Abstract
Rhesus rhadinovirus (RRV) is a gamma-2 herpesvirus and is the rhesus macaque homologue of human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus. DNA sequence analysis of RRV indicates that it shares numerous open reading frames (ORFs) with HHV-8, including one (ORF74) encoding a seven-transmembrane-spanning G protein-coupled receptor (GPCR) with similarity to cellular chemokine receptors. Examination of the predicted amino acid sequence of RRV ORF74 reveals that it encodes a seven-transmembrane-spanning GPCR sharing 40.8% amino acid sequence identity with HHV-8 ORF74 and 24.1% amino acid sequence identity with rhesus macaque CXCR2. In addition, immunofluorescence studies indicate that an epitope-tagged version of RRV ORF74 is expressed on the surfaces of transfected cells, suggesting that this protein is in fact a membrane receptor. In in vitro cell culture assays, RRV ORF74 possesses transforming potential, as NIH 3T3 clones stably expressing the receptor demonstrate an increased ability to grow in soft agarose and to induce tumor formation in nude mice. Further analysis of RRV ORF74 indicates that expression of the receptor in NIH 3T3 cells causes an increased secretion of vascular endothelial growth factor and activation of the ERK1/2 (p44/42) mitogen-activated protein kinase signaling pathway. The results of these studies suggest that RRV ORF74 encodes a GPCR with properties similar to those of its homologue in HHV-8 and that this gene may play a role in RRV-associated pathogenesis.
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is a recently identified herpesvirus associated with several AIDS- and non-AIDS-related malignancies. In particular, HHV-8 is the etiological agent of KS, the most common AIDS-associated neoplasm, as well as primary effusion lymphoma and multicentric Castleman's disease (MCD) (8, 9, 28). The genome of HHV-8 has been sequenced and has been found to contain several unique open reading frames (ORFs) postulated to play roles in the transforming potential of the virus (23). Several of these ORFs appear to be viral homologues of cellular genes encoding proteins involved in cell cycle control (v-cyclin D), chemokines (vMIP-I, -II, and -III), cytokines (viral interleukin 6 [vIL-6]), apoptotic regulators (vBcl2 and vFLIP), interferon regulators (vIRFs), and a G protein-coupled receptor (vGPCR).
The HHV-8 vGPCR, encoded by ORF74, has been the focus of numerous studies. HHV-8 ORF74 appears to be similar to CXCR2, the cellular chemokine receptor for IL-8, and has been demonstrated to be a constitutively active receptor which can activate numerous signaling pathways in different cell types (5, 6, 10, 18, 26, 27). Although the GPCR seems to possess constitutive signaling abilities in the absence of exogenous ligand, it has been found that chemokines can modulate this activity. Chemokines containing an N-terminal ELR motif, such as IL-8, appear to activate the receptor above basal levels, while chemokines lacking this motif, such as IP-10, appear to inhibit constitutive signaling (12, 13, 14). The exact reason for the constitutive activity of ORF74 is not known, although it has been postulated that the presence of a VRY motif instead of a highly conserved DRY motif often found in the second intracellular loop of many GPCRs may play a role (7). However, despite this finding, more recent evidence suggests that this single base substitution is likely not responsible for the receptor's constitutive activity (22).
The signaling pathways activated by ORF74 appear to be extremely diverse, and numerous other pathways not yet examined are also likely affected by this receptor. One of the signaling pathways initially shown to be activated by the receptor is the phospholipase C (PLC) pathway (5), which results in the production of soluble inositol-1,4,5-triphosphate and membrane-associated 1,2-diacylglycerol and causes the activation of various downstream signaling events. In addition to PLC, signaling through this GPCR appears to activate members of the mitogen-activated protein kinase (MAPK) family, although the data on exactly which members of this family are being activated by the receptor are somewhat conflicting and activation appears to be cell type dependent (6, 19, 26, 27). Recently, it has been shown that ORF74 can also activate the prosurvival kinase Akt (also known as PKB) in endothelial cells in a PI3K-dependent manner, resulting in their increased resistance to apoptosis (18). The receptor also appears capable of activating transcription of NF-κB- and AP-1-responsive promoters, resulting in the increased production of several cytokines and chemokines in transfected cells of epithelial, monocytic, endothelial, and T-cell origin (21, 24). Moreover, there is likely a significant amount of cross talk between signaling pathways which are directly activated by the receptor and other cellular signaling pathways, further extending the signaling capabilities of the protein.
The signaling activities of HHV-8 ORF74 appear to confer oncogenic properties on the protein. NIH 3T3 mouse fibroblasts expressing ORF74 form foci in in vitro assays, exhibit growth in soft agar, and form tumors in nude mice (6, 7), indicating the transforming potential of the protein. In addition, cells expressing this receptor secrete increased levels of vascular endothelial growth factor (VEGF), an angiogenic cytokine which has been suggested to play a role in the development of KS (6). The increased secretion of VEGF by ORF74-expressing cells has been partially attributed to the activation of the VEGF promoter by the MAPK family members p38 and ERK1/2 (27). It also appears that ORF74 expression may play a direct role in the development of KS, since expression of the receptor has been demonstrated to induce endothelial cells to become spindle-shaped cells, a characteristic cell type found in KS lesions, and it is expressed in KS, primary effusion lymphoma, and MCD (16). Further, studies conducted with transgenic mice expressing ORF74 in hematopoietic cells have shown that these animals develop angioproliferative lesions resembling KS in various tissues, suggesting that vGPCR expression alone may be able to promote the development of KS-like disease (30).
