Abstract
Lysophosphatidic acid (LPA) is a pluripotent lipid mediator acting through plasma membrane-associated LPAx receptors that transduce many, but not all, of its effects. We identify peroxisome proliferator-activated receptor γ (PPARγ) as an intracellular receptor for LPA. The transcription factor PPARγ is activated by several lipid ligands, but agonists derived from physiologic signaling pathways are unknown. We show that LPA, but not its precursor phosphatidic acid, displaces the drug rosiglitazone from the ligand-binding pocket of PPARγ. LPA and novel LPA analogs we made stimulated expression of a PPAR-responsive element reporter and the endogenous PPARγ-controlled gene CD36, and induced monocyte lipid accumulation from oxidized low-density lipoprotein via the CD36 scavenger receptor. The synthetic LPA analogs were effective PPARγ agonists, but were poor ones for LPA1, LPA2, or LPA3 receptor transfected cells. Transfection studies in yeast, which lack nuclear hormone and LPAx receptors, show that LPA directly activates PPARγ. A major growth factor of serum is LPA generated by thrombin-activated platelets, and media from activated platelets stimulated PPARγ function in transfected RAW264.7 macrophages. This function was suppressed by ectopic LPA-acyltransferase expression. LPA is a physiologic PPARγ ligand, placing PPARγ in a signaling pathway, and PPARγ is the first intracellular receptor identified for LPA. Moreover, LPA produced by stimulated plasma platelets activates PPARγ in nucleated cells.
Lysophosphatidic acid (LPA) is a pluripotent lipid mediator controlling growth, motility, and differentiation (1, 2). It is the ovarian cancer-activating factor that is elevated in the serum of ovarian cancer patients (3), and it controls adipogenesis (4). LPA also is generated during platelet activation (5) to become a major growth factor of serum. LPA stimulates three G protein-linked, plasma membrane-associated receptors [LPA1, LPA2, and LPA3, formerly edg2, edg4, and edg7 (6)] that recognize extracellular LPA (7). However, control of complex processes including growth and differentiation is difficult to reconcile with these receptors (8), suggesting that undiscovered receptors for LPA may exist. LPA is a central component of cellular phospholipid metabolism and, because a role for intracellular LPA beyond this is unknown, the plasma membrane separates signaling LPA from metabolic LPA.
The transcription factor peroxisome proliferator-activated receptor γ (PPARγ) regulates genes that in general control energy metabolism (9). PPARγ, like other members of its extended nuclear hormone receptor superfamily, is activated by binding an appropriate lipid ligand (10). Synthetic compounds, including the widely prescribed drug rosiglitazone, target PPARγ and activate transcription with high affinity. Anionic fatty acids and their oxidized derivatives also bind and activate PPARγ (11), but they do so through low-affinity interactions (12). Although a number of biologic ligands for PPARγ are known, these ligands do not provide signal amplification because the products are about as potent as their precursors.
PPARγ agonists arising from physiologic signaling pathways remain elusive, but a pathologic agonist is known. Uncontrolled free radical oxidation of low-density lipoprotein (LDL) fragments its phospholipids, and one of these phospholipid oxidation products, hexadecyl azelaic phosphatidylcholine (azPC), binds and activates PPARγ as potently as the drug rosiglitazone (13). azPC differs from its inactive phospholipid precursor in that it has a shortened sn-2 residue that is also anionic. Oxidatively fragmented phospholipids are found in atherosclerotic lesions (14) and in the circulation after exposure to primary (15) or second-hand (16) cigarette smoke, but are unlikely to constitute PPARγ ligands under normal conditions. Knowing that an anionic phospholipid was a PPARγ agonist, we examined other anionic phospholipids integrated in signaling pathways for this property.
Materials and Methods
Binding Analysis.
[3H]azPC and [3H]rosiglitazone were synthesized and then incubated with immobilized His6-PPARγ1 in the presence or absence of a 20-fold molar excess (6.7 μM) of the stated phospholipids (Avanti Polar Lipids, Alabaster, AL) or oxidized fatty acids (Cayman Chemical, Ann Arbor, MI) as described (13).
Transfection Assays.
