Abstract
A double-guanine-insertion mutation within a run of guanines in the herpes simplex virus gene encoding thymidine kinase (TK) was previously found in an acyclovir-resistant clinical isolate. This mutation was engineered into strain KOS, and stocks were generated from single plaques. Plaque autoradiography revealed that most plaques in such stocks exhibited low levels of TK activity, while ∼3% of plaques exhibited high levels of TK activity, indicating a remarkably high frequency of phenotypic reversion. This virus was able to reactivate from latency in mouse ganglia; a fraction of the reactivating virus expressed a high level of TK activity due to an additional G insertion, suggesting that the observed genetic instability contributed to pathogenicity.
Herpes simplex virus (HSV) thymidine kinase (TK) activates a number of highly effective antiviral drugs, such as acyclovir (ACV); however, resistance to these drugs can hamper therapy. It has been estimated that ACV-resistant (ACVr) disease occurs in ∼5% of immunocompromised patients undergoing antiviral treatment (3, 9). The most common mutations in isolates from these patients occur on homopolymeric sequences in tk, especially on a run of 7 guanine (G) bases (G string) (12, 24, 27). Isolates that contain a single-G insertion within the G string express a low level of full-length active TK as the result of a ribosomal frameshift (16, 17). This observation provoked the hypothesis that the low levels of TK synthesized by these ACVr isolates were sufficient to permit disease but inadequately activate ACV. Less frequently, ACVr isolates containing insertions of two Gs within the G string have been recovered (12). Interestingly, one of these viruses has been described previously as expressing low levels of TK (12). We wanted to examine the effect of this mutation on TK expression, and on viral latency in a mouse model of HSV infection, by engineering the mutation into a well-studied laboratory strain, KOS.
Construction of recombinant virus TKG7+2G.
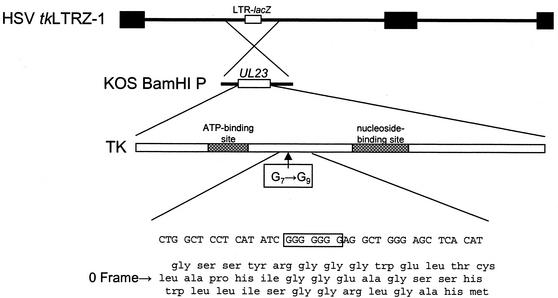
As an initial step in the engineering, a double-G-insertion mutant, plasmid pTKG7+2G, was constructed from plasmid pAG5, which contains the entire BamHI P fragment of HSV-1 strain KOS in pBluescript SK+ (Promega) such that tk is in the same orientation as the T7 promoter. The wild-type sequence G7 was mutated to G9, using two complementary oligonucleotides with the sequence CTGGCTCCTCATATCGGGGGGGGGAGGCTGGGAGCT (only the forward primer is shown) and a site-directed mutagenesis kit (QuickChange; Stratagene) according to the manufacturer's instructions. Correct introduction of the mutation was confirmed by sequencing through the mutation.
To generate two independently isolated recombinant viruses (Fig. 1), plasmid midi-prep DNA (Wizard Prep; Promega) and infectious virion mini-prep (6) tkLTRZ1 DNA, together with transfection reagent (Effectene; Qiagen), were added to Vero cells previously seeded to be 50% confluent. tkLTRZ1, a recombinant virus made from laboratory strain KOS (7), contains an insertion within the tk gene of lacZ downstream of the Moloney murine leukemia virus long terminal repeat sequence, which permits a blue-white screening procedure for isolation of recombinant viruses. Control experiments transfecting tkLTRZ1 DNA alone failed to demonstrate the presence of any white plaques among >1,000 blue plaques, compared to a recombination efficiency of between 0.3 and 2% following cotransfection of a variety of tk mutant plasmids (A. Griffiths and D. M. Coen, unpublished data). It was therefore unlikely that we would follow a “white herring.” tkLTRZ1 does not reactivate from the mouse model of latency (references 2 and 19 and this study), and this phenotype is reversed in a virus in which the KOS BamHI P fragment has been restored (19). Furthermore, the BamHI P fragment of tkLTRZ1 has been sequenced and was shown, except for the inserted LTR-lacZ, to be identical to KOS (G. Mulamba and D. M. Coen, unpublished data). Crucially for this study, using a TK-negative mutant as a starting point for isolation of recombinants eliminated a source of contaminating TK+ virus.
FIG. 1.