Despite the abundance of information on HHV-8 ORF74, the role this protein plays in HHV-8 pathogenesis is difficult to elucidate without an appropriate animal model for HHV-8. An alternative approach is to utilize a closely related animal model, such as rhesus macaques, that can be infected with a closely related herpesvirus, referred to as rhesus rhadinovirus (RRV) (11). Our laboratory independently isolated a related strain, RRV17577, from a rhesus macaque that was infected with the simian immunodeficiency virus (SIV) and developed widespread lymphoproliferative disorder (29). Analysis of the entire genome of RRV17577, and the related isolate H26-95 (2), revealed that RRV shows high sequence similarity to HHV-8, and phylogenetic analysis of several ORFs indicated that RRV is the rhesus macaque homologue of HHV-8 (2, 25). More importantly, experimental inoculation of SIV-infected rhesus macaques with RRV17577 resulted in the induction of B-cell hyperplasia and persistent lymphadenopathy resembling MCD in the macaques (29). Recently, some SIV- and RRV17577-infected macaques have developed B-cell lymphoma or a proliferative mesenchymal lesion referred to as retroperitoneal fibromatosis that possesses histomorphological features resembling KS (unpublished data). The utility of this animal model is intriguing, given the fact that RRV shares several unique genes with HHV-8, including homologues of HHV-8 genes potentially involved in pathogenesis, such as those for vIL-6, vFLIP, vIRF, vMIP, and a vGPCR. Like HHV-8 vIL-6, RRV vIL-6 has been shown to be a functional homologue of cellular IL-6 (15); however, the functions of many of the other HHV-8-like genes in RRV have not yet been examined. In an attempt to further characterize more of these genes, we decided to examine the activities of the putative GPCR of RRV, also encoded by ORF74. Our studies with RRV ORF74 aim to characterize the activities of this protein to determine if it in fact behaves like ORF74 of HHV-8. If so, this may allow RRV to be used as an animal model to study the contributions of the viral GPCR to HHV-8-associated disease.
MATERIALS AND METHODS
Protein alignments.
Protein alignment was performed using ClustalW with MacVector version 6.5.3 software (Accelrys, Inc., Madison, Wis.). The Blosum 30 scoring matrix was used in pairwise alignment of each sequence, with a gap introduction penalty of 10 and a gap extension penalty of 0.1.
Cell lines and transfections.
NIH 3T3 mouse fibroblasts were maintained in Dulbecco's modified Eagle's medium (Mediatech, Herndon, Va.) containing 5% bovine calf serum (HyClone, Logan, Utah). The DNA sequence encoding ORF74 was isolated from RRV17577 by PCR with primers specific for ORF74 which also contained restriction sites for XhoI (primer 1, 5′-CCGCTCGAGAACAACATGGACGCC-3′) and EcoRI (primer 2, 5′-CGGAATTCCTATAAACTACCTGAAGTGGA-3′). The resulting product of the PCR was sequenced to confirm its identity. Stable ORF74 cell lines were produced by cloning the ORF74 sequence into the retroviral vector plasmid pLNCX (Clontech, Palo Alto, Calif.) and using this vector to transfect the amphotropic retrovirus-packaging cell line PA317 (17) with Lipofectin reagent (Invitrogen, San Diego, Calif.). Retroviral particles containing the inserted gene were then collected from the PA317 cell supernatants and used to infect NIH 3T3 cells. Stable cells which had integrated the retrovirus were then selected for neomycin resistance using G418 at 1 mg/ml, and individual cell clones were isolated using cloning cylinders and maintained in medium with G418 at 750 μg/ml. Cells transduced by the pLNCX vector only were created in a similar manner. ORF74 clones were tested for ORF74 gene expression by reverse transcription-PCR (RT-PCR) analysis of total RNA utilizing the Titan One Tube RT-PCR System (Roche, Indianapolis, Ind.) and ORF74-specific primers. RNA was isolated from cells with a High Pure RNA Isolation Kit (Roche). For transient-transfection assays, the ORF74 sequence was cloned into the expression vector pcDNA3.1(−) (Invitrogen), and NIH 3T3 cells were transfected in 35-mm-diameter six-well dishes using Transit-LT1 reagent (Mirus, Madison, Wis.) following the manufacturer's protocol.
HA-tagged RRV-ORF74 protein expression.
A hemagglutinin (HA) tag was introduced onto the N terminus of RRV ORF74 by performing PCR with primers specific for the full-length ORF74 sequence as described above, using a primer containing the sequence for the HA epitope inserted after the initiating methionine (5′-CCGCTCGAGATGTACCCATACGACGTCCCAGACTACGCTGACGCCTTGAACAATAACCTT-3′) in place of primer 1. The product of this reaction was purified and sequenced to confirm the presence of the HA epitope sequence in the correct reading frame, as well as the absence of mutations in the ORF74 sequence. The tagged sequence was then cloned into the expression vector pcDNA3.1(−) and used to transfect NIH 3T3 cells plated on chambered slides (Nunc, Inc., Naperville, Ill.) for use in immunofluorescence analysis. Empty pcDNA3.1(−) vector was used as a control. Briefly, cells were transfected with 1 μg of DNA per chamber using Transit-LT1 reagent, and 48 h posttransfection, the cells were fixed with either 2% paraformaldehyde (nonpermeabilized) to detect only surface-expressed receptor or 100% cold methanol (permeabilized) to detect both surface and total cellular expression. The fixed cells were then probed with mouse anti-HA antibody (Sigma, St. Louis, Mo.) in Tris-buffered saline (TBS)-1% bovine serum albumin overnight at 4°C. The following day, the cells were washed with TBS and probed with anti-mouse fluorescein isothiocyanate (FITC) antibody (Sigma) in TBS-1% bovine serum albumin for 30 min at room temperature. Visualization of FITC staining of nonpermeabilized cells was performed using a Zeiss Axiovert 25 microscope, and confocal images of permeabilized cells were acquired using a TCS SP confocal system (Leica Microsystems, Heidelberg, Germany) with a Plan Apo 40× NA 1.25 objective.
Soft-agarose assays.
Five thousand stably transfected NIH 3T3 cells were plated in 1.5 ml of Dulbecco's modified Eagle's medium with 5% bovine calf serum and 0.3% melted agarose onto a 1.5-ml bottom layer of 0.6% agarose medium in a well of a six-well dish. Each clone tested was plated in triplicate wells. The cells were fed every 3 days with several drops of medium, and foci were photographed after 2 to 3 weeks.
Nude-mouse tumor formation.
Four- to 5-week old athymic nude (nu/nu) mice were obtained from Harlan (Indianapolis, Ind.) and maintained according to the Division of Animal Resources Standard Operating Procedures at the Oregon National Primate Research Center. Stable cell clones were resuspended in sterile phosphate-buffered saline (PBS) to 106/100 μl, and 100 μl of the suspension was injected subcutaneously into the right flank of individual nude mice. The mice were observed daily for signs of tumor formation at the site of injection. The mice were euthanized once the tumors had reached ∼1 cm in diameter or when the tumors appeared to stop growing rapidly. The mice were also euthanized if they appeared unhealthy or injured. The tumors were measured, photographed, and then excised from the mice, and samples of each tumor were saved for further analysis. Total RNA was extracted from the tumor samples using Tri-Reagent (Sigma) according to the manufacturer's protocol. The tumor samples were analyzed for ORF74 expression using 500 ng of total RNA for RT-PCR with the Titan One Tube RT-PCR system and primers specific for ORF74. Reactions with GAPDH (glyceraldehyde-3-phosphate dehydrogenase)-specific primers with and without reverse transcription served as positive and negative controls, respectively.
VEGF ELISA.
Enzyme-linked immunosorbent assay (ELISA) analysis was performed with a commercial mouse VEGF ELISA kit (Oncogene Research Products, San Diego, Calif.) following the manufacturer's protocol. Supernatants were obtained from transiently transfected NIH 3T3 cells. The cells were transfected in 35-mm-diameter wells in triplicate with 2 μg of DNA/well and serum starved at 24 h posttransfection, and the supernatants from individual wells were collected at 48 h for analysis of VEGF levels. The VEGF levels of individual wells were measured, and an average level of VEGF was then calculated from triplicate samples. Statistical significance was determined using a t test analysis program available from http://www.graphpad.com, and the unpaired two-tailed P values are reported.
Western blot analysis.
For transient transfections, cells were transfected in 35-mm-diameter wells in triplicate with 2 μg of DNA per well, serum starved at 24 h posttransfection for an additional 24 h, and then lysed at 48 h for analysis. For stable clones, cells were plated in 35-mm-diameter wells and serum starved the day following plating for 24 h before lysis. Where appropriate, stimulation with GROα (Peprotech, Rocky Hill, N.J.) was done for exactly 5 min prior to lysis by adding it to designated wells. For cell lysis, medium was removed from the wells and the cells were rinsed with 1× PBS; RIPA buffer (1× PBS, 1% NP-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate) containing a mixture of 1× protease and phosphatase inhibitors (Sigma) was added to each well, and the cells were removed from the dishes by scraping. The lysates were cleared of cellular debris by centrifugation, and the protein concentrations were measured by Bradford analysis. Equivalent amounts of all protein samples were loaded and run on a 10% polyacrylamide gel, transferred to a nitrocellulose membrane, probed with antibody against phospho-ERK (Cell Signaling Technology, Inc., Beverly, Mass.), and detected by chemiluminescence. To control for total ERK levels, the membranes were then stripped and reprobed in a similar manner using antibody recognizing total ERK protein (Cell Signaling Technology, Inc.). Quantitation was performed by using a Kodak Image Station 440 CF to obtain images of exposed films and using relative band intensities obtained from the accompanying Kodak 1D image analysis software to calculate relative phospho-ERK induction versus total ERK levels.
RESULTS
Alignment of RRV ORF74, HHV-8 ORF74, and rhesus CXCR2.
