Abstract
Double-stranded RNAs ≈21 nucleotides long [small interfering RNA (siRNA)] are recognized as powerful reagents to reduce the expression of specific genes. To use them as reagents to protect cells against viral infection, effective methods for introducing siRNAs into primary cells are required. Here, we describe success in constructing a lentivirus-based vector to introduce siRNAs against the HIV-1 coreceptor, CCR5, into human peripheral blood T lymphocytes. With high-titer vector stocks, >40% of the peripheral blood T lymphocytes could be transduced, and the expression of a potent CCR5-siRNA resulted in up to 10-fold inhibition of CCR5 expression on the cell surface over a period of 2 weeks in the absence of selection. In contrast, the expression of another major HIV-1 coreceptor, CXCR4, was not affected. Importantly, blocking CCR5 expression by siRNAs provided a substantial protection for the lymphocyte populations from CCR5-tropic HIV-1 virus infection, dropping infected cells by 3- to 7-fold; only a minimal effect on infection by a CXCR4-tropic virus was observed. Thus, our studies demonstrate the feasibility and potential of lentiviral vector-mediated delivery of siRNAs as a general means of intracellular immunization for the treatment of HIV-1 and other viral diseases.
Although the idea of protecting cells against HIV-1 infection by the internal production of a protective molecule (“intracellular immunization”) was suggested 14 years ago (1), it has not been realized as a clinical procedure, partly because no macromolecule has proved potent enough and because of limitations in gene delivery vehicles. Recently, it has been appreciated that small, double-stranded RNAs (siRNA) can be powerful sequence-specific catalysts for targeted RNA destruction by means of an evolutionarily conserved mechanism known as RNA interference (RNAi) (2–5). The properties of siRNA suggest its potential as an “intracellular immunogen.” With the discovery that siRNAs can be effectively produced as hairpin transcripts from RNA polymerase III (Pol III) promoters (6–11), intracellular synthesis of siRNAs has become feasible. The best vector for delivering an siRNA template would be a lentivirus vector derived from HIV-1, because such vectors stably infect nondividing cells and are not subject to the silencing imposed on other retrovirus vectors (a “turning the tables” approach; refs. 12 and 13). Furthermore, lentiviral vectors have proven to be effective in expressing transgenes within multiple lineages over prolonged periods of time and safe in SCID-hu and non-human primate hematopoietic stem cell transplants (14, 15). HIV-1 infection could be prevented by either an siRNA directed against viral RNA, as has been done in several in vitro models (16–20), or by targeting the mRNA for the primary HIV-1 coreceptor, CCR5. CCR5 suggests itself as a target because people who lack both genes for CCR5 (CCR5Δ32 homozygotes) are resistant to HIV-1 infection but are otherwise apparently normal (21–24). We report here the successful use of this approach.
Materials and Methods
Vector Construction.
A human U6-RNA pol III promoter (−328 to +1) was amplified from HEK-293 genomic DNA with primers 5′-gggaattcccccagtggaaagacgcgcag-3′ and 5′-cggaagcttgaagaccacggtgtttcgtcctttccacaa-3′, in which a BbsI site was introduced at the 3′ end allowing the insertion of siRNA sequences at the +1 position of the U6 transcript. The PCR fragment was cloned at EcoRI–HindIII sites of pBS-SKII plasmid (Stratagene). Sequencing analysis showed all of the individual clones recovered had a GCGCG insertion at the −267 position comparing to the published human U6-RNA promoter sequence (GenBank accession nos. X07425 and M14486). This difference might originate either from a PCR error or a natural polymorphism. Nevertheless, transient transfection assays showed that this promoter fragment was fully functional (X.-F.Q. and D.B., unpublished data). To construct the hairpin siRNA expression cassette, two complementary DNA oligos (see below) were synthesized, annealed, and inserted between BbsI and XhoI sites immediately downstream of the U6 promoter: 5′- accg(n)18ttcaagaga(n)18ctttttc-3′; 3′-(n)18aagttctct(n)18gaaaaagagct-5′. The 19-nt sense and reverse complementary targeting sequences are highlighted in bold. Note that the sense targeting sequence always starts with G at position 1 (thus, the reverse complementary sequence ends with C), as required for the efficient transcription initiation from the U6 promoter (25). The siRNA cassette also features a TTCAAGAGA loop situated between the sense and reverse complementary targeting sequences and a TTTTT terminator at the 3′ end. The CCR5-siRNA (186) contains the sense targeting sequence of gagcatgactgacatctac corresponding to the 186–204 nucleotide positions of human CCR5 coding sequence (GenBank accession no. U57840), whereas the CCR5-siRNA (809) has the targeting sequence of gtagctctaacaggttgga to the 809–827 nucleotide positions. The targeting sequence for lacZ-siRNA is gtgaccagcgaatacctgt, which is directed to the 1915–1933 region of the bacterial galactosidase gene.