Construction of recombinant virus. Below the top two lines, which represent the tkLTRZ1 genome and the location of the tk gene (UL23), is a schematic diagram of the functional domains of TK and the location of the G string. The bottom four lines show the wild-type nucleotide sequence and a three-frame translation of this sequence, with the wild-type frame marked as the 0 frame.
Using a two-step process, we isolated white recombinants containing the double-G insertion following limiting dilution. First, to increase the concentration of recombinants to a more manageable level, several 96-well dishes were infected with a total of approximately 500 PFU each, such that there would be about 5 plaques per well. Following the appearance of plaques, the medium in each well was harvested and this medium was added to the corresponding well in a second tray. The cells in the first tray were stained for β-galactosidase activity with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (Promega) and then counterstained with neutral red (Sigma), as described previously (13). Any wells that contained a white plaque were noted, and the virus in the corresponding medium was amplified on Vero cells. To purify the white plaques, a 96-well tray was infected with approximately 30 PFU, such that one plaque appeared approximately every 3 wells. This time, wells that contained only a single white plaque were amplified. Viral DNA from this amplified virus was sequenced to confirm the presence of the double-G insertion. Two independently isolated recombinant viruses, TKG7+2G.1 and TKG7+2G.2, were generated from separate transfections.
Heterogeneity of TK activity of virus carrying a double-G insertion.
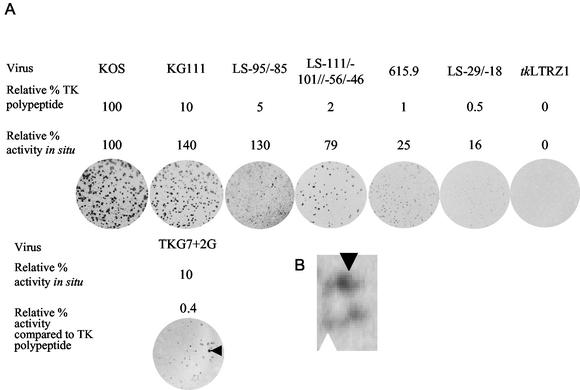
Semiquantitative plaque autoradiography was employed to measure in situ TK activity expressed by TKG7+2G.1 and TKG7+2G.2 by modifying previously described protocols (2, 15) so that increased sensitivity was achieved. The modifications were that the dishes were seeded with 8 × 105 143B cells (American Type Culture Collection), which lack cytosolic TK, 24 h prior to addition of the inoculum and that radioactive labeling with [3H]thymidine (methyl-3H, 64.5 Ci/mmol; Moravek) lasted 14 h. Viruses that had previously been shown to have TK activities at levels lower than those of the wild type were used to permit estimation of TK activities: KG111 (10% of wild-type TK activity), LS-95/-85 (5%), LS-111/-101//-56/-46 (2%), 615.9 (1%), and LS-29/-18 (0.5%) (1, 4-6, 17, 18) (Fig. 2A). Several of these viruses were made in a temperature-sensitive TK backbone; therefore, for quantitative experiments the cells were incubated at 34°C and in nonquantitative experiments the cells were incubated at 37°C. Because of the increased sensitivity of the assay, LS-95/-85 exhibited activity similar to that of KOS and LS-29/-18 exhibited detectable activity (Fig. 2A).
FIG. 2.
(A) Semiquantitative plaque autoradiography of viruses. The top line of values shows the approximate amounts of active TK expressed by each mutant as a percentage of that expressed by the wild-type strain KOS. The next line shows the average amount of radioactivity measured per plaque as a percentage relative to that measured for KOS. The next line presents the images of the plates. The relative level of TK activity associated with TKG7+2G is shown above the image of the plate used to ascertain this value. Relative TK activity levels were estimated from a graph (data not shown) of the relative percentage of TK polypeptide plotted against the relative percentage of TK activity in situ for LS-111/-101//-56/-46, 615.9, LS-29/-18, and tkLTRZ1 (y = 0.034x + 0.2684; R2 = 0.91). The arrowhead on the TKG7+2G autoradiograph indicates a plaque with TK+ activity. (B) Enlarged image of a single plaque that appears to have a mixed phenotype. The white arrowhead indicates a cell with a low level of TK activity, and the black arrowhead indicates a cell with a high level of TK activity.