RAW264.7 monocytic cells (American Type Culture Collection) were transiently transfected (13) for 4 h with 0.1 μg of a simian virus 40 (SV40)-β-galactosidase reporter and 1 μg of an acyl-CoA oxidase-luciferase, a CD36−273-luciferase reporter with its peroxisomal proliferator-responsive element (PPRE), or a CD36−261 reporter that lacks just this element before stimulation overnight with 5 μM agonist. Some experiments included 0.25 μg of empty pcDNAI, pcDNAI expression plasmids encoding LPA acyltransferase (17), or PPARγ (13). Saccharomyces cerevisiae was transfected with lithium acetate and rat acyl-CoA oxidase PPRE-β-galactosidase reporter, PPARγ, and retinoid X receptor (RXR) before selection in nutrient-deficient media (18). Overnight cultures were supplemented with the stated lipid for 16 h before harvesting and analyzing β-galactosidase levels with o-nitrophenyl β-d-galactopyranoside.
LPAx Receptor Activation.
LPA receptor activation was evaluated by examining Ca2+ flux in fura-2-loaded Sf9 cells expressing LPA1, LPA2, or LPA3 by excitation at 340 and 380 nm with emission monitored at 500 nm as described (19).
Chemical Synthesis.
Hexadecyl lysophosphatidylcholine was prepared as described (20). Translocase-3 {Tris[2-(2-naphthalenesulfonamido)ethyl]amine} was prepared as described (21). Total synthesis of enantiomerically pure XY4 [1,1-difluorodeoxy-(2R)-palmitoyl-sn-glycero-3-phosphate] was achieved in nine steps, with 21% overall yield, from protected d-mannitol. XY4 is an analog of sn-2-palmitoyl LPA where the sn-1-hydroxyl group is replaced by two electronegative fluorine atoms to prevent acyl migration. Its synthesis (22) involves difluorination of an aldehyde intermediate, selective deprotection of a glyceryl bissilyl ether, and deprotection of dimethyl phosphate by trimethylsilyl bromide, after neutralization the free phosphoric acid was obtained and characterized by 1H-, 13C-, and 31P-NMR and by high-resolution mass spectrometry. XY8 is an analog of sn-1-palmitoyl LPA. Synthesis of enantiomerically pure XY8 [1-palmitoyl-(2R)-fluorodeoxy-sn-glycero-3-phosphate] involves selective protection of the primary alcohol and deoxyfluorination of the secondary alcohol; after deprotection as with XY4, an ion exchange step afforded the sodium salt after seven steps in 18% overall yield from (R)-isopropylideneglycerol (Y.X., L. Qian, and G.D.P., unpublished work).
Monocyte Manipulation.
Monocytes were elutriated from human blood (23). For oxidized LDL accumulation, the cells were immobilized on anti-ICAM3 (BBA29, R & D Systems) on plastic chamber slides for 2 h as before (24) to induce PPARγ, and then stimulated, or not, with 5 μM oleoyl LPA for 16 h. After this, the cells were exposed to 50 μg/ml oxidized LDL (25) for 30 min before fixation and staining with oil red O. Total cellular cholesterol was determined with a Sigma60 cholesterol oxidase kit (Sigma), whereas protein content was determined with Coomassie blue (Pierce). Flow cytometry of surface CD36 protein used FITC-conjugated CLB-IVC7 antibody (Accurate Chemicals) (24). CD36 function was blocked by pretreating monocytes with 5 μg/ml 185-1G2 antibody (Neomarkers, Fremont, CA) for 30 min, before cholesterol loading and analysis as described above.
Platelet Stimulation.
Platelet-rich plasma was isolated from the blood of healthy volunteers by centrifugation for 20 min at 200 × g, and 3 × 108 platelets per ml were then stimulated with 0.1 units/ml thrombin. Prostaglandin E1 (0.1 μM) was added to control platelets to avoid activation. After this, platelets were removed by centrifugation at room temperature for 10 min at 500 × g, and the supernatant was tested on transfected RAW264.7 cells at a dilution of either 1:10 or 1:100.
Results
LPA Binds and Activates PPARγ.
