Abstract
Mouse mammary tumor virus (MMTV) infection establishes chronic germinal centers and a lifelong neutralizing Ab response. We show that removal of the draining lymph node after establishment of the germinal center reaction led to complete loss of neutralizing Abs despite comparable infection levels in peripheral lymphocytes. Importantly, in the absence of neutralization, only the exocrine organs mammary gland, salivary gland, pancreas, and skin showed strikingly increased infection, resulting in accelerated mammary tumor development. Induction of stronger neutralization did not influence chronic infection levels of peripheral lymphoid organs but strongly inhibited mammary gland infection and virus transmission to the next generation. Taken together, we provide evidence that a tight equilibrium in virus neutralization allows limited infection of exocrine organs and controls cancer development in susceptible mouse strains. These experiments show that a strong neutralizing Ab response induced after infection is not able to control lymphoid MMTV infection. Strong neutralization, however, is capable of blocking amplification of mammary gland infection, tumor development, and virus transmission to the next generation. The results also indicate a role of neutralization in natural resistance to MMTV infection.
Many pathogens such as HIV, Epstein–Barr virus (EBV), and mouse mammary tumor virus (MMTV) infect cells of the immune system, leading to the establishment of chronic infection. Infection of antigen-presenting cells and T cells gives the immune system an advantage for initiating immune responses but such viruses have adapted to the immune response and developed strategies that allow chronic infection. Therefore, viruses that have coevolved with their host have found an equilibrium that allows chronic infection and viral spread and maintains the host in good health until virus has spread to the next host.
MMTV initially infects dendritic cells, leading to efficient superantigen (SAg)-mediated T cell priming accompanied by B cell infection (1). The infected B cells receive cognate SAg-dependent T cell help from the primed T cells. SAg recognition is determined by the expression of SAg-reactive T cell receptor Vβ elements, and up to 30% of peripheral T cells can react with such infected SAg-presenting cells (2, 3). The SAg response is comparable to classical T cell–B cell interactions and leads to both follicular and extrafollicular B cell responses in the lymph nodes (LN) draining the site of injection. In the draining LN an ongoing germinal center (GC) reaction is observed, which is maintained for >120 days (4). MMTV has a small genome and a simple genome organization. Besides Env it uses only one gene product, the SAg to become fixed in the B cell population, to evade cytotoxic T lymphocyte (CTL) function and to induce a classical long-lasting GC reaction in the draining LN predominantly harboring infected B cells and SAg-reactive T cells (5). After the initial establishment of a chronic infection in the lymphoid compartment, lymphocytes carry the virus to the mammary gland as well as other secretory epithelial compartments (6–9). Thus, in addition to lymphocyte infection, MMTV can spread to epithelial cells in mammary gland, salivary gland, skin, sebaceous glands, as well as reproductive organs (for review see ref. 10). Finally, mammary carcinomas can be initiated by chance MMTV-integration close to protooncogenes (11). The goal of the current study was to directly assess the contribution of virus-specific Abs in controlling MMTV spread, virus transmission, and tumor development.
Here we show that the draining LN plays an important role in maintenance of the neutralizing anti-Env Ab response and control of MMTV infection in epithelial cells and mammary gland cancerogenesis. High neutralizing Ab titers induced shortly after infection did not inhibit infection of peripheral lymphoid organs as efficiently as initial infection of epithelial compartments but did dramatically reduce amplification of infection in the epithelial target organs. Importantly, this Ab response interrupts the viral life cycle. These results explain the reason for reduced mammary tumor incidence in mouse strains producing strong neutralization and shed light on a tight equilibrium in virus–host interaction that prevents early cancer development and allows virus transmission to the next generation.
Experimental Procedures
Mice, Viruses, and Immunization.
Six- to 7-week-old BALB/c mice were obtained from Harlan Olac (Bicester, U.K.). Mtv-7 congenic BALB/c mice (BALB.D2) were bred at the Swiss Institute for Cancer Research, Epalinges, Switzerland. Mtv-free mice were backcrossed six times with BALB/c mice with an intercross at each stage to select for Mtv-free mice (12). As controls Mtv-8- and Mtv-9-expressing littermates were used. MMTV(SW) and MMTV(C3H) were recovered from milk of virus-infected BALB/c mice. It was diluted 1:3 in PBS, centrifuged at 600 × g for 10 min, and stored at −70°C. Mice were injected into one hind footpad. Alternatively, virus was transmitted to the pups via milk. For surgical LN removal, mