Abstract
β-Arrestin 1 is required for internalization and mitogen-activated protein (MAP) kinase activation by the β2 adrenergic receptor (β2AR). Our previous studies have shown that chronic insulin treatment down-regulates cellular β-arrestin 1 levels, leading to a marked impairment in G protein-coupled receptor and insulin-like growth factor-1 receptor-mediated MAP kinase and mitogenic signaling. In this study, we show that chronic insulin-treated, β-arrestin 1depleted 3T3-L1 adipocytes display (i) increased isoproterenol-induced cAMP generation (53 ± 38% at 1.5 min, 25 ± 19% at 5 min, 63 ± 14% at 30 min, and 59 ± 2% at 60 min), a Gαs-associated pathway; (ii) impaired isoproterenol-induced β2AR internalization (reduced by 98 ± 4%), which is required for MAP kinase signaling, a Gαi-associated pathway; and (iii) increased β-arrestin 1 phosphorylation at Ser-412. Taken together, these findings represent a hitherto unknown mechanism (degradation and phosphorylation of β-arrestin, whereby the activation of the insulin receptor, belonging to the family of receptor tyrosine kinases, causes supersensitization of Gαs-associated signaling and inhibition of Gαi-associated signaling by the β2AR, a prototypical G protein-coupled receptor.
The β2 adrenergic receptor (β2AR) is a prototypical G protein-coupled receptor (GPCR), and its mechanism of action has been studied extensively (1, 2). After ligand binding, the intracellular domains of GPCRs interact with regulatory heterotrimeric G proteins, which then dissociate into a GTP-bound α-subunit and a βγ-subunit. After isoproterenol stimulation of the β2AR, the Gα-subunit Gαs, through exchange of GTP for GDP, activates adenylyl cyclase, generating cyclic adenosine monophosphate (cAMP). In the adipocyte, cAMP activates protein kinase A, which, in turn, phosphorylates and activates hormone-sensitive lipase, the rate-limiting enzyme for triglyceride hydrolysis (3).
Another important feature of the β2AR, and GPCRs in general, is desensitization after repeated or prolonged ligand stimulation. One mechanism by which desensitization is accomplished is termed homologous desensitization (4, 5). A GPCR kinase (GRK) rapidly phosphorylates the agonist-occupied β2AR on serine and threonine residues. Once phosphorylated by the GRK, the receptor can bind β-arrestin 1, a regulatory protein that prevents further β2AR/Gαs coupling, reducing the amount of cAMP generated in response to a β2AR ligand. β2AR-bound β-arrestin 1 also displays a high binding affinity for clathrin, targeting the β2AR to clathrin-coated pits for internalization after ligand stimulation (6). β-Arrestin 1 also recruits Src kinase to the β2AR. The β-arrestin 1-mediated association of Src with the β2AR is required for activation of the mitogen-activated protein kinase (MAPK) pathway by β2AR ligands (7).
We have shown (8) that chronic insulin treatment leads to proteasomal-dependent down-regulation of β-arrestin 1 protein levels in rat fibroblasts and in 3T3-L1 adipocytes. Because β-arrestin 1 brings Src to the GPCR and initiates signaling to the Ras/MAPK pathway, we predicted that down-regulation of β-arrestin 1 would cause impaired GPCR-mediated MAPK activation. Indeed, this was the case, with marked attenuation of MAPK phosphorylation by ligands of two β-arrestin 1-regulated GPCRs (the β2AR and the lysophosphatidic acid receptor) in chronic insulin-treated, β-arrestin 1-depleted cells. The other major function of β-arrestin 1 is to desensitize the receptor to further heterotrimeric G protein signaling. Therefore, we hypothesized that β2AR/Gαs coupling, leading to activation of adenylyl cyclase and the generation of cAMP, would be enhanced in β-arrestin 1 down-regulated cells because of impaired receptor desensitization. In support of this idea, we now report that chronic insulin-treated, β-arrestin 1-depleted 3T3-L1 adipocytes display enhanced cAMP production in response to isoproterenol. Furthermore, we have found that isoproterenol-induced internalization of the β2AR and dephosphorylation of β-arrestin 1 at Ser-412 are blunted in β-arrestin 1 down-regulated cells.