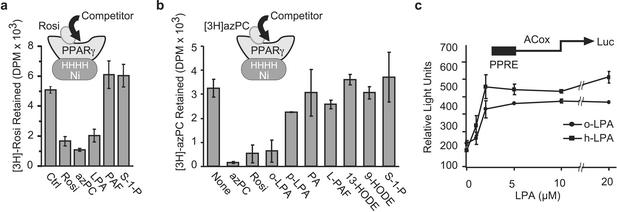
We used competition binding to test for PPARγ ligands. We immobilized recombinant human PPARγ1 on Ni+ chelate beads and allowed [3H]rosiglitazone to bind [crystallography shows it immobilized in the ligand binding pocket (26, 27)] in the absence or presence of a 20-fold molar excess of various unlabeled lipids. Unlabeled rosiglitazone and azPC, a synthetic anionic oxidized phospholipid that is a PPARγ ligand (13), displaced [3H]rosiglitazone from PPARγ (Fig. 1a). We found that the anionic phospholipid LPA also competed with [3H]rosiglitazone for binding to PPARγ, but that neither sphingosine 1-phosphate nor the water-soluble phospholipid platelet-activating factor did so. We obtained similar results when we tested for ligand competition using [3H]azPC. LPA effectively displaced [3H]azPC from immobilized PPARγ (Fig. 1b), with a preference for the more soluble oleoyl homolog. The diacyl phospholipid phosphatidic acid did not compete for [3H]rosiglitazone binding, nor did the zwitterionic lipids lysoPAF (hexadecyl phosphatidylcholine) or sphingosine 1-phosphate. Neither 9- nor 13-hydroxyoctadecadienoic acid was an effective competitor, at just a 20-fold excess, as anticipated from their affinity relative to rosiglitazone.
Figure 1.
LPA is a PPARγ ligand and agonist. LPA competes for [3H]rosiglitazone (a) or [3H]azPC (b) binding to immobilized PPARγ. His6-PPARγ1 was immobilized on Ni+ beads and incubated with [3H]rosiglitazone or [3H]azPC in the presence or absence of a 20-fold molar excess of the stated lipids. Rosi, rosiglitazone; o-LPA, oleoyl LPA; p-LPA, palmitoyl LPA; PA, phosphatidic acid; PAF, 1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine; L-PAF, 1-O-hexadecyl-sn-glycero-3-phosphocholine; HODE, hydroxyoctadecadienoic acid; S-1-P, sphingosine 1-phosphate. (c) LPA induces a concentration-dependent increase in PPRE-luciferase reporter expression. RAW 264.7 monocytic cells were transfected with rat acyl-CoA oxidase PPRE-luciferase and SV40-β-galactosidase reporter plasmids. The transfected cells were treated with the stated amount of oleoyl LPA (o-LPA) or sn-1-hexadecyl LPA (h-LPA) for 16 h before the relative amounts of luciferase and β-galactosidase were determined. All luciferease data are reported as relative light units after normalization for transfection efficiency with β-galactosidase.
We determined whether the binding of 1-oleoyl LPA to PPARγ initiated transcription from a PPRE. We transiently transfected the RAW264.7 macrophage-like cell line with a luciferase reporter gene controlled by the canonical acyl-CoA oxidase PPRE to find (Fig. 1c) that LPA stimulated this reporter. The effect of LPA depended on its concentration and was maximally effective by 2 μM, a concentration well below that found in serum (28). There was a slight preference for the sn-1 ether over the ester homolog, but both LPAs were agonists. The PPAR subfamily contains PPARα and PPARβ/δ that also act on PPREs, but only cotransfection with PPARγ enhanced the effect of LPA or rosiglitazone (data not shown).
Exogenous LPA Has an Intracellular Site of Action.
LPA1, LPA2, and LPA3 are plasma membrane receptors that recognize extracellular LPA (7), whereas PPARγ requires an intracellular, indeed intranuclear, agonist. To distinguish between intracellular and extracellular sites of action, we first enhanced access of LPA to the intracellular compartment. We found that a tridentate sulfonamid (Fig. 2a), with the potential to hydrogen bond with the anionic phosphate group and thereby mask it, enhanced LPA stimulation of the PPRE reporter (Fig. 2b) just as a related Tris-sulfonamid translocase enhanced the effect of azPC (13). The LPA translocase by itself did not stimulate reporter expression, nor did it affect reporter induction when the agonist was rosiglitazone.
Figure 2.
