Abstract
Adenovirus vectors have been targeted to different cell types by genetic modification of the capsid or by using recombinant or chemically engineered adaptor molecules. However, both genetic capsid modifications and bridging adaptors have to be specifically tailored for each particular targeting situation. Here, we present an efficient and versatile strategy allowing the direct use of monoclonal antibodies against cell surface antigens for targeting of adenovirus vectors. A synthetic 33-amino-acid immunoglobulin G (IgG)-binding domain (Z33) derived from staphylococcal protein A was inserted into the adenovirus fiber protein. The fiber retained the ability to assemble into trimers, bound IgG with high affinity (Kd = 2.4 nM), and was incorporated into vector particles. The transduction efficiency of the Z33-modified adenovirus vector in epidermal growth factor receptor (EGFR)-expressing cells was strongly and dose-dependently enhanced by combination with an EGFR-specific monoclonal antibody. The antibody-mediated increase in cellular transduction was abolished in the presence of competing protein A. In targeting experiments with differentiated primary human muscle cells, up to a 77-fold increase in reporter gene transfer was achieved by preincubation of the vector with monoclonal antibodies directed against neuronal cell adhesion molecule or integrin α7, respectively. The IgG-binding adenovirus vector holds promise for directed gene transfer to a wide variety of cell types by simply changing the target-specific antibody.
Adenoviruses (Ad) are nonenveloped viruses with a DNA genome of about 36 kb. Recombinant Ad have been widely used as gene transfer vehicles in preclinical and clinical studies (14). Infection with Ad vectors requires expression of separate cell receptors for attachment and entry. While the attachment of the virus to the cell is mediated by high-affinity binding of the knob domain of the Ad fiber to the 46-kDa coxsackie- and Ad receptor (CAR) (2, 48), internalization of the virus in clathrin-coated vesicles occurs through endocytosis upon interaction of the penton base protein with αv integrins (28, 54). In spite of a wide tissue distribution, CAR expression is low or absent in many cell types and tissues which are of interest for experimental or therapeutic gene transfer, including skeletal muscle, endothelium, hematopoietic cells, and tumor cells. Therefore, considerable effort has recently been directed to the retargeting of Ad vectors toward those cell types.
Genetic modification of the Ad fiber protein through incorporation of small peptide motifs into the HI loop (12, 24), a flexible, protruding region in the globular knob domain, through the addition of short peptide sequences at the C terminus of the fiber protein (6, 55), or through more radically reengineering knobless fiber molecules (30), improved the Ad-mediated transduction of cell types expressing ligand binding cell surface receptors. For example, incorporation of an RGD motif into the HI loop of first-generation Ad vectors (12) and high-capacity Ad vectors (4, 23) has been shown to enhance the transduction of CAR-negative integrin-expressing target cells. Similarly, the hexon protein has been modified by incorporation of an RGD peptide (49). Due to structural constraints of the capsid proteins, however, this approach seems to be restricted to small peptide ligands.
In an alternative approach, bispecific adaptor molecules composed of chemically cross-linked monoclonal antibodies (MAbs) (53) or fusion proteins containing a peptide ligand and a capsid-specific single-chain antibody or a soluble CAR domain (11, 50) have been employed to bridge Ad vector capsid proteins to cell surface receptor molecules. This strategy of tropism modification has also proved to be successful in vivo (40). However, it requires recombinant overexpression or chemical synthesis and modification, as well as extensive purification steps for the adaptor molecule, which may be time-consuming, costly, and difficult to scale up. Therefore, it was highly desirable to design a system based on the binding of unmodified MAbs to Ad vector particles, rendering the adaptor concept considerably more versatile and easy to apply.
A stable variant of the immunoglobulin (Ig)-binding B domain of the staphylococcal protein A (46), the so-called Z domain, has been described as a three-helix, 59-amino-acid (aa)-residue module that binds the Fc portion of IgGs with high affinity (9, 36). The entire Z domain or derivatives thereof have been genetically incorporated into envelope proteins of baculovirus (34, 38) and Sindbis virus (21, 37) and into the capsid of adeno-associated virus type 2 (41) and have been shown to retain IgG-binding activity (33, 37, 41). In this study, we describe the construction of an Ad vector displaying a short modified version of the Z domain, Z33 (7), in the HI loop of the fiber knob and the application of this vector in targeting experiments with specific MAbs directed against cell surface antigens. The Z33-modified Ad vector could be very efficiently targeted to epidermal growth factor receptor (EGFR)-expressing tumor cells, as well as to skeletal muscle cells, by complexation with cell-type-specific MAbs.
MATERIALS AND METHODS
Primary cells and cell lines.
A431 cells were purchased from Cell Lines Services (Heidelberg, Germany) and were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and penicillin-streptomycin (Invitrogen Life Technologies, Karlsruhe, Germany). HeLa cells were cultivated in Alpha-MEM medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. C2C12 mouse myoblasts and primary human myoblasts (PHM) were obtained from the Muscle Tissue Culture Collection at the Friedrich-Baur-Institut (Munich, Germany) and grown in skeletal muscle cell growth medium (Promocell, Heidelberg, Germany) at 37°C in 5% CO2. For fusion and differentiation of myoblasts, the growth medium was replaced by Dulbecco's modified Eagle medium containing 2% horse serum (fusion medium), and the cells were cultivated for a further 10 days until all myoblasts had fused to form multinucleated myotubes.
MAbs and polyclonal antibodies.
The murine IgG2a-type MAb LA22 against human EGFR was obtained from Upstate Biotechnology (Lake Placid, N.Y.), the MAb HIT3a against human CD3 was obtained from Pharmingen BD (Hamburg, Germany), and the MAb DO-1 against the p53 protein was obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.) (both IgG2a isotype). The MAbs 3C12 and 6A11 (both IgG1) against mouse α7 integrin have been described previously (31, 45). The MAb 5.1H11 against human neural cell adhesion molecule (NCAM) (39) was a kind gift from E. Shoubridge (Montreal Neurological Institute, McGill University, Montreal, Canada). Anti-NCAM and anti-α7 integrin antibodies were affinity-purified from hybridoma cell culture supernatant with protein G-agarose (Roche Diagnostics, Mannheim, Germany). The purity of antibody preparations was higher than 90% as assessed by polyacrylamide gel electrophoresis (PAGE) followed by silver staining. Protein concentrations of purified antibody preparations were determined with a colorimetric bicinchoninic acid-based protein detection and quantitation assay (Pierce, Rockford, Ill.). A polyclonal antibody against the Ad type 5 (Ad5) fiber knob was raised in rabbits by immunization with recombinant purified knob protein (Charles River Deutschland, Kisslegg, Germany).
Construction of recombinant plasmids.
Restriction endonucleases and DNA-modifying enzymes were purchased from New England Biolabs (Beverly, Mass.), and Pfu polymerase was from Stratagene (La Jolla, Calif.). For cloning purposes, the ClaI restriction site in the lacZ gene of the plasmid pCMVbeta (Clontech, Heidelberg, Germany) was deleted by introducing a silent nucleotide exchange (C to T) at nucleotide position 909 of the lacZ open reading frame, resulting in plasmid pCMVbeta-Cla−. Details of the genetic reengineering procedure will be provided upon request.
In order to insert the lacZ expression cassette with ClaI deleted into the E1 region of a first-generation Ad5 vector plasmid, pCMVbeta-Cla− was digested with HindIII and EcoRI and 5′ overhangs were filled in with Klenow polymerase. The plasmid pGS60, which contains the Ad5 sequences 1 to 440 and 3,523 to 21,562 separated by a unique PacI cloning site in the deleted E1 region, was digested with PacI. Overhanging ends were removed using T4 DNA polymerase, and the purified HindIII/EcoRI fragment from pCMVbeta-Cla− was ligated to the cleaved pGS60, resulting in pVBβgal2. The right part of the adenovirus genome was derived from pVB4 (4). A BstBI/PmeI fragment from pVBβgal2 was inserted into the BstBI/PmeI-cleaved plasmid pVB4, yielding pVB6. Thus, pVB6 contains the genome of a first-generation Ad vector with a cytomegalovirus (CMV) promoter-lacZ expression cassette replacing the E1 region and unique PacI and ClaI restriction sites at Ad5 nucleotide position 32,670 in the HI loop region of the fiber gene. The proper expression of the CMV promoter-lacZ cassette was tested by transfection of 293 cells and staining for β-galactosidase activity.
For insertion of the nucleotide sequence encoding the Z33 peptide into the fiber HI loop region of pVB6, three pairs of 5′-phosphorylated oligonucleotides, 5′-TAAGTTTAACATGCAGCAGCAGCGCCGCTTTTAC-3′ and 5′-GCGGCGCTGCTGCTGCATGTTAAACTTAAT-3′, 5′-GAGGCCCTGCACGACCCCAACCTGAACGAGGAGCAG-3′ and 5′-CTCGTTCAGGTTGGGGTCGTGCAGGGCCTCGTAAAA-3′, and 5′-CGCAACGCCAAGATTAAGAGCATTCGCGACGACAT-3′ and 5′-CGATGTCGTCGCGAATGCTCTTAATCTTGGCGTTGCGCTGCTC-3′, were annealed and ligated to the PacI/ClaI-cleaved expression plasmid pAH58 (4). The insert was removed from the resulting plasmid, pVB45, with PacI and ClaI and ligated to the dephosphorylated PacI/ClaI-cleaved pVB6, generating pVB44. The correct sequence and position of the insert were confirmed by cycle sequencing.
The baculovirus transfer plasmid pFB-Knob was generated by PCR amplification of the fiber gene region encoding knob domain aa 387 to 581 by use of forward and reverse primers containing nonhybridizing EcoRI and PstI restriction site sequences, respectively, and adding a 6-His tag-encoding sequence to the 5′ end. The PCR product was purified, cut with EcoRI and PstI, and ligated to EcoRI/PstI-cleaved pFastBac1 (Invitrogen Life Technologies, Karlsruhe, Germany). The plasmid pFB-KnobZ33 was constructed in the same way, using pVB45 as a template for PCR. The transfer plasmid pFB-CAR was obtained by PCR amplification of the extracellular CAR region encoding aa 1 to 236 with primers containing BamHI and XbaI restriction site sequences. After cloning of the PCR product into BamHI/XbaI-cut pFastBac1, oligonucleotides encoding a Flag tag and a subsequent stop codon were annealed and inserted into the XbaI site. The plasmids pFB-Fiber and pFB-FiberZ33 were generated by insertion of a 1.4-kb AccI-fragment from pFB-Fibermyc (C. Volpers, unpublished data) encoding the complete fiber tail and shaft region into AccI-cut, dephosphorylated pFB-Knob and pFB-KnobZ33, respectively.
Expression and purification of recombinant proteins.
The transfer plasmids pFB-Knob, pFB-KnobZ33, and pFB-CAR were used to rescue recombinant baculoviruses according to the Bac-to-Bac protocol (Invitrogen Life Technologies). Culture supernatants from transfected insect cells were harvested, and recombinant baculoviruses were propagated by two rounds of amplification. For expression of the Knob protein and the KnobZ33 protein, baculovirus-infected Sf9 cells were harvested 72 h postinfection, washed with phosphate-buffered saline, and resuspended in 5 ml of Dounce buffer (10 mM sodium phosphate buffer [pH 8.0], 10 mM NaCl, 1.5 mM MgCl2) per 1.8 × 108 cells. After incubation on ice for 10 min, the cells were lysed by 50 strokes in a tight-pestle glass-Teflon homogenizer. The lysate was cleared by centrifugation at 5,000 × g for 15 min and adjusted to equilibration buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 10 mM imidazole). His-tagged recombinant proteins were purified from the lysate in a batch procedure by incubation with Ni-nitrilotriacetic acid agarose beads (Qiagen, Hilden, Germany) for 2 h at 8°C. After the beads were washed three times for 10 min each time, the protein was eluted with one bead volume of elution buffer (equilibration buffer with 250 mM imidazole) and dialyzed against phosphate-buffered saline. For expression of soluble CAR (sCAR) protein, Hi-Five cells in serum-free medium were infected with the recombinant baculovirus, and the Flag-tagged sCAR protein was purified from the cell culture supernatant by affinity chromatography on anti-Flag agarose (Sigma, Deisenhofen, Germany) (Volpers et al., unpublished data). A recombinant 15-kDa fragment of staphylococcal protein A expressed in Escherichia coli was purchased from Sigma (Taufkirchen, Germany).
Surface plasmon resonance measurements.
Surface plasmon resonance instrumentation (BIAcoreX), CM5 sensor chips, and amine-coupling reagents containing N-hydroxysuccinimide, N-ethyl-N′-(3,3-diethylaminopropyl)-carbodiimide, and ethanolamine-HCl were obtained from BIAcore AB (Freiburg, Germany). Recombinant purified fiber knob proteins with and without a protein A binding site (KnobZ33 and Knob), as well as an anti-EGFR MAb (LA22), were coupled to the dextran-modified gold surface of a CM5 sensor chip according to the BIAcore manual using amine-coupling chemistry. Flow cell 2 was treated in all experiments in the same manner except for the ligand injection step and served as a nonbinding control (reference flow cell). Briefly, both flow cells were activated with 0.2 M N-ethyl-N′-(3-dimethyl-aminopropyl)-carbodiimide hydrochloride and 5 mM N-hydroxysuccinimide (35-μl injection; flow, 5 μl/min) prior to injection of the ligands (in 10 mM sodium acetate, pH 4.0). Three different sensor chips were coupled, in which either anti-EGFR antibody (50 ng/μl; flow, 5 μl/min; 1,380 resonance units [RU]), wild-type fiber knob protein (25 ng/μl; flow, 5 μl/min; 1,180 RU), or the knob protein containing the Z33 domain (25 ng/μl; flow, 5 μl/min; 1,050 RU) was immobilized to flow cell 1. The remaining activated groups in both flow cells were blocked by injection of 1 M ethanolamine, pH 8.5, for 7 min. After immobilization, the flow cells were washed two times with 1-min pulses of 100 mM glycine, pH 2.5 (regeneration buffer), followed by a continuous flow using HBS-EP buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.0005% surfactant P20). For determination of the kinetic parameters, the sensorgram of the reference flow cell (without immobilized ligand) was subtracted from each sensorgram prior to data processing using BIAevaluation software version 3.1.
Western blot analysis.
Recombinant proteins were incubated in sample buffer at 98°C for 5 min and subjected to 10% sodium dodecyl sulfate (SDS)-PAGE (27). Lysates from baculovirus-infected Sf9 cells were prepared (Volpers et al., unpublished data) and run on an SDS-7.5% polyacrylamide gel. For seminative electrophoresis, samples were not boiled and sample buffer without β-mercaptoethanol was used. The proteins were blotted onto nitrocellulose membranes, and the blots were developed as described before (4) using a knob-specific polyclonal rabbit antiserum (1:5,000 in Tris-buffered saline with 0.1% Tween 20 [TTBS]) and peroxidase-coupled goat anti-rabbit Ig antibody (Jackson ImmunoResearch, West Grove, Pa.) (1:30,000 in TTBS) as a secondary reagent.
Rescue and propagation of Ad vectors.
Ten micrograms each of pVB6 and pVB44 DNAs were cleaved with SwaI, followed by phenol extraction and ethanol precipitation. The DNA was transfected into N52.E6 cells (44) by the calcium phosphate method (18), and the cells were harvested after 14 days when they showed cytopathic effect. After three cycles of freeze-thawing to release vector particles, the N52.E6 cells were infected with half of the cell lysate, and the cell monolayer was overlaid with 0.5% agarose mix. After plaque purification, the rescued Ad vectors were propagated in N52.E6 cells as previously described (18). Following CsCl equilibrium density gradient centrifugation, the infectious titers of AdFβGal and AdFZ33βGal were determined by standard β-galactosidase assay on 293 cells (42), and the total particle contents of the preparations were determined by slot blot assay (26).
Virus precipitation with IgG Sepharose.
A total of 5 × 108 virus particles in 180 μl of phosphate-buffered saline was incubated with a 30-μl bead volume of IgG-Sepharose and glutathione-S-transferase (GST)-Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany), respectively, in an end-over-end shaker for 2 h at 8°C. The beads were washed five times with phosphate-buffered saline containing 0.1% Tween 20, resuspended in sample buffer, and boiled for 5 min. Samples were subjected to SDS-10% PAGE and analyzed by Western blotting.