FG12 lentiviral vector was derived from FUGW (26). The extra nucleotides from the HindIII site downstream of the Ubiquitin-C promoter (UbiC) promoter to the NcoI site in front of the initiation codon of GFP were deleted by a HindIII–NcoI adapter ligation. Further, XbaI, EcoRI, and XhoI sites at the 3′ end of GFP and WRE were eliminated, followed by a polylinker oligonucleotide ligation at the PacI site between the Flap element and the UbiC promoter, to generate a set of new restriction sites, XbaI–HpaI–XhoI–BstXI–PacI, that is optimal for accommodating the siRNA expression cassette (X.-F.Q. and D.B., unpublished data). To construct the siRNA-expressing lentiviral vectors, the siRNA expression cassette was subcloned into FG12 between the XbaI and XhoI sites. The resulting plasmid was confirmed by restriction enzyme digestion and DNA sequencing.
Lentiviral Vector Production.
All vesicular stomatitis virus (VSV)-G pseudotyped lentiviral vector stocks were produced by calcium phosphate-mediated transient transfection of HEK-293 T cells. Briefly, HEK-293 T cells were cultured in either Iscov's modified Eagle's medium or DMEM (GIBCO Invitrogen) containing 10% FCS (HyClone), 100 units of penicillin, and 100 μg/ml streptomycin. The cells were cotransfected with appropriate amounts of vector plasmid, the HIV-1 lentiviral packaging constructs pRSVREV (27) and pMDLg/pRRE (27), and the VSV-G expression plasmid pHCMVG (28). The viruses were collected from the culture supernatants on days 2 and 3 posttransfection and concentrated 100- to 1,000-fold by ultracentrifugation (28). The concentrated virus stocks were titered on HEK-293 T cells based on GFP expression. Titers for the siRNA expression constructs were only slightly reduced compared with the parental vector.
Cell Culture and Lentiviral Vector Transduction.
Magi-CCR5 cells (29) (AIDS Research and Reference Reagent Program of the National Institutes of Health) were maintained in DMEM, 10% FCS containing 200 μg of G418 (GIBCO/BRL), 100 μg of hygromycin, and 1 μg/ml puromycin (Sigma). The stable expression of human CD4 and CCR5 on the cell surface was confirmed by fluorescence-activated cell sorter (FACS) analysis. The cells were transduced with concentrated lentiviral vector stocks at a multiplicity of infection (moi) of 10–25 (note that Magi-CCR5 cells have a considerable lower transducing efficiency) in the presence of 8 μg/ml polybrene (Sigma). The transduced cells were harvested 4 days later and stained with Cy-Chrome-labeled mouse anti-human CCR5 mAb (2D7, PharMingen) or a mouse IgG2a/k isotype control (OX-35, PharMingen), according to the manufacturer's instructions. Human peripheral blood lymphocytes (PBLs) were isolated from leukopacks by Histopaque (Sigma) and cultured in RPMI medium 1640/20% FCS with 2.5 μg of phytohemagglutinin (PHA) (Murex Biotech, Dartford, U.K.)/100 units of penicillin/100 μg/ml streptomycin for 2 days. After 2 days of PHA stimulation, CD8+ cells were depleted by M450 CD8 Dynabeads (Dynal, Great Neck, NY) and the residual amount of CD8+ cells was <1%, as confirmed by FACS analysis. The CD8+-depleted PBLs were used for lentiviral vector transduction. Briefly, 4 × 105 cells were incubated with various lentiviral vectors at a moi of 5 for 2 h in the presence of 8 μg/ml polybrene. After the incubation, virus supernatants were removed and replaced with 1.5 ml of fresh RPMI medium 1640/20% FCS containing 20 units/ml IL-2 (Roche Molecular Biochemicals). GFP, CD4, CCR5, and CXCR4 expression was analyzed by FACS at multiple time points after transduction. Note that, although a moi of 5 was used for transduction with different lentivectors, actual transduction efficiency appeared to vary from sample to sample, depending on the initial titers of the virus preparations.
HIV-1 Virus Production and Infection.
Stocks for the murine heat-stable antigen (HSA)-expressing HIV-1 reporter viruses, NFNSX-r-HSAS (CCR5-tropic; ref. 30) and NL-r-HSAS (CXCR4-tropic; ref. 31), were produced by calcium phosphate transfection with the infectious proviral plasmid in HEK-293 T cells. The virus supernatants were filtered with 0.22-μm filters and stored at −70°C. The p24 value of the virus stocks was 6,966 ng/ml for NFNSX-r-HSAS and 1,077 ng/ml for NL-r-HSAS. Four days after lentivector or mock transduction, the PBLs (5 × 105 cells) were infected with 100 μl of NFNSX-r-HSAS or NL-r-HSAS virus in the presence of 8 μg/ml polybrene for 2 h. After the incubation, the cells were washed and replated with 1.5 ml of RPMI medium 1640/20% FCS and 20 units/ml IL-2. The rate of infection was determined by FACS analysis for HSA expression on the cell surface at various times points as indicated. p24 levels in the culture supernatants were measured by ELISA.
FACS Analysis.
Cells (5 × 105) were stained with monoclonal antibodies to human CCR5 [2D7, allophycocyanin (APC)-labeled, BD Biosciences], CXCR4 (CXCR4, APC-labeled, BD Biosciences), and CD4 [RPAT4, phycoerythrin (PE)-labeled, eBioscience], according to the manufacturer's instructions. For measuring HIV-1 reporter virus infection, a PE-labeled anti-murine HSA mAb (M1/69, PharMingen) was used. The cells were also stained with isotype controls for each of the specific antibodies. The stained cells were fixed with 2% formaldehyde and acquired on a FACScan or FACSCalibur (Becton Dickinson). The data analysis was performed with cellquest (Becton Dickinson) or flowjo (Tree Star, San Carlos, CA) software.
Results
The backbone of the siRNA expression vector was derived from FUGW, an HIV-1-based lentivirus vector originally designed for germ-line gene transduction (26). The short hairpin form of siRNA template was transcribed from a human U6-RNA polymerase III (Pol III) promoter, and the expression cassette was inserted in the forward orientation at the junction of the HIV-1 DNA Flap element (Flap) and UbiC of the vector FG12 (Fig. 1A). This configuration permits high-titer virus production and the functional expression of siRNA templates in murine splenocytes, bone marrow cells, and transgenic animals (X.-F.Q., C. Lois, and D.B., unpublished data). The vector also expresses a human UbiC-driven GFP gene to provide a marker for tracking transduced cells (Fig. 1A). To identify an effective siRNA targeted to CCR5, we first used Magi-CCR5 cells (29) carrying a transfected human CCR5 gene as a target. Mock-transduced Magi-CCR5 cells expressed no GFP but had a high level of CCR5 expression on the cell surface (Fig. 1B). Cells transduced by a vector lacking the siRNA cassette (FG12) or a control siRNA cassette against lacZ (lacZ-siRNA) contained many GFP+ cells (positively transduced cells), but CCR5 expression was unaltered in these cells (Fig. 1B). However, with an anti-CCR5-specific siRNA-expressing vector targeting to the 809–827 region of the human CCR5 mRNA coding sequence [CCR5-siRNA (809)], the surface expression of CCR5 on the GFP+ cells was dropped to 30–40% the level of the controls, judging by the mean fluorescence intensity of CCR5 antibody staining (Fig. 1B). More significantly, the anti-CCR5 siRNA construct directed to the 186–204 region [CCR5-siRNA (186)] resulted in >90% reduction of CCR5 expression (Fig. 1B). In addition to CCR5-siRNA (809) and CCR5-siRNA (186), we also tested several other constructs targeting to different regions of CCR5 mRNA; these exhibited variable degrees of effectiveness but none >90% inhibition (not shown). A systematic approach might in the future identify even more potent siRNA templates, but the 90% inhibition provided by the CCR5-siRNA (186) vector appeared to be sufficient for our further studies described below.
Figure 1.
Construction of a lentivirus-based vector for delivering anti-human CCR5 siRNAs. (A) Schematic diagram of the siRNA-expressing lentiviral vector. The short hairpin form of siRNA is expressed under the control of a human U6-RNA Pol III promoter (Pol III). The vector also contains a human UbiC promoter driving the GFP marker gene for tracking transduced cells. 5′ LTR, HIV-1 5′LTR; Δ3′ LTR, HIV-1 self-inactivating 3′LTR; Flap, HIV-1 DNA flap element; WRE, woodchuck hepatitis B virus RNA regulatory element. (B) Selection of siRNA constructs that can effectively inhibit CCR5 expression in Magi-CCR5 cells. Magi-CCR5 cells were transduced with various lentiviral vectors. The cells were harvested 4 days after virus transduction and analyzed by FACS with anti-human CCR5 or isotype control antibody staining. The productively transduced (GFP+) and nontransduced (GFP−) cells were gated based on their GFP signals. The CCR5 staining is represented by the open curve and the isotype control by the shaded curve. Note that some lower GFP-expressing cells were included in the GFP− gate and, thus, there appeared to be a slight decrease of CCR5 staining in this population of the anti-CCR5 vector-transduced samples. CCR5-siRNA(186), the vector expressing a potent anti-CCR5 siRNA; CCR5-siRNA(809), the vector expressing a weaker anti-CCR5 siRNA; lacZ-siRNA, a nonspecific siRNA vector; FG12, empty vector; Mock, mock transduction control.
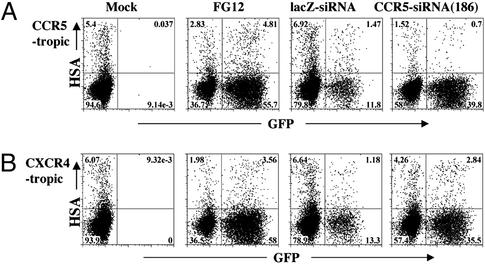
To test the efficacy of the vector against HIV-1 infection in primary human CD4+ T cells, PBLs depleted of CD8+ cells were isolated, stimulated with phytohemagglutinin (PHA) for 2 days, and transduced or mock transduced with various vectors. After the transduction, the cells were cultured in the presence of human IL-2. IL-2 elicits the synthesis and surface expression of CCR5 as well as the other major HIV-1 coreceptor CXCR4 on activated T cells (32, 33). We monitored CCR5 and CXCR4 expression levels at various time points by FACS. Fig. 2 shows FACS plots from a representative experiment (Donor B) at day 8 post-vector transduction. Quantitative analyses of the FACS results from two donors (A and B) are shown in Table 1, and more extensive time course studies with four different donors (A, B, C, and D) will be presented in Table 3. The mock-transduced cells in Fig. 2A showed that ≈30% of the cells were CCR5+. The cells transduced with the vector control (FG12) had ≈60% GFP+, and among those positively transduced cells, there were still ≈29% cells expressing CCR5 (Table 1, Donor B). lacZ-siRNA-transduced cells had a similar percentage of CCR5 positivity (Table 1), but there were fewer GFP positive cells, presumably because of a lower titer of the viral stock. Over 40% of PBLs were productively transduced by the CCR5-siRNA (186) vector, and within this GFP+ population only 3.4% of the cells scored positive for CCR5 expression, suggesting that siRNA expression caused an ≈10-fold reduction in CCR5 positivity compared with a lacZ-siRNA control (Table 1). Of the remaining CCR5-siRNA (186)-transduced cells that still expressed CCR5, the mean fluorescence intensity as a measure of relative abundance of CCR5 surface expression was substantially reduced. For the GFP− population, levels of CCR5 expression were slightly higher than in controls (Table 1), showing that down-regulation of CCR5 expression only occurred in the productively transduced cells. In contrast to CCR5, Fig. 2B and the data in Table 1 show that CXCR4 expression was not affected by the anti-CCR5 siRNA-expressing vector. Thus, lentivector-mediated expression of an appropriate siRNA in primary human T cells can specifically reduce the expression of CCR5.
Figure 2.
Reduction of CCR5 surface expression on human PBLs transduced by anti-CCR5 lentiviral vector. PHA-stimulated and CD8+-depleted PBLs were transduced with various lentiviral vectors as described in Fig. 1. The transduced cells were further cultured in IL-2-containing medium for 8 days before FACS analysis for CCR5 or CXCR4 expression on the cell surface. A representative experiment with Donor B is shown. (A) Cells were stained with allophycocyanin (APC)-labeled anti-human CCR5 mAb (2D7, PharMingen). The results are exhibited as CCR5 vs. GFP dotplots with cell populations in the live lymphocyte gate (typically >75%). The quadrant lines were defined by mock-transduction and isotype-control staining, and the percentage numbers are indicated. (B) Cells were stained with APC-labeled anti-human CXCR4 mAb (CXCR4, PharMingen), and the results are plotted in the same manner as in A.
Table 1.
Selective down-regulation of CCR5 expression by lentiviral vector-mediated delivery of anti-CCR5 siRNA in human PBLs
| Donor | Treatment* | CCR5 expression
|
CXCR4 expression
|
||||
|---|---|---|---|---|---|---|---|
| % of CCR5+ cells in the GFP− population† | % of CCR5+ cells in the GFP+ population† | Fold of reduction‡ | % of CXCR4+ cells in the GFP− population† | % of CXCR4+ cells in the GFP+ population† | Fold of reduction‡ | ||
| A | Mock | 36.83 | N/A | 37.43 | N/A | ||
| FG12 | 43.03 | 27.96 | 37.07 | 46.38 | |||
| lacZ-siRNA | 50.20 | 25.36 | 33.94 | 49.24 | |||
| CCR5-siRNA | 42.87 | 3.63 | 6.99 | 39.95 | 52.22 | 0.94 | |
| B | Mock | 30.30 | N/A | 48.80 | N/A | ||
| FG12 | 41.62 | 29.00 | 39.68 | 47.09 | |||
| lacZ-siRNA | 35.48 | 32.63 | 45.44 | 47.64 | |||
| CCR5-siRNA | 49.16 | 3.38 | 9.65 | 31.27 | 51.30 | 0.93 | |
N/A, not applicable.
Lentiviral vector transduction was performed as described in Fig. 2. Eight days after the transduction, the cells were analyzed by FACS for the surface expression of CCR5 or CXCR4 (Materials and Methods).
Calculated from the quadrant percentage numbers from the CCR5 or CXCR4 vs. GFP FACS plots shown in Fig. 2.
Expressed as the ratio of the percentage of CCR5+ or CXCR4+ cells scored in the GFP+ population between lacZ-siRNA and CCR5-siRNA [CCR5-siRNA(186)]-transduced samples.
Table 3.