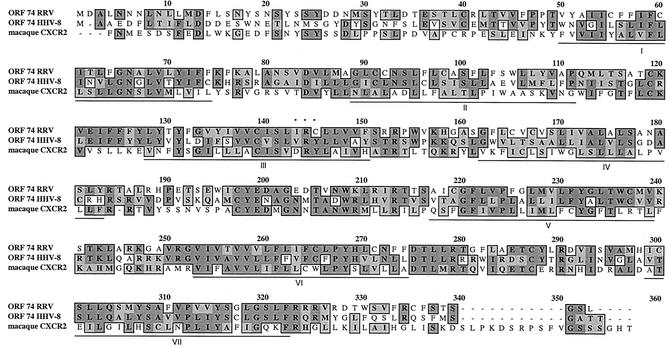
The amino acid sequences of RRV ORF74 and HHV-8 ORF74 were aligned with that of rhesus macaque CXCR2 and analyzed with the ClustalW analysis program to determine the degree of amino acid sequence identity. Alignment of RRV ORF74 with HHV-8 ORF74 reveals high similarity between the two ORFs (Fig. 1). At the protein level, the two ORFs show ∼62% similarity and ∼40% identity. These sequence similarities suggest that RRV ORF74 encodes a GPCR and that this receptor may in fact function in a manner similar to that of ORF74 of HHV-8. Moreover, alignment of RRV ORF74 with the published rhesus macaque sequence for CXCR2 shows that these proteins are ∼41% similar and ∼24% identical, suggesting that RRV ORF74 may be a viral homologue of the cellular macaque receptor for IL-8. Further examination of the sequences reveals that a DRY motif located in the second intracellular loop of many GPCRs is conserved in CXCR2 but is converted to VRY in HHV-8 ORF74 and to IRC in RRV ORF74. The constitutive activity of HHV-8 ORF74 has previously been attributed to the presence of this VRY motif, suggesting that there may in fact be significant differences in the sequences of the two viral receptors which could potentially affect their functions.
FIG. 1.
Amino acid alignment of RRV ORF74 with HHV-8 ORF74 and macaque CXCR2. Alignment was performed with ClustalW using the Blosum scoring matrix. The darkly shaded boxes indicate identical residues, and the lightly shaded boxes indicate residues with similarity. The dashes represent regions not shared by the proteins, and the underlined regions are putative transmembrane domains.
Cell surface expression of RRV ORF74.
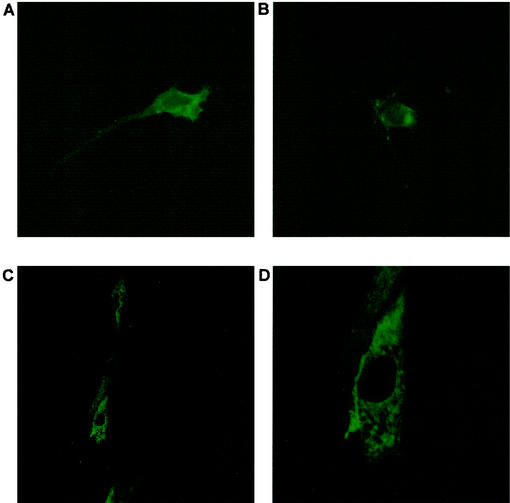
NIH 3T3 cells were chosen as the cell type to address the transforming potential of RRV ORF74. First, ORF74 protein expression was evaluated to determine whether the protein could be detected on the surfaces of these cells. Unfortunately, initial attempts to generate polyclonal antisera to RRV ORF74 were unsuccessful. Therefore, to facilitate detection of the protein, an HA epitope tag sequence was attached to the N terminus, which is a predicted extracellular region of the protein. Transient transfection of NIH 3T3 cells with HA-ORF74-expressing vector, followed by immunofluorescence analysis of nonpermeabilized cells using anti-HA antibody and a FITC-labeled secondary antibody, revealed that the protein is expressed on the surfaces of these cells and that the receptor displays a clustered pattern of expression on the surfaces of the cells (Fig. 2A and B). In addition, confocal analysis of permeabilized cells transfected with HA-ORF74 revealed that, as expected, the protein could be detected throughout the cytoplasm, as well as on the cell surface (Fig. 2C and D). In both cases, similar staining done on cells transfected by vector only did not show any significant signal above background (data not shown), indicating that the observed staining patterns are specific for cells expressing the HA-tagged receptor. These data demonstrate that NIH 3T3 cells transfected with RRV ORF74 do in fact express this protein and that like HHV-8 ORF74, RRV ORF74 is also a membrane receptor.
FIG. 2.
Immunofluorescence analysis of transfected NIH 3T3 cells. NIH 3T3 cells were transfected with an expression vector encoding N-terminally HA-tagged RRV ORF74 and stained with primary anti-HA antibody and FITC-labeled secondary antibody. (A and B) Nonpermeabilized cells (magnification, ×317). (C and D) Permeabilized cells visualized by confocal microscopy. The field shown in panel C is 250 by 250 μm. Panel D is an enlarged image (×3.5) of the same field shown in panel C and is 74.1 by 74.1 μm.
Transforming potential of RRV ORF74.