Ad transduction and competition assays.
One day before transduction, 3 × 104 A431 cells per well and 1 × 104 (each) PHM and C2C12 cells per well were seeded in 96-well flat-bottom tissue culture plates (Corning Glass Works, Corning, N.Y.). In order to form antibody-virus complexes, Ad vector for infection of the cells at a multiplicity of infection (MOI) of 10 in three parallel wells (triplicate experiments) (A431 cells) or six parallel wells (PHM, C2C12 cells, and myotubes) was preincubated with or without MAbs at different concentrations in Opti-MEM I (Invitrogen Life Technologies) for 1 h at room temperature. The cell culture medium was exchanged with 70 μl of Opti-MEM (75 μl for muscle cells), and infection with 10 μl of the preincubated Ad vector per well (4 μl for muscle cells) was performed for 1 to 1.5 h on ice. The cells were washed twice with Opti-MEM and incubated with full medium for 48 h at 37°C before quantification of transgene expression. For competition studies, preincubation of the Ad vector with MAb and infection were done in the presence of 20 μg of recombinant Knob protein, KnobZ33 protein, or staphylococcal protein A fragment/ml. Transduction efficiencies were evaluated by luminometric quantification of β-galactosidase activity and by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. Lysates of infected cells were incubated with Galacto-Star reagent (PE Biosystems, Weiterstadt, Germany) according to the manufacturer's instructions, and light emission was measured in a Berthold (Bad Wildbad, Germany) plate luminometer. Total cell protein was determined by Micro BCA Protein Assay (Pierce). X-Gal staining of transduced cells was performed according to standard procedures (42), and pictures of stained cells were recorded with AxioCam HRC and analyzed with AxioVision version 3.1 software (Zeiss, Jena, Germany).
RESULTS
Incorporation of Z33 domain into fiber. (i) Trimerization.
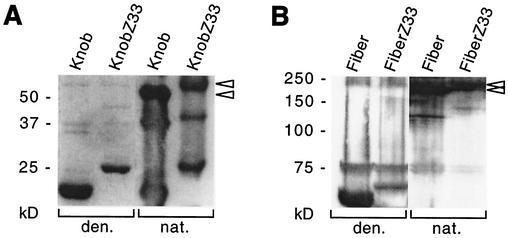
For conferring IgG-binding activity on the Ad capsid, a small synthetic version of the staphylococcal protein A-derived Z domain, Z33 (7), was incorporated into the C-terminal knob region of the fiber protein. The recombinant fiber molecule containing the Z33 insertion in the protruding, flexible HI loop of the globular knob domain was required to retain basic features of both protein moieties: trimerization of the knob domain, which is essential for fiber incorporation into the capsid and proper virus assembly, and IgG-binding of the Z33 domain, which would allow the complexation of the Ad vector with the targeting antibody. In order to assess these essential activities at the level of recombinant proteins, baculovirus vectors were constructed either expressing wild-type Ad5 knob protein carrying a 6-His tag and including the last fiber shaft repeat at the N terminus or expressing KnobZ33 protein containing the 33-aa-long Z33 domain inserted at fiber amino acid position 543. After expression in baculovirus-infected Sf9 insect cells and purification by affinity chromatography, the recombinant proteins were analyzed by Western blotting under denaturing and seminative conditions (Fig. 1A). Protein bands for denatured Knob and KnobZ33 were detected, as expected, at molecular masses of 22 and 25 kDa, respectively. Under seminative conditions, specific bands were visible in both protein preparations which were shifted to higher positions in the gel and corresponded to a trimeric state of the proteins. The trimers were found to run slightly faster than calculated from their molecular weights due to their compact globular structure. This result indicated that the Z33 insertion did not compromise the trimerization of the fiber knob domain. This was confirmed by analysis of baculovirus-expressed full-length wild-type Fiber and Z33-modified FiberZ33 proteins, both of which quantitatively trimerized (Fig. 1B).
FIG. 1.
Trimerization of recombinant knob and fiber proteins. Purified wild-type Knob protein and Z33-modified KnobZ33 protein expressed from recombinant baculovirus vectors in Sf9 insect cells (A) and extracts from Sf9 cells infected with baculovirus vectors expressing wild-type Fiber protein and Z33-modified FiberZ33 protein (B) were analyzed by Western blotting with a knob-specific polyclonal rabbit antiserum under denaturing (den.) or seminative (nat.) conditions. The arrowheads indicate the positions of the protein trimers. A nonspecific band at ∼75 kDa in panel B was stained in denaturing and seminative blots.
(ii) IgG binding.
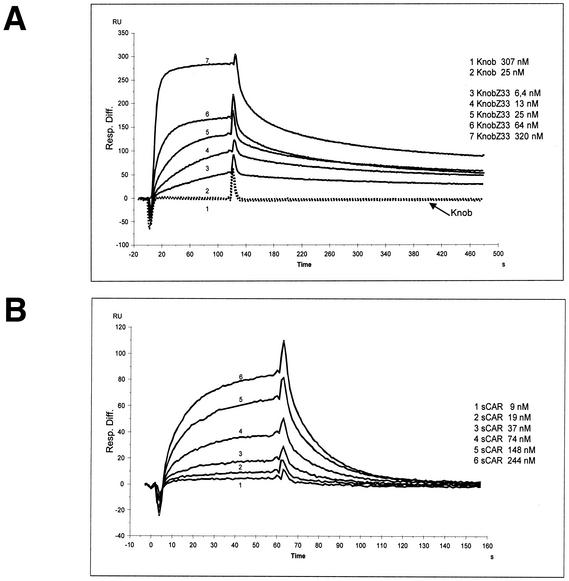
The IgG Fc-binding activity of the Z33 domain in the context of the fiber knob region was quantitatively assessed by surface plasmon resonance measurements. A murine MAb against human EGFR (LA22) was immobilized on the surface of a BIAcore CM5 sensor chip, and baculovirus-expressed recombinant Knob and KnobZ33 proteins were tested as analytes for binding. Figure 2A shows an overlay of the sensorgrams obtained when different concentrations of KnobZ33 between 6.4 and 320 nM were injected. KnobZ33 bound to the anti-EGFR MAb with high affinity. Binding constants calculated from the primary data using a simple one-to-one model for interaction led to an average dissociation constant (Kd) of 2.4 nM (χ2 = 3) (Table 1), which was consistent with the Kd calculated from the equilibrium data using a Scatchard plot (Kd = 5.7 nM). In contrast, the wild-type Knob protein did not bind to the anti-EGFR MAb even at higher concentrations (25 and 307 nM) (Fig. 2A), as expected. The affinity of the interaction between KnobZ33 and MAb LA22 was almost as high as that found for binding of a 15-kDa recombinant protein A fragment to the same MAb (Kd = 2.0 nM) (Table 1) and as reported by others for binding of full-length protein A to murine MAbs of the same isotype (Kd = 1 nM) (25).
FIG. 2.
Determination of binding rate constants by surface plasmon resonance. The resonance signal in RU is given as the response difference (Resp. Diff.) between the reference cell (without immobilized ligand) and the sample cell. Injection of the analyte starts at time zero and ends at 120 (A) or 65 (B) s. (A) Overlay of sensorgrams measured by injection of KnobZ33 (6.4 to 320 nM) and Knob (25 and 307 nM) into immobilized anti-EGFR MAb LA22 on a CM5 sensor chip (50 ng/μl; 1,380 RU). (B) Overlay of sensorgrams measured by the injection of sCAR (9 to 244 nM) into immobilized KnobZ33 (25 ng/μl; 1,050 RU).
TABLE 1.
Association rate (ka), dissociation rate (kd), and dissociation constant (Kd) of interactions between anti-EGFR MAb LA22, KnobZ33, Knob, a recombinant 15-kDa protein A fragment (“Protein A”), and sCARa
| Ligand | Analyte | ka (1/Ms) | kd (1/s) | Kd (nM) |
|---|---|---|---|---|
| Anti-EGFR MAb LA22 | KnobZ33 | 7.5 × 105 | 1.8 × 10−3 | 2.4 |
| Anti-EGFR MAb LA22 | Knob | No binding | No binding | No binding |
| Anti-EGFR MAb LA22 | Protein A | 1.9 × 106 | 3.7 × 10−3 | 2.0 |
| KnobZ33 | sCAR | 1.1 × 105 | 5.3 × 10−2 | 494 |
| Knob | sCAR | 1.6 × 105 | 6.6 × 10−2 | 409 |
As determined by surface plasmon resonance (sensorgrams for LA22-protein A and Knob-sCAR interactions are not shown in Fig. 2).
