Abstract
Cytomegalovirus (CMV) infections have been shown to dramatically affect solid organ transplant graft survival in both human and animal models. Recently, it was demonstrated that rat CMV (RCMV) infection accelerates the development of transplant vascular sclerosis (TVS) in both rat heart and small bowel graft transplants. However, the mechanisms involved in this process are still unclear. In the present study, we determined the kinetics of RCMV-accelerated TVS in a rat heart transplant model. Acute RCMV infection enhances the development of TVS in rat heart allografts, and this process is initiated between 21 and 24 days posttransplantation. The virus is consistently detected in the heart grafts from day 7 until day 35 posttransplantation but is rarely found at the time of graft rejection (day 45 posttransplantation). Grafts from RCMV-infected recipients had upregulation of chemokine expression compared to uninfected controls, and the timing of this increased expression paralleled that of RCMV-accelerated neointimal formation. In addition, graft vessels from RCMV-infected grafts demonstrate the increased infiltration of T cells and macrophages during periods of highest chemokine expression. These results suggest that CMV-induced acceleration of TVS involves the increased graft vascular infiltration of inflammatory cells through enhanced chemokine expression.
With the escalation in the number of immunosuppressed patients undergoing immunosuppressive therapy following solid organ or bone marrow transplantation, human cytomegalovirus (HCMV) disease has now become a major clinical problem. While the majority of HCMV infections result in subclinical disease in healthy individuals, HCMV is a significant pathogen in immunocompromised patients (1, 3, 4, 9, 26, 33, 34, 50, 51, 56). Primary HCMV infection is followed by lifelong persistence of the virus in a latent state, and reactivation of the latent virus is considered to be the major source of virus in these immunocompromised individuals. HCMV has been linked to the development of atherosclerosis, arterial restenosis following angioplasty, and solid organ transplant vascular sclerosis (TVS) (26-28, 47). HCMV infection doubles the 5- and 3-year rates of graft failure due to accelerated TVS in cardiac and liver transplant patients, respectively (15; L. W. Miller, Editorial, J. Heart Lung Transplant. 11:S1-S4, 1992). The observation that HCMV infects many of the cell types involved in vascular disease, including monocyte-derived macrophages, endothelial cells, and smooth muscle cells (SMC), suggests that HCMV may play a role in these disease processes. Because of the similarities between the CMV-species-specific viruses, animal models provide an ideal tool to study the association between CMV and TVS.
The most compelling evidence that herpesviruses play a role in vascular disease comes from studies in animal models. Using a rat CMV (RCMV) solid organ transplant model, researchers have demonstrated, in light of the RCMV-associated acceleration of TVS, an association of herpesvirus infection with vascular disease, leading to graft failure (7, 21, 24, 36, 39). Studies by Bruggeman and colleagues have shown that treatment of CMV-infected graft recipients with ganciclovir, a potent inhibitor of viral replication and CMV disease, resulted in the elimination of virus-induced TVS and prolonged graft survival (22, 23). In a study by Merigan et al., treatment of human recipients of heart transplants with ganciclovir delayed the time to allograft rejection (31). A subsequent prospective analysis of these data demonstrated that prophylactic treatment with ganciclovir also delayed graft rejection compared to the untreated group (53), implicating HCMV as a causative agent in chronic rejection (CR). These results suggest that virus replication is necessary to induce this virus-accelerated vascular disease; however, the mechanisms behind subsequent virus-host interactions leading to the development of TVS are unclear.
Studies have shown that immunosuppressive drug therapy (20, 25) can also reduce the RCMV-accelerated development of TVS, demonstrating the importance of the immune response in this process. However, these studies do not distinguish between the role of anti-CMV immunity and the role of anti-donor alloreactivity in the development of CMV-accelerated TVS. In previous studies, researchers used alloantigen-specific tolerance induced by bone marrow chimerism, which preserves anti-CMV immunity and other immune-mediated events while specifically eliminating anti-donor alloreactivity (37). In this model, donor-specific tolerance prevented the RCMV-induced acceleration of TVS in the allografts, demonstrating that the alloreactive immune response is critical for initiating the events that are necessary for the CMV-mediated acceleration of TVS.
Chemokines (chemotactic cytokines) and their receptors play a major role in the development of vascular disease (30). Chemokines are a group of inducible cytokines that promote cellular migration and activation through binding to their respective G-protein-coupled receptors (for a review, see reference 14). The chemokines are grouped into four families based on a conserved Cys motif and include the C chemokine (Lymphotactin), the CC chemokines (monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein 1α [MIP-1α], MIP-1β, and RANTES, among others), the CXC chemokines, (interleukin-8, INF-γ-induced protein 10 [IP-10], and stromal-cell-derived factor 1α, among others), and the CX3C chemokine (Fractalkine). Chemokines are produced at sites of inflammation and act by attracting monocytes, T cells, and B cells to these sites. Chemokine binding increases the cellular production of other cytokines and growth factors and increases the expression of integrins promoting cellular adhesion to the vascular endothelium and extravasation of leukocytes. Chemokines are present in vascularized grafts at all stages posttransplantation, including during ischemia/reperfusion injury, acute rejection, chronic rejection, and the healing processes (30). By contrast, long-term graft acceptance has been attributed to the absence of chemokines, thus substantiating a major role for chemokines in allogeneic graft rejection and during the development of TVS (43).
CMV infection modifies a number of host cellular functions that are involved in the development of TVS. For example, CMV infection increases endothelial cell adhesive properties caused by increasing the levels of adhesion molecules and major histocompatibility complex class I and II molecules on their surface (19, 48, 52, 54), which may increase immune-cell infiltration and activation. Furthermore, HCMV infection upregulates the expression of RANTES in SMC and fibroblasts (32, 49), promoting cellular infiltration at sites of vascular injury. We recently demonstrated that infection of human SMC with HCMV induces migration, which is dependent upon the expression of the virus-encoded chemokine receptor US28 and binding of the CC chemokines RANTES or MCP-1 (49). Not only does HCMV upregulate host chemokines and cytokines but the virus also encodes for a number of homologues to cellular inflammatory molecules including a viral interleukin 10 and a viral CC chemokine (18, 44, 55). While CMV encodes chemokines and chemokine receptors and can upregulate chemokine expression in cell culture, the effects of CMV on chemokine expression in vivo and the associated relationship of altered expression to TVS are unclear.
In this report, we determined the kinetics of graft vascular lesion development with and without RCMV infection and associated chemokine expression in a rat heart transplantation model. We have determined that RCMV accelerates neointimal formation in the heart grafts between 21 and 28 days posttransplantation. This virus-induced increase in TVS development is accompanied by a parallel increase in graft chemokine expression and by an increase in immune-cellular infiltrates. These data suggest that a link between CMV and chronic rejection involves a complex dynamic interplay between the virus and the host immune and inflammatory response, which is translated through the upregulation of chemokines in the graft.
MATERIALS AND METHODS
Animals.
Adult male inbred rats (Harlan Sprague-Dawley, Indianapolis, Ind.) were used. Fisher 344 (F344) rats served as allogeneic heart donors, while Lewis rats served as solid organ transplant recipients. All animals were housed in the Portland Veterans Affairs Medical Center animal facilities or, if CMV-infected, in a specific-pathogen-free room in the Oregon Health Sciences University animal facilities. Both facilities are Association for Assessment and Accreditation of Laboratory Animal Care accredited and comply with the requirements for animal care as stipulated by the U.S. Departments of Agriculture and Health and Human Services.
Heart transplantation.
Heart grafts from F344 rat donors were transplanted heterotopically into naïve Lewis recipients as previously described (37). Briefly, after 300 IU of heparin was infused intravenously into the inferior vena cava, the anterior chest wall was opened via the sternum. An iced saline gauze pad was placed on the heart, and the ascending aorta and main pulmonary artery were divided. The superior and inferior vena cava and the confluence of the pulmonary veins were ligated and divided. The heart was removed and placed in iced saline while the recipient was prepared for implantation. Transplantation was achieved by anastomosing the graft aorta to the recipient aorta and the graft pulmonary artery to the recipient inferior vena cava in an end-to-side fashion by a 9-0 running nylon suture. Lewis recipients were treated with low-dose cyclosporine for 10 days (5 mg/kg of body weight/day; Sandoz, Inc., East Hanover, N.J.). In all animals, the native heart remained intact. Acute RCMV infection was accomplished by injecting 105 PFU of RCMV intraperitoneally on postoperative day (POD) 1 after the heart transplant operation. The inoculum was diluted in 1 ml of Eagle's minimal essential medium (EMEM, catalog no. 41600-016; Gibco BRL, Rockville, Md.) containing 2% fetal calf serum (FCS). Uninfected allograft recipients served as controls. Animals were monitored daily for overall health, appetite, and clinical condition. In order to determine the events during the development of TVS, graft and native hearts were harvested on days 7, 14, 21, 24, 28, 32, 35, and 45 posttransplantation (45 days is the mean time to develop CR and TVS in CMV-infected allograft recipients [37]). Animals not surviving POD 5 were considered technical failures and were excluded from further study.
Histology.
At predetermined time points, the recipients were euthanized and the heart graft was harvested for histologic assessment. The tissue was immediately cross-sectioned and fixed in 10% buffered formalin for 24 h. Paraffin-embedded tissue blocks were serially sectioned at 4 μm and stained with hematoxylin and eosin. Sections were evaluated by the light microscopic method in a blinded fashion to assess the presence of CR and TVS.
NI measurements.
To determine the degree of TVS development in heart graft coronary arteries, multiple 4-μm-thick paraffin-embedded tissue sections were cut and stained with von Gieson stain for elastic fibers. A minimum of eight vessels over 85 μm in diameter were analyzed under a Zeiss Axioplan 2 microscope coupled to a camera that was interfaced with a computer. The extent of neointimal formation (TVS) was determined by the neointimal index [NI = (intimal area/lumen + intimal area) × 100 (2)]. All data were expressed as the mean ± the standard error of the mean. Statistical analyses were performed by using SPSS version 6.1 (SPSS, Inc., Chicago, Ill.). The severity of TVS (NI scores) in the uninfected graft recipients compared to that in the RCMV-infected graft recipients was analyzed by using Student's t test and Fisher's exact test. P values of <0.05 were considered statistically significant.
Immunohistochemistry.
Immunohistochemistry was performed on 4-μm-thick paraffin or frozen sections as previously described (38). Monoclonal antibodies to rat CD4 (catalog no. 550296; BD Pharmingen, San Diego, Calif.), CD8 (catalog no. 550298; BD Pharmingen), alpha-SMC actin (catalog no. m085101; Dakopatts, Glostrup, Denmark), and ED1 (macrophage marker, catalog no. NC MCA341R; Serotec) were used to assess cellular infiltration into rat heart graft tissues. The primary antibody was diluted in phosphate-buffered saline (PBS)-0.05% Tween 20 solution and incubated with the sections for 45 min at room temperature in a Dako Immunostainer. Subsequently, slides were incubated for 30 min at room temperature with a species-specific, peroxidase-conjugated secondary antibody diluted in PBS solution and developed by using a MAB kit (Dako). Slides were counterstained with hematoxylin and visualized on a Nikon microscope. The degree of intimal versus medial cellular infiltration by cells expressing various markers was scored by using a semiquantitative scale: 0, no staining; 1, few cells and faint staining; 2, moderate, multifocal staining; 3, intense, diffuse staining. Immunohistochemical staining results were analyzed by Student's t test. P values of <0.05 were considered statistically significant.
Rat cytomegalovirus.
The Maastricht strain of RCMV (6) used for this study was derived from the homogenized salivary glands of acutely infected rats (5). Briefly, 10- to 12-week-old Lewis rats were irradiated with 5 Gy of total body irradiation and infected with 105 PFU of RCMV. At 4 weeks postinfection, the salivary glands were harvested and homogenized in EMEM supplemented with 2% FCS (Gibco BRL) and 100 U of penicillin/ml-100 μg of streptomycin/ml in a Dounce tube, sonicated three times for 15 s, and finally centrifuged at 1,000 × g at 20 to 25°C for 15 min to yield a clear supernatant. The virus suspension was stored at −70°C until use. The titer of the RCMV stock was determined by plaque assays (5). Confluent rat embryo fibroblasts (REFs) in 24-well plates were infected with an appropriate serial virus dilution in 0.2 ml and then incubated at 37°C for 90 min. Following incubation, the infected cells were rinsed with PBS and overlaid with 1 ml of MEM-10-C (EMEM supplemented with 10% FCS, nonessential amino acids, penicillin-streptomycin, and 20 mM l-glutamine; Gibco BRL) with a final concentration of 0.6% agarose (Sigma, St. Louis, Mo.). After 7 days, the cells were fixed in 10% formalin in PBS for 15 min at room temperature. The solid base layer was removed, the cell monolayers were stained with 1% aqueous methylene blue (catalog no. 180-8; Sigma), and the plaques were counted by light microscopy.
Serologic testing for RCMV antibodies.
In the animals whose grafts were harvested at 45 days posttransplantation, serum was isolated from the blood and assessed for anti-RCMV antibodies as previously described (37). Briefly, serological testing was performed on RCMV-infected, formaldehyde-fixed REFs grown in 96-well plates. Serial dilutions of serum antibody samples were incubated with uninfected and infected REFs overnight at 4°C. The cells were washed with PBS and then incubated with a secondary rabbit anti-rat antibody conjugated to fluorescein isothiocyanate (1:50 dilution; DAKO catalog no. F0234) for 2 h at 4°C. The cells were washed and assessed with an inverted fluorescence microscope (Leica). Nuclear or cytoplasmic staining of RCMV-infected, but not uninfected, REFs was considered a positive result.
TaqMan PCR detection of RCMV.
For the detection of viral DNA, total genomic DNA was extracted from 0.8 g of rat tissues (native and graft hearts and submandibular glands [SMG]) by the DNAzol method (Life Technologies). In addition, DNA was extracted from total peripheral blood mononuclear cells isolated from 3 ml of whole blood by using Ficoll-Hypaque. A total of 0.5 μg of DNA was analyzed by TaqMan PCR techniques with a probe-primer set recognizing a RCMV DNA polymerase sequence. Forward (5′-CCT CAC GGG CTA CAA CAT CA-3′) and reverse (5′-GAG AGT TGA CGA AGA ACC GAC C-3′) primers were used. The probe used was 5′-CGG CTT CGA TAT CAA GTA TCT CCT GCA CC-3′ labeled on the 5′ end with the reporter FAM and on the 3′ end with the quencher TAMRA (Applied Biosystems, Foster City, Calif.). PCRs were set up using TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's specifications. Following thermal activation of AmpliTaq Gold (10 min at 95°C), a total of 40 cycles were performed (15 s at 95°C and 1 min at 58°C) by using a Prism 7700 TaqMan apparatus (Applied Biosystems). DNA isolated from sucrose-gradient-purified RCMV was used as a positive control. TaqMan results were analyzed using ABI Prism model 7700 Sequence Detector software. The sensitivity of detection for this assay was <100 copies.
TaqMan RT-PCR detection of chemokines.