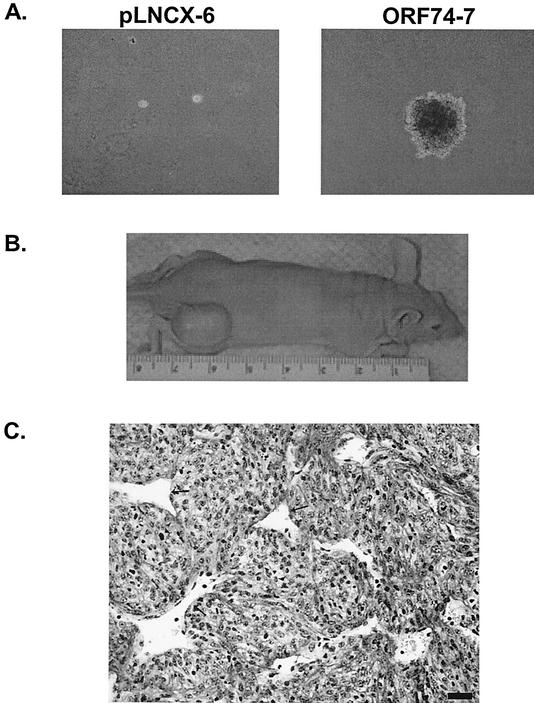
To initiate studies of the function of RRV ORF74, individual clones of NIH 3T3 mouse fibroblasts that stably express the gene were created and used to assess the ability of ORF74 to cause transformation of the cells. Control clones which contained empty pLNCX vector or HHV-8 ORF74 were also created. Stable clones were initially tested for anchorage-independent growth in soft agarose medium, a common property of transformed cells. Cells were seeded in soft agarose medium, and after ∼3 weeks in culture, both pLNCX clones tested exhibited no significant growth while both pLNCX-RRV ORF74 clones proliferated well in the soft agarose and had formed numerous foci (Fig. 3A). An HHV-8 ORF74-expressing clone was capable of growth in soft agarose, as expected, and grew in a fashion similar to that of RRV ORF74 clones (data not shown).
FIG. 3.
RRV ORF74-transduced cells exhibit transformation potential. (A) Cell growth in soft agarose (magnification, ×40). (B) RRV ORF74-transformed NIH 3T3 cells induce tumor formation in nude mice. (C) Histological analysis of tumor stained with hematoxylin and Lee stain. The arrows indicate endothelial-cell-lined blood vessels. Bar = 20 μm.
The results of the soft-agarose assays suggest that RRV ORF74 has transforming potential similar to that of HHV-8 ORF74. To test the transforming ability of RRV ORF74 in vivo, stable NIH 3T3 clones were injected into nude mice to assess their ability to form tumors. The results of this experiment are summarized in Table 1, and a representative mouse is shown in Fig. 3B. In this case, all of the mice injected with ORF74-expressing clones developed tumors after only 11 weeks, while only two of five mice injected with one vector clone had developed tumors at this same time point. All of the tumors were collected and analyzed for ORF74 mRNA expression by RT-PCR, and as expected, only the tumors from mice initially injected with ORF74-expressing cells were positive for the transcript (data not shown). The development of tumors in mice injected with a vector-only clone is not uncommon in these types of experiments and has been reported by numerous others performing similar studies with nude mice (4). In addition, the fact that ORF74-expressing cells could induce tumor formation in all injected mice, and that these tumors appeared more rapidly than the vector clone tumors, suggests that ORF74 expression promotes the transforming potential of stably transfected NIH 3T3 cells in vivo. Analysis of tissue samples taken from tumors induced in mice injected with stable clones expressing ORF74 revealed that they possess histomorphological features of mesenchymal tumors and are comprised of pleomorphic spindle-shaped cells, with mitotic cells evident throughout the tissue section (Fig. 3C).
TABLE 1.
Tumorigenic activity of RRV ORF74
| Transduced cellsa | No. of mice developing tumorsb | Average time (wk) | No. ORF74 positivec |
|---|---|---|---|
| pLNCX-6 | 2/5 | 10.4 | 0/2 |
| pLNCX-8 | 0/1 | NAd | NA |
| ORF74-3 | 5/5 | 5.8 | 5/5 |
| ORF74-7 | 5/5 | 8.7 | 5/5 |
NIH 3T3 cells transduced with retroviral vector were grown in the presence of 1.0 mg of G418/ml, and individual colonies were isolated.
Number with tumors/total. Athymic nu/nu mice were inoculated with 106 cells as described in Materials and Methods and observed for the formation of tumors.
Number positive/total. ORF74-expressing tumors were determined by RT-PCR as described in Materials and Methods.
NA, not applicable.
RRV ORF74 can induce VEGF secretion.
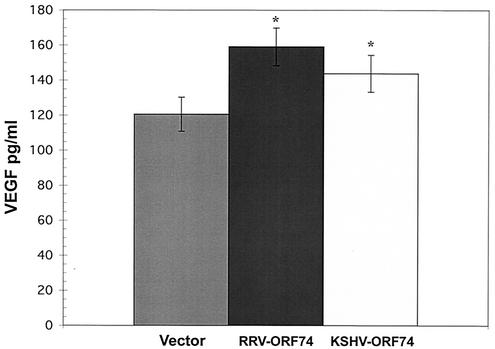
HHV-8 ORF74 has been shown to induce the secretion of VEGF in transfected cells (6, 24, 27). To determine if RRV ORF74 expression was also capable of up-regulating the production of VEGF in NIH 3T3 cells, stable NIH 3T3 clones expressing RRV ORF74 were analyzed and found to have various levels of VEGF but consistently higher levels of VEGF production than vector clones (data not shown). In an attempt to eliminate some of the variability of these results and to directly compare HHV-8 ORF74 with RRV ORF74, the abilities of both of these genes to induce VEGF secretion were examined in transiently transfected NIH 3T3 cells. Since each transfection is done identically for all vectors in each experiment, transient transfections eliminate some of the variability seen with stable clones and allow a better comparison of the activities of these two viral genes. In these experiments, cells were transfected in triplicate, and individual wells were measured for VEGF levels. Figure 4 shows that cells transiently transfected with either RRV ORF74 or HHV-8 ORF74 secrete 159.1 (standard deviation [SD], ±10.75) and 143.8 (SD, ±10.56) pg/ml, respectively, versus 120.6 (SD, ±9.67) pg/ml for the cells transfected with vector alone. These values correspond to 31.9 and 19.2% mean higher levels of VEGF than in cells transfected with vector alone. Although small, the differences compared to the vector are statistically significant (RRV ORF74, P = 0.01; HHV-8 ORF74, P = 0.0486), and similar results were obtained in three independent experiments. Thus, like its HHV-8 counterpart, RRV ORF74 is capable of inducing the increased secretion of VEGF in cells in which it is expressed.