Intracellular LPA acyltransferase expression blocks LPA stimulation of a PPRE reporter, and novel nonacylatable LPA analogs are PPAR agonists. (a) Structure of: XY4, 1,1-difluorodeoxy-(2R)-oleoyl-sn-glycero-3-phosphate; XY8, 1-palmitoyl-(2R)-fluorodeoxy-sn-glycero-3-phosphate; (Left) translocase-3 Tris[2-(2-naphthalenesulfonamido)ethyl]amine interacting with oleoyl LPA. (b) A small organic cationic translocase enhances exogenous LPA stimulation of a PPRE reporter. RAW264.7 cells were transfected with an acyl-CoA oxidase-PPRE reporter and treated with 5 μM rosiglitazone, oleoyl LPA, or an sn-2 (XY4) or sn-1 (XY8) LPA analog. Some wells were additionally treated with 35 μM translocase-3. Normalized luciferase expression was determined as before. (c) LPA acyltransfersase suppresses LPA, but not nonacylatable LPA analog, stimulation of a PPRE reporter. RAW264.7 cells were transfected with acyl-CoA oxidase-PPRE and SV40-β-galactosidase reporters, and, for some cells, an LPA acyltransferase (LPAAT) pcDNAI expression construct before their response to the stated agonist was determined as above.
Support for an intracellular site of action for LPA was provided by a converse approach in which we suppressed intracellular levels of LPA through the overexpression of LPA acyltransferase (17). This metabolic enzyme enhances the flux of LPA to phosphatidic acid, a lipid that was not a PPARγ ligand (vide supra), by catalyzing the condensation of LPA with intracellular acyl-CoA. LPA acyltransferase expression completely suppressed LPA, but not rosiglitazone or azPC, stimulation of the acyl-CoA oxidase PPRE reporter (Fig. 2c).
Synthetic LPA Analogs Activate PPRE Function.
The tumor promoter ovarian cancer-activating factor is sn-2 LPA (29) rather than the more common sn-1 LPA isomer we used in the experiments above. sn-2 LPA activates LPA2 and LPA3 receptors (19), but studies of positional specificity are complicated by a rapid chemical equilibrium favoring the sn-1 isomer by almost 6-fold. To circumvent this intramolecular rearrangment, we synthesized sn-1 and sn-2 LPA analogs where one hydroxyl group was conservatively changed to a fluoro moiety to give LPA analogs XY4 and XY8 (Fig. 2a) that cannot undergo acyl migration or further acylation. We found (Fig. 2 b and c) that the fluorodeoxy LPA analogs induced luciferase expression from the acyl-CoA oxidase PPRE reporter. We established that the effective concentrations of XY4, XY8, and LPA were equivalent (data not shown), so substitution of fluorine for the hydroxyl of LPA was an effective strategy to create stabilized LPA mimetics. There was no positional specificity for the acyl chain because both the sn-1-like (XY8) and sn-2-like (XY4) analogs were equally effective PPARγ agonists. We also found oleoyl analogs of XY4 and XY8 were effective PPARγ agonists (data not shown), so there was no specificity for the acyl residue. The Tris-sulfonamid translocase enhanced the effect of XY4 and XY8 in stimulating the PPRE reporter (Fig. 2b), as anticipated from the free phosphoryl group of XY4 and XY8. XY4 and XY8 cannot be acylated, so intracellular expression of LPA acyltransferase did not change the response to either of these two stable LPA analogs (Fig. 2c).
LPA Is a Direct, Rather Than Indirect, PPARγ Agonist.
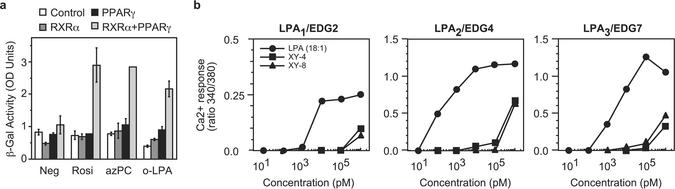
We sought a way to show that LPA directly acts on PPARγ, rather than stimulating the formation of an unknown endogenous ligand through an LPAx receptor. We also sought a way to circumvent the possibility that other nuclear hormone receptors, which might physically interact with PPARγ, might bind LPA and then indirectly stimulate PPARγ function. We accomplished these goals in two ways; the first was through the use of S. cerevisiae, an organism that lacks all nuclear hormone receptors and contains only a single G protein-linked receptor for mating factor. Yeast will accumulate intact anionic phospholipids from their media (30) and can be stably transfected with a PPARγ reporting system (18). We found (Fig. 3a) that yeast engineered to contain PPARγ, RXR, and the β-galactosidase PPRE reporter expressed the reporter in response to the PPARγ agonists rosiglitazone and azPC, and they expressed the reporter in response to LPA. Strains transfected with just the PPRE reporter, or the reporter and just PPARγ or just RXR, showed no basal reporter expression. The three strains lacking the PPARγ/RXR heterodimer also did not respond to rosiglitazone, azPC, or LPA, confirming the lack of confounding receptors in S. cerevisiae.