The vast majority of plaques from TKG7+2G exhibited low, but detectable, levels of TK activity that averaged ∼0.4% of those of the wild type (TK-low phenotype [TKL]), which was somewhat less than that observed with the single-G insertion into the G string (615.9). However, a surprisingly high number of plaques (∼3%) with high levels of TK activity (TK+) (Fig. 2A) were present; such plaques were not observed in >500 plaques from 615.9, which carries a run of 8 Gs, or from a recombinant KOS strain with the same G7-to-G8 mutation (Griffiths and Coen, unpublished). Moreover, the mutation frequency was far higher than the estimated occurrence of ACVr viruses within a wild-type population (0.01 to 0.2%), which was seen as an indicator of polymerase fidelity and may have been due to any of the many mutations associated with ACVr, either from strain KOS or from clinical isolates (14, 25). Interestingly, we observed several plaques that appeared to consist of cells that had low levels of TK activity as well as cells with high levels of TK activity (Fig. 2B), presumably reflecting a reversion to the TK+ phenotype during the growth of the plaque. No such plaques were observed in ∼300 plaques of 615.9 (Griffiths and Coen, unpublished). Thus, not only the virus stock but individual plaques exhibited heterogeneity for TK activity, i.e., phenotypic reversion.
Enrichment of virus with high levels of TK activity during acute replication in the mouse.
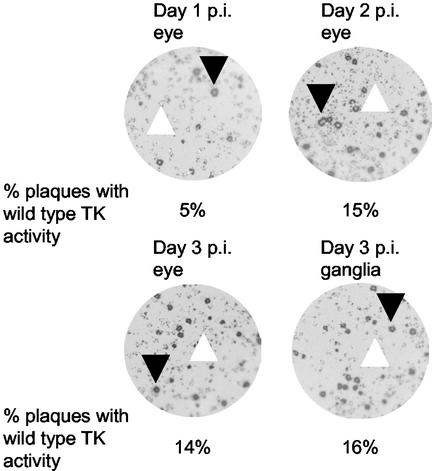
Male 8-week-old CD-1 mice (Charles River Laboratories) were infected via the cornea with 2 × 106 PFU of KOS or 7 × 107 PFU of TKG7+2G.1, TKG7+2G.2, or tkLTRZ1 as previously described (4, 22). Acute viral replication was assessed by sampling and determining the titers of virus in the tear film at 1, 2, and 3 days postinfection (p.i.) and in homogenized trigeminal ganglia excised at 3 days p.i. (Table 1). Both TKG7+2G isolates replicated in the eye to levels roughly similar to those of tkLTRZ1. No replication of tkLTRZ1 in ganglia was detected; however, substantial viral replication in ganglia was observed in animals infected with either of the TKG7+2G isolates, although it was less than that observed in KOS-infected animals. The viruses isolated from the mice during acute replication of one of the TKG7+2G isolates were amplified on Vero cells, in which there should not have been any selection pressure in favor of TK+ viruses. Plaque autoradiography demonstrated that the TK+ population appeared to have been enriched, during replication in the eye as well as in the ganglia (∼15%), compared to the TK+ population in the TKG7+2G stock (∼3%) (Fig. 3). This suggests a selective advantage for TK+ viruses in the eye, which is consistent with several reports that indicate that TK− viruses replicate less efficiently in the eyes of infected animals during acute infection, especially at later times (2, 5, 29). This may be due to the presence in the eye of cells that have limited nucleotide pools.
TABLE 1.
Virus titers in eye swabs and ganglia during acute infections of mice
| Virus | Titer (log meana ± SE) at indicated location and time p.i.b
|
|||
|---|---|---|---|---|
| Eye
|
Trigeminal ganglia | |||
| Day 1 | Day 2 | Day 3 | Day 3 | |
| KOS | 4.3 ± 0 (3) | 5.1 ± 0.2 (4) | 4.3 ± 0.3 (4) | 5.3 ± 0.3 (3) |
| tkLTRZ1 | 5.4 ± 0.2 (3) | 4.0 ± 0.3 (4) | 2.6 ± 0.2 (4) | 0.3 ± 0 (4) |
| TKG7+2G.1 | 5.6 ± 0.5 (2) | 5.5 ± 0.2 (2) | 2.3 ± 0.2 (2) | 3.9 ± 0.2 (2) |
| TKG7+2G.2 | 4.6 (1) | 4.4 ± 0 (2) | 3.5 ± 0.3 (2) | 3.8 ± 0.3 (2) |
Calculated by averaging the logs of the titers.
The number of samples titrated for each group is shown in parentheses.
FIG. 3.
Plaque autoradiography of viruses isolated during the acute phase of infection with TKG7+2G. The percentage of plaques with high levels of TK activity among those with low levels of TK activity is noted. In each image, one example each of a plaque with a high level of TK activity and a TKL plaque is indicated with a black and a white arrowhead, respectively.
Reactivation from latency.
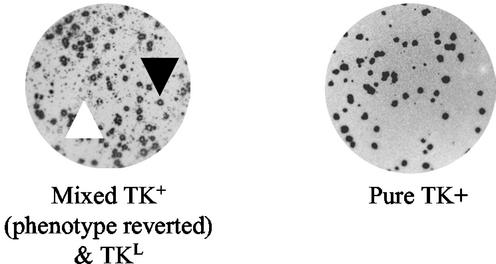
At 30 days p.i., the mice were sacrificed and the ganglia were harvested, enzymatically dissociated, and plated onto Vero cells as previously described (22). Of 24 KOS-infected ganglia, all 24 reactivated compared to none of 28 tkLTRZ1 ganglia; reactivation of both TKG7+2G.1 (6 of 10 ganglia) and TKG7+2G.2 (14 of 15 ganglia) was intermediate, with ∼70% (on average) of the ganglia reactivating. Two of the reactivated TKG7+2G.1 samples were amplified and assayed by plaque autoradiography. These viruses exhibited two phenotypes with respect to TK activity; one sample exhibited a mixture of parental (TKL) and TK+ phenotypes, and the other was exclusively TK+ (Fig. 4). The tk gene from virus with the TK+ phenotype was sequenced, and the parental G9 sequence was shown to be altered by the insertion of an additional G into the G-rich sequence, giving G10.
FIG. 4.
Plaque autoradiography of viruses isolated following reactivation of TKG7+2G. In the image of the mixed population, one example each of a plaque with a high level of TK activity and a TKL plaque is indicated with a black and a white arrowhead, respectively.
What accounts for the low level of TK activity of most TKG7+2G plaques?
This laboratory has previously reported that the low levels of TK associated with virus 615.9, which contains a single-G insertion in the G string, were the result of a net +1 frameshift (17). Further analysis demonstrated that the recoding event was unusual in that there was not a requirement for a downstream structure and paused ribosomes were not detected; rather, the efficiency of frameshifting correlated with the ability of the sequence to form non-Watson-Crick base pairs (16). The observation of low levels of TK activity expressed by TKG7+2G seems likely also to be due to frameshifting on the G string. If so, this would be very interesting, as it would entail directing ribosomes into the −1 frame with an efficiency similar to that with the +1 frame. Consistent with this, a virus that had a wild-type G string (G7) but had a single-base deletion immediately downstream of the G string, such that a net −1 shift in frame must occur for synthesis of active TK, synthesized similar levels of active TK, as measured by plaque autoradiography (A. Griffiths, M. A. Link, and D. M. Coen, unpublished observations).
Hwang et al. (17) were careful to describe the frameshift on G8 as a net +1 event, as the shift in frame might have been through a +1 shift or a −2 frameshift; indeed, a mammalian frameshift signal has been observed to utilize either +1 or −2 frameshifting, depending upon the system in which it was analyzed (23). While both +1 and −2 frameshifting would result in the synthesis of full-length TK peptide, following a −2 shift an additional glycine codon would be synthesized. Given that active TK was observed to be synthesized from a tk gene with a run of 10 Gs in the G string (Fig. 4), we conclude that the enzyme can tolerate the insertion of an additional glycine in this region. Therefore, the possibility that the net +1 frameshift occurs through a −2 frameshift remains.
What causes the high frequency of phenotypic reversion?
As mentioned above, there are many reports demonstrating that the frequency with which errors occur on a homopolymeric sequence during DNA synthesis increases as the length of the homopolymeric run increases (reviewed in reference 21). Misalignment of the template and primer DNA strands during synthesis of homopolymeric sequences has been proposed to explain these errors (28). An unpaired base (or bases) would be the result of the misalignment, resulting in a deletion if the unpaired base(s) is in the template strand or in an addition if it is (they are) in the primer strand. The increased frequency of reversion to TK+ that was observed with TKG7+2G compared to that of a mutant containing a string of 8 Gs is consistent with this hypothesis, assuming that the viral polymerase has an equal probability of being induced to give an addition or deletion mutation. As this is frequently not the case (reviewed in reference 20), a better comparison may be to the aforementioned virus that has a 7-base G string but a deletion immediately downstream and that does not appear to have the same high frequency of reversion to TK+ as TKG7+2G (Griffiths et al., unpublished).