Materials and Methods
Materials.
Anti-β-arrestin 1 antibody was purchased from Transduction Laboratories (Lexington, KY). Anti-phosphorylated β-arrestin 1 (Ser-412) was purchased from Cell Signaling Technology (Beverly, MA). Anti-β2AR antibody and horseradish peroxidase-linked anti-mouse antibody were purchased from Santa Cruz Biotechnology. The cAMP enzyme immunoassay system was from Amersham Pharmacia Biotech (Buckinghamshire, England). [3H]CGP-12177 was from Perkin–Elmer. All other reagents were from Sigma.
Cell Culture.
3T3-L1 adipocytes were cultured and differentiated as described (9) and were used between day 10 and day 14.
cAMP Immunoassay.
3T3-L1 adipocytes were placed in serum-free media for 8 h in the presence or absence of 100 ng/ml insulin. Isoproterenol (10 μM) was then added to the media for the indicated times. The reaction was terminated by rapid dilution and washing with ice-cold PBS. Intracellular cAMP protein content was then determined as recommended by the manufacturer. Final cAMP concentrations per well were corrected for total protein per well, as determined by the Bradford assay.
Ligand Binding Assay.
Ligand binding was determined by using the hydrophilic β2AR ligand [3H]CGP-12177 as described (10), with some modification. 3T3-L1 adipocytes were starved for 12 h in the presence or absence of insulin. Isoproterenol (10 μM) was then added to the media for an additional 15 min, and the reaction was terminated by rapid dilution and washing with PBS. After the final wash, cells were placed in 500 μl of binding buffer (DMEM with 20 μM Hepes, pH 7.4, and 10 nM CGP-12177) with 1 μl/ml [3H]CGP-12177 per well, and incubated at 4°C for 6 h. Cells were then washed again on ice with 3× PBS. Acid extraction of membrane-bound ligand was performed by two successive 10-min incubations with 1 ml of acidic buffer (10 mM Hepes/2.5 mM NaH2PO4/130 mM NaCl/5 mM KCl/1.2 mM MgSO4/2.5 mM CaCl2, pH 4.0) at 4°C. The final supernatant volume of 2 ml, containing acid-dissociable membrane proteins, was then assayed for [3H] content by scintillation counting. Nonspecific binding (NSB) was determined by comparing [3H]CGP-12177 binding in the presence and absence of 20 μM propanolol, and it was found to be <20%. The NSB was subtracted from each result before the final calculations. The “% surface receptors” was determined by using the formula (E/C) × 100, where E represents counts obtained after the various experimental treatments, and C represents counts obtained under control conditions.
Western Blotting.
3T3-L1 adipocytes were placed in serum-free media in the presence or absence of insulin for 12 h. After the various treatments with ligands, cells were rapidly washed on ice with PBS and lysed in RIPA buffer containing 50 mM Tris⋅HCl pH 7.4, 1% Nonidet P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM sodium vanadate, and 50 mM NaF. For each experimental condition, equivalent amounts of cellular proteins, as determined by Bradford assay, were dissolved in Laemmli buffer, boiled for 5 min, and separated by SDS/PAGE.
Results
Insulin Enhances cAMP Generation in Response to Isoproterenol.