Time course study on the reduction of CCR5 expression and inhibition to CCR5-tropic HIV-1 infection with the CCR5-siRNA lentivector-transduced PBLs from different donors
| Donor | Days after HIV-1 challenge | Reduction of CCR5 | HIV-1 inhibition |
|---|---|---|---|
| A | 4 | 6.99 | 3.64 |
| 7 | 6.61 | 2.64 | |
| B | 4 | 9.65 | 6.41 |
| 6 | 10.54 | 7.04 | |
| 8 | 4.88 | 5.81 | |
| C | 4 | 10.09 | 1.25 |
| 6 | 8.7 | 3.48 | |
| 8 | 9.56 | 3.73 | |
| D | 4 | 13.26 | 2.59 |
| 6 | 13.75 | 3.68 | |
| 8 | 5.68 | 4.09 |
CD8+-depleted PBLs from four different donors were transduced with various lentiviral vectors, as described in Fig. 2. Four days after the transduction, the cells were challenged with the CCR5-tropic HIV-1 reporter virus (as in Fig. 3). The virus-infected cells were analyzed by FACS for CCR5 and HSA expression at the different time points indicated. The reduction of CCR5 expression and inhibition of CCR5-tropic HIV-1 infection were determined in the same manner as in Tables 1 and 2. Equivalent degrees of CCR5 down-regulation were obtained with the similarly transduced cells but not being challenged with the HIV-1 virus.
To examine the effect of blocking CCR5 expression by the anti-CCR5 siRNA lentivector on HIV-1 infection, the lymphocyte populations were challenged with a CCR5-tropic HIV-1 reporter virus. This reporter virus is modified to express the HSA marker in place of the HIV-1 accessory gene Vpr, which allows the assay for HIV-1 infection at the single-cell level by enumerating HSA+ cells (30). Table 2 shows the quantitative results of FACS analysis with two different donor samples and Fig. 3 shows representative FACS plots from Donor B. With the empty vector (FG12) and the lacZ-siRNA vector (lacZ-siRNA), 8% and 11% of the GFP+ cells were infected by the virus, as measured by the expression of the HSA marker (see Table 2 for percentages). With the anti-CCR5 siRNA vector [CCR5-siRNA (186)] treatment, only 1.7% of the GFP+ cells were detectably positive for HIV-1 infection, Thus, for this donor, expression of the specific siRNA led to a >6-fold drop in the level of cells infected by a CCR5-tropic HIV-1 virus. A slightly lower level of protection (3.64-fold) was also obtained from another donor (Table 2, Donor A). More extensive time course experiments with additional donors (Table 3) showed that effective CCR5 down-regulation and inhibition to CCR5-tropic HIV-1 infection occurred with all of the donors over a period of 7–8 days, although the degree of effectiveness varied from donor to donor (within the range of 3- to 7-fold of protection). In addition to the GFP+-transduced cells, we found that the frequency of the HIV-1-infected HSA+ cells in the GFP− population was also decreased 2- to 3-fold in comparison with the mock, empty vector (FG12), and lacZ-siRNA controls (Fig. 3A; Table 2, Donors A and B). Consistent with this observation, supernatant p24 levels, a measurement of virus production, were reduced ≈3-fold (Fig. 4), despite the fact that cells without siRNA to CCR5 outnumber the siRNA-transduced population. Thus, the anti-CCR5 siRNA was not only effective at protecting the transduced cells in a mixed population, but also resulted in an overall reduction of virus load and decreased infection of nontransduced cells over the course of HIV-1 infection. As a control, the lymphocyte populations were also infected with a CXCR4-tropic HIV-1 reporter virus similarly modified to express HSA (31). Infection of the CXCR4-tropic virus was not significantly inhibited by the transduction of an anti-CCR5 siRNA vector (Fig. 3B, Table 2), confirming the specificity of the siRNA-mediated inhibition. Taken together, we conclude that a lentivirus vector expressing a potent siRNA against CCR5 can substantially reduce CCR5 expression on the cell surface in a specific manner and render the transduced cells relatively resistant to CCR5-tropic HIV-1 infection.
Table 2.
Inhibition of CCR5-tropic HIV-1 infection by lentiviral vector-mediated delivery of anti-CCR5 siRNA in human PBLs
| Donor | Treatment* | Response to CCR5-tropic HIV-1 challenge
|
Response to CXCR4-tropic HIV-1 challenge
|
||||
|---|---|---|---|---|---|---|---|
| % of HSA+ cells in the GFP− population† | % of HSA+ cells in the GFP+ population† | Fold of inhibition‡ | % of HSA+ cells in the GFP− population† | % of HSA+ cells in the GFP+ population† | Fold of inhibition‡ | ||
| A | Mock | 4.07 | N/A | 4.82 | N/A | ||
| FG12 | 4.26 | 5.64 | 7.26 | 8.70 | |||
| lacZ-siRNA | 4.16 | 5.39 | 7.94 | 8.33 | |||
| CCR5-siRNA | 2.02 | 1.48 | 3.64 | 5.90 | 5.76 | 1.45 | |
| B | Mock | 5.05 | N/A | 6.07 | N/A | ||
| FG12 | 7.16 | 7.95 | 5.15 | 5.78 | |||
| lacZ-siRNA | 7.98 | 11.08 | 7.76 | 8.15 | |||
| CCR5-siRNA | 2.55 | 1.73 | 6.41 | 6.91 | 7.41 | 1.10 | |
N/A, not applicable.