Given the importance of TK to viral pathogenicity and its role in activating ACV, it is perhaps not surprising that in the clinic, ACVr viruses have been isolated that synthesize limited amounts of active TK. Previous work has alluded to three potential mechanisms that permit HSV to evade antiviral chemotherapy and yet remain pathogenic following frameshift mutations within tk. Each proposes that the mutant virus synthesizes a little TK, either by exploiting the reduced fidelity of the DNA polymerase when replicating homopolymeric runs (giving rise to a heterogeneous population) (26) or as the result of ribosomal frameshifting, during which low levels of active TK are synthesized (17). It is worth noting that the mutation suggested to confer increased heterogeneity is the same as that reported to permit the low levels of TK via ribosomal frameshifting (27). There is also evidence for another mechanism in which the virus appears to circumvent any requirement for TK in pathogenesis, possibly by a compensatory mutation elsewhere in the viral genome (15). In this study we have attempted to avoid the influence of the third mechanism by reconstituting the mutation into a laboratory strain, KOS, which has an absolute requirement for TK for reactivation from latency. For the 7G+2G mutation studied, its ability to evade chemotherapy, yet remain pathogenic, is most likely explained by the first model, in which phenotypic reversion provides TK activity in trans for viruses that lack sufficient TK for pathogenicity, particularly as significant levels of virus with wild-type activity levels were observed during acute replication of the viruses. It has been previously reported that resistant viruses complement sensitive viruses for resistance and that sensitive viruses complement resistant viruses for pathogenicity (8, 10, 11); the latter scenario is consistent with the behavior of TKG7+2G described in this report. It is possible to imagine two scenarios explaining how TKG7+2G reactivated as a mixed population: TK activity was provided in trans for the TKL virus by a TK+ population that established latency (in the same neuron); alternatively, the TKL population synthesized sufficient TK to permit reactivation, and subsequently, reversion to the TK+ phenotype occurred as the result of genetic instability. These ideas are presently under investigation.
Acknowledgments
We thank Jean Pesola for assistance with the animal experiments and G. Mulamba for sequencing part of tkLTRZ.
This work was supported by grants PO1 NS35138, RO1 AI26126, and T32 AI07245 from the National Institutes of Health.
REFERENCES
- 1.Böni, J., and D. M. Coen. 1989. Examination of the roles of transcription factor Sp1-binding sites and an octamer motif in trans induction of the herpes simplex virus thymidine kinase gene. J. Virol. 63:4088-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, S. H., W. J. Cook, K. L. Grove, and D. M. Coen. 1998. Human thymidine kinase can functionally replace herpes simplex virus type 1 thymidine kinase for viral replication in mouse sensory ganglia and reactivation from latency upon explant. J. Virol. 72:6710-6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christophers, J., J. Clayton, J. Craske, R. Ward, P. Collins, M. Trowbridge, and G. Darby. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42:868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coen, D. M., A. F. Irmiere, J. G. Jacobson, and K. M. Kerns. 1989. Low levels of herpes simplex virus thymidine-thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology 168:221-231. [DOI] [PubMed] [Google Scholar]
- 5.Coen, D. M., M. Kosz Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coen, D. M., S. P. Weinheimer, and S. L. McKnight. 1986. A genetic approach to promoter recognition during trans induction of viral gene expression. Science 234:53-59. [DOI] [PubMed] [Google Scholar]
- 7.Davar, G., M. F. Kramer, D. Garber, A. L. Roca, J. K. Andersen, W. Bebrin, D. M. Coen, M. Kosz Vnenchak, D. M. Knipe, X. O. Breakefield, and O. Isacson. 1994. Comparative efficacy of expression of genes delivered to mouse sensory neurons with herpes virus vectors. J. Comp. Neurol. 339:3-11. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, M. N., R. Waters, E. L. Hill, D. C. Lobe, D. W. Selleseth, and D. W. Barry. 1989. Orofacial infection of athymic mice with defined mixtures of acyclovir-susceptible and acyclovir-resistant herpes simplex virus type 1. Antimicrob. Agents Chemother. 33:304-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englund, J. A., M. E. Zimmerman, E. M. Swierkosz, J. L. Goodman, D. R. Scholl, and H. H. Balfour, Jr. 1990. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann. Intern. Med. 112:416-422. [DOI] [PubMed] [Google Scholar]