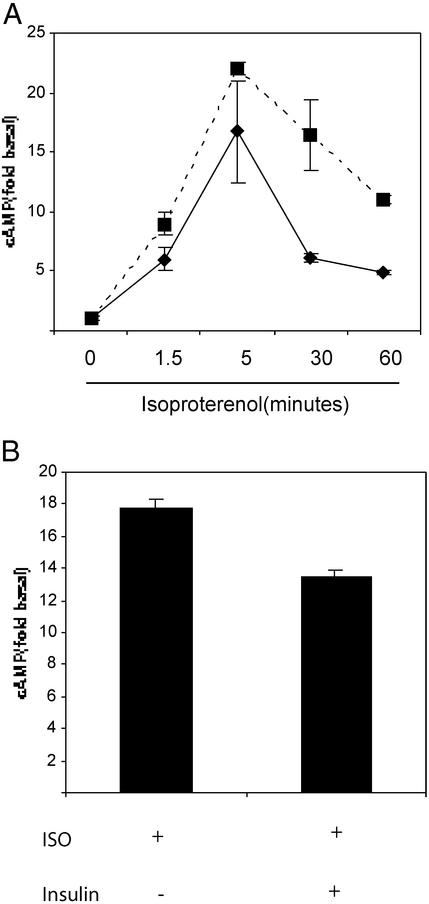
We have recently shown that chronic insulin treatment of 3T3-L1 adipocytes causes an ≈50% reduction of β-arrestin 1 protein content. Because β-arrestin 1 binds to the ligand-activated β2AR and sterically inhibits further receptor signaling through Gαs, we hypothesized that reduced cellular levels of β-arrestin 1 could lead to supersensitization to β2AR Gαs signaling. In 3T3-L1 adipocytes exposed to insulin for 8 h, β-arrestin 1 levels are decreased by 50% (data not shown), and, as seen in Fig. 1A, isoproterenol-mediated cAMP generation was increased at 1.5 min (53 ± 38%), 5 min (25 ± 19%), 30 min (63 ± 14%), and 60 min (56 ± 2%) compared with non-insulin-exposed cells. This result is also in marked contrast to adipocytes exposed to insulin acutely for 15 min, which have a reduced ability to generate cAMP in response to isoproterenol (Fig. 1B, 30% reduction), which is consistent with published studies (11, 12). Insulin treatment alone had no effect on cAMP levels in the absence of isoproterenol (data not shown).
Figure 1.
Insulin enhances cAMP generation in response to isoproterenol. (A) 3T3-L1 adipocytes were incubated in serum-free media at 37°C with (dashed line) or without (solid line) 100 ng/ml insulin for 8 h. Isoproterenol (10 μM) was then added for the indicated time course at 37°C, and intracellular cAMP was determined by enzyme immunoassay, as described in Materials and Methods. Data are from a typical experiment done in triplicate wells ± SEM and are representative of three separate experiments. (B) 3T3-L1 adipocytes were incubated in serum-free media for 8 h. Where indicated, 100 ng/ml insulin was added for 15 min before isoproterenol treatment. Cells were then treated with 10 μM isoproterenol for 5 min at 37°C, and intracellular cAMP was determined by enzyme immunoassay, as described in Materials and Methods. Data are from a typical experiment done in triplicate wells ± SEM and are representative of three separate experiments.
Insulin Enhances Isoproterenol-Induced cAMP Generation in “Desensitized” 3T3-L1 Cells.
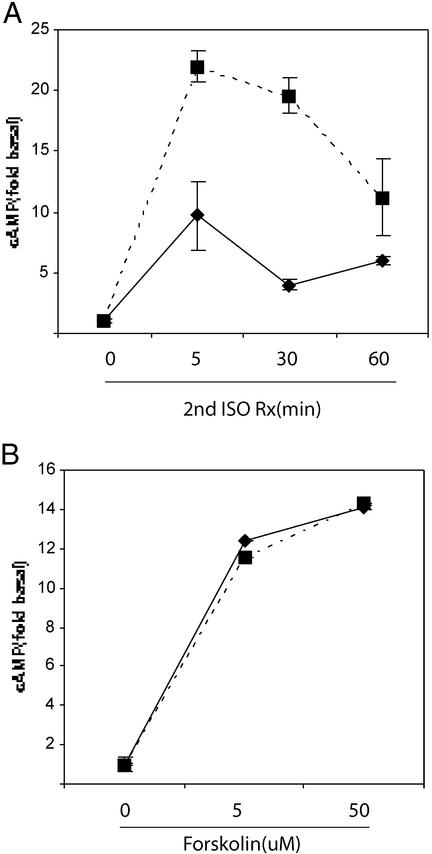
Previous studies have demonstrated that cells pretreated with isoproterenol display an ≈50% reduction in their cAMP generative response to a second isoproterenol exposure (13, 14). This is caused by rapid desensitization of the β2AR initiated by receptor phosphorylation and β-arrestin binding, resulting in both reduced β2AR/Gαs coupling and increased β2AR internalization. We found a similar ≈50% reduction in cAMP generation in 3T3-L1 adipocytes that were pretreated with 10 μM isoproterenol for 5 min and then rechallenged with 10 μM isoproterenol for an additional 5 min (compare solid lines in Figs. 1A and 2A). However, in cells pretreated with insulin for 8 h, the desensitizing effect of isoproterenol was abolished (Fig. 2A, solid vs. dashed lines).