To further investigate whether the interaction with CAR, another function of the fiber knob domain, was affected by incorporation of the Z33 domain, we coupled Knob and KnobZ33 proteins on the surfaces of sensor chips and tested for binding of a purified recombinant sCAR protein. When various concentrations of sCAR (9 to 244 nM) were injected over surfaces containing 1,050 RU of immobilized KnobZ33 (Fig. 2B) and 1,180 RU of Knob (not shown), both KnobZ33 and wild-type Knob protein bound to sCAR protein with comparable affinities (Kd [KnobZ33] = 494 nM [χ2 = 0]; Kd [Knob] = 409 nM [χ2 = 1]) (Table 1). Thus, insertion of the Z33 peptide into the knob domain obviously had no influence on its binding to CAR.
Construction and rescue of Z33-modified Ad vector.
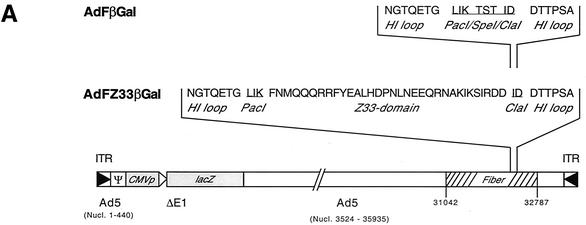
Based on a helper virus plasmid for generation of fiber knob HI loop-modified high-capacity Ad vectors (4), an infectious first-generation Ad vector plasmid (pVB6; for reasons of clarity referred to in this manuscript as pAdFβGal) was constructed to contain a lacZ reporter gene under the control of a CMV promoter in the deleted E1 region. In addition, pAdFβGal carries unique PacI and ClaI restriction sites at nucleotide position 32,670 of the Ad5 genome for insertion of peptide ligands into the HI loop of the knob domain at the same position as in the recombinant fiber proteins. After insertion of a Z33 domain-encoding sequence via annealed oligonucleotides into the PacI/ClaI restriction sites (pVB44; for reasons of clarity referred to here as pAdFZ33βGal), both constructs were released from the plasmid backbone by SwaI digestion. Following transfection into N52.E6 producer cells (44), the Ad vectors AdFβGal (control vector) and AdFZ33βGal (Z33 modified) were rescued (Fig. 3 A). The infectious titers of the propagated and purified Ad vectors determined by X-Gal staining of infected 293 cells were comparable (AdFβGal, 4 × 108 blue-forming units (BFU)/ml; AdFZ33βGal, 5 × 108 BFU/ml).
FIG. 3.
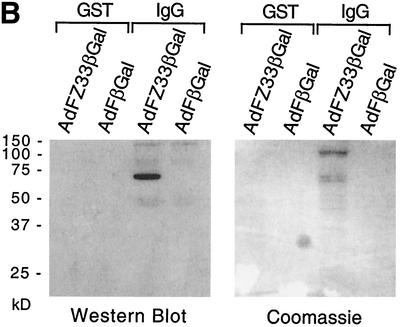
(A) Genomic organization of the Ad5-based fiber-modified vectors AdFZ33βGal, containing the Z33 peptide-encoding sequence inserted into PacI/ClaI restriction sites in the HI loop, and the negative control vector AdFβGal, containing only the PacI/ClaI restriction site sequence in the HI loop. The numbers refer to nucleotide (Nucl.) positions in the Ad5 genome. ITR, inverted terminal repeat. (B) IgG binding of the Z33-modified Ad vector. Purified AdFZ33βGal and AdFβGal vector particles were incubated with IgG-Sepharose (IgG) and GST-Sepharose (GST), and after washing of the Sepharose beads, precipitates were subjected to SDS-10% PAGE and analyzed by Coomassie staining or Western blotting developed with a knob-specific polyclonal rabbit antibody and peroxidase-coupled goat anti-rabbit Ig as a secondary reagent.
To confirm that the IgG-binding activity of the Z33 domain was retained in assembled vector particles, pull-down experiments with IgG-Sepharose were performed. Purified vector preparations of AdFZ33βGal and AdFβGal were incubated with IgG-Sepharose, and fiber proteins bound to the beads after washing were detected by immunoblotting them with a knob-specific polyclonal antibody (Fig. 3B, left). The fiber protein band at 65 to 68 kDa was readily detected in IgG-Sepharose precipitates of AdFZ33βGal but not in precipitates of AdFβGal, whereas no bands were detected after precipitation with GST-Sepharose, which was used as a control to exclude nonspecific binding to the bead material. After Coomassie blue staining of the gel, additional viral protein bands, especially the hexon band at ∼110 kDa, were visible in IgG-Sepharose precipitates of AdFZ33βGal (Fig. 3B, right). No viral proteins were visible, however, in the Coomassie-stained gel when an excess of a 15-kDa recombinant protein A fragment was included as a competitor during incubation with the IgG-Sepharose beads (not shown). Thus, IgG specifically bound to the Z33-modified fiber protein within Ad vector particles.
Targeting to EGFR-positive cells.
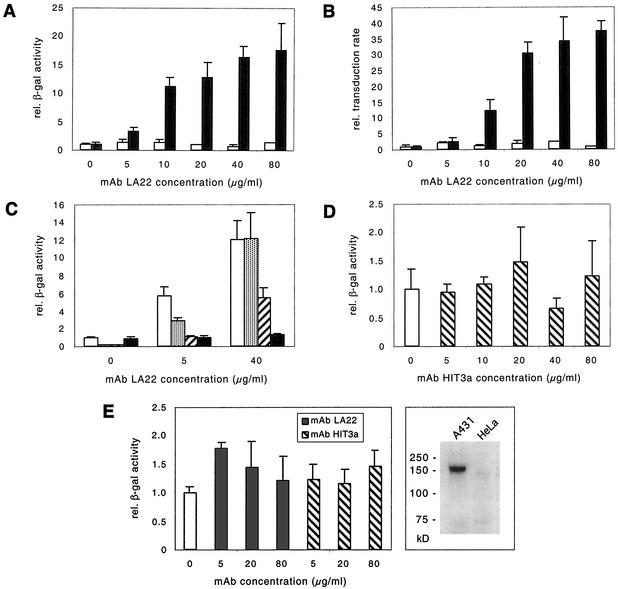
As EGFR is highly overexpressed on many epithelium-derived tumors (51), the carcinoma cell line A431 was used as a model system to validate the concept of targeting the Z33-reengineered Ad vector via unmodified MAbs to an abundant cell surface antigen. The Ad vectors AdFZ33βGal, AdFβGal, and AdβGal (a β-galactosidase-expressing Ad vector with E1 deleted and without any capsid modification) were incubated for 1 h at room temperature with different concentrations of affinity-purified MAb LA22 directed against human EGFR and were then used for infection of A431 cells as described in Materials and Methods. Reporter gene expression levels as assessed by a quantitative luminometric β-galactosidase assay in AdFZ33βGal-infected A431 cells were significantly enhanced in a dose-dependent manner after vector pretreatment with MAb LA22 (Fig. 4A). Incubation of the vector prior to infection with 5 μg of MAb LA22/ml increased the transduction efficiency 3- to 4-fold, and preincubation with 80 μg/ml, the highest concentration tested, increased the efficiency 18-fold compared to AdFZ33βGal infection without MAb. In contrast, no significant effect on transduction efficiency was observed when AdFβGal (Fig. 4A) or AdβGal (not shown) was incubated with the same MAb concentrations prior to infection. The few additional amino acids in the HI loop of AdFβGal due to the insertion of PacI and ClaI restriction sites (Fig. 3A), therefore, did not affect the performance of the Ad vector in regard to IgG binding and transduction.
FIG. 4.