Figure 3.
LPA is a direct PPARγ agonist. (a) LPA stimulates PPRE reporter transcription in S. cerevisiae after ectopic expression of PPARγ and RXR. S. cerevisiae was selected to stably express a PPRE-β-galactosidase reporter alone or the reporter and RXR, PPARγ, or both PPARγ and RXR. These cells were then incubated with the stated agonist for 16 h before the amount of β-galactosidase produced was quantified. (b) XY4 and XY8 are ineffective LPA1, LPA2, or LPA3 agonists. Sf9 cells were stably transfected with one of the three LPAx receptors, loaded with the Ca2+-sensitive dye fura-2, and then stimulated with the stated amount of LPA, XY4, or XY8 before changes in intracellular Ca2+ levels were determined from the ratio of fluorescence after excitation at two wavelengths.
We next tested whether G protein-linked receptors for LPA could be excluded from a role in PPARγ activation. We stably transfected Sf9 insect cells, derived from an organism that lacks all G protein-linked LPA receptors, with messages for LPA1, LPA2, or LPA3. The transfected cells responded to LPA with a transient intracellular Ca2+ flux as anticipated (Fig. 3b). In contrast, the migration-stabilized LPA analogs XY4 and XY8 were not effective agonists for these receptors and were two to four orders of magnitude less active than oleoyl LPA. Because XY4 and XY8 were effective PPARγ agonists, these LPA analogs did not act through LPAx receptors.
LPA Induces CD36 Expression and Foam Cell Formation.
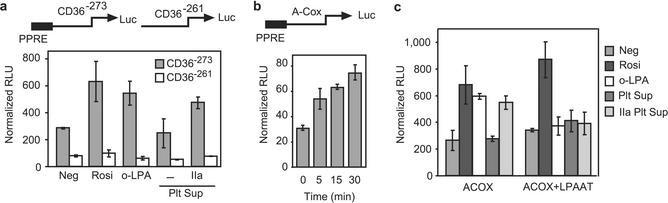
LPA accumulates in atherosclerotic lesions (31), a location enriched with PPARγ (32), and PPARγ is required for regulated CD36 expression (33, 34). Monocyte accumulation of oxidized LDL by CD36 (35, 36) leads to intracellular lipid droplet accumulation and foam cell formation that are characteristic of these lesions. We found that human monocytes stimulated with LPA and then exposed to oxidized LDL as a cholesterol source rapidly accumulated excessive intracellular neutral lipid stores that stained with oil red O (Fig. 4a). Human monocytes express a low level of CD36 on their surface, which increases after exposure to troglitazone (33, 34) or azPC (13). We found (Fig. 4b) that LPA also stimulated the expression of CD36 on the surface of human monocytes. The CD36 promoter contains a functional PPRE (13, 35, 37); and a reporter construct containing this PPRE, but not a truncated version lacking just this element, responded to LPA stimulation (Fig. 4c). The LPA analogs XY4 and XY8 also induced expression of the luciferase reporter controlled by the CD36 promoter when it contained the PPRE, but failed to do so when the construct lacked this element (Fig. 4c). Chemical quantitation of cellular cholesterol levels showed that LPA or rosiglitazone treatment doubled cellular cholesterol content (Fig. 4d), and that a blocking anti-CD36 antibody prevented this increase.
Figure 4.
LPA stimulates lipid accumulation, CD36 expression, and oxidized LDL uptake through a PPAR-responsive element. (a) LPA stimulates monocyte uptake of oxidized LDL. Freshly elutriated human monocytes were allowed to interact with an anti-ICAM3-coated well, which leads to rapid PPARγ expression (13), and then stimulated, or not (negative, oxLDL), with oleoyl LPA. Some cells were then briefly exposed to oxidized LDL before intracellular lipid stores were visualized with oil red O stain. (b) LPA increases the expression of CD36 on the surface of primary human monocytes. Monocytes engaging anti-ICAM3 were treated or not with LPA, and then recovered by gentle scraping and washing by centrifugation before their surface CD36 was assessed by flow cytometry. (c) LPA and the LPA analogs XY4 and XY8 stimulate CD36 promoter function only when the PPRE is present. RAW264.7 cells were transfected with the human CD36 promoter containing the PPRE (CD36−273) or a reporter that lacks only this element (CD36−261) and then stimulated with oleoyl LPA, azPC, XY4, or XY8. Expression of luciferase normalized to β-galactosidase was determined as above. (d) Anti-CD36 blocks LPA-stimulated accumulation of cholesterol from oxidized LDL. Freshly isolated human monocytes were treated as in a, but after being preincubated with a blocking anti-CD36 antibody before exposure to oxidized LDL.