Figure 2.
Insulin enhances cAMP generation in response to isoproterenol in desensitized cells. (A) 3T3-L1 adipocytes were incubated at 37°C in serum-free media with (dashed lines) or without (solid lines) 100 ng/ml insulin for 8 h. After this, cells were treated with 10 μM isoproterenol for 5 min at 37°C. Cells were then washed with PBS at 37°C and restimulated with a second 10 μM dose of isoproterenol for an additional 5 min at 37°C; intracellular cAMP was determined by enzyme immunoassay. (B) 3T3-L1 adipocytes were incubated at 37°C in serum-free media for 8 h in the presence (dashed lines) or absence (solid lines) of 100 ng/ml insulin. Forskolin was added for the final 5 min, and intracellular cAMP levels were determined by enzyme-linked immunoassay. Data are from typical experiments done in triplicate wells and are representative of three separate experiments.
Forskolin stimulates cAMP production through direct activation of adenylyl cyclase (15), independent of β2AR/Gαs signaling events. Because forskolin requires functional adenylyl cyclase to generate cAMP, and forskolin-generated cAMP is sensitive to changes in phosphodiesterase activity, we used forskolin as a control to evaluate the activity of adenylyl cyclase and phosphodiesterase. As seen in Fig. 2B, chronic insulin exposure had no effect on cAMP generation by either submaximal or maximal concentrations of forskolin.
Chronic Insulin Treatment Prevents Isoproterenol-Induced Loss of β2AR from the Cell Surface.
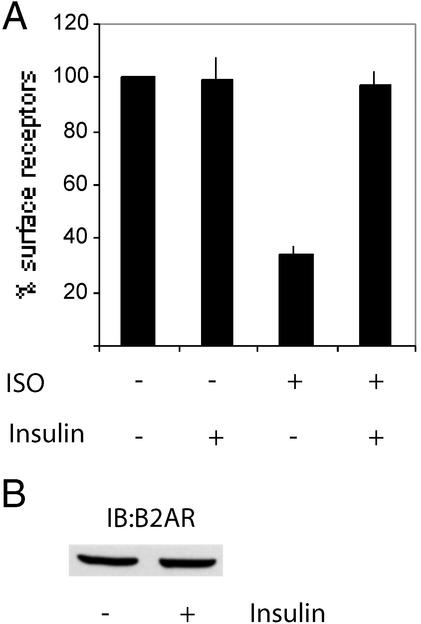
After ligand exposure, the β2AR is phosphorylated on several C-terminal serine residues by GPCR kinase, enhancing β-arrestin binding and leading to clathrin-mediated internalization (16, 17). CGP-12177 is a hydrophilic, noninternalized ligand for the β2AR, and its binding to the cell surface correlates quantitatively with the presence of surface β2AR (10). In 3T3-L1 adipocytes, a 15-min isoproterenol exposure led to rapid internalization of the β2AR, as measured by a 70% reduction of CGP-12177 binding (Fig. 3A). In contrast, isoproterenol-induced internalization of the β2AR was abolished by a 12-h pretreatment with 100 ng/ml insulin.
Figure 3.