For the detection of host chemokine expression within the native and graft hearts, total RNA was extracted from 0.8 g of heart tissue by the RNAzol method (Life Technologies). cDNA was generated by using the Omniscript reverse transcription (RT) kit (Qiagen) and analyzed by TaqMan PCR techniques with a probe and primer set recognizing chemokine sequences. Primers used were MIP-1α forward (5′-AGC TGA CAC CCC GAC TGC), reverse (5′-CCA AAG GCT GCT GGT CTC AA), and probe (5′-CTG CTT CTC CTA TGG ACG GCA AAT TCC); MCP-1 forward (5′-CCC CAC TCA CCT GCT GCT A), reverse (5′-AGC TTC TTT GGG ACA CCT GCT), and probe (5′-TCG GCT GGA GAA CTA CAA GAG AAT CAC CA); IP-10 forward (5′-TTC CAT GAA CAG CCG CTG A), reverse (5′-CTC AAC ATG CGG ACA GGA TAG A), and probe (5′-ACC CAG GGC CAT AGG AAA ACT TGA AAT CA); Fractalkine forward (5′-CCA CAA GAT GAC CTC GCC A), reverse (5′-GCT GTC TCG TCT CCA GGA TGA), and probe (5′-CAC TAT CAA CTG AAC CAG GAG TCC TGC GG); Lymphotactin forward (5′-CAA GGA GGG AGC CAT GAG AG), reverse (5′-ACC CAT TTG GCT TGT GGA TC), and probe (5′-CGT GTT TAA GAA TGA GGA GCA AAC CAC TGT T); RANTES forward (5′-CCA TAT GGC TCG GAC ACC AC), reverse (5′-CAA AGA CGA CTG CAA GGT TGG), and probe (5′-CTG CTT TGC CTA CCT CTC CCT CGC ACT); and gamma interferon (IFN-γ) forward (5′-AAG TTC GAG GTG AAC AAC CCA), reverse (5′-TTA GGC TAG ATT CTG GTG ACA GCT G), and probe (5′-AGA TCC AGC ACA AAG CTG TCA ATG AAC TCA). To normalize samples, detection of the mRNA for L32 (ribosomal protein) was used as a control with the primers forward (5′-GAA GAT TCA AGG CAG ATC C), reverse (5′-GTG GAC CAG AAA CTT CCG GA), and probe (5′-CGG GAG TAA CAA GAA AAC CAA GCA CAT GC-3′). The probes were labeled on the 5′ end with the reporter FAM and on the 3′ end with the quencher TAMRA (Applied Biosystems). RT-PCRs were set up using TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's specifications. Following thermal activation of AmpliTaq Gold (10 min at 95°C), a total of 40 cycles were performed (15 s at 95°C and 1 min at 58°C) with the Prism model 7700 TaqMan apparatus (Applied Biosystems). Plasmid clones containing each gene fragment were used as positive controls and quantitation standards. TaqMan results were analyzed with ABI Prism model 7700 Sequence Detector software. The sensitivity of detection of this assay was <100 copies for all of the tested chemokines. The normalized native or graft heart chemokine expression for infected recipients was compared to that for the uninfected animals by using Student's t test. P values of <0.05 were considered statistically significant.
RESULTS
Kinetics of RCMV-accelerated TVS in rat graft hearts.
Previously, it was determined that RCMV infection accelerates the time to develop CR and severity of TVS in a rat heart transplant model (37). However, in that study, grafts were analyzed at the time of rejection or the study endpoint. In this model of F344-to-Lewis rat heart transplants infected with RCMV on POD 1, mean graft survival was shortened from 90 to 45 days and the mean NI was increased from 50 to 84 in time-matched grafts. However, the sequence of events and factors involved in this acceleration process are unclear. To study the role of CMV infection in the kinetics of the development of TVS, we measured the graft NIs at days 7, 14, 21, 28, 35, and 45 in infected mice and the uninfected controls. At days 7 and 14, graft TVS was not yet evident; however, histologically, endothelialitis was observed in the RCMV-infected grafts and the corresponding uninfected grafts were normal (Fig. 1A and B). TVS was first detected at POD 21 with little difference between infected and uninfected allogeneic recipients (NI = 38 ± 4 compared to 35 ± 8, P = not significant). However, beginning at POD 28, RCMV infection in the recipient grafts resulted in a stepwise increase in the severity of TVS in heart graft vessels continuing up until terminal rejection at day 45 (NI = 78 ± 8) compared to that in uninfected allografts (NI = 40 ± 6, P < 0.001) (Fig. 1A and B). A significant difference in NI between infected and uninfected graft vessels was first manifested on day 28 (NI = 49 ± 5 compared to 31 ± 3, P < 0.01). By day 35, the mean graft NI in the infected group was greater than twice that of the uninfected grafts (NI = 64 ± 3 compared to 30 ± 3, P < 0.001). These data suggest that the effect of RCMV on the acceleration of TVS was first evident histologically between PODs 21 and 28.
FIG. 1.
Kinetics of RCMV acceleration of rat cardiac allograft TVS. (A) Heterotopic heart allografts were transplanted into Lewis recipients, with or without RCMV infection, and graft tissues were harvested at the predetermined endpoints of PODs 7, 14, 21, 28, 35, and 45. Graft vessels stained with elastin showed endothelialitis in the RCMV-infected, but not in the uninfected, allogeneic recipients at PODs 7 and 14. Average NIs are given below each representative photo. TVS was detected at POD 21 with little difference between infected and uninfected allogeneic recipients. However, at PODs 28, 35, and 45, RCMV-infected-recipient heart graft vessels showed a dramatic increase in the severity of TVS compared to uninfected controls. These data suggest that RCMV accelerates the time to graft rejection between PODs 21 and 35; however, this might be due to events occurring within the first 7 to 14 PODs. Original magnification, ×200. END, endothelium; NH, neointimal hyperplasia; IEL, internal elastic lamina. (B) Graphical comparison of average NIs of graft heart vessels of uninfected and RCMV-infected animals. The x axis shows the days posttransplantation; the y axis shows the mean NI (n is at least four animals).
RCMV DNA is present in heart allografts during TVS formation.