Antibody-mediated transduction of A431 cells. (A and B) Cells were transduced with AdFZ33βGal (▪) and AdFβGal (□) at an MOI of 10 (A) or 1 (B) after preincubation with increasing concentrations of the anti-EGFR MAb LA22. (C) Cells were transduced with AdFZ33βGal at an MOI of 10 after preincubation with MAb LA22 (5 and 40 μg/ml) in the absence of reagent (□) or in the presence of 20 μg of Knob protein ([░⃞]), KnobZ33 protein (▨), and protein A (▪)/ml. (D) Cells were transduced with AdFZ33βGal after preincubation with increasing concentrations of the anti-CD3 MAb HIT3a. (E) HeLa cells were transduced with AdFZ33βGal after preincubation with MAb LA22 (anti-EGFR; ▪) or MAb HIT3a (anti-CD3; ▧) in different concentrations. On the right is a Western blot of A431 and HeLa cell lysates developed with anti-EGFR MAb LA22. For panels A, C, D, and E, transgene expression levels were luminometrically quantified and normalized to total cellular protein; for panel B, transduction rates were assessed by counting blue cells after X-Gal staining, and the values were expressed in relation to results obtained with the respective vectors not preincubated with MAb, which were set to 1.0. Each value was obtained from triplicate determinations. The error bars represent standard deviations. rel., relative; β-gal, β-galactosidase.
Assessment of transduction rates by X-Gal staining of infected cells yielded similar results: the number of transduced cells was dose-dependently enhanced by preincubation of AdFZ33βGal, but not AdFβGal, with MAb LA22; with 80 μg/ml used for preincubation of AdFZ33βGal, the transduction rate was increased 37-fold (Fig. 4B). When cells were incubated with AdFZ33βGal at an MOI of 10, we estimated that <1% of the cells stained blue without pretreatment of the vector but ∼20% stained blue with vector pretreated with 80 μg of MAb LA22/ml (not shown).
Specificity of anti-EGFR MAb-mediated targeting.
Competitive inhibition assays were performed in order to confirm that the MAb-mediated transduction of A431 cells by AdFZ33βGal actually required the specific binding of the MAb to the vector and occurred via a CAR-independent cell entry pathway. In these experiments, preincubation of AdFZ33βGal with or without MAb LA22 and infection of A431 cells were performed in the presence of 20 μg of recombinant Knob protein, KnobZ33 protein, and protein A/ml (Fig. 4C). Knob protein largely blocked infection by uncomplexed Ad vector and reduced the transduction efficiency of Ad vector pretreated with 5 μg of MAb LA22/ml but had no effect on the transduction efficiency of Ad vector pretreated with 40 μg of MAb LA22/ml, indicating that at this MAb concentration infection was completely redirected to a receptor different from CAR without affinity for competing soluble Knob protein. As expected, KnobZ33 protein reduced infection by uncomplexed Ad vector but also competitively inhibited MAb-mediated transduction, especially when the Ad vector was pretreated with the lower MAb concentration (5 μg/ml). In the presence of 20 μg of protein A/ml, which competes for IgG Fc binding sites with the Z33 domain of the vector, the MAb-mediated increase in transduction efficiency was completely abolished.
A control experiment with an isotype-matched irrelevant MAb confirmed that the IgG-mediated transduction enhancement was specific for the MAb used. No significant, concentration-correlated effect on transduction efficiency was observed when AdFZ33βGal was pretreated with the anti-CD3 MAb HIT3a (Fig. 4D).
Compared to A431 cells, HeLa cells are known to express considerably fewer EGFR molecules per cell (3, 50); in fact, we were able to detect EGFR as a strong band in Western blots of total A431 cell lysate but not at all in HeLa cell lysate (Fig. 4E, right). The deficient EGFR expression in HeLa cells correlated with a lack of specific MAb-mediated transduction enhancement: preincubation of AdFZ33βGal with MAb LA22 only slightly increased transduction efficiency in HeLa cells at all MAb concentrations tested. However, the same effect was found with an isotype-matched irrelevant control antibody directed against human CD3 (Fig. 4E) and therefore was regarded as nonspecific.
In conclusion, these data demonstrated that the Z33 modification of the Ad vector allowed an antibody-mediated enhancement of transduction efficiency. This targeting concept required specific interaction between vector and antibody, as well as sufficient cellular expression of the respective target antigen.
Targeting to primary human muscle cells.
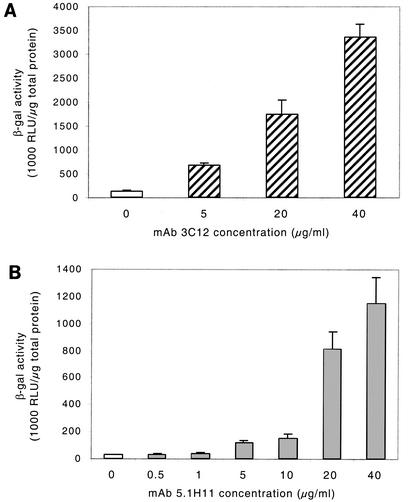
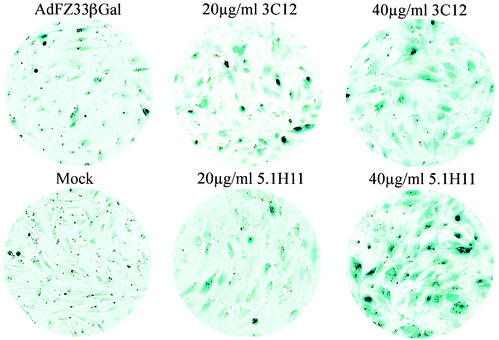
Ad-mediated gene transfer into skeletal muscle has been observed to be of very limited efficiency. Two cell surface antigens were selected for targeting of AdFZ33βGal to skeletal muscle cells: integrin α7β1, which is a laminin receptor almost exclusively expressed on myoblasts and myofibers (8, 56), and neural cell adhesion molecule (NCAM), which is involved in mediating intercellular contacts in muscle and in a variety of other tissues and cell types (15). In initial experiments with C2C12 mouse myoblasts, preincubation of AdFZ33βGal with the murine integrin α7-specific MAb 6A11 was found to enhance transduction efficiency by up to eightfold (not shown), indicating that this surface antigen was indeed suitable for a MAb-mediated targeting strategy. Therefore, primary human myoblasts (PHM) were analyzed for an increase in gene transfer by pretreatment of the Z33-modified vector with the MAb 3C12 directed against human integrin α7. AdFZ33βGal was incubated for 1 h with increasing concentrations of up to 40 μg of MAb 3C12/ml prior to infection of the cells, and transgene expression levels were assessed in a luminometric β-galactosidase assay. As shown in Fig. 5A, the antibody enhanced AdFZ33βGal-mediated gene transfer in a dose-dependent manner, with a 24-fold increase at the highest MAb concentration tested. In agreement with these data, X-Gal staining of PHM after infection demonstrated a much higher proportion of transduced cells when the Ad vector had been loaded with MAb 3C12 (Fig. 6, upper row). As assessed by counting blue cells in three different microscopic areas of the well, on average ∼80% of PHM stained blue after transduction with AdFZ33βGal pretreated with 40 μg of MAb 3C12/ml, as opposed to ∼10% with untreated vector.
FIG. 5.
Antibody concentration-dependent transduction of PHM. Cells were transduced with the Z33-modified vector AdFZ33βGal at an MOI of 10 after preincubation with or without increasing concentrations of anti-integrin α7 MAb 3C12 (A) or anti-NCAM MAb 5.1H11 (B). Transgene expression levels were luminometrically determined as relative light units (RLU) of β-galactosidase (β-gal) activity and normalized to total cellular protein. Each value was obtained from six parallel infections. The error bars represent standard deviations.
FIG. 6.
Antibody-mediated transduction of PHM. Cells were mock infected (Mock) or infected with AdFZ33βGal at an MOI of 10 alone or after preincubation with two different concentrations of either the anti-integrin α7 MAb 3C12 or the anti-NCAM MAb 5.1H11 and stained for β-galactosidase expression after 48 h. The microscopic fields shown contain equal total numbers of cells.
