are induced to differentiate with methylisobutylxanthine, dexamethasone, and insulin, the cells undergo two sequential rounds of mitosis over the next 2 days. These mitoses, referred to as MCE, precede expression of the adipocyte genes that produce the terminally differentiated phenotype. As illustrated in Fig. 1A, the first round of mitosis is completed 24–36 h after induction, and the second round is completed 48–60 h after induction. Together, these mitoses result in an ≈4-fold increase in cell number. After 60 h, the cell number remains constant, and by day 8, virtually every cell has undergone adipogenesis (see Fig. 3F, captioned MDI; refs. 5 and 26). Exposure to the differentiation inducers (M, D, or I) individually provokes only a small increase in cell number and a correspondingly low extent of differentiation (not shown).
Figure 1.
Synchronous reentry of the cell cycle by growth-arrested 3T3-L1 preadipocytes after induction of differentiation. (A) Day 0 postconfluent 3T3-L1 preadipocytes were induced to differentiate into adipocytes by using the standard differentiation protocol. Cell number was determined by using a Coulter counter, and the relative cell number was plotted. At the time of induction, there were 1.4 × 106 cells per 6-cm culture dish. (B) Day 0 postconfluent 3T3-L1 preadipocytes were induced to differentiate. At the times indicated, cells were trypsinized, fixed, and labeled with propidium iodide, and the relative DNA content per cell was determined by FACS analysis. For comparison, FACS analysis data are shown (as % of cells with 2n DNA content) with [3H]thymidine incorporation data from an earlier paper (11).
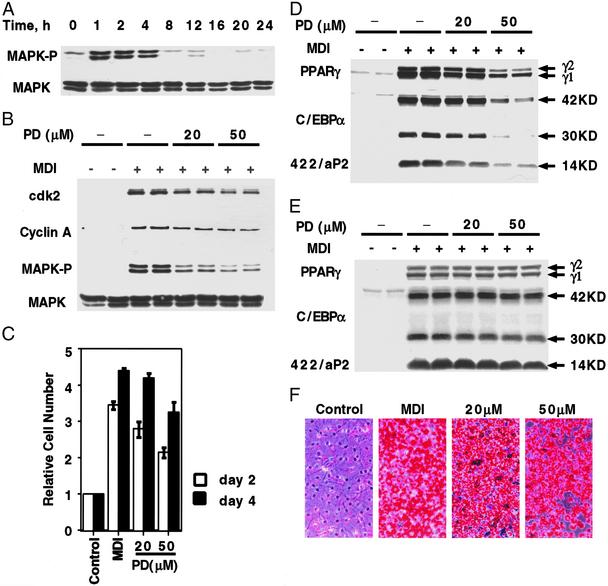
Figure 3.
Effect of PD98059, a MEK inhibitor, on MAPK kinase activity, cell cycle markers, MCE, and adipogenesis. (A) Two-day postconfluent 3T3-L1 preadipocytes were induced to differentiate by using the standard differentiation protocol. At various time points, cell lysates were prepared and subjected to SDS/PAGE and immunoblotting with antibodies to MAPK and phospho-MAPK. (B) Two-day postconfluent 3T3-L1 preadipocytes were exposed to 20 or 50 μM PD98059 for 30 min before induction and again at the time of induction. Cell lysates were prepared 1 h later for MAPK and phospho-MAPK and 20 h later for cyclin A and cdk2 immunoblot analyses. (C) Two-day postconfluent 3T3-L1 preadipocytes were exposed to 20 or 50 μM PD98059 for 30 min before induction and again at the time of induction. On days 2 and 4, cell number was determined. MDI refers to the differentiation inducers. (D) Postconfluent 3T3-L1 preadipocytes were treated as in B. Cell lysates were prepared on day 3, and 50 μg of lysate protein were subjected to SDS/PAGE and immunoblotted with antibodies against C/EBPα, PPARγ, and 422/aP2. (E) Cells were treated as in B, above, except that cell lysates were prepared on day 6. (F) Cells were treated as B, except that on day 8, cells were fixed and cytoplasmic triacylglycerol was stained with Oil Red-O. PD98059 concentrations are given (20 and 50 μM).