LPA Is a Transcellular Lipid Mediator.
Aggregating platelets generate LPA through the release of lipases that act on plasma lipids, creating the micromolar levels of LPA in serum (28). We stimulated human platelets in platelet-rich plasma with thrombin, or not, and then collected cell-free supernatants by centrifugation. RAW264.7 monocytic cells previously transfected with the PPRE-containing CD36 reporter responded to these platelet supernatants by expressing the luciferase reporter just as they did when stimulated with synthetic LPA or rosiglitazone (Fig. 5a). None of these agents stimulated reporter expression when the CD36 regulatory region lacked the PPRE. The supernatants collected from activated platelets also stimulated reporter expression from the acyl-CoA oxidase reporter (Fig. 5b), and the formation of this PPARγ agonist from activated platelets was rapid; the PPARγ agonist appeared within 5 min after thrombin stimulation, with a modest increase thereafter. Thrombin by itself had no effect on the transfected RAW264.7 cells (data not shown). The response to the PPARγ agonist generated by activated platelets, like the response to LPA, was completely abolished when the reporter cells had been cotransfected with LPA acyltransferase (Fig. 5c). We conclude that platelets, like ovarian cancer cells (3) and adipocytes (4), produce LPA as a paracrine signal that can modulate transcription in distal target cells through PPARγ.
Figure 5.
Extracellular LPA generated by platelet activation stimulates PPRE reporter expression in monocytic cells. (a) Activated platelets generate an agonist that stimulates a CD36 reporter through its PPRE. Human platelet-rich plasma was treated, or not, with activated thrombin, and platelet-free serum was recovered and tested, as a 10% addition to the medium, for the ability to stimulate luciferase expression in RAW264.7 cells previously transfected with CD36−273 or CD36−261 reporters that do or do not, respectively, contain the PPRE. Rosiglitazone and oleoyl LPA were the positive controls for PPARγ activation. (b) Accumulation of a PPARγ agonist(s) in platelet-rich plasma is time-dependent. Human platelet-rich plasma was treated with activated thrombin for the stated times before addition, at a 100-fold dilution, to RAW264.7 cells transfected with acyl-CoA oxidase PPRE luciferase and SV40-β-galactosidase reporters. (c) LPA acyltransferase expression suppresses the response of a PPRE reporter to the supernatants of thrombin-activated platelets. RAW264.7 cells were transfected with acyl-CoA oxidase PPRE and SV40-β-galactosidase reporters and, for some cells, with an LPA acyltransferase (LPAAT) expression construct. These cells were treated with the supernatant from unactivated or thrombin-activated platelets as in a.
Discussion
PPARγ is not an orphan nuclear hormone receptor, but neither is it currently integrated in a pathway displaying signal amplification. The primary mode of PPARγ activation is the conformational change induced by a lipid ligand, and many lipids that bind and stimulate PPARγ function are known, but all are low-affinity ligands that are unlikely to be bona fide ligands (10). Thus various arachidonate and linoleate oxygenated metabolites bind and activate PPARγ, but because their Kd values are in the same low micromolar range (38) as their unmodified parent compounds there is little signal amplification achieved by their modification (12). Cellular metabolism of LPA (to free fatty acids, for instance) was not responsible for the PPARγ activation we observed, because a phospholipase A1-insensitive sn-1 ether homolog of LPA was an effective PPARγ agonist, as were LPA analogs unable to undergo acyl migration or acylation to phosphatidic acid.