Similar results were obtained when the NCAM-specific MAb 5.1H11 was used to redirect AdFZ33βGal to PHM (Fig. 5B). Whereas preincubation of the vector with MAb concentrations of 0.5 and 1 μg/ml had no significant effect on transduction efficiency, concentrations of 5 μg/ml and higher improved reporter gene transfer into these cells in a dose-dependent manner. A 37-fold-enhanced β-galactosidase expression was observed with the vector pretreated at a concentration of 40 μg of MAb 5.1H11/ml. Correspondingly, the transduction rate detected by X-Gal staining was drastically increased with NCAM-targeted vector at higher antibody concentrations (Fig. 6, lower row). AdFZ33βGal pretreated with 40 μg of MAb 5.1H11/ml transduced ∼85% of the cells as determined by counting three microscopic areas in the experiment shown in Fig. 6. In contrast, no targeting effect was obtained in PHM when AdFβGal was pretreated with either of the MAbs, even at high concentrations (not shown).
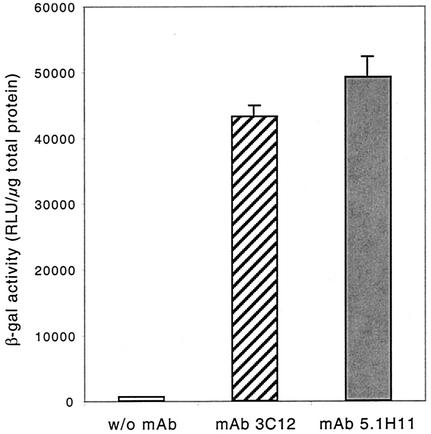
Compared to undifferentiated myoblasts, myotubes differentiated in cell culture are even less susceptible to infection by subgroup C Ad. Myotubes express differentiation-specific antigens and have a rudimentary extracellular matrix more closely resembling the situation in vivo. Since integrin α7 and NCAM are abundantly expressed on the surfaces of myotubes, we studied the adaptor functions of both MAbs with AdFZ33βGal in these cells (Fig. 7). Expression levels of β-galactosidase in myotubes after infection with AdFZ33βGal pretreated with 40 μg of either MAb 3C12 or MAb 5.1H11/ml were 68- and 77-fold higher, respectively, than in control infections with untreated vector. These results demonstrate that the Z33 domain displaying Ad vector can be efficiently targeted to differentiated human skeletal muscle cells via integrin α7- and NCAM-specific MAbs.
FIG. 7.
Antibody-mediated transduction of primary human myotubes. Differentiated, multinucleated myotubes were transduced with AdFZ33βGal at an MOI of 10 alone or after complexation with 40 μg of the anti-integrin α7 MAb 3C12 or the anti-NCAM MAb 5.1H11/ml, and β-galactosidase (β-gal) expression levels were quantified. The values are means of six parallel infections. The error bars represent standard deviations. RLU, relative light units.
DISCUSSION
In this work, we have developed a versatile strategy for Ad vector targeting based on the incorporation of an IgG-binding peptide into the capsid, allowing the use of unmodified MAbs to redirect the vector to specific cell surface molecules. The Z33 domain incorporated into the HI loop of the fiber knob retained high-affinity IgG-binding activity. In surface plasmon resonance measurements, the binding affinity of KnobZ33 protein to a murine MAb was determined to have a Kd of 2.4 nM, suggesting that the binding affinity of Z33 in the context of the knob domain was in the same range or even better than the affinities of Z33 and the full-length Z domain as isolated polypeptides, which have been determined to have Kds of 43 (Z33) and 10 (Z) nM (7); however, our measurements were performed with a BIAcore sensor chip carrying immobilized mouse IgG2a, in contrast to human IgG1 used in the previous study (7). The Z33 domain, which is a 33-aa-long two-helix version (7) of the 59-aa-long three-helix Z module derived from staphylococcal protein A (9, 36), might potentially be stabilized by either flanking amino acid residues or the tertiary structure of the knob domain. In addition, the homotrimeric nature of the fiber knob carrying three Z33 domains per molecule most probably accounts for the higher overall IgG affinity of KnobZ33 in the BIAcore experiments as opposed to the monomeric peptide. Insertion of Z33 into the knob domain did not significantly affect CAR binding, as BIAcore affinity measurements using a sCAR protein as an analyte binding to immobilized Knob and KnobZ33 yielded similar binding rate constants (association rate [ka] and dissociation rate [kd]). Confirming this result, the modified vector AdFZ33βGal readily infected CAR-expressing producer cells. The dissociation constants obtained for interaction between our sCAR protein containing both extracellular CAR domains, D1 and D2, and the knob proteins were high compared to values previously reported by others for the association of the CAR D1 domain alone with wild-type knob protein (Kd = 25 nM) (29) and for the binding of fiber protein to full-length CAR-expressing cells in vitro (Kd = 2 nM) (54). This could be due to a modifying or masking effect of the D2 domain in our recombinant sCAR protein on the knob binding site in the D1 domain, which might not be exerted if D1 is expressed alone or the full-length CAR protein is anchored in the cellular membrane. In addition, our surface plasmon resonance experiments definitely do not reflect the complex binding situation of multivalent vector particles interacting with high avidity with mobile, dimerizing CAR receptors on a target cell; in fact, the concentration of our sCAR preparation did not allow saturating conditions to be reached in the BIAcore measurements with immobilized knob proteins in order to determine the exact stoichiometry of the interaction.
The human EGFR-specific MAb LA22 of isotype IgG2a, which had been shown in surface plasmon resonance experiments to bind to Z33 with high affinity in the context of the fiber knob domain, was used to validate the concept of MAb-mediated Ad vector targeting to A431 cells. Gene transfer by AdFZ33βGal into these carcinoma cells expressing a high number (3 × 106) of EGFR molecules per cell (3, 52) was enhanced in a dose-dependent manner by preincubation with MAb LA22, while gene transfer into HeLa cells was not affected. HeLa cells express 40 times fewer EGFR molecules per cell (3)—in fact, EGFR expression was not detectable by immunoblotting—and probably considerably more CAR receptor molecules than A431 cells, as deduced from the much better transduction by unmodified control vector (not shown). These results were in agreement with previous findings by Watkins et al. (50), who obtained a 13-fold increase in transduction by an Ad vector preincubated with an anti-knob-EGF fusion protein in A431 cells but <2-fold increase in HeLa cells. A targeting effect was also observed when untreated AdFZ33βGal was added to A431 cells which had been preincubated with EGFR-specific MAb on ice (not shown). Competition experiments with protein A demonstrated that binding of the MAb to AdFZ33βGal, and not just its presence during infection, was actually required to exert the targeting effect. The slight increase in transduction observed after preincubation with irrelevant MAb (Fig. 4E) could be due to a nonspecific binding of the MAb to the cell surface or to the formation of small aggregates composed of several Ad particles still capable of infection via the CAR pathway. In contrast to this weak nonspecific enhancement, preincubation of AdFZ33βGal with murine preimmune serum was found to slightly reduce infectivity in 293 cells (not shown).
The efficiency of transduction enhancement obtained by preincubation of the Z33-modified Ad vector with MAb LA22 was well within the range previously achieved by the use of different kinds of EGFR-targeting bispecific adaptor molecules in A431 cells, such as fusion proteins of EGF with either a knob-specific single-chain antibody (50) or a soluble CAR domain (11), as well as in other EGFR-positive tumor cells with genetically fused or chemically cross-linked knob- and EGFR-directed antibody specificities (16, 17, 32). Direct targeting of Ad vectors to EGFR via adaptor molecules has been shown to circumvent the need for penton base-integrin interactions to trigger internalization (50). As EGFR is overexpressed on many epithelial tumors (16, 51) with various levels of CAR and integrin αv expression, it appears to be a suitable target molecule for MAb-mediated therapeutic gene transfer by Z33-modified Ad vectors locally injected into such tumors.