After induction of differentiation, progression through the cell cycle, monitored by changes in cellular DNA content, coincides precisely with changes in the rate of [3H]thymidine incorporation into DNA (Fig. 1B). During the first 12 h after induction, no change in DNA content occurred, as judged by the fraction of cells exhibiting a 2n level of DNA. However, between 12 and 16 h, a large fraction of the cell population acquired 2n status, peaking at ≈20 h, and then reverting to 1n status by 28–30 h, indicating completion of one round of DNA replication. These results show that upon induction, growth-arrested 3T3-L1 preadipocytes reenter and progress synchronously through the cell cycle. Further evidence shown below verifies that the preadipocytes cross the G1-S checkpoint phase between 12 and 16 h.
Previous studies (7, 11) revealed that C/EBPβ, a key transcriptional activator of the C/EBPα and PPARγ genes (7, 12–15), is expressed immediately upon treatment of preadipocytes with differentiation inducers (11). However, acquisition of DNA-binding function by C/EBPβ is delayed until 12–16 h after induction (11). This timing corresponds to the entry of S phase. Progression from G1 to S requires down-regulation of p27/kip1 in 3T3-L1 preadipocytes (29) and the activation of cdk2 by cyclins E and A in most cell types (30). The kinetics of these events (Fig. 2 A–D) correlate closely with the acquisition of DNA-binding activity by C/EBPβ (11).
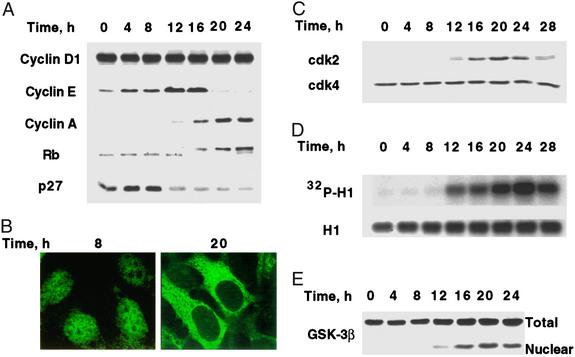
Figure 2.
Changes in the expression and translocation of cell-cycle markers and GSK-3β during MCE. (A) Day 0 postconfluent 3T3-L1 preadipocytes were induced to differentiate into adipocytes. At the times indicated, whole-cell lysates (50 μg of protein) were subjected to SDS/PAGE and immunoblotted. The decreased mobility of Rb after 16 h was shown to be caused by hyperphosphorylation by incubating nuclear extracts with alkaline phosphatase, which increased the mobility of Rb (not shown). (B) 3T3-L1 preadipocytes on glass coverslips were cultured to postconfluence in DMEM containing 10% calf serum and induced to differentiate. At 8 and 20 h, cells were fixed and exposed to cyclin D1 antibody followed by FITC-labeled secondary antibody. (C) At the indicated times after induction of differentiation, cell lysates (50 μg of protein) were subjected to SDS/PAGE immunoblotted with antibodies to cdk2 and cdk4. (D) Nuclear extracts were prepared at the indicated times after induction. Cdk2 was immunoprecipitated and incubated with purified histone H1 and [γ-32P]ATP. After SDS/PAGE, [32P]histone H1 was detected by autoradiography. Total histone H1 was detected by Coomassie blue staining. (E) At the indicated times after induction of differentiation, preadipocytes were lysed and resolved into nuclear and cytoplasmic fractions. The whole-cell lysate and nuclear fractions (identical cell equivalents) were subjected to SDS/PAGE and then immunoblotted with antibody to GSK-3β.
As evidenced by expression of cyclin D1 (Fig. 2A, time 0 h), growth-arrested 3T3-L1 preadipocytes are in G1 phase at the time of induction of differentiation. Although both cyclin D1 and cdk4 are expressed throughout the cell cycle (Fig. 2 A and C, respectively), cyclin D1 translocates from nucleus to cytosol at the G1-S checkpoint (12–16 h after induction; Fig. 2B) as occurs in other cell types (31). Another process known to take place at the G1-S checkpoint, i.e., the translocation of GSK-3β from cytoplasm to the nucleus, also occurs during this time window (Fig. 2E). Cyclin E is up-regulated late in G1 (at 12–16 h) and then is dramatically down-regulated at 16–20 h upon the entry of S phase (Fig. 2A).
Consistent with entry into S phase, expression of cyclin A is activated between 12 and 16 h and is maintained at this level through 24 h (Fig. 2A). Expression of cdk2 is activated with similar kinetics to cyclin A, reaching a maximum at 20–24 h and then undergoing down-regulation at the end of the first cell cycle (Fig. 2C). It should be noted that cdk2 has a unique expression pattern in the cell cycle of 3T3-L1 preadipocytes during the MCE of adipogenesis. In contrast, most cell types express cdk2 throughout the cell cycle (see introduction of ref. 32). During MCE of the adipocyte differentiation program cdk2 is not expressed during G1. However, as preadipocytes enter S phase, expression of cdk2 is activated, like that of cyclin A. This change in expression of cdk2 is paralleled by its catalytic activity as measured in vitro by phosphorylation of Histone H1 (Fig. 2D). Conversely, p27/kip1, an inhibitor of the cyclin E/A–cdk2 complexes, is down-regulated in the same time frame during which expression of cyclin A (Fig. 2A) and cdk2 (Fig. 2C) are up-regulated. Consistent with the synchronous events that lead to activation of cdk2, Rb, a pivotal cell-cycle substrate of cdk2, becomes hyperphosphorylated, facilitating S-phase progression (30).
Effect of MAPK/Extracellular Signal-Regulated Kinase Kinase (MEK) Inhibitors on MCE and Adipogenesis.
In a recent report, Qiu et al. (6) concluded that DNA synthesis and MCE are not required for differentiation of 3T3-L1 preadipocytes into adipocytes. In view of considerable circumstantial evidence to the contrary (29, 33), we reinvestigated this issue. Qiu et al. based their conclusions primarily on the effects of 20 μM PD98059, an inhibitor of MEK, which catalyzes the phosphorylation of erk-1 and erk-2 (MAPK). MAPK is expressed constitutively by growth-arrested 3T3-L1 preadipocytes and is rapidly (within 1 h after induction) and transiently phosphorylated (Fig. 3A). Treatment of preadipocytes with 20 or 50 μM PD98059 (added 30 min before the differentiation inducers) markedly, but not completely, inhibited phosphorylation of MAPK (Fig. 3B). A higher level, i.e., 100 μM, caused substantial cell death (not shown). Although expression of both cyclin A and cdk2 (measured at 20 h after induction) was reduced, 40% remained at the highest level of the inhibitor (Fig. 3B). Likewise, cell number, an indicator of the extent of MCE, was only partially reduced 2 days after induction of differentiation, but then recovered to nearly the control level by day 4 (Fig. 3C).
By day 3, cells treated with PD98059, particularly at the 50 μM level, expressed lower levels of the adipocyte differentiation marker proteins, i.e., PPARγ, C/EBPα, and 422/aP2 (Fig. 3D). By day 6, however, the levels of these markers recovered to levels comparable to those of untreated control cells (Fig. 3E). The extent of differentiation, indicated by the accumulation of cytoplasmic triacylglycerol, was only partially decreased, reflecting the partial decrease in the levels of the adipocyte markers (Fig. 3F). These findings show that although PD98059 partially reduces MCE, the extent to which mitotic clonal expansion is reduced corresponds closely to the reduced expression of adipocyte markers and accumulation of cytoplasmic triacylglycerol. Importantly, substantial recovery of MCE, as indicated by cell number, occurred between days 2 and 4 (Fig. 3 C–F). These findings support the view, but do not prove, that differentiation depends on MCE.
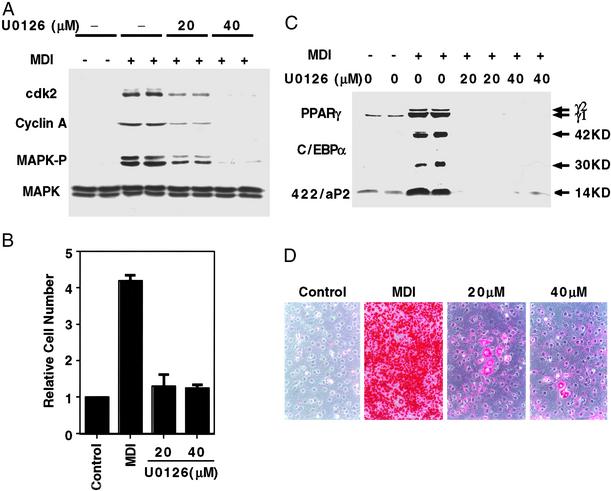
Use of the more specific and potent MEK inhibitor U0126 (34) at 40 μM prevented phosphorylation of MAPK and expression of cyclin A and cdk2 (Fig. 4A) and completely blocked MCE (Fig. 4B). Likewise, U0126 blocked expression of adipocyte marker proteins (Fig. 4C) and acquisition of the adipocyte phenotype as indicated by failure to accumulate cytoplasmic triacylglycerol (Fig. 4D). Moreover, addition of U0126 after MCE had been completed had no effect on the expression of adipocyte markers (i.e., C/EBPα, PPARγ, and 422/aP2) or the accumulation of cytoplasmic triacylglycerol (not shown). Taken together, these results indicate that terminal differentiation depends upon MCE.
Figure 4.
Effect of U0126 (a MEK inhibitor) on MCE, expression of cell cycle and adipocyte markers, and adipogenesis. (A) Two-day postconfluent 3T3-L1 preadipocytes were incubated with 20 or 40 μM U0126 for 30 min and then induced to differentiate with the same amount of U0126; U0126 was added again 24 h later. Cell lysates were prepared 1 h later for MAPK and phospho-MAPK analysis and at 20 h for cyclin A and cdk2 analysis. Cell lysates (50 μg of protein) were subjected to SDS/PAGE and immunoblotting as described in Fig. 3. (B) Two-day postconfluent 3T3-L1 preadipocytes were treated as A, and cell number was determined on day 4. (C) Two-day postconfluent 3T3-L1 preadipocytes were treated as in A; cell lysates were prepared on day 6 and subjected to analysis as in A with antibodies to C/EBPα, PPARγ, and 422/aP2. (D) Two-day postconfluent 3T3-L1 preadipocytes were treated as in A; on day 8, cells were fixed, and cytoplasmic triacylglycerol was stained with Oil Red-O. U0126 concentrations are given (20 and 40 μM).
Effect of a cdk2 Inhibitor on MCE and Adipogenesis.
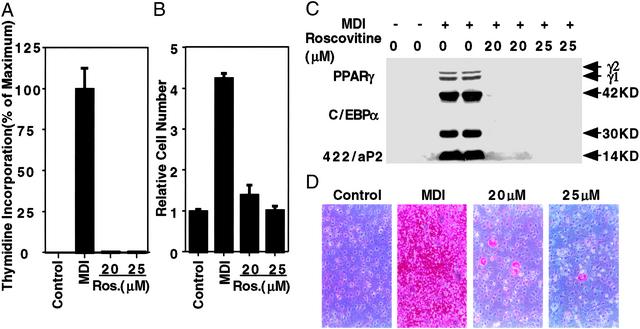
To determine the effect of blocking S-phase entry on MCE and adipogenesis, 3T3-L1 preadipocytes were treated with roscovitine, a potent and specific cdk inhibitor (35), at the time of induction of differentiation. Roscovitine-treated preadipocytes failed to enter S phase, as indicated by the complete inhibition of [3H]thymidine incorporation into DNA (Fig. 5A) and failure to proliferate (Fig. 5B). Consequently, expression of adipocyte marker proteins and the accumulation of cytoplasmic triacylglycerol were also blocked (Fig. 5 C and D). Addition of roscovitine after MCE had no effect on terminal differentiation (not shown). These findings also indicate that blocking the cell cycle at the G1-S checkpoint, thereby preventing MCE, derails subsequent events in the adipocyte differentiation program.
Figure 5.