LPA is formed and released from cells in response to physiologic signals, and, because its precursor phosphatidic acid was not a PPARγ ligand, provides signal amplification for PPARγ activation just as it does for LPAx receptors. We found PPARγ, like the LPA receptors LPA2 and LPA3 (19), was activated by either sn-1 or sn-2 LPA homologs but also by alkyl LPA with an sn-1 ether bond. PPARγ also did not display specificity for an unsaturated acyl residue, so it appears that an anionic phosphoryl group is a primary determinant for PPARγ activation by LPA. PPARγ, therefore, responds to a wider range of LPA homologs than any single LPAx receptor (19).
Our experiments show extracellular LPA has access to nuclear PPARγ, so externally generated LPA can affect PPARγ-controlled genes in surrounding cells. LPA is produced extracellularly by lipoprotein oxidation (31) or by the action of secretory phospholipases A2 on microvesicles released from activated cells (39). LPA also is produced in plasma by thrombin-activated platelets (5) through the stimulated release of phospholipase A1 (5, 40), phospholipase A2 (5), and lysophospholipase D (autotaxin) (41, 42), which act on plasma lipids or secreted lysophosphatidylcholine (42). Whereas plasma contains undetectable amounts of LPA, its concentration in serum is several micromolar (28). Serum levels of LPA increase over gestation (43) and in ovarian tumorigenesis (44), although this also may reflect platelet activation and attendant LPA production (45). Extracellular LPA also derives from stimulated ovarian cancer cells (29) and potentially other cancer cells (42). LPA, therefore, can couple activated tumor cells or platelets (28) to PPARγ stimulation and gene regulation in neighboring as well as distal target cells.
Acknowledgments
We thank John Capone (McMaster University, Hamilton, ON, Canada) for providing the S. cerevisiae reporter and appropriate RXR and PPARγ expression plasmids, Li-in Wang for performing the S. cerevisiae selections, and David Stillman (University of Utah) for providing expertise and help with this system. We are grateful to Larry Tjoelker (ICOS Corp., Bothell, WA) for generously providing the LPA acyltransferase plasmids and a careful reading of the manuscript. We appreciate the technical aid of Donnie Benson, Jennifer Eyre, and Margaret Vogel, and hexadecyl LPA preparation by Haiying Zhang. We thank Diana Lim for graphic artwork. This work was supported by National Institutes of Health Grants HL 44513 and HL 44525, a PEW Latin American Fellowship, the Utah Center of Excellence, and the Human Frontier Science Program.
Abbreviations
- LPA
lysophosphatidic acid
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
peroxisomal proliferator-responsive element
- RXR
retinoid X receptor
- LDL
low-density lipoprotein
- azPC
hexadecyl azelaic phosphatidylcholine
- SV40
simian virus 40
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goetzl E J, Lee H, Dolezalova H, Kalli K R, Conover C A, Hu Y L, Azuma T, Stossel T P, Karliner J S, Jaffe R B. Ann NY Acad Sci. 2000;905:177–187. doi: 10.1111/j.1749-6632.2000.tb06549.x. [DOI] [PubMed] [Google Scholar]
- 2.Moolenaar W H. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 3.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, et al. Ann NY Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 4.Pages G, Girard A, Jeanneton O, Barbe P, Wolf C, Lafontan M, Valet P, Saulnier-Blache J S. Ann NY Acad Sci. 2000;905:159–164. doi: 10.1111/j.1749-6632.2000.tb06547.x. [DOI] [PubMed] [Google Scholar]
- 5.Sano T, Baker D L, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G J. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 6.Chun J, Goetzl E J, Hla T, Igarashi Y, Lynch K R, Moolenaar W, Pyne S, Tigyi G. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 7.Goetzl E J, An S. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- 8.Hooks S B, Santos W L, Im D S, Heise C E, Macdonald T L, Lynch K R. J Biol Chem. 2000;276:4611–4621. doi: 10.1074/jbc.M007782200. [DOI] [PubMed] [Google Scholar]
- 9.Auwerx J. Diabetologia. 1999;42:1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 10.Rosen E D, Spiegelman B M. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 11.Desvergne B, Wahli W. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 12.Spiegelman B M. Cell. 1998;93:153–155. doi: 10.1016/s0092-8674(00)81567-6. [DOI] [PubMed] [Google Scholar]