The lentivector-transduced PBLs were challenged with the CCR5- or CXCR4-tropic reporter HIV-1 viruses as described in Fig. 3. Four days after virus challenge, the cells were analyzed by FACS for HSA expression to determine the number of cells infected by the HIV-1 virus (Materials and Methods).
Calculated from the quadrant percentage numbers from the HSA vs. GFP FACS plots shown in Fig. 3.
Expressed as the ratio of the percentage of HSA+ cells in the GFP+ population between lacZ-siRNA and CCR5-siRNA [CCR5-siRNA(186)]-transduced samples.
Figure 3.
Inhibition of CCR5-tropic HIV-1 infection in human PBLs transduced by lentiviral vector expressing anti-CCR5 siRNA. PBLs were transduced by various lentiviral vectors as described in Fig. 2. The transduced PBLs were cultured in IL-2-containing medium for 4 days before being challenged with either the CCR5-tropic reporter virus (A) or the CXCR4-tropic reporter virus (B). The cells were harvested 4 days after the virus challenge, and the virus-infected cells were quantitated by FACS analysis for the expression of the HSA marker. The FACS results are presented as HSA vs. GFP dotplots with cell populations in the live lymphocyte gate. The quadrant lines were defined by mock-infection and isotype-control staining, and the percentage numbers are indicated. One representative experiment with Donor B is shown.
Figure 4.
Decrease of p24 production in HIV-1-infected cultures of anti-CCR5 lentiviral vector-transduced human PBLs. Culture supernatants were collected 6 days after the HIV-1 virus challenge, as described in Fig. 3, and p24 levels were measured by ELISA in triplicates. Again, the representative result from Donor B is shown.
Discussion
These results demonstrate that an siRNA directed to a cellular gene that is required for HIV-1 infection, namely CCR5, can inhibit HIV-1 replication. With HIV-1 replication as a model system, our studies also demonstrate the power and general utility of using lentiviral vectors for the stable expression of siRNAs in primary human cells to inhibit the expression of cellular genes.
The human chemokine receptor gene, CCR5, was chosen as a target for these studies for several reasons. First, CCR5 is a necessary coreceptor for infection by most strains of HIV-1 (34, 35). After binding of the HIV-1 envelope protein gp120 to CD4, interaction with gp120 and CCR5 induces a conformational change that then leads to HIV-1 envelope gp41 fusion with the cell membrane (36). This is an obligatory step for the infection process of CCR5-tropic strains of HIV-1; the absence of CCR5 prevents HIV-1 infection to cells. Not all strains of HIV-1 require CCR5. CXCR4, for example, is another major coreceptor (37). However the majority of naturally occurring strains of HIV-1 use CCR5 as a coreceptor for primary infection (38–41). Second, CCR5 is apparently dispensable for normal human growth, differentiation, and immune functions (42). Among the white population, the occurrence of a CCR5-null allele, known as CCR5Δ32, is ≈1% (21–24). The CCR5Δ32 allele is a deletion resulting in a frameshift truncation of CCR5 that prevents the mutant protein from appearing on the cell surface (21, 33). Individuals homozygous for the CCR5Δ32 do not show any apparent adverse phenotypic effects, although a recent report linked CCR5Δ32 homozygosity with higher viral loads in hepatitis C infection (43). In heterozygous CCR5Δ32 individuals, cell surface CCR5 is reduced to 20–30% of wild-type levels (33, 44, 45). Again, no adverse effects are observed in these individuals (42). It will be important to confirm that acquired loss of CCR5 expression through siRNA inhibition also has no adverse phenotypic effects.
Cohort studies indicate that individuals that are homozygous for the CCR5 mutation are almost completely resistant to infection by CCR5-tropic strains of HIV-1 (38–41, 46, 47). To date, there have been only eight reports of HIV-1 infection in CCR5Δ32 homozygous individuals (48–51). These individuals appeared to have been infected with a CXCR4-tropic strain of HIV-1 and did develop AIDS. Individuals that are heterozygous for CCR5Δ32 are infected at rates similar to CCR5+/CCR5+ individuals. However, their disease course is prolonged, presumably because of reduced spread of virus within the individuals over time (23). Thus, the 3- to 7-fold inhibition produced by our anti-CCR5 siRNA vector could already have a marked clinical effect and, with an optimized system, a greater inhibition might even be obtained. However, it is possible that in a patient producing HIV-1, anti-CCR5 siRNA could select for viral variants that use CXCR4 and then cause progression to AIDS. Therefore, a CCR5 inhibitory therapy would be best used in combination with other antiviral approaches.