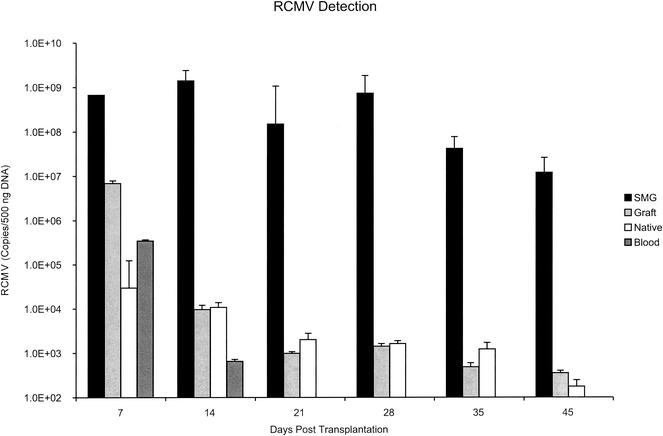
Previously, it was reported that all of the RCMV-infected heart transplant recipients developed anti-RCMV antibodies, as determined by serologic testing (37). These findings were confirmed in the present study, as all of the RCMV-infected graft recipients developed a positive serology for RCMV at day 45 posttransplantation (data not shown). To determine whether RCMV was present in the recipient grafts and their native hearts during the time course of virus-accelerated TVS and chronic rejection, we used quantitative TaqMan PCR to analyze viral loads in native and graft hearts, salivary glands, and blood leukocytes at 7, 14, 21, 28, 35, and 45 days posttransplantation. It was previously demonstrated by TaqMan PCR that graft RCMV DNA detection at the time of graft rejection was sporadic but limited to RCMV-infected animals (37). Here, we demonstrate the consistent presence of viral DNA in the heart graft tissues at 7, 14, 21, and 28 days posttransplantation (Fig. 2). Day 7 viral loads in heart grafts were >10-fold higher than those detected in blood leukocytes (1 × 107 ± 5 × 105 versus 6 × 105 ± 1 × 104 copies/μg of total DNA, respectively), suggesting that the graft tissue is an initial site of virus replication and may correlate with the heart graft endothelialitis (Fig. 1A) observed at days 7 and 14 in only the infected recipients. Unlike the disappearance of viral DNA in the blood after 14 days posttransplantation, RCMV DNA levels in heart grafts consistently remained above the background up to 35 days for all of the animals tested. However, similar to the previous findings, virus detection in graft hearts was sporadic at 35 and 45 days posttransplantation, which may be due to tissue death associated with graft failure and/or chronic rejection or immune clearance. In the infected transplant recipients, the native hearts showed the presence of virus at all times tested (7, 14, 21, 35, and 45 days posttransplantation [data not shown]). Again, virus detection was sporadic at 35 and 45 days posttransplantation. The amount of viral DNA detected in the native hearts was similar to that in grafts at all of the time points tested (Fig. 2). Viral DNA was present in the salivary glands (SMG) at all the times tested, including 7, 14, 21, 28, 35, and 45 days posttransplantation (ranging from 1 × 109 to 2 × 107 copies/μg of total DNA), which suggests that SMG tissues may be a potential viral reservoir throughout the TVS disease process (Fig. 2). These data demonstrate that RCMV is present in graft heart tissues during the time of virus-accelerated TVS (between days 21 and 28 posttransplantation).
FIG. 2.
RCMV DNA is detectable in native and graft heart tissues, blood, and SMG. Total DNA was prepared from graft and native hearts, SMG, and peripheral blood leukocytes at PODs 7, 14, 21, 28, 35, and 45. Viral DNA was quantitated by using TaqMan PCR techniques specific for RCMV DNA polymerase. These data suggest that RCMV is present within the graft tissues and that SMG is present during all of the stages of RCMV-accelerated TVS. The presence of virus in the blood was detected only through 14 days posttransplantation.
RCMV infection increases cellular infiltrates during early stages of graft rejection.
Since researchers previously reported that the alloreactive environment was crucial for the RCMV-mediated acceleration of TVS, we next determined whether the RCMV-induced acceleration was due to an enhancement of immune-cell infiltration into graft vessels. For these experiments, the subset and distribution of infiltrating leukocytes was analyzed by the immunohistochemical staining of either frozen or paraffin sections of allograft hearts harvested at PODs 24, 28, and 32. Serial sections were stained for ED1 (monocyte/macrophage marker), CD4 (T-helper-cell marker), CD8 (cytotoxic-T-cell marker), or SMC actin. Using the internal elastic lamina as a guide to vessel architecture and to distinguish between the vessel intima and media, we compared leukocyte staining of the neointimal space (intimal staining) to that of the vessel media (medial staining) (Table 1).
TABLE 1.
Immunohistochemical staining of heart allograft frozen or paraffin sections for analysis of vessel intimal and medial infiltrating leukocyte subsetsa
| Cellular marker | POD | Intima
|
Media
|
||
|---|---|---|---|---|---|
| RCMV | Mock | RCMV | Mock | ||
| ED1 (monocyte/macrophage) | 24 | 0.2 ± 0.1 | 0.1 ± 0 | 0.2 ± 0.1* | 0 |
| 28 | 1.7 ± 0.5* | 0 | 1 ± 0.4* | 0.2 ± 0 | |
| 32 | 1.8 ± 0.3* | 1.1 ± 0.6 | 1.4 ± 0.4* | 0.4 ± 0.2 | |
| CD4 (T helper cell) | 24 | 0 | 0 | 0 | 0 |
| 28 | 0 | 0 | 0 | 0 | |
| 32 | 1.4 ± 0.2* | 0 | 0.4 ± 0.2 | 0.3 ± 0.2 | |
| CD8 (cytotoxic T cell) | 24 | 0.6 ± 0.2* | 0 | 0.4 ± 0.2* | 0 |
| 28 | 0.7 ± 0.3* | 0 | 0.2 ± 0.1* | 0 | |
| 32 | 0.9 ± 0.2* | 0.3 ± 0.6 | 0.7 ± 0.2* | 0.2 ± 0.2 | |
∗, P < 0.05 compared to mock uninfected controls.
As expected, native hearts from either infected or uninfected recipients failed to stain for ED1, CD4, or CD8 at all of the times tested (data not shown). Vessel intimal and medial staining for the macrophage marker ED1 was minimal in sections from uninfected allografts until day 32 posttransplantation (Table 1). However, grafts from RCMV-infected recipients showed faint staining for ED1 as early as 24 days posttransplantation, with moderate to intense staining for macrophages at days 28 and 32 (Fig. 3). Similarly, uninfected controls demonstrated little to no CD8-T-cell staining at days 21 and 28 posttransplantation but at day 32, there was a slight increase in CD8-T-cell staining, which was less intense compared to that for vessels from RCMV-infected allografts. The infected grafts demonstrated the presence of CD8+ T cells at days 24, 28, and 32 (staining ranged from faint to moderate [Table 1]). Little to no intimal- and medial-vessel CD4+-T-cell staining was observed at all times posttransplantation in the uninfected allografts. However, at day 32, the CD4+-T-cell staining of vessels from the infected allografts was moderate to intense (Table 1 and Fig. 3). Together, these findings demonstrate that RCMV-infected allograft vessels contain increased cellular infiltration compared to that in uninfected controls.
FIG. 3.
Host cellular infiltrates are higher in RCMV-infected graft tissues. Heterotopic heart allografts, with or without RCMV infection, were harvested at the times of RCMV-accelerated TVS formation (PODs 24, 28, and 32), and subsequently, tissue sections were immunohistochemically stained to determine the levels and types of infiltrating cells. Average staining indices are given below each picture. NH, neointimal hyperplasia. (A) Immunohistochemical staining of graft heart tissues for the macrophage marker ED1 at day 28 posttransplantation. (B) Immunohistochemical staining of graft heart tissues for T-helper-cell marker CD4 at day 32 posttransplantation. (C) Immunohistochemical staining of graft heart tissues for cytotoxic-T-cell marker CD8 at day 28 posttransplantation. RCMV infection increases the proportion of cellular infiltrates in both the intima and media of graft vessels, which corresponds to the timing of virus-enhanced chemokine expression. Original magnification, ×200.
Allograft chemokine expression is enhanced during RCMV infection.
Chemokines are expressed to varying degrees during all stages of transplantation, and the inhibition or lack of certain chemokines promotes graft survival (30). HCMV infections in vivo and of cultured cells have been shown to alter host chemokine expression, which is hypothesized to be a contributing factor in CMV-related diseases (8, 10, 35, 40, 42, 49). To determine whether the RCMV-induced enhancement of immune-cell infiltration into graft vessels was due to infection-mediated alterations in chemokine expression, we used quantitative RT-PCR TaqMan techniques to compare the mRNA levels of the host chemokines (MCP-1, RANTES, MIP-1α, IP-10, Fractalkine, and Lymphotactin) and the cytokine IFN-γ in the grafts and native hearts from infected and uninfected transplant recipients at days 7, 14, 21, 24, 28, 32, 35, and 45 after transplantation.