Ad-mediated gene transfer for the treatment of muscular dystrophies has been hampered by the low transduction efficiency of adult skeletal muscle, mostly due to low CAR expression levels (35, 47). To date, only few muscle-specific and sufficiently expressed surface markers which could be used for transductional targeting of virus vectors have been characterized. We believe that integrin α7β1 is an excellent candidate for muscle-specific vector targeting, since the α7 subunit is almost exclusively expressed in skeletal, smooth, and cardiac muscle and is increased on the surfaces of muscle fibers in Duchenne muscular dystrophy patients and mdx mice (8, 19, 56). Another promising target receptor is NCAM, which is expressed in activated satellite cells and myogenic precursor cells during regeneration (20, 22). The concept of targeting regenerating and newly forming myofibers, or even muscle satellite cells (“targeting the next generation”), may prove particularly useful for very young patients with limited fibrosis. In this study, we showed that both integrin α7β1 and NCAM are able to serve as attachment receptors for targeted Ad vectors and to promote their internalization in myoblasts, as well as in differentiated myotubes. The finding that transduction of myotubes with integrin α7β1- and NCAM-targeted AdFZ33βGal was up to 77-fold enhanced compared to the untargeted Ad vector is of particular interest for further development of an Ad vector-based gene therapy for muscular dystrophies. Since myotubes cultivated in vitro have a rudimentary extracellular matrix and are difficult to infect with Ad, they reflect the behavior of muscle fibers in vivo quite well (1, 43). Additional candidate receptors for MAb-mediated Ad vector targeting might include the Fe-transferrin receptor that is overexpressed in regenerating fibers (13), as well as the c-Met receptor (receptor for hepatocyte growth factor) (10) and M-cadherin (22), which are expressed in satellite cells in adult muscle (5). In future experiments, several of these receptors will have to be evaluated for the ability to allow MAb-mediated gene transfer by AdFZ33βGal in vivo.
In previous studies, the Sindbis virus E2 envelope glycoprotein was modified by incorporation of two copies of the Z domain (ZZ) for MAb-mediated targeting of Sindbis virus (21, 37) and of retrovirus vectors (33) to hematopoietic and certain tumor cell lines. In contrast to the ZZ-modified Sindbis virus vector (21, 37), and in contrast to a recently described adeno-associated virus-based vector displaying a 34-aa-long Z domain derivative on the capsid (41), AdFZ33βGal was not found to have significantly reduced infectivity in cells expressing the native virus receptor, in spite of the Z33 domain modification. Preincubation of a ZZ-modified Sindbis virus vector with 20 μg of anti-NCAM MAb/ml, a concentration well within the range used in our experiments for preincubation of AdFZ33βGal, drastically increased gene transfer to a small-cell lung cancer cell line (21). Similarly, a concentration of 10 μg (each) of anti-HLA and anti-CD4 MAb/ml was used for the preincubation of ZZ-modified retrovirus vectors in targeting experiments with peripheral blood mononuclear cells (33). Furthermore, with a concentration of 0.5 μg of anti-EGFR MAb LA22/ml used for preincubation of the Sindbis virus vector, transduction enhancement was achieved in A431 cells (37). Due to the technical method of Sindbis virus and retrovirus vector production and transduction, however, these concentrations were also the final concentrations during infection, whereas in this work, MAb-preincubated AdFZ33βGal was diluted 8-fold (10 into 70 μl) for infection of A431 cells and 20-fold (4 into 80 μl) for infection of muscle cells, yielding final MAb concentrations of ∼0.2 to 10 μg/ml on the cells during infection. Thus, although different vector systems and IgG-binding domains are difficult to compare, the MAb concentrations used for targeting of the Z33-modified Ad vector in our study appear to be in a reasonable range. From the dissociation constant of the KnobZ33-IgG interaction, it could be calculated that within this range of effective MAb concentrations (0.2 to 10 μg/ml), the proportion of IgG-binding Z33 sites actually occupied by IgG varies between 35 and 96%. Considering that binding of the first IgG molecule to the trimeric fiber molecule might be sterically favored compared to binding of a second and third IgG molecule, the threshold level of one-third-occupied IgG-binding sites would suggest that all fiber molecules of an Ad particle have to be evenly loaded with one IgG molecule each for a targeting effect to be detected.
Z33-modified Ad vectors could be used for specific therapeutic applications, as well as for systematic screening and detection of new virus uptake-promoting entry receptors on the surfaces of cell types refractory to Ad infection. The straightforward and universal targeting strategy developed in this work does not require specific modifications of the capsid for each cellular target and at the same time avoids expensive and laborious expression, purification, and chemical modification steps often required to provide particular adaptor molecules. Increasing knowledge of cell-type-specific surface antigen expression patterns, as well as a growing set of differentiating diagnostic antibodies available, suggest a wide potential for application of this approach.
Acknowledgments
C.V. and C.T. contributed equally to this work.
We thank Ursula Klutzny and Eva Schmidtmeyer for expert technical assistance. Human myoblast cultures were obtained from the Muscle Tissue Culture Collection at the Friedrich Baur Institute (Department of Neurology, Ludwig Maximilians University, Munich, Germany).
The Muscle Tissue Culture Collection is supported by grants from the Deutsche Gesellschaft für Muskelkranke (Freiburg, Germany) and the Association Francaise contre les Myopathies (Paris, France). This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to H.L. and S.K. and from the German Duchenne Parents Project (Aktion Benni und Co.) to H.L. and by grant FKZ 01KS9502 from the Federal Ministry of Education and Research and the Center for Molecular Medicine Cologne (ZMMK) to S.K.
REFERENCES
- 1.Acsadi, G., A. Jani, J. Huard, K. Blaschuk, B. Massie, P. Holland, H. Lochmuller, and G. Karpati. 1994. Cultured human myoblasts and myotubes show markedly different transducibility by replication-defective adenovirus recombinants. Gene Ther. 1:338-340. [PubMed] [Google Scholar]
- 2.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 3.Berkers, J. A. M., P. P. M. van Bergen en Henegouwen, and J. Boonstra. 1992. The effects of receptor density and cell shape on epidermal growth factor binding. J. Receptor Res. 12:71-100. [DOI] [PubMed] [Google Scholar]
- 4.Biermann, V., C. Volpers, S. Hussmann, A. Stock, H. Kewes, G. Schiedner, A. Herrmann, and S. Kochanek. 2001. Targeting of high-capacity adenoviral vectors. Hum. Gene Ther. 12:1757-1769. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff, R. 1994. The satellite cell and muscle regeneration, p. 97-118. In A. G. Engel and C. Franzini-Armstrong (ed.), Myogenesis. McGraw-Hill, New York, N.Y.