Effect of roscovitine (a cyclin-dependent kinase inhibitor) on DNA synthesis, MCE, and adipogenesis. (A) Two-day postconfluent 3T3-L1 preadipocytes were treated with 20 or 25 μM roscovitine at the time of induction of differentiation. After 20 h, cells were pulse-labeled with [3H]thymidine for 30 min and lysed; DNA was precipitated, and 3H incorporation into DNA was determined. (B) Two-day postconfluent 3T3-L1 preadipocytes were treated as in A. Roscovotine was added with medium changes (every other day) until day 4, at which time cell number was determined. (C) Two-day postconfluent 3T3-L1 preadipocytes were treated as in B, and cell lysates were prepared on day 6 and subjected to immunoblotting with antibodies to C/EBPα, PPARγ, and 422/aP2. (D) Two-day postconfluent 3T3-L1 preadipocytes were treated as in C; on day 8, they were fixed, and cytoplasmic triacylglycerol was stained with Oil Red-O. Roscovitine concentrations are given (20 and 25 μM).
Discussion
MCE is an event associated with adipocyte differentiation (3–5, 11, 29, 33, 36). Whether MCE is required for adipocyte differentiation, however, has remained controversial. The recent reported finding (6) that treatment of 3T3-L1 preadipocytes with 20 μΜ PD98059, a MEK inhibitor, prevented both DNA replication and MCE without affecting differentiation could not be reproduced in our laboratory. This finding may be accounted for by our observation that 20 μΜ PD98059 (or even a level of 50 μΜ) delays, but does not block, MCE (Fig. 3C), expression of cell cycle (cyclin A and cdk2; Fig. 3B) and adipocyte (C/EBPα, PPARγ, and 422/aP2; Fig. 3E) markers, or cytoplasmic triacylglycerol accumulation (Fig. 3F). Importantly, the magnitude of the decreases in differentiation markers and cytoplasmic triacylglycerol accumulation with PD98059 were proportional to the decreases in MCE and of cell-cycle markers. Treatment of 3T3-L1 preadipocytes with the more specific and potent MEK inhibitor UO126 almost totally blocked the expression of cell-cycle markers (cyclin A and cdk2) and as a consequence prevented MCE, the expression of adipocyte gene markers, and the accumulation of cytoplasmic triacylglycerol (Fig. 4). Furthermore, blocking entry of S phase with the potent cdk inhibitor, roscovitine, also prevented MCE, the expression of adipocyte markers, and the accumulation of cytoplasmic triacylglycerol (Fig. 5). Consistent with these results is our previous finding (29, 37) that by specifically blocking the down-regulation of p27/kip1 (a cyclin E/A-cdk2 inhibitor), both MCE and differentiation were prevented. We conclude that 3T3-L1 preadipocytes must undergo MCE for terminal adipocyte differentiation.
Evidence reported in this paper suggests that the cell cycle of 3T3-L1 preadipocytes during adipogenic MCE differs from that of other preconfluent proliferating cells. Whereas most cell types express cdk2 throughout the cell cycle including G1 (32), 3T3-L1 preadipocytes do not express cdk2 in the G1 phase of MCE (Fig. 2C). Rather, expression of cdk2 occurs as the cells synchronously enter S phase and reaches a maximum level of expression concomitant with the incorporation of [3H]thymidine into DNA (Fig. 1B). The kinetics of expression of cdk2 is also coincident with the expression of cyclin A (Fig. 2A) and the acquisition of DNA-binding activity by C/EBPβ (11). This difference in the expression pattern of cdk2 distinguishes preadipocytes undergoing MCE from other cell types during proliferation.
In other studies, we showed that C/EBPβ is required for MCE and adipogenesis (38). Thus, mouse embryo fibroblasts (MEFs) from C/EBPβ(−/−) mice neither undergo MCE or differentiation into adipocytes. This result is consistent with the findings described above showing that MCE is required for the differentiation of 3T3-L1 preadipocytes into adipocytes. Importantly, MCE and adipogenesis by C/EBPβ(−/−) MEFs could be rescued by enforced expression of WT C/EBPβ. Like 3T3-L1 preadipocytes, MEFs (WT C/EBPβ) exhibited similar kinetics of expression of C/EBPβ and delayed acquisition of DNA-binding activity after induction of differentiation. Taken together, these results further support the findings reported in the present paper that MCE is a prerequisite for adipogenesis.
Acknowledgments
This research was supported by National Institutes of Health Research Grant DK38418, by K01 Award DK61355 (to Q.-Q.T.), and by National Research Service Award DK61840 (to T.C.O.).