In general, the CC chemokines were upregulated at sites of inflammation, as seen during solid organ transplantation, and mediated monocyte, T-cell, and B-cell infiltration and activation. As shown in Fig. 4A, expression of the CC chemokines RANTES, MCP-1, and MIP-1α was upregulated after transplantation. Expression of each of these chemokines was low at days 7 and 14, which may have been due to the cyclosporine-mediated immunosuppression used to prevent acute allograft rejection. At day 21, chemokine RNA expression levels increased in both the uninfected and infected grafts (Fig. 4A). However, by day 24, these levels dropped in the uninfected recipient grafts but remained elevated in the RCMV-infected allografts (Fig. 4A). For instance, RANTES was >3-fold higher in the infected grafts at day 24 than in the uninfected controls and this virus-induced enhancement persisted until day 32 posttransplantation (Fig. 4B). RANTES levels were highest in the infected grafts at days 24 and 32. While MCP-1 expression in virus-infected grafts was enhanced approximately threefold between days 28 and 32 (Fig. 4B), expression of this chemokine was highest between days 32 and 45 (Fig. 4A). At this later time, the uninfected grafts also demonstrated similar levels. MIP-1α expression levels were increased at day 21 in both the infected and uninfected heart graft tissues but dropped in the uninfected grafts at day 24. At this time, virus-infected grafts demonstrated a nearly 2.5-fold increase in MIP-1α expression levels compared to that in uninfected controls (Fig. 4B). Levels were reduced in both groups at day 28 but were enhanced in virus-infected grafts at day 32. Virus infection did not significantly alter RANTES or MIP-1α expression levels in native hearts compared to those in uninfected native hearts (Fig. 4A and B). The levels of MCP-1 in the infected native hearts were increased twofold at day 14 over those in the uninfected native hearts; however, the amount detected at this time was fivefold lower than that of the infected grafts (Fig. 4A and B). Overall, these data demonstrate that expression of the CC chemokines in heart grafts is specifically increased between days 21 and 32 posttransplantation by acute RCMV infection and that the kinetics of chemokine enhancement corresponds to the time of virus acceleration of TVS in the heart allograft.
FIG. 4.
Host CC chemokine expression is enhanced in rat heart graft tissues infected with RCMV. (A) RT-PCR TaqMan was used to detect expression of the CC chemokines (MCP-1, MIP-1α, and RANTES) in heart allografts and native hearts. Graft and native hearts from RCMV-infected and uninfected animals were analyzed at PODs 7, 14, 21, 24, 28, 32, 35, and 45 (n = 4). cDNA was produced from total RNA isolated from the graft tissues. RT-PCR TaqMan detection of the ribosomal protein L32 was used as a normalizing control. (B) Virus-specific induction of host CC chemokine expression in native and graft hearts is shown as a fold increase compared to that for uninfected controls. These data suggest that host CC chemokine expression is specifically enhanced during the time that RCMV accelerates graft TVS (days 21 to 32) but resolves as the graft fails (days 35 to 45).
The CXC chemokine IP-10 is a potent chemoattractant for active CXCR3-expressing T cells during inflammation and may be an important contributor to the progression of chronic rejection, as rejecting allografts demonstrated high expression levels of IP-10. In addition, recipient mice lacking the chemokine receptor for IP-10 failed to develop chronic rejection (12, 13, 29, 57). As shown in Fig. 5A, expression of IP-10 was significantly upregulated at days 21, 28, and 45 posttransplantation, specifically at days 21 and 28, in infected animals compared to that in uninfected controls. In fact, at day 21, expression was >3-fold higher in CMV-infected recipients and at day 28, expression of IP-10 was >20-fold higher (Fig. 5B). Virus infection did not alter IP-10 expression in native hearts until day 45 (fourfold); however, the levels at this time were eightfold lower than those in the infected graft hearts. Because IP-10 expression is driven by the cytokine IFN-γ, we hypothesized that expression of this cytokine must also be upregulated between days 21 and 32 and specifically enhanced in the infected recipients. Using RT-PCR TaqMan, we found an increase in the expression of IFN-γ in RCMV-infected allografts beginning after day 21 and persisting through day 32 (Fig. 5A). Similar to the IP-10 expression profile, IFN-γ levels were nearly fivefold higher in the infected grafts at day 24 posttransplantation and 20-fold higher at day 28 (Fig. 5B), which parallels the timing of virus-accelerated allograft TVS. IFN-γ expression was not altered in infected native hearts compared to that for the uninfected controls (Fig. 5A and B).
FIG. 5.
RCMV infection dramatically increases IFN-γ and IP-10 expression in heart allografts. (A) RT-PCR TaqMan was used to detect expression of the cytokine IFN-γ and the CXC chemokine IP-10 in native hearts and heart allografts. Graft and native hearts from RCMV-infected and uninfected animals were analyzed at PODs 7, 14, 21, 24, 28, 32, 35, and 45 (n = 4). cDNA was produced from total RNA isolated from the graft tissues. RT-PCR TaqMan detection of the ribosomal protein L32 was used as a normalizing control. (B) Virus-specific induction of host IFN-γ and IP-10 expression in native and graft hearts from RCMV-infected recipients compared to that for uninfected controls is shown. These data suggest that IFN-γ induces IP-10, and this occurs at days 28 and 32, corresponding to the acceleration of TVS in the grafts from RCMV-infected recipients.
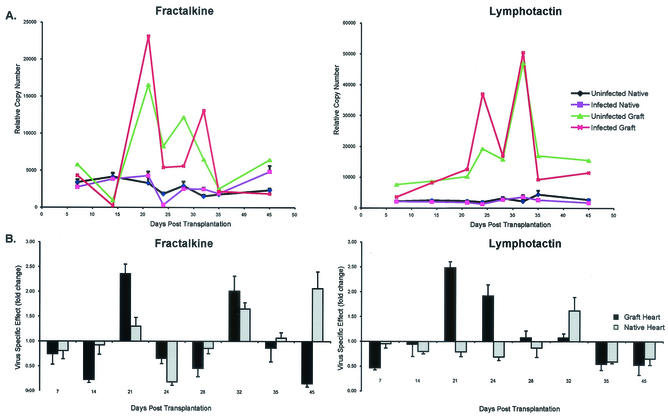
Robinson and colleagues have demonstrated that antibody blocking of the Fractalkine receptor (CX3CR1) prolongs graft survival in a mouse cardiac transplantation model (41). In our rat heart transplant model, the expression of the ligand for this receptor, Fractalkine (CX3C chemokine), was highest at 21 days posttransplantation and expression was >2-fold higher in the infected grafts than in the uninfected grafts at days 21 and 32 (Fig. 6A and B). Interestingly, Fractalkine expression was higher in the uninfected grafts at both 24 and 28 days posttransplantation. Fractalkine expression in the infected grafts dropped dramatically at the time of CR and graft failure; however, this was the only time that Fractalkine was upregulated in the infected native hearts. The role of the potent T-cell chemoattractant Lymphotactin (C chemokine) in chronic rejection is unclear. However, here, we demonstrate that allograft expression of this chemokine was elevated at days 24 and 32 posttransplantation. In the RCMV-infected recipients, Lymphotactin expression was increased >2-fold compared to that in uninfected controls at day 24 but then dropped to the levels observed in control graft tissues (Fig. 6A and B). At day 32, Lymphotactin was again upregulated in grafts from infected and uninfected recipients. Lymphotactin expression was not altered in the infected native hearts compared to that in uninfected controls.
FIG. 6.