- 6.Bouri, K., W. G. Feero, M. M. Myerburg, T. J. Wickham, I. Kovesdi, E. P. Hoffman, and P. R. Clemens. 1999. Polylysine modification of adenoviral fiber protein enhances muscle cell transduction. Hum. Gene Ther. 10:1633-1640. [DOI] [PubMed] [Google Scholar]
- 7.Braisted, A. C., and J. A. Wells. 1996. Minimizing a binding domain from protein A. Proc. Natl. Acad. Sci. USA 93:5688-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkin, D. J., and S. J. Kaufman. 1999. The α7β1 integrin in muscle development and disease. Cell Tissue Res. 296:183-190. [DOI] [PubMed] [Google Scholar]
- 9.Cedergren, L., R. Andersson, B. Jansson, M. Uhlen, and B. Nilsson. 1993. Mutational analysis of the interaction between staphylococcal protein A and human IgG1. Protein Eng. 6:441-448. [DOI] [PubMed] [Google Scholar]
- 10.Cornelison, D. D., and B. J. Wold. 1997. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191:270-283. [DOI] [PubMed] [Google Scholar]
- 11.Dmitriev, I., E. Kashentseva, B. E. Rogers, V. Krasnykh, and D. T. Curiel. 2000. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J. Virol. 74:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dmitriev, I., V. Krasnykh, C. R. Miller, M. Wang, E. Kashentseva, G. Mikheeva, N. Belousova, and D. T. Curiel. 1998. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 72:9706-9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feero, W. G., S. Li, J. D. Rosenblatt, N. Sirianni, J. E. Morgan, T. A. Partridge, L. Huang, and E. P. Hoffman. 1997. Selection and use of ligands for receptor-mediated gene delivery to myogenic cells. Gene Ther. 4:664-674. [DOI] [PubMed] [Google Scholar]
- 14.Ferber, D. 2001. Gene therapy: safer and virus-free? Science 294:1638-1642. [DOI] [PubMed] [Google Scholar]
- 15.Goridis, C., and J.-F. Brunet. 1992. NCAM: structural diversity, function and regulation of expression. Semin. Cell Biol. 3:189-197. [DOI] [PubMed] [Google Scholar]
- 16.Grill, J., V. W. van Beusechem, P. van der Valk, C. M. F. Dirven, A. Leonhart, D. S. Pherai, H. J. Haisma, H. M. Pinedo, D. T. Curiel, and W. R. Gerritsen. 2001. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin. Cancer Res. 7:641-650. [PubMed] [Google Scholar]
- 17.Haisma, H. J., J. Grill, D. T. Curiel, S. Hoogeland, V. W. van Beusechem, H. M. Pinedo, and W. R. Gerritsen. 2000. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 7:901-904. [DOI] [PubMed] [Google Scholar]
- 18.Hitt, M., A. J. Bett, C. L. Addison, L. Prevec, and F. L. Graham. 1995. Techniques for human adenovirus vector construction and characterization. Methods Mol. Genet. 7:13-30. [Google Scholar]
- 19.Hodges, B. L., Y. K. Hayashi, I. Nonaka, W. Wang, K. Arahata, and S. J. Kaufman. 1997. Altered expression of the α7β1 integrin in human and murine muscular dystrophies. J. Cell Sci. 110:2873-2881. [DOI] [PubMed] [Google Scholar]
- 20.Hurko, O., and F. S. Walsh. 1983. Human fetal muscle-specific antigen is restricted to regenerating myofibers in diseased adult muscle. Neurology 33:737-743. [DOI] [PubMed] [Google Scholar]
- 21.Iijima, Y., K. Ohno, H. Ikeda, K. Sawai, B. Levin, and D. Meruelo. 1999. Cell-specific targeting of a thymidine kinase/ganciclovir gene therapy system using a recombinant Sindbis virus vector. Int. J. Cancer 80:110-118. [DOI] [PubMed] [Google Scholar]
- 22.Irintchev, A., M. Zeschnigk, A. Starzinski-Powitz, and A. Wernig. 1994. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 199:326-337. [DOI] [PubMed] [Google Scholar]
- 23.Kochanek, S. 1999. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 10:2451-2459. [DOI] [PubMed] [Google Scholar]
- 24.Krasnykh, V., I. Dmitriev, G. Mikheeva, C. R. Miller, N. Belousova, and D. T. Curiel. 1998. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 72:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kravchuk, Z. I., A. A. Chumanevich, A. P. Vlasov, and S. P. Martsev. 1998. Two high-affinity monoclonal IgG2a antibodies with differing thermodynamic stability demonstrate distinct antigen-induced changes in protein A-binding affinity. J. Immunol. Methods 217:131-141. [DOI] [PubMed] [Google Scholar]
- 26.Kreppel, F., V. Biermann, S. Kochanek, and G. Schiedner. 2002. A DNA-based method to assay total and infectious particle contents and helper virus contamination in high-capacity adenoviral vector preparations. Hum. Gene Ther. 13:1151-1156. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lortat-Jacob, H., E. Chouin, S. Cusack, and M. J. van Raaij. 2001. Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor-ligand interactions. J. Biol. Chem. 276:9009-9015. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson, M. K., S. S. Hong, P. Boulanger, and L. Lindholm. 2001. Genetic retargeting of adenovirus: novel strategy employing “deknobbing” of the fiber. J. Virol. 75:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer, U., G. Saher, R. Fassler, A. Bornemann, F. Echtermeyer, H. von der Mark, N. Miosge, E. Poschl, and K. von der Mark. 1997. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat. Genet. 17:318-323. [DOI] [PubMed] [Google Scholar]
- 32.Miller, C. R., D. J. Buchsbaum, P. N. Reynolds, J. T. Douglas, G. Y. Gillespie, M. S. Mayo, D. Raben, and D. T. Curiel. 1998. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 58:5738-5748. [PubMed] [Google Scholar]
- 33.Morizono, K., G. Bristol, Y.-M. Xie, S. K.-P. Kung, and I. S. Y. Chen. 2001. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75:8016-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mottershead, D. G., K. Alfthan, K. Ojala, K. Takkinen, and C. Oker-Blom. 2000. Baculoviral display of functional scFv and synthetic IgG-binding domains. Biochem. Biophys. Res. Commun. 275:84-90. [DOI] [PubMed] [Google Scholar]
- 35.Nalbantoglu, J., N. Larochelle, E. Wolf, G. Karpati, H. Lochmuller, and P. C. Holland. 2001. Muscle-specific overexpression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J. Virol. 75:4276-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsson, B., T. Moks, B. Jansson, L. Abrahmsen, A. Elmblad, E. Holmgren, C. Henrichson, T. A. Jones, and M. Uhlen. 1987. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1:107-113. [DOI] [PubMed] [Google Scholar]
- 37.Ohno, K., K. Sawai, Y. Iijima, B. Levin, and D. Meruelo. 1997. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15:763-767. [DOI] [PubMed] [Google Scholar]
- 38.Ojala, K., D. G. Mottershead, A. Suokko, and C. Oker-Blom. 2001. Specific binding of baculoviruses displaying gp64 fusion proteins to mammalian cells. Biochem. Biophys. Res. Commun. 284:777-784. [DOI] [PubMed] [Google Scholar]
- 39.Patel, K., S. E. Moore, G. Dickson, R. J. Rossell, P. C. Beverley, J. T. Kemshead, and F. S. Walsh. 1989. Neural cell adhesion molecule (NCAM) is the antigen recognized by monoclonal antibodies of similar specificity in small-cell lung carcinoma and neuroblastoma. Int. J. Cancer 44:573-578. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds, P. N., S. A. Nicklin, L. Kaliberova, B. G. Boatman, W. E. Grizzle, I. V. Balyasnikova, A. H. Baker, S. M. Danilov, and D. T. Curiel. 2001. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 19:838-842. [DOI] [PubMed] [Google Scholar]
- 41.Ried, M. U., A. Girod, K. Leike, H. Buning, and M. Hallek. 2002. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 76:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Sanes, J. R. 1994. The extracellular matrix, p. 242-260. In A. G. Engel and C. Franzini-Armstrong (ed.), Myogenesis. McGraw-Hill, New York, N.Y.
- 44.Schiedner, G., S. Hertel, and S. Kochanek.2000. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 11:2105-2116. [DOI] [PubMed] [Google Scholar]
- 45.Schober, S., D. Mielenz, F. Echtermeyer, S. Hapke, E. Poschl, H. von der Mark, H. Moch, and K. von der Mark. 2000. The role of extracellular and cytoplasmic splice domains of α7-integrin in cell adhesion and migration on laminins. Exp. Cell Res. 255:303-313. [DOI] [PubMed] [Google Scholar]
- 46.Surolia, A., D. Pain, and M. I. Khan. 1982. Protein A: nature's universal anti-antibody. Trends Biochem. Sci. 7:74-76. [Google Scholar]
- 47.Thirion, C., N. Larochelle, C. Volpers, P. Holland, J. Nalbantoglu, S. Kochanek, and H. Lochmüller. 2002. Strategies for muscle-specific targeting of adenoviral gene transfer vectors. Neuromuscul. Disord. 12:S30-S39. [DOI] [PubMed] [Google Scholar]
- 48.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigne, E., I. Mahfouz, J.-F. Dedieu, A. Brie, M. Perricaudet, and P. Yeh. 1999. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J. Virol. 73:5156-5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins, S. J., V. V. Mesyanzhinov, L. P. Kurochkina, and R. E. Hawkins. 1997. The ′adenobody' approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 4:1004-1012. [DOI] [PubMed] [Google Scholar]
- 51.Wells, A. 1999. EGF receptor. Int. J. Biochem. Cell. Biol. 31:637-643. [DOI] [PubMed] [Google Scholar]
- 52.West, M. A., M. S. Bretscher, and C. Watts. 1989. Distinct endocytic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wickham, T. J., D. Segal, P. W. Roelvink, M. E. Carrion, A. Lizonova, G. M. Lee, and I. Kovesdi. 1996. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J. Virol. 70:6831-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 55.Wickham, T. J., P. W. Roelvink, D. E. Brough, and I. Kovesdi. 1996. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 14:1570-1573. [DOI] [PubMed] [Google Scholar]
- 56.Yao, C. C., J. Breuss, R. Pytela, and R. H. Kramer. 1997. Functional expression of the α7 integrin receptor in differentiated smooth muscle cells. J. Cell Sci. 110:1477-1487. [DOI] [PubMed] [Google Scholar]