- 13.Davies S S, Pontsler A V, Marathe G K, Harrison K A, Murphy R C, Hinshaw J C, Prestwich G D, St. Hilaire A, Prescott S M, Zimmerman G A, McIntyre T M. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 14.Watson A D, Leitinger N, Navab M, Faull K F, Horkko S, Witztum J L, Palinski W, Schwenke D, Salomon R G, Sha W, et al. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 15.Imaizumi T, Satoh K, Yoshida H, Kawamura H, Hiramoto M, Takamatsu S. Atherosclerosis. 1991;87:47–55. doi: 10.1016/0021-9150(91)90231-q. [DOI] [PubMed] [Google Scholar]
- 16.Lehr H A, Weyrich A S, Saetzler R K, Jurek A, Arfors K E, Zimmerman G A, Prescott S M, McIntyre T M. J Clin Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhardt C, Gray P W, Tjoelker L W. Adv Exp Med Biol. 1999;469:351–356. doi: 10.1007/978-1-4615-4793-8_51. [DOI] [PubMed] [Google Scholar]
- 18.Kassam A, Hunter J, Rachubinski R A, Capone J P. Mol Cell Endocrinol. 1998;141:153–162. doi: 10.1016/s0303-7207(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 19.Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. FEBS Lett. 2000;478:159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D J, Weintraub S T, Friedberg S J, Tokumura A, Ayer D E. J Lipid Res. 1985;26:1345–1355. [PubMed] [Google Scholar]
- 21.Valiyaveettil S, Engbersen J F J, Verboom W, Reinhoudt D N. Angew Chem Int Ed Engl. 1993;32:900–901. [Google Scholar]
- 22.Xu Y, Prestwich G D. J Org Chem. 2002;67:7158–7161. doi: 10.1021/jo0203037. [DOI] [PubMed] [Google Scholar]
- 23.Elstad M R, Prescott S M, McIntyre T M, Zimmerman G A. J Immunol. 1988;140:1618–1624. [PubMed] [Google Scholar]
- 24.Pontsler A V, St. Hilaire A, Marathe G K, Zimmerman G A, McIntyre T M. J Biol Chem. 2002;277:13029–13036. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 25.Heery J M, Kozak M, Stafforini D M, Jones D A, Zimmerman G A, McIntyre T M, Prescott S M. J Clin Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolte R T, Wisely G B, Westin S, Cobb J E, Lambert M H, Kurokawa R, Rosenfeld M G, Willson T M, Glass C K, Milburn M V. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 27.Gampe R T, Montana V G, Lambert M H, Miller A B, Bledsoe R K, Milburn M V, Kliewer S A, Wilson T M, Xu H E. Mol Cell. 2000;5:545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 28.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar W H. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Gaudette D C, Boynton J D, Frankel A, Fang X J, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, et al. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 30.Trotter P J. Traffic. 2000;1:425–434. doi: 10.1034/j.1600-0854.2000.010507.x. [DOI] [PubMed] [Google Scholar]
- 31.Siess W, Zangl K J, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum J L, Auwerx J, Palinski W, Glass C K. Proc Natl Acad Sci USA. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans R M. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 34.Moore K J, Rosen E D, Fitzgerald M L, Randow F, Andersson L P, Altshuler D, Milstone D S, Mortensen R M, Spiegelman B M, Freeman M W. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 35.Teboul L, Febbraio M, Gaillard D, Amri E Z, Silverstein R, Grimaldi P A. Biochem J. 2001;360:305–312. doi: 10.1042/0264-6021:3600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Febbraio M, Hajjar D P, Silverstein R L. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tontonoz P, Nagy L, Alvarez J G, Thomazy V A, Evans R M. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 38.Xu H E, Lambert M H, Montana V G, Parks D J, Blanchard S G, Brown P J, Sternbach D D, Lehmann J M, Wisely G B, Willson T M, et al. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 39.Fourcade O, Simon M F, Viode C, Rugani N, Leballe F, Ragab A, Fournie B, Sarda L, Chap H. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 40.Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, Taguchi R, Inoue K, Arai H. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- 41.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 42.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills G B, Inoue K, Aoki J, Arai H. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokumura A, Kanaya Y, Miyake M, Yamano S, Irahara M, Fukuzawa K. Biol Reprod. 2002;67:1386–1392. doi: 10.1095/biolreprod.102.004051. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Shen Z, Wiper D W, Wu M, Morton R E, Elson P, Kennedy A W, Belinson J, Markman M, Casey G. J Am Med Assoc. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 45.Baker D L, Morrison P, Miller B, Riely C A, Tolley B, Westermann A M, Bonfrer J M, Bais E, Moolenaar W H, Tigyi G. J Am Med Assoc. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]