Because of the importance of CCR5 to HIV-1 infection and disease development, a number of approaches have been investigated to inhibit the utilization of CCR5 as a coreceptor by HIV-1. The chemokines, RANTES, MIP-1α, and MIP1-β, are physiological ligands for CCR5 and have been shown to inhibit HIV-1 infection both in vitro and in vivo (52). However, these chemokines and their pharmacological derivatives have not been used clinically because they have a short half-life, are not orally bioavailable, and may have other effects (53, 54). Other investigators have inhibited CCR5 in vitro by using “intrakines,” fusions of RANTES and MIP1-α to endoplasmic reticulum retention signals (55, 56), and single-chain antibodies directed to CCR5 (30).
We have shown here that it is possible to obtain at least partial inhibition of a viral infection by lentiviral vector-mediated expression of an siRNA targeting a cellular gene. Within the transduced population of T cells (GFP+ fraction), we observed an up to 10-fold inhibition of CCR5 expression. As a consequence, HIV-1 infection within the transduced population was also substantially reduced, relative to mock-transduced cells, cells transduced with an irrelevant siRNA (lacZ-siRNA), or nontransduced cells in the same culture (GFP− fraction). However, there remained transduced cells that were infected by HIV-1 (HSA+). Residual infection of transduced cells may occur because of incomplete inhibition, variability of inhibition over time, or infection through a CCR5-independent mechanism (57). It is important to remember that in this culture system, the cells are bathed continuously in virus produced by the unprotected cells.
Although a 3-fold reduction of the total virus load in our in vitro model is rather modest, an effective clinical application of this technology would be through a hematopoietic stem cell transplant. In this manner, therapeutic siRNA-expressing stem cells would give rise to mature progeny T cells, macrophages, and/or dendritic cells that would be relatively protected from infection by HIV-1. Furthermore, the rapid demise of HIV-1-infected T cells (58) should lead to rapid selection for the CCR5-negative cells that are relatively protected from the effects of HIV-1. We did not observe selection in cell culture, possibly because the HIV-1 viruses that we used are lacking the Vpr gene as a result of substitution by the HSA reporter gene. Vpr induces cell-cycle arrest, followed by apoptosis, which would lead to rapid selection against HIV-1-infected cells (59). Selection of transduced cells expressing a therapeutic transgene has played an important role in the successful human clinical trials of gene therapy, the treatment of X-linked SCID-X1 and ADA-SCID genetic disorders (60, 61).
In summary, our studies provide proof of principle for the idea that siRNA directed to a gene essential for viral replication can serve as a potential therapeutic agent for human infectious disease. The utilization of siRNA as a therapeutic reagent in the clinical setting is advantageous to previously reported approaches in that siRNA is a small nucleic acid reagent as opposed to relatively large chimeric or antibody proteins. A small nucleic acid reagent should be less likely to elicit an immune response. Furthermore, the relative simplicity of the siRNA approach lends itself to easy combination with other siRNAs directed to different regions of CCR5 and/or other cellular or HIV-1-specific genes, which can result in additive or synergistic effects, and will help to prevent the escape of mutant variants. It seems possible that further modifications in vector design and choice of siRNAs simultaneously targeting multiple essential components for viral replication could provide an effective treatment against HIV-1.
Acknowledgments
We thank Carlos Lois, Yi-ming Xie, Si Hua Mao, Vaheideh Gudeman, and Kathie Grovit-Ferbas for reagents and helpful discussions. This work was supported by grants from the National Institutes of Health to D.B. (AI42549-04) and I.S.Y.C. (AI39975-05 and AI28697). X.-F.Q. was supported by Damon Runyon–Walter Winchell Fellowship DRG-1568.
Abbreviations
- siRNA
small interfering RNA
- PBLs
peripheral blood lymphocytes
- FACS
fluorescence-activated cell sorter
- HSA
murine heat-stable antigen
- UbiC
Ubiquitin-C promoter
References
- 1.Baltimore D. Nature. 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharp P A. Genes Dev. 2001;15:485–490. doi: 10.1101/gad.880001. [DOI] [PubMed] [Google Scholar]
- 3.Tuschl T. Nat Biotechnol. 2002;20:446–448. doi: 10.1038/nbt0502-446. [DOI] [PubMed] [Google Scholar]
- 4.Zamore P D. Science. 2002;296:1265–1269. doi: 10.1126/science.1072457. [DOI] [PubMed] [Google Scholar]
- 5.Hannon G J. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 6.Brummelkamp T R, Bernards R, Agami R. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 7.Paddison P J, Caudy A A, Bernstein E, Hannon G J, Conklin D S. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J Y, DeRuiter S L, Turner D L. Proc Natl Acad Sci USA. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester W C, Shi Y. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyagishi M, Taira K. Nat Biotechnol. 2002;20:497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- 11.Paul C P, Good P D, Winer I, Engelke D R. Nat Biotechnol. 2002;20:505–508. doi: 10.1038/nbt0502-505. [DOI] [PubMed] [Google Scholar]
- 12.Naldini L, Verma I M. Adv Virus Res. 2000;55:599–609. doi: 10.1016/s0065-3527(00)55020-9. [DOI] [PubMed] [Google Scholar]
- 13.Kay M A, Glorioso J C, Naldini L. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 14.An D S, Wersto R P, Agricola B, Metzger M, Lu S, Amado R G, Chen I S Y, Donahue R E. J Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An D S, Kung S K, Bonifacino A, Wersto R P, Metzger M E, Agricola B A, Mao S H, Chen I S, Donahue R E. J Virol. 2001;75:3547–3555. doi: 10.1128/JVI.75.8.3547-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N S, Dohjima T, Bauer G, Li H, Li M J, Ehsani A, Salvaterra P, Rossi J. Nat Biotechnol. 2002;20:500–505. doi: 10.1038/nbt0502-500. [DOI] [PubMed] [Google Scholar]
- 17.Novina C D, Murray M F, Dykxhoorn D M, Beresford P J, Riess J, Lee S K, Collman R G, Lieberman J, Shankar P, Sharp P A. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- 18.Jacque J M, Triques K, Stevenson M. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn G A, Cullen B R. J Virol. 2002;76:9225–9231. doi: 10.1128/JVI.76.18.9225-9231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Myers C, Kilzer J, Pfaff S, Bushman F. Curr Biol. 2002;12:1301–1311. doi: 10.1016/s0960-9822(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 22.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 23.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 25.Paule M R, White R J. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lois C, Hong E J, Pease S, Brown E J, Baltimore D. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 27.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee J K, Friedmann T, Burns J C. Methods Cell Biol. 1994;43:99–112. doi: 10.1016/s0091-679x(08)60600-7. [DOI] [PubMed] [Google Scholar]
- 29.Chackerian B, Long E M, Luciw P A, Overbaugh J. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberger P, Andris-Widhopf J, Buhler B, Torbett B E, Barbas C F., III Proc Natl Acad Sci USA. 2000;97:805–810. doi: 10.1073/pnas.97.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamieson B D, Zack J A. J Virol. 1998;72:6520–6526. doi: 10.1128/jvi.72.8.6520-6526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 35.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 36.Berger E A. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 37.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 38.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 39.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, Degoede R E Y, Vansteenwijk R P, Lange J M A, Schattenkerk J K M E, Miedema F, Tersmette M. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos M T L, Lange J M A, Degoede R E Y, Coutinho R A, Schellekens P T A, Miedema F, Tersmette M. J Infect Dis. 1992;165:427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- 41.Richman D D, Bozzette S A. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien S J, Moore J P. Immunol Rev. 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 43.Woitas R P, Ahlenstiel G, Iwan A, Rockstroh J K, Brackmann H H, Kupfer B, Matz B, Offergeld R, Sauerbruch T, Spengler U. Gastroenterology. 2002;122:1721–1728. doi: 10.1053/gast.2002.33660. [DOI] [PubMed] [Google Scholar]
- 44.Moore J P. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 45.Benkirane M, Jin D Y, Chun R F, Koup R A, Jeang K T. J Biol Chem. 1997;272:30603–30606. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman P A, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, et al. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 47.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 48.Theodorou I, Meyer L, Magierowska M, Katlama C, Rouzioux C. Lancet. 1997;349:1219–1220. [PubMed] [Google Scholar]
- 49.Biti R, Ffrench R, Young J, Bennetts B, Stewart G, Liang T. Nat Med. 1997;3:252–253. doi: 10.1038/nm0397-252. [DOI] [PubMed] [Google Scholar]
- 50.Balotta C, Bagnarelli P, Violin M, Ridolfo A L, Zhou D, Berlusconi A, Corvasce S, Corbellino M, Clementi M, Clerici M, et al. AIDS. 1997;11:F67–F71. doi: 10.1097/00002030-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Michael N L, Nelson J A, KewalRamani V N, Chang G, O'Brien S J, Mascola J R, Volsky B, Louder M, White G N, Littman D R, et al. J Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 53.Marozsan A J, Torre V S, Johnson M, Ball S C, Cross J V, Templeton D J, Quinones-Mateu M E, Offord R E, Arts E J. J Virol. 2001;75:8624–8638. doi: 10.1128/JVI.75.18.8624-8638.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons G, Reeves J D, Hibbitts S, Stine J T, Gray P W, Proudfoot A E, Clapham P R. Immunol Rev. 2000;177:112–126. doi: 10.1034/j.1600-065x.2000.17719.x. [DOI] [PubMed] [Google Scholar]
- 55.Yang A G, Bai X, Huang X F, Yao C, Chen S. Proc Natl Acad Sci USA. 1997;94:11567–11572. doi: 10.1073/pnas.94.21.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroers R, Davis C M, Wagner H J, Chen S Y. Gene Ther. 2002;9:889–897. doi: 10.1038/sj.gt.3301711. [DOI] [PubMed] [Google Scholar]
- 57.Pang S, Yu D, An D S, Baldwin G C, Xie Y, Poon B, Chow Y H, Park N H, Chen I S. J Virol. 2000;74:10994–11000. doi: 10.1128/jvi.74.23.10994-11000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 59.Stewart S A, Poon B, Jowett J B, Chen I S. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova J L, et al. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 61.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, et al. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]