FIG. 4.
RRV ORF74 induces VEGF secretion. Supernatants were collected from transiently transfected NIH 3T3 cells and subjected to ELISA using a commercial kit. *, statistically significant difference from vector control as measured by a t test. RRV-ORF74, P = 0.01; Kaposi's sarcoma-associated herpesvirus ORF74, P = 0.0486. The error bars indicate SDs. One representative experiment of three is shown.
RRV ORF74 activates ERK signaling.
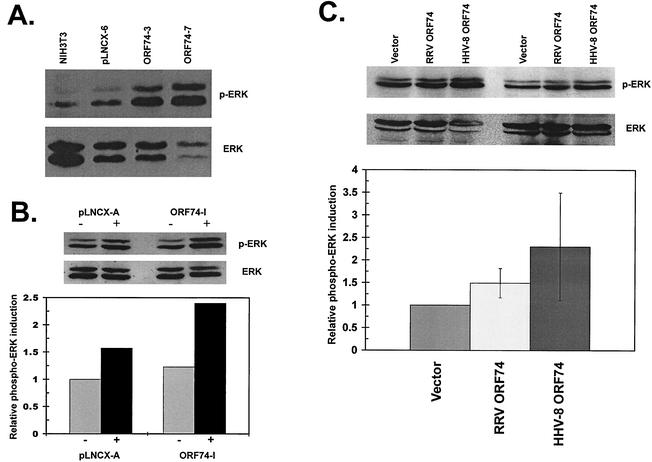
The increased activation of the MAPK pathway family member ERK1/2 (p44/p42) is often involved in cellular transformation and has been implicated in various cancers, including KS (3). More importantly, data from various studies show that ERK1/2 is activated by HHV-8 ORF74 in different types of transfected cells (26, 27); however, there seems to be no consensus on exactly which other MAPK family members (i.e., p38 and JNK) might also be activated by the receptor, although evidence for both exists (6, 19, 27). To elucidate the potential signaling capabilities of RRV ORF74, NIH 3T3 cells expressing the receptor that grew in soft-agarose assays and formed tumors in nude-mouse studies were examined for increased ERK activation. Lysates from these stable clones possess higher levels of activated ERK than a vector-only clone or wild-type cells, as measured by an increased level of phosphorylation of ERK1/2 in Western blot analysis (Fig. 5A). The activation seen in these experiments appears to be constitutive in that no addition of exogenous ligand was needed to stimulate ERK activity, although the presence in the culture medium of stimulating ligand(s) that activates receptor signaling cannot be ruled out.
FIG. 5.
Analysis of RRV ORF74-induced ERK1/2 activation in NIH 3T3 cells. The blots were probed with phospho-ERK (p-ERK) antibody and then stripped and reprobed with antibody recognizing total ERK protein. (A) Lysates from wild-type NIH 3T3 cells and stable clones (pLNCX-6, RRV ORF74-3, and RRV ORF74-7). (B) Lysates from stable clones (pLNCX-A and RRV ORF74-I) either untreated (−) or stimulated with 30 nM GROα 5 min prior to lysis (+). A single blot with quantitation is shown, representative of two independent experiments with similar results. (C) Lysates from cells transiently transfected with equivalent amounts of empty vector (Vector), RRV ORF74, or HHV-8 ORF74. Representative blots from two independent transfections are shown, and the quantitation represents the average of three independent experiments ± SD.
To determine if receptor signaling was in fact responsive to the presence of exogenous ligand, a stable RRV ORF74-expressing clone which demonstrated growth in soft agarose was treated with GROα prior to lysis and examination by Western analysis for ERK activation (Fig. 5B). GROα was chosen as a potential ligand, since it is known to bind to HHV-8 ORF74 and to further stimulate its constitutive signaling abilities (14), including signaling through the ERK pathway (26). Although the constitutive ERK activation of the particular RRV ORF74 clone examined in these experiments seemed lower than those of some clones tested in previous assays, it did demonstrate slightly elevated basal ERK activation (∼1.23-fold above vector), and the addition of exogenous GROα stimulated the ERK-activating abilities of this clone above basal levels (∼1.95-fold induction versus untreated vector) to a much greater extent than a similarly treated vector clone (∼1.57-fold induction versus untreated vector). This demonstrates that RRV ORF74-expressing NIH 3T3 cells are more responsive to GROα than cells not expressing the receptor. Therefore, like HHV-8 ORF74, RRV ORF74 is capable of interacting with a stimulating exogenous ligand, and signaling by the receptor, at least through the ERK pathway, appears to be responsive to these interactions.
Since the RRV and HHV-8 GPCRs can signal through ERK, the ERK-signaling properties of RRV ORF74 were directly compared with those of HHV-8 ORF74 to determine whether these receptors displayed similar constitutive signaling capabilities. To achieve this, NIH 3T3 cells were transiently transfected with equivalent amounts of plasmid DNA, and lysates were then collected and analyzed for ERK activation by Western blot analysis. In these experiments, both RRV ORF74 and HHV-8 ORF74 were capable of constitutively activating ERK signaling compared to a vector control (Fig. 5C). Quantitation of ERK activation revealed that on average, the levels of phospho-ERK in RRV ORF74-transfected cells were 1.49-fold higher than in vector-transfected cells (SD, ±0.325), while HHV8 ORF74-transfected cells displayed a 2.3-fold-higher level (SD, ±1.19) than vector-transfected cells. Interestingly, from these experiments, it appears that RRV ORF74 is not as potent a stimulator of the ERK pathway as the HHV-8 receptor, since the levels of phospho-ERK tended to be somewhat less elevated in RRV ORF74-expressing cells than in those expressing HHV-8 ORF74. These observations were made in multiple experiments, and similar patterns were observed. Although the differences in activation did vary between transfections, the trend consistently demonstrated a slightly stronger activation of ERK by HHV-8 ORF74 than by RRV ORF74. This demonstrates that despite a similar constitutive activation of ERK signaling, variations in the signaling abilities of these two receptors are likely to exist.
DISCUSSION
Several herpesviruses are known to carry GPCRs with sequence similarity to cellular chemokine receptors, including cytomegalovirus (US27, US28, UL33, and UL78), Herpesvirus saimiri (ECRF3), and HHV-8 (ORF74). Here, we have demonstrated that RRV ORF74 encodes a protein with high sequence similarity to ORF74 of HHV-8 and that these receptors display some similar activities in vitro. Like its HHV-8 counterpart, RRV ORF74 possesses transforming potential, given that RRV ORF74-expressing cells can grow in soft agarose and can induce tumor formation in nude mice. Also, we have examined the ability of RRV ORF74 to induce the up-regulation of VEGF production in transfected NIH 3T3 cells, and in transient-transfection assays we have found that RRV ORF74 is capable of inducing a slight increase in VEGF secretion, similar to that seen with HHV-8 ORF74. Despite these similarities, we have been able to detect constitutive activation of the ERK signaling pathway only in NIH 3T3 cells, and this activation appears to be somewhat less potent than what is observed with HHV-8 ORF74. Due to a lack of antibodies against these receptors, the levels of RRV and HHV-8 ORF74 protein expression in transfected cells could not be examined, and indeed, variations in expression could affect the levels of ERK activation seen in these experiments. Alternatively, it is also very likely that the observed differences in ERK signaling are in fact due to the sequence variations between these two viral receptors, which may ultimately result in differences in their abilities to activate certain signaling pathways. In addition to ERK, we have also examined several other signaling pathways known to be activated by HHV-8 ORF74 in various cell types (i.e., PLC/PKC, JNK, p38, and Akt/PKB) and have found no evidence that these pathways are similarly activated by RRV ORF74, at least in NIH 3T3 cells. However, further studies, possibly using other cell types, would be necessary to completely rule out the involvement of any of these pathways in RRV ORF74 signaling and activities. Nevertheless, our data seem to indicate that the signaling abilities of these two receptors may actually vary, even though they display similar activities in other assays.
Since cells expressing RRV ORF74 possess transforming ability and can induce VEGF secretion, it is likely that the receptor retains some of the signaling properties found in the HHV-8 receptor which are responsible for these activities. For example, the induction of ERK1/2 activity by RRV ORF74 appears to correlate with its ability to increase VEGF secretion in transfected cells. In fact, cells that were transiently transfected with RRV ORF74 and found to secrete increased levels of VEGF also demonstrated increased phospho-ERK levels. The same was true of cells transfected with HHV-8 ORF74. This observation is not surprising, since induction of VEGF secretion by HHV-8 ORF74 has been partially attributed to the ability of this receptor to activate the MAPK family members ERK1/2 and p38, resulting in the activation of the VEGF promoter (27). However, all of the signaling pathways RRV ORF74 may be activating to produce its various activities are unknown, as is the case for HHV-8 ORF74. Many of these pathways could be the same as or differ from those activated by the HHV-8 receptor but may still ultimately result in similar activities, such as cellular transformation.
As mentioned previously, it is highly possible that sequence variations in the receptor carried by RRV could result in differences in activity from the HHV-8 receptor, due to alterations in the signaling capabilities of the protein. Of particular interest is the presence of an IRC motif in the second intracellular loop instead of the VRY motif found in the HHV-8 GPCR, a motif that has been speculated to be responsible for the constitutive activity of this receptor. In mutagenesis studies with RRV ORF74, we have found that alterations in this motif which more closely resemble CXCR2 (DRY) and HHV-8 ORF74 (VRY), specifically, 143I/D and 145C/Y, do not appear to alter the transforming or ERK-signaling ability of the receptor (data not shown). Interestingly, the CXCR2-like receptor encoded by ECRF3 of Herpesvirus saimiri contains an LRC motif at this same position which is structurally quite similar to the IRC motif. This receptor does not appear to be constitutively active, although it is responsive to chemokines (1).