Abbreviations
- MCE
mitotic clonal expansion
- PPAR
peroxisome proliferator-activated receptor
- cdk
cyclin-dependent kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/extracellular signal-regulated kinase kinase
References
- 1.Shepherd P R, Gnudi L, Tozzo E, Yang H, Leach F, Kahn B B. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 2.Gnudi L, Shepherd P R, Kahn B B. Proc Nutr Soc. 1996;55:191–199. doi: 10.1079/pns19960020. [DOI] [PubMed] [Google Scholar]
- 3.Bernlohr D A, Bolanowski M A, Kelly T J, Lane M D. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- 4.Cornelius P, MacDougald O A, Lane M D. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 5.MacDougald O A, Lane M D. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Z, Wei Y, Chen N, Jiang M, Wu J, Liao K. J Biol Chem. 2001;276:11988–11995. doi: 10.1074/jbc.M011729200. [DOI] [PubMed] [Google Scholar]
- 7.Yeh W-C, Cao Z, Classon M, McKnight S L. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelman B M, Flier J S. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 9.Brun R P, Tontonoz P, Forman B M, Ellis R, Chen J, Evans R M, Spiegelman B M. Genes Dev. 1996;10:974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 10.Rosen E D, Walkey C J, Puigserver P, Spiegelman B M. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 11.Tang Q-Q, Lane M D. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christy R J, Kaestner K H, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q-Q, Jiang M-S, Lane M D. Mol Cell Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Qi C, Korenberg J R, Chen X N, Noya D, Rao M S, Reddy J K. Proc Natl Acad Sci USA. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke S L, Robinson C E, Gimble J M. Biochem Biophys Res Commun. 1997;240:99–103. doi: 10.1006/bbrc.1997.7627. [DOI] [PubMed] [Google Scholar]
- 16.Umek R M, Friedman A D, McKnight S L. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 17.Lin F-T, MacDougald O A, Diehl A M, Lane M D. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timchenko N, Wilde M, Nakanishi M, Smith J, Darlington G. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler W J, Timchenko N A. Mol Cell. 2001;8:817–828. doi: 10.1016/s1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- 20.Altiok S, Xu M, Spiegelman B M. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera R, Ro H S, Robinson G S, Xanthopoulos K G, Spiegelman B M. Mol Cell Biol. 1989;9:5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaestner K H, Christy R J, Lane M D. Proc Natl Acad Sci USA. 1990;87:251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheneval D, Christy R J, Geiman D, Cornelius P, Lane M D. Proc Natl Acad Sci USA. 1991;88:8465–8469. doi: 10.1073/pnas.88.19.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christy R J, Yang V W, Ntambi J M, Geiman D E, Landschulz W H, Friedman A D, Nakabeppu Y, Kelly T J, Lane M D. Genes Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 25.Hwang C-S, Mandrup S, MacDougald O M, Geiman D E, Lane M D. Proc Natl Acad Sci, USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Student A K, Hsu R Y, Lane M D. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 27.MacDougald O A, Cornelius P, Lin F-T, Chen S S, Lane M D. J Biol Chem. 1994;269:19041–19047. [PubMed] [Google Scholar]
- 28.Phelps D E, Xiong Y. Cell Growth Differ. 1998;9:595–610. [PubMed] [Google Scholar]
- 29.Patel Y M, Lane M D. J Biol Chem. 2000;275:17653–17660. doi: 10.1074/jbc.M910445199. [DOI] [PubMed] [Google Scholar]
- 30.Sherr C J, Roberts J M. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 31.Diehl J A, Cheng M, Roussel M F, Sherr C J. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeh W, Bierer B E, McKnight S L. Proc Natl Acad Sci USA. 1995;92:11086–11090. doi: 10.1073/pnas.92.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, et al. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 35.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala L A, Scovassi A I, Meijer L, Prosperi E. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 36.Green H, Kehinde O. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 37.Patel Y M, Lane M D. Proc Natl Acad Sci USA. 1999;96:1279–1284. doi: 10.1073/pnas.96.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang, Q.-Q., Otto, T. C. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA, in press.