Insulin prevents isoproterenol-induced loss of β2AR from the cell surface. (A) 3T3-L1 adipocytes were incubated at 37°C in serum-free media ± 100 ng/ml insulin for 12 h. Isoproterenol (10 μM) was added for the final 15 min at 37°C. β2AR internalization was determined by the CGP-12177 binding assay, as described in Materials and Methods. (B) 3T3-L1 adipocytes were incubated in serum-free media at 37°C ± 100 ng/ml insulin for 12 h. Cells were washed with ice-cold PBS and lysed, and soluble protein was resolved by SDS/PAGE and immunoblotted by using a polyclonal β2AR antibody, as described in Materials and Methods.
In theory, the preserved CGP-12177 binding in insulin-treated cells could be caused by an insulin-mediated increase in β2AR expression. However, as shown in Fig. 3B, 12 h of insulin exposure had no effect on the expression of cellular β2AR protein.
Chronic Insulin Treatment Inhibits the Isoproterenol-Induced Dephosphorylation of β-Arrestin 1.
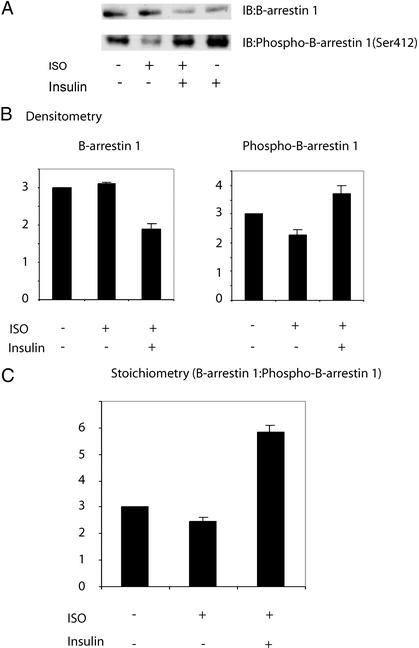
In response to isoproterenol, β-arrestin 1 undergoes dephosphorylation at Ser-412 (18). This event, mediated by an unknown phosphatase, enhances the ability of β-arrestin 1 to bind both clathrin and Src and is required for both β2AR internalization and MAPK signaling. We have previously shown that, in 3T3-L1 cells treated with insulin for 12 h, where β-arrestin 1 levels were reduced by 40–50%, isoproterenol- induced MAPK phosphorylation was nonetheless inhibited nearly 100%. Therefore, we speculated that after insulin treatment, the cellular β-arrestin 1 that remained was rendered dysfunctional in some way, as would occur with excessive Ser-412 phosphorylation. In 3T3-L1 adipocytes, we observed a 40% decrease in β-arrestin 1 protein level after 12 h of insulin treatment, whereas isoproterenol alone had no effect (Fig. 4A Upper). Although isoproterenol had no effect on total β-arrestin protein level, it led to a 24% reduction in β-arrestin 1 phosphorylation, as measured by Western blotting of cell lysates with a phosphospecific (Ser-412) β-arrestin 1 antibody. This effect was abolished when cells were pretreated with insulin for 12 h (Fig. 4A Lower). Furthermore, compared with control cells, the overall phosphorylation of β-arrestin 1 at Ser-412 was enhanced by ≈25% in insulin-treated cells. Because chronic insulin treatment led to a reduction in total β-arrestin 1 content, the insulin-induced increase in the stoichiometry of Ser-412 phosphorylation is even more striking. The phosphorylation stoichiometry is estimated in Fig. 4C, where the amount of phosphorylated β-arrestin 1 is calculated in relation to total cellular β-arrestin 1 protein content.
Figure 4.
Insulin inhibits isoproterenol-induced dephosphorylation of β-arrestin 1 at Ser-412. (A) 3T3-L1 adipocytes were incubated in serum-free media at 37°C ± 100 ng/ml insulin for 12 h. Isoproterenol (10 μM) was added for the final 15 min at 37°C. Cell lysates were then immunoblotted by using a monoclonal β-arrestin 1 antibody, as described in Materials and Methods. The displayed blot is representative of five separate experiments. (B) Quantitation of signal intensities in A was performed by using nih image and represents cumulative results of five separate experiments ± SEM. Data are normalized to basal conditions, which are set at 100%. (C) Phosphorylation stoichiometry was determined by finding the ratio of intensities from phospho-β-arrestin 1 blots:β-arrestin 1 blots.