- 10.Field, H. J. 1982. Development of clinical resistance to acyclovir in herpes simplex virus-infected mice receiving oral therapy. Antimicrob. Agents Chemother. 21:744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field, H. J., and E. Lay. 1984. Characterization of latent infections in mice inoculated with herpes simplex virus which is clinically resistant to acyclovir. Antivir. Res. 4:43-52. [DOI] [PubMed] [Google Scholar]
- 12.Gaudreau, A., E. Hill, H. H. Balfour, Jr., A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths, A., S. Renfrey, and T. Minson. 1998. Glycoprotein C-deficient mutants of two strains of herpes simplex virus type 1 exhibit unaltered adsorption characteristics on polarized or non-polarized cells. J. Gen. Virol. 79:807-812. [DOI] [PubMed] [Google Scholar]
- 14.Hall, J. D., D. M. Coen, B. L. Fisher, M. Weisslitz, S. Randall, R. E. Almy, P. T. Gelep, and P. A. Schaffer. 1984. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology 132:26-37. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh, B. C., S. H. Chen, A. Hu, G. B. Mulamba, W. H. Burns, and D. M. Coen. 1998. Recurrent acyclovir-resistant herpes simplex in an immunocompromised patient: can strain differences compensate for loss of thymidine kinase in pathogenesis? J. Infect. Dis. 178:618-625. [DOI] [PubMed] [Google Scholar]
- 16.Horsburgh, B. C., H. Kollmus, H. Hauser, and D. M. Coen. 1996. Translational recoding induced by G-rich mRNA sequences that form unusual structures. Cell 86:949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang, C. B., B. C. Horsburgh, E. Pelosi, S. Roberts, P. Digard, and D. M. Coen. 1994. A net +1 frameshift permits synthesis of thymidine kinase from a drug-resistant herpes simplex virus mutant. Proc. Natl. Acad. Sci. USA 91:5461-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irmiere, A. F., M. M. Manos, J. G. Jacobson, J. S. Gibbs, and D. M. Coen. 1989. Effect of an amber mutation in the herpes simplex virus thymidine kinase gene on polypeptide synthesis and stability. Virology 168:210-220. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, J. G., S. H. Chen, W. J. Cook, M. F. Kramer, and D. M. Coen. 1998. Importance of the herpes simplex virus UL24 gene for productive ganglionic infection in mice. Virology 242:161-169. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel, T. A. 1990. Misalignment-mediated DNA synthesis errors. Biochemistry 29:8003-8011. [DOI] [PubMed] [Google Scholar]
- 21.Kunkel, T. A., and K. Bebenek. 2000. DNA replication fidelity. Annu. Rev. Biochem. 69:497-529. [DOI] [PubMed] [Google Scholar]
- 22.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsufuji, S., T. Matsufuji, N. M. Wills, R. F. Gesteland, and J. F. Atkins. 1996. Reading two bases twice: mammalian antizyme frameshifting in yeast. EMBO J. 15:1360-1370. [PMC free article] [PubMed] [Google Scholar]
- 24.Morfin, F., D. Thouvenot, M. Aymard, and G. Souillet. 2000. Reactivation of acyclovir-resistant thymidine kinase-deficient herpes simplex virus harbouring single base insertion within a 7 Gs homopolymer repeat of the thymidine kinase gene. J. Med. Virol. 62:247-250. [PubMed] [Google Scholar]
- 25.Parris, D. S., and J. E. Harrington. 1982. Herpes simplex virus variants resistant to high concentrations of acyclovir exist in clinical isolates. Antimicrob. Agents Chemother. 22:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasadeusz, J. J., and S. L. Sacks. 1996. Spontaneous reactivation of thymidine kinase-deficient, acyclovir-resistant type-2 herpes simplex virus: masked heterogeneity or reversion? J. Infect. Dis. 174:476-482. [DOI] [PubMed] [Google Scholar]
- 27.Sasadeusz, J. J., F. Tufaro, S. Safrin, K. Schubert, M. M. Hubinette, P. K. Cheung, and S. L. Sacks. 1997. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 71:3872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streisinger, G., and J. Owen. 1985. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics 109:633-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson, R. L., and N. M. Sawtell. 2000. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J. Virol. 74:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]