Another reason for the potential differences in signaling between these receptors may be that HHV-8 ORF74 is not truly a constitutively active receptor and that it may in fact require the presence of exogenous ligand(s) to become fully stimulated. This ligand(s) could be a factor that is present in cell culture media, that is normally secreted by cells used to analyze the receptor, or whose expression is up-regulated by cells expressing the receptor (i.e., an autocrine signaling mechanism). If this were the case for HHV-8 ORF74, it could be that RRV ORF74 is either less responsive to similar ligands or is less capable of inducing their expression to stimulate the receptor in an autocrine fashion, thus resulting in weaker constitutive activity of the receptor. We have shown that when GROα is added to cells expressing RRV ORF74, the constitutive ERK activation of the receptor is elevated, suggesting responsiveness to exogenous ligand(s). Although we have not determined whether the RRV and HHV-8 receptors respond identically to GROα or whether other ligands might also alter signaling of the RRV receptor, it appears that both of these receptors are capable of being regulated by exogenous ligands.
Despite the differences mentioned above, it appears that RRV ORF74 encodes a protein that demonstrates activities similar to those of HHV-8 ORF74. It should be noted, however, that this receptor likely does not function in mouse fibroblasts as it might in other, more relevant cell types. Indeed, studies recently done with HHV-8 ORF74 in endothelial cells have shown that some of the constitutive signaling properties and activation of pathways seen in cells such as NIH 3T3 and COS do not occur in endothelial cells (10). Therefore, it will be extremely important to test the activities of the RRV GPCR in different cell types as well, particularly in those cell types that may be naturally infected by RRV, such as B cells and endothelial cells.
The fact that RRV ORF74 has activities similar to those of HHV-8 ORF74 suggests that this gene may be involved in the development of HHV-8-like diseases seen in RRV-infected macaques. Although the RRV receptor may in fact have some activities that differ from those of the HHV-8 GPCR, it is likely that these viral proteins both provide some similar functions that are crucial to the survival and replication of these two highly homologous viruses. The exact functions that these receptors may provide to the viruses are unknown; however, possibilities include homing of virus-infected cells and regulation of cellular activities that affect viral growth and replication. In addition, the fact that these viral receptors possess transforming activity is likely not reflective of the true functions they provide for the virus but rather may be an unintended side effect of GPCR expression in infected cells. Further analysis of RRV ORF74 should provide greater insight into the contributions of herpesvirus GPCRs to virus-induced disease and the role they may play in the viral life cycle.
Acknowledgments
This work was supported by NIH grants AI07472 (R.D.E.), RR00163 (M.K.A. and S.W.W.), and CA75922 (S.W.W.).
We thank Carly Pratt for helpful suggestions and discussions and Anda Cornea for assistance with confocal microscopy.
REFERENCES
- 1.Ahuja, S. K., and P. M. Murphy. 1993. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem. 268:20691-20694. [PubMed] [Google Scholar]
- 2.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral, M. C., S. Miles, and A. E. Nel. 1993. Oncostatin-M stimulates tyrosine protein phosphorylation in parallel with the activation of p42MAPK/ERK-2 in Kaposi's cells. Evidence that this pathway is important in Kaposi cell growth. J. Clin. Investig. 92:848-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 5.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus HHV-8 encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 6.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. Geras-Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gershengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 7.Burger, M., J. A. Burger, R. C. Hoch, Z. Oades, H. Takamori, and I. U. Schraufstatter. 1999. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 163:2017-2022. [PubMed] [Google Scholar]
- 8.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 10.Couty, J.-P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 11.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geras-Raaka, E., A. Varma, I. Clark-Lewis, and M. C. Gershengorn. 1998. Kaposi's sarcoma-associated herpesvirus (HHV-8) chemokine vMIP-II and human SDF-1α inhibit signaling by HHV-8 G protein-coupled receptor. Biochem. Biophys. Res. Commun. 253:725-727. [DOI] [PubMed] [Google Scholar]
- 13.Geras-Raaka, E., A. Varma, H. Ho, I. Clark-Lewis, and M. C. Gershengorn. 1998. Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J. Exp. Med. 188:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershengorn, M. C., E. Geras-Raaka, A. Varma, and I. Clark-Lewis. 1998. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J. Clin. Investig. 102:1469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaleeba, J. A., E. P. Bergquam, and S. W. Wong. 1999. A rhesus macaque rhadinovirus related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J. Virol. 73:6177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, A. D., and C. Buttimore. 1986. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol. Cell. Biol. 6:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 19.Munshi, N., R. K. Ganju, S. Avraham, E. A. Mesri, and J. E. Groopman. 1999. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor activation of c-jun amino-terminal kinase/stress-activated protein kinase and lyn kinase is mediated by related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2. J. Biol. Chem. 274:31863-31867. [DOI] [PubMed] [Google Scholar]
- 20.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Sald, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656. [PubMed] [Google Scholar]
- 21.Pati, S., M. Cavrois, H.-G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkilde, M. M., T. M. Kledal, P. J. Holst, and T. W. Schwartz. 2000. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. J. Biol. Chem. 275:26309-26315. [DOI] [PubMed] [Google Scholar]
- 23.Russo, J. J., R. A. Bohenzhy, M.-C. Chein, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi's sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-κB and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 25.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit, M. J., D. Verzijl, P. Casarosa, M. Navis, H. Timmerman, and R. Leurs. 2002. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via Gi and phospholipase C-dependent signaling pathways. J. Virol. 76:1744-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 28.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, and F. Sigaux. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 29.Wong, S. W., E. P. Bergquam, R. M. Swanson, F. W. Lee, S. M. Shiigi, N. A. Avery, J. W. Fanton, and M. K. Axthelm. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, T.-Y., S.-C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C.-H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]