RCMV infection increases Fractalkine and Lymphotactin expression in heart allografts. (A) RT-PCR TaqMan was used to detect expression of the chemokines Lymphotactin and Fractalkine in the heart allografts. Native and graft hearts from RCMV-infected and uninfected animals were analyzed at PODs 7, 14, 21, 24, 28, 32, 35, and 45 (n = 4). cDNA was produced from total RNA isolated from the graft tissues. RT-PCR TaqMan detection of the ribosomal protein L32 was used as a normalizing control. (B) Virus-specific induction of the Lymphotactin (C chemokine) and Fractalkine (CX3C chemokine) expression in native and graft hearts is shown (fold increase is the expression of chemokines in grafts from infected recipients compared to that for uninfected controls). These data suggest that expression of both Fractalkine and Lymphotactin is highest during the RCMV acceleration phase of TVS.
Overall, our allograft chemokine expression profiling demonstrated that RCMV infection dramatically enhances graft chemokine expression with kinetics that match the timing of the virus-mediated acceleration of graft TVS that was observed in this model. Accordingly, the virus-induced acceleration of TVS likely occurred through increased inflammatory cell infiltration and activation in the RCMV-infected graft tissues due to an increase in host chemokine expression.
DISCUSSION
In this study, we examined the role of RCMV in the acceleration of transplant vascular sclerosis in a rat heart transplant model. Through kinetic analysis of this disease process, we have established that RCMV infection of allograft recipients induces an early endothelialitis at day 7, which is followed by an acceleration of TVS formation occurring between 21 and 28 days posttransplantation. The severity of TVS was greater in the infected allografts than in the control allografts starting sometime between days 21 and 28, and persisting through 45 days posttransplantation, which was the mean time to allograft failure in the infected recipients. The allografts from RCMV-infected recipients developed chronic rejection and/or graft failure substantially earlier compared to allografts from uninfected recipients (day 45 versus day 90 [Fig. 1A and B]). Viral DNA was consistently detected in the grafts between 7 and 32 days posttransplantation, suggesting a link between the presence of virus and accelerated TVS (Fig. 2). At later times after transplantation (>35 days), the detection of RCMV DNA became sporadic, which is consistent with the hypothesis that the CMV-induced acceleration of vascular disease occurs during the early stages of the disease process and not during the terminal phases. Other groups have reported a hit-or-miss detection of CMV in the development of vascular disease when looking at the end stages of disease development, suggesting CMV's early involvement in the virus-mediated acceleration of TVS.
Previously, it was demonstrated that the alloreactive environment was crucial for the RCMV-mediated acceleration of TVS, as heart allograft recipients given autologous bone marrow failed to accelerate TVS even when infected with RCMV (37). Upregulation of chemokines occurs in the early stages of allograft transplantation, and this environment may be necessary and required for the activation of CMV in virus-infected recipients. This activation, in turn, may cause the further upregulation of chemokines, resulting in an acceleration of the inflammatory process. Indeed, allograft chemokine expression profiling demonstrates that the RCMV infection of cardiac allograft recipients dramatically enhances host chemokine mRNA levels in the grafts with kinetics that parallel the timing of the virus-mediated acceleration of TVS observed in this model (between days 21 and 32 posttransplantation). This enhancement of chemokine expression promotes an increase in macrophage and T-cell (CD4+ and CD8+ cells) infiltration at these times (Fig. 3 and Table 1). Macrophage infiltration is critical in the early stages of the development of allograft rejection. Macrophages, which migrate and become activated in response to the proinflammatory CC chemokines RANTES, MCP-1, and MIP-1α, were found in the infected graft vessels at 24, 28, and 32 days posttransplantation (Fig. 4). Expression of these CC chemokines was enhanced in allografts from virus-infected recipients at days 24 and 28, corresponding to the timing of virus-accelerated TVS. Genetically engineered mouse strains containing a deletion in CCR2, a chemokine receptor found predominately on monocytes/macrophages, was shown to reduce graft rejection and the related vascular disease atherosclerosis (11). Not only is macrophage recruitment and activation central to vascular disease but these cells are also important vehicles for CMV dissemination and replication. Researchers have reported that allogeneic stimulation of human monocytes bearing latent HCMV promotes their differentiation, which leads to virus reactivation and replication in these monocyte-derived macrophages (16, 17, 45, 46). Interestingly, the cytokine IFN-γ was necessary for the production of the HCMV reactivation macrophage phenotype and replication of HCMV (46). In the present study, we found that IFN-γ levels are nearly 20-fold higher in the graft hearts of RCMV-infected recipients than in the uninfected controls at day 24 (Fig. 5), which may enhance monocyte/macrophage recruitment and activation and/or differentiation, promoting virus dissemination to the allografts and replication in these tissues.
Chemokines are critical mediators of T-cell infiltration into allograft tissues during acute and chronic rejection (for a review, see reference 30). Alloreactive T cells are arguably the most crucial cell type involved in acute and chronic rejection, but in CMV-infected recipients, the role of virus-specific T cells in the acceleration of TVS is still unclear. Differentiation of the effects from each of these two types of antigen-specific T cells in this process may be extremely difficult to achieve experimentally. Moreover, the general T-cell effect on virus-induced acceleration of TVS, whether virus specific or allospecific, may simply be due to the massive increase in the numbers of infiltrating T cells and the damaging effects that they may have on the graft tissue during both virus clearance and the cytopathology associated with alloreactivity. In our model, we detected CD8+ T cells by day 24 posttransplantation and CD4+ T cells by day 32 posttransplantation. However, we failed to observe detectable levels of T-cell infiltration in the native hearts despite the presence of virus in this tissue. Hence, the T-cell response is not generalized to uninfected nontransplanted tissues, suggesting the importance of the allospecific T cells in conjunction with the virus-specific T cells in promoting this disease process. In our model, the combined development of both allo- and virus-specific responses may augment or exacerbate the overall immune response to the transplanted graft. Chemokine profiling in our rat heart transplantation model demonstrates this enhanced effect on the immune response, as mRNA levels for all of the chemokines we tested were increased in the infected recipients compared with those in the uninfected controls in the course of allograft rejection. However, human recipients that have previously been exposed to HCMV have a more favorable outcome when receiving a CMV+ allograft than seronegative recipients, suggesting that preexisting virus-specific responses can also be beneficial to the outcome of disease. The control of virus replication in the graft by T cells or other means, such as drug therapy, and the resultant decrease in tissue viral load may reduce the amount of T cells infiltrating the grafts and thus reduce the T-cell-mediated pathology. We are currently testing this hypothesis with our rat heart transplant model of chronic rejection.
T cells express many different types of CC and CXC chemokine receptors. Thus, these cells are capable of responding to many different types of chemokines. Of the many T-cell chemoattractants, we have detected increased RANTES, MIP-1α, Fractalkine, IP-10, and Lymphotactin expression in the allografts from infected recipients compared to that in the allografts from uninfected controls (Fig. 4 through 6). The impact of Lymphotactin in rejection is unknown; however, IP-10 is a major mediator of acute allograft rejection. Using a mouse model of cardiac allograft rejection, Hancock et al. demonstrated that IP-10 receptor (CXCR3)-deficient recipients failed to develop acute rejection and showed dramatically improved allograft survival (13). These mice had fewer infiltrating alloreactive T cells in their grafts. In addition, donor hearts derived from IP-10 knockout mice also failed to develop allograft rejection, suggesting that the CXCR3-positive T cells' reacting to donor-derived IP-10 is a major pathway involved in rejection (12). We have determined that IP-10 expression is nearly 20-fold higher in the allografts from infected recipients than in the allografts from uninfected controls at day 28, which corresponds to the timing of CD8+-T-cell infiltration in these infected recipient grafts. IP-10 expression is mediated by the cytokine IFN-γ, which was nearly 20-fold higher in the infected grafts at days 24 and 28. Thus, upregulation of IFN-γ corresponds to the enhanced expression of IP-10, which may be associated with the increase in CD8+-T-cell infiltration. IP-10 has also been shown to be a growth factor for vascular SMC, causing the activation, proliferation, and migration of these cells. The timing of increased IP-10 expression matches the timing of RCMV-accelerated neointimal formation, consisting in large part of SMC. Thus, increased IP-10 expression between days 24 and 28 in the CMV-infected allografts may have an additional impact on TVS, other than merely causing T-cell infiltration. The generation of accelerated TVS by CMV infection was associated with the chemokine expression profile of rejecting allografts, and this profile was not paralleled by the native hearts in the infected recipients. These data demonstrate the importance of allograft rejection in addition to CMV infection for the accelerated TVS process to occur.