Discussion
β-Arrestin 1 is a vital component of the multiprotein complex that assembles on the β2AR after ligand stimulation. Acting as a protein scaffold, it recruits Src kinase to the activated β2AR, an event that is required for β2AR-mediated activation of Ras. β-arrestin 1 also binds to clathrin, which serves to target the activated β2AR to clathrin-coated pits for internalization. These two events (Src binding and clathrin-mediated receptor internalization) are required for β2AR-mediated MAPK signaling. β-Arrestin 1 also induces rapid homologous desensitization of the β2AR by binding to the receptor and sterically inhibiting further β2AR–Gαs interaction, leading to a progressive decline in cAMP generation. We have previously reported that chronic insulin treatment leads to a reduction in cellular β-arrestin 1 protein content and have studied the consequences of this on GPCR signaling. We found that β-arrestin 1 down-regulation leads to a marked decrease in GPCR signaling through the Ras/MAPK pathway. In the present study, we show that insulin-induced down-regulation of β-arrestin 1 supersensitizes β2AR signaling through Gαs to cAMP, inhibits β2AR internalization, and leads to phosphorylation of β-arrestin 1 on Ser-412.
In β-arrestin 1 down-regulated 3T3-L1 adipocytes, we found that isoproterenol-induced generation of cAMP was greatly enhanced, and this effect was evident throughout the cAMP response time course. This result is consistent with the findings using fibroblasts from β-arrestin 1 knockout mice, which display enhanced cAMP responses to isoproterenol (19). In parallel experiments, we pretreated cells with a “desensitizing” dose of isoproterenol before washing and isoproterenol restimulation. Consistent with previous studies in other cell lines (13, 14), desensitized 3T3-L1 adipocytes showed an ≈50% reduction in isoproterenol-stimulated cAMP production. However, when these cells were pretreated with insulin to cause β-arrestin 1 down-regulation, isoproterenol-induced desensitization was abolished. Thus, β-arrestin 1 depletion impairs the normal process of β2AR desensitization, leading to enhanced β2AR-mediated cAMP generation, a plasma membrane event requiring Gαs signaling to adenylyl cyclase.
Next, we measured β2AR internalization, as determined by binding of [3H]CGP-12177. As CGP-12177 is not internalized after binding to the β2AR because of its hydrophilic nature, the amount of CGP-12177 bound to the cell after treatment and washing reflects the number of β2ARs present at the cell surface. We found that isoproterenol-induced β2AR internalization was completely inhibited in insulin-treated, β-arrestin 1 down-regulated cells. This finding suggests that chronic insulin treatment may enhance β2AR/Gαs/cAMP signaling at the expense of β2AR/Gαi/MAPK signaling, at least in part by effectively “trapping” the β2AR at the plasma membrane. The insulin-induced reduction in β-arrestin 1 protein content alone should reduce β2AR internalization. However, as insulin treatment leads to a 40–50% reduction in β-arrestin 1 protein, it is difficult to explain how isoproterenol-induced β2AR internalization is reduced by 100% after insulin treatment at 100 ng/ml. A potential explanation for these results has to do with the functional properties of phosphorylated vs. dephosphorylated β-arrestin 1.
Most of the cytosolic β-arrestin 1 is phosphorylated on Ser-412, and phosphorylated β-arrestin 1 binds very poorly to clathrin and Src. Thus, isoproterenol treatment, which induces dephosphorylation of β-arrestin 1 at Ser-412, as well as transfection of the S412A β-arrestin 1 mutant, which mimics dephosphorylated β-arrestin 1, greatly enhances clathrin and Src binding to β-arrestin 1, resulting in enhanced β2AR internalization and MAPK signaling (7, 18). We found that chronic insulin treatment not only inhibits isoproterenol-induced dephosphorylation of β-arrestin 1 at Ser-412, but it also increases phosphorylation of β-arrestin 1 to levels 25% above control. Because total cellular β-arrestin 1 content is reduced after chronic insulin treatment, the stoichiometry of β-arrestin 1 phosphorylation is markedly increased (≈2-fold) after insulin treatment. Thus, the cellular β-arrestin 1 remaining after insulin treatment is highly likely to be phosphorylated at Ser-412, rendering it inefficient for Src and clathrin binding. This finding could explain why reducing β-arrestin 1 protein levels by 50% with insulin leads to near complete inhibition of β2AR internalization (Fig. 3) and MAPK signaling (8).
Our results shed further light on the function of Ser-412 phosphorylated vs. nonphosphorylated β-arrestin 1. The βarrestin 1 mutant S412D, which mimics phosphorylated β-arrestin 1, is unable to associate with clathrin and mediate β2AR internalization. However, it can still bind to the β2AR and inhibit further Gαs signaling (18). Consistent with the idea that phosphorylated β-arrestin 1 can bind to the β2AR, we have shown that β-arrestin 1:β2AR association is reduced by only 50% in insulin-treated 3T3-L1 adipocytes, similar to the 50% reduction in β-arrestin 1 protein levels (8), even though the phosphorylation of β-arrestin 1 was greatly enhanced (Fig. 4). Because one would expect greater inhibition (>50%) of β2AR:β-arrestin 1 association if phosphorylated β-arrestin 1 could not interact normally with the β2AR, we conclude that phosphorylated β-arrestin 1 can bind efficiently to the β2AR. Thus, although increased Ser-412 phosphorylation of β-arrestin 1 contributes to the inhibition of β2AR internalization and MAPK signaling in insulin-treated cells, it is not a factor in β-arrestin 1-mediated desensitization of β2AR/Gαs signaling, which only depends on β2AR:β-arrestin 1 binding.
At least two forms of β-arrestin exist (β-arrestin 1 and 2), and both forms are ubiquitously expressed (20). The relative contribution of each isoform to GPCR desensitization is largely unknown. In β-arrestin 1 knockout fibroblasts, β2AR-mediated cAMP generation is supersensitized, but β2AR internalization is normal (19). In contrast, the β-arrestin 2 knockout cells showed both supersensitized β2AR-mediated cAMP generation as well as a pronounced loss of β2AR internalization. These findings suggest that β-arrestin 1 and 2 have different roles in the trafficking of the β2AR, at least in mouse fibroblasts. Thus, β-arrestin 1 is critical for desensitization to Gαs signaling, whereas β-arrestin 2 seems to be capable of directing receptor internalization in the absence of β-arrestin 1. The expression level, if any, of β-arrestin 2 in adipocytes has not been reported, and it will also be of interest to determine the effect of insulin treatment on β-arrestin 2 protein levels.
Chronic hyperinsulinemia is characteristic of the insulin resistance syndrome, which includes type II diabetes mellitus, obesity, hyperlipidemia, hypertension, and accelerated atherosclerosis. Taking the assumption that our findings might be generalized from the β2AR to any GPCR which uses β-arrestin 1 as part of its signaling apparatus, it is tempting to speculate that supersensitization of GPCR Gαs signaling caused by chronic hyperinsulinemia-mediated down-regulation of β-arrestin 1 could play a role in some of the defects seen in the insulin resistance syndrome. The β2AR is the major regulator of adipocyte lipolysis, and excessive free fatty acid release from visceral adipocytes is associated with insulin resistance as well as the hypertriglyceridemic/low-HDL lipid profile common in diabetes. Peripheral vascular tone is regulated by the α-1 adrenergic receptor (α-AR) family of GPCRs, and excessive α-AR activity could contribute to hypertension (21). Polycystic ovarian syndrome (PCOS) is characterized by oligomenorrhea, obesity, and hyperandrogenism and is also strongly associated with insulin resistance and hyperinsulinemia. Excessive activity of the leutinizing hormone (LH) receptor, a GPCR which stimulates androgen production, is often implicated in the pathophysiology of PCOS. Indeed, in porcine ovarian follicular membranes, antibodies to β-arrestin 1 block desensitization of the LH receptor (22).
In summary, we have shown that chronic insulin exposure supersensitizes 3T3-L1 adipocytes to β2AR-mediated cAMP production, while inhibiting the internalization of the β2AR. We also show that, in addition to causing down-regulation of βarrestin 1 protein levels, chronic insulin treatment leads to increased phosphorylation of β-arrestin 1 at Ser-412. These findings provide a mechanism whereby chronic stimulation of the insulin receptor leads to heterologous supersensitization of Gαs signaling by the β2AR while simultaneously leading to heterologous desensitization of Gαi signaling by the same receptor.
Acknowledgments
We thank Elizabeth Hansen for excellent editorial assistance. This work was supported by National Institutes of Health Grant DK 33651 (to J.M.O.), the Veterans Administration San Diego Health Care System Research Service, and the Whittier Institute for Diabetes.
Abbreviations
- β2AR
β2 adrenergic receptor
- GPCR
G protein-coupled receptor
- MAPK
mitogen-activated protein kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ji T H, Grossman M, Ji I. J Biol Chem. 1998;273:17299–17302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 2.Gether U, Kobilka B K. J Biol Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 3.Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpene C, Bousquet-Meleu A, Berlan M. Hum Reprod. 1997;12:S6–S20. doi: 10.1093/humrep/12.suppl_1.6. [DOI] [PubMed] [Google Scholar]
- 4.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–353. [PubMed] [Google Scholar]
- 5.Lefkowitz R J. J Biol Chem. 1998;30:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon A W, Kallal L, Benovic J L. J Biol Chem. 1998;12:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 7.Luttrell L M, Ferguson S S G, Daaka Y, Miller W M, Moudsley S, Della Rocca G J, Lin F T, Kawakatsu H, Owada K, Luttrell D K, et al. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 8.Dalle S, Imamura T, Rose D W, Sears Worrall D, Ugi S, Hupfeld C J, Olefsky J O. Mol Cell Biol. 2002;22:6272–6285. doi: 10.1128/MCB.22.17.6272-6285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haruta T A, Morris A J, Rose D W, Nelson J G, Mueckler M, Olefsky J M. J Biol Chem. 1995;270:27991–27994. doi: 10.1074/jbc.270.47.27991. [DOI] [PubMed] [Google Scholar]
- 10.Staehlin M, Simons P. EMBO J. 1982;1:187–190. doi: 10.1002/j.1460-2075.1982.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadcock J R, Port J D, Gelman M S, Malbon C C. J Biol Chem. 1992;267:26017–26022. [PubMed] [Google Scholar]
- 12.Doronin S, Wang H, Malbon C C. J Biol Chem. 2001;277:10698–10703. doi: 10.1074/jbc.M109432200. [DOI] [PubMed] [Google Scholar]
- 13.Fan G, Shumay E, Malbon C C, Wang H. J Biol Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 14.Freedman N J, Liggett S B, Drachman D E, Pei G, Caron M G, Lefkowitz R J. J Biol Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 15.Seamon K B, Daly J W. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- 16.Lin F S, Miller W E, Luttrell L M, Lefkowitz R J. J Biol Chem. 1999;274:15971–15974. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- 17.Karoor V, Wang L, Wang H, Malbon C C. J Biol Chem. 1998;273:33035–33041. doi: 10.1074/jbc.273.49.33035. [DOI] [PubMed] [Google Scholar]
- 18.Lin F S, Kreuger K M, Kendall H E, Daaka Y, Fredericks Z L, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 19.Kohout T A, Lin F S, Perry S J, Conner D A, Lefkowitz R J. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attramada H, Arriza J L, Aoki C, Dawson T M, Codina J, Kwatra M M, Snyder S H, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 21.McCune D F, Edelmann S E, Olgas J R, Post G R, Waldrop B A, Waugh D J J, Perez D M, Piascik M T. Mol Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Palczewski K, Gurevich V, Benovic J L, Banga P, Hunzicker-Dunn M. Proc Natl Acad Sci USA. 1999;96:493–498. doi: 10.1073/pnas.96.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]