All viral pathogens have evolved to use the host inflammatory response for their benefit or have developed methods to quell these antiviral responses. Many of these adaptations have included using or modulating the expression of chemokines and chemokine receptors. In addition to modulating the host expression of chemokines, viruses such as CMV encode their own chemokines and chemokine receptors. The results presented here suggest that RCMV-accelerated TVS and chronic rejection result from the virus-induced enhancement of host chemokine expression and inflammatory cell infiltration. For evolutionary benefit, the virus manipulates the host inflammatory response in order to propagate and maintain itself, an additional consequence of which is damage to the host tissues. Expression of other host mediators of inflammation and wound repair may be similarly upregulated by CMV, which is a focus of our ongoing research.
Acknowledgments
We acknowledge Andrew Townsend for graphic work.
This work was supported by research grants from the Department of Veterans Affairs, a Public Health Service grant from the National Institutes of Health (HL 66238-01), and the Roche Organ Transplantation Research Foundation (S.L.O. and D.N.S.). J.A.N. was supported by Public Health Service grants AI 21640 and HL 65754 from the National Institutes of Health.
REFERENCES
- 1.Adler, S. P. 1983. Transfusion-associated cytomegalovirus infections. Rev. Infect. Dis. 5:977-993. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, A. T., A. R. Strauch, R. C. Starling, D. D. Sedmak, and C. G. Orosz. 1997. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation 63:941-947. [DOI] [PubMed] [Google Scholar]
- 3.Ballard, R. A., W. L. Drew, K. G. Hufnagle, and P. A. Riedel. 1979. Acquired cytomegalovirus infection in preterm infants. Am. J. Dis. Child. 133:482-485. [DOI] [PubMed] [Google Scholar]
- 4.Boehler, A., A. Schaffner, F. Salomon, and G. Keusch. 1994. Cytomegalovirus disease of late onset following renal transplantation: a potentially fatal entity. Scand. J. Infect. Dis. 26:369-373. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman, C. A., H. Meijer, F. Bosman, and C. P. van Boven. 1985. Biology of rat cytomegalovirus infection. Intervirology 24:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Bruggeman, C. A., H. Schellekens, G. Grauls, W. M. Debie, and C. P. van Boven. 1983. Rat cytomegalovirus: induction of and sensitivity to interferon. Antivir. Res. 3:315-324. [DOI] [PubMed] [Google Scholar]
- 7.Bruning, J. H., M. C. J. Persoons, K. B. Lemstrom, F. S. Stals, E. De Clercq, and C. A. Bruggeman. 1994. Enhancement of transplantation-associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transplant. Int. 7(Suppl. 1):S365-S370. [DOI] [PubMed] [Google Scholar]
- 8.Cheeran, M. C., S. Hu, S. L. Yager, G. Gekker, P. K. Peterson, and J. R. Lokensgard. 2001. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J. Neurovirol. 7:135-147. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn, L., and A. Ost. 1984. Cytomegalovirus infection of human blood cells. J. Infect. Dis. 149:207-214. [DOI] [PubMed] [Google Scholar]
- 10.Froberg, M. K., A. Adams, N. Seacotte, J. Parker-Thornburg, and P. Kolattukudy. 2001. Cytomegalovirus infection accelerates inflammation in vascular tissue overexpressing monocyte chemoattractant protein-1. Circ. Res. 89:1224-1230. [DOI] [PubMed] [Google Scholar]
- 11.Gu, L., Y. Okada, S. K. Clinton, C. Gerard, G. K. Sukhova, P. Libby, and B. J. Rollins. 1998. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2:275-281. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, W. W., W. Gao, V. Csizmadia, K. L. Faia, N. Shemmeri, and A. D. Luster. 2001. Donor-derived IP-10 initiates development of acute allograft rejection. J. Exp. Med. 193:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock, W. W., B. Lu, W. Gao, V. Csizmadia, K. Faia, J. A. King, S. T. Smiley, M. Ling, N. P. Gerard, and C. Gerard. 2000. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horuk, R. 2001. Chemokine receptors. Cytokine Growth Factor Rev. 12:313-335. [DOI] [PubMed] [Google Scholar]
- 15.Hosenpud, J. D., G. D. Shipley, and C. R. Wagner. 1992. Cardiac allograft vasculopathy: current concepts, recent developments and future directions. J. Heart Lung Transplant. 11:9-23. [PubMed] [Google Scholar]
- 16.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo, K., J. Xu, and E. S. Mocarski. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. USA 93:11137-11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautenschlager, I., A. Soots, L. Krogerus, K. Inkinen, J. Kloover, R. Loginov, K. Holma, H. Kauppinen, C. Bruggeman, and J. Ahonen. 1999. Time-related effects of cytomegalovirus infection on the development of chronic renal allograft rejection in a rat model. Intervirology 42:279-284. [DOI] [PubMed] [Google Scholar]
- 20.Lemstrom, K., J. Bruning, P. Koskinen, C. Bruggeman, I. Lautenschlager, and P. Hayry. 1994. Triple-drug immunosuppression significantly reduces chronic rejection in noninfected and RCMV-infected rats. Transpl. Proc. 26:1727-1728. [PubMed] [Google Scholar]
- 21.Lemstrom, K., P. Koskinen, L. Krogerus, M. Daemen, C. A. Bruggeman, and P. J. Hayry. 1995. Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection. Circulation 92:2594-2604. [DOI] [PubMed] [Google Scholar]
- 22.Lemstrom, K., R. Sihvola, C. Bruggeman, P. Hayry, and P. Koskinen. 1997. Cytomegalovirus infection-enhanced cardiac allograft vasculopathy is abolished by DHPG prophylaxis in the rat. Circulation 95:2614-2616. [DOI] [PubMed] [Google Scholar]
- 23.Lemstrom, K. B., J. H. Bruning, C. A. Bruggeman, P. Koskinen, P. T. Aho, S. Yilmaz, I. T. Lautenschlager, and P. J. Hayry. 1994. Cytomegalovirus infection-enhanced allograft arteriosclerosis is prevented by DHPG prophylaxis in the rat. Circulation 90:1969-1978. [DOI] [PubMed] [Google Scholar]
- 24.Lemstrom, K. B., J. H. Bruning, C. A. Bruggeman, I. T. Lautenschlager, and P. J. Hayry. 1993. Cytomegalovirus infection enhances smooth muscle cell proliferation and intimal thickening of rat aortic allografts. J. Clin. Investig. 92:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemstrom, K. B., J. H. Bruning, C. A. Bruggeman, I. T. Lautenschlager, and P. J. Hayry. 1994. Triple drug immunosuppression significantly reduces immune activation and allograft arteriosclerosis in cytomegalovirus-infected rat aortic allografts and induces early latency of viral infection. Am. J. Pathol. 144:1334-1347. [PMC free article] [PubMed] [Google Scholar]
- 26.Macher, A. M., C. M. Reichert, S. E. Straus, D. L. Longo, J. Parrillo, H. C. Lane, A. S. Fauci, A. H. Rook, J. F. Manischewitz, and G. V. Quinnan, Jr. 1983. Death in the AIDS patient: role of cytomegalovirus. N. Engl. J. Med. 309:1454.. [DOI] [PubMed] [Google Scholar]
- 27.Melnick, J. L., E. Adam, and M. E. DeBakery. 1998. The link between CMV and atherosclerosis. Infect. Med. 479-486.
- 28.Melnick, J. L., B. L. Petrie, G. R. Dreesman, J. Burek, C. H. McCollum, and M. E. DeBakey. 1983. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet 644-647. [DOI] [PubMed]
- 29.Melter, M., A. Exeni, M. E. Reinders, J. C. Fang, G. McMahon, P. Ganz, W. W. Hancock, and D. M. Briscoe. 2001. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation 104:2558-2564. [DOI] [PubMed] [Google Scholar]
- 30.Melter, M., G. McMahon, J. Fang, P. Ganz, and D. M. Briscoe. 1999. Current understanding of chemokine involvement in allograft transplantation. Pediatr. Transplant. 3:10-21. [DOI] [PubMed] [Google Scholar]
- 31.Merigan, T. C., D. G. Renlund, S. Keay, et al. 1992. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. N. Engl. J. Med. 326:1182-1186. [DOI] [PubMed] [Google Scholar]
- 32.Michelson, S., P. Dal Monte, D. Zipeto, B. Bodaghi, L. Laurent, E. Oberlin, F. Arenzana-Seisdedos, J. Virelizier, and M. P. Landini. 1997. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J. Virol. 71:6495-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neiman, P., P. B. Wasserman, B. B. Wentworth, G. F. Kao, K. G. Lerner, R. Storb, C. D. Buckner, R. A. Clift, A. Fefer, L. Fass, H. Glucksberg, and E. D. Thomas. 1973. Interstitial pneumonia and cytomegalovirus infection as complications of human marrow transplantation. Transplantation 15:478-485. [PubMed] [Google Scholar]
- 34.Nelson, J. A., J. W. Gnann, Jr., and P. Ghazal. 1990. Regulation and tissue-specific expression of human cytomegalovirus. Curr. Top. Microbiol. Immunol. 154:75-100. [DOI] [PubMed] [Google Scholar]
- 35.Nordoy, I., F. Muller, K. P. Nordal, H. Rollag, P. Aukrust, and S. S. Froland. 2000. Chemokines and soluble adhesion molecules in renal transplant recipients with cytomegalovirus infection. Clin. Exp. Immunol. 120:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orloff, S. L. 1999. Elimination of donor-specific alloreactivity by bone marrow chimerism prevents cytomegalovirus accelerated transplant vascular sclerosis in rat small bowel transplants. J. Clin. Virol. 12:G3-G25.
- 37.Orloff, S. L., D. N. Streblow, C. Soderberg-Naucler, Q. Yin, C. Kreklywich, C. L. Corless, P. A. Smith, C. B. Loomis, L. K. Mills, J. W. Cook, C. A. Bruggeman, J. A. Nelson, and C. R. Wagner. 2002. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation 73:679-688. [DOI] [PubMed] [Google Scholar]
- 38.Orloff, S. L., Q. Yin, C. L. Coreless, C. B. Loomis, J. M. Rabkin, and C. R. Wagner. 1999. A rat small bowel transplant model of chronic rejection: histopathologic characteristics. Transplantation 68:766-779. [DOI] [PubMed] [Google Scholar]
- 39.Orloff, S. L., Q. Yin, C. L. Corless, M. S. Orloff, J. M. Rabkin, and C. R. Wagner. 2000. Tolerance induced by bone marrow chimerism prevents transplant vascular sclerosis in a rat model of small bowel transplant chronic rejection. Transplantation 69:1295-1303. [DOI] [PubMed] [Google Scholar]
- 40.Redman, T. K., W. J. Britt, C. M. Wilcox, M. F. Graham, and P. D. Smith. 2002. Human cytomegalovirus enhances chemokine production by lipopolysaccharide-stimulated lamina propria macrophages. J. Infect. Dis. 185:584-590. [DOI] [PubMed] [Google Scholar]
- 41.Robinson, L. A., C. Nataraj, D. W. Thomas, D. N. Howell, R. Griffiths, V. Bautch, D. D. Patel, L. Feng, and T. M. Coffman. 2000. A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J. Immunol. 165:6067-6072. [DOI] [PubMed] [Google Scholar]
- 42.Rott, D., J. Zhu, M. S. Burnett, Y. F. Zhou, A. Wasserman, J. Walker, and S. E. Epstein. 2001. Serum of cytomegalovirus-infected mice induces monocyte chemoattractant protein-1 expression by endothelial cells. J. Infect. Dis. 184:1109-1113. [DOI] [PubMed] [Google Scholar]
- 43.Russel, M. E., W. W. Hancock, A. F. Wallace, L. R. Wyner, and M. J. Karnovsky. 1995. Modulation of inflammatory activation pathways in the Lewis-to-F344 rat chronic cardiac rejection model. Transplant. Proc. 27:2100-2104. [PubMed] [Google Scholar]
- 44.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Söderberg-Naucler, C., K. Fish, and J. A. Nelson. 1997. Reactivation of human cytomegalovirus in a novel dendritic cell phenotype from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 46.Söderberg-Nauclér, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 48.Steinhoff, G., X. M. You, C. Steinmuller, K. Boeke, F. S. Stals, C. A. Bruggeman, and A. Haverich. 1995. Induction of endothelial adhesion molecules by rat cytomegalovirus in allogeneic lung transplantation in the rat. Scand. J. Infect. Dis. Suppl. 99:58-60. [PubMed] [Google Scholar]
- 49.Streblow, D., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 50.Tegtmeier, G. E. 1988. The use of cytomegalovirus-screened blood in neonates. Transfusion 28:201-203. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, E. D., R. Storb, R. A. Clift, A. Fefer, L. Johnson, P. E. Neiman, K. G. Lerner, H. Glucksberg, and C. D. Buckner. 1975. Bone-marrow transplantation. N. Engl. J. Med. 292:895-902. [DOI] [PubMed] [Google Scholar]
- 52.Ustinov, J. A., T. T. Lahtinen, C. A. Bruggeman, P. J. Hayry, and I. T. Lautenschlager. 1994. Direct induction of class II molecules by cytomegalovirus in rat heart microvascular endothelial cells is inhibited by ganciclovir (DHPG). Transplantation 58:1027-1031. [DOI] [PubMed] [Google Scholar]
- 53.Valantine, H. A., S. Z. Gao, G. Santosh, et al. 1999. Impact of prophylactic immediate post-transplant ganciclovir on development of transplant atherosclerosis. A post-hoc analysis of a randomized, placebo-controlled study. Circulation 100:61-66. [DOI] [PubMed] [Google Scholar]
- 54.van Dorp, W. T., E. Jonges, C. A. Bruggeman, M. R. Daha, L. A. van Es, and F. J. van Der Woude. 1989. Direct induction of MHC class, I, but not class II, expression on endothelial cells by cytomegalovirus infection. Transplantation 48:469-472. [DOI] [PubMed] [Google Scholar]
- 55.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 74:7656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weller, T. H. 1971. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. N. Engl. J. Med. 285:203-214. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Z., L. Kaptanoglu, W. Haddad, D. Ivancic, Z. Alnadjim, S. Hurst, D. Tishler, A. D. Luster, T. A. Barrett, and J. Fryer. 2002. Donor T cell activation initiates small bowel allograft rejection through an IFN-gamma-inducible protein-10-dependent mechanism. J. Immunol. 168:3205-3212. [DOI] [PubMed] [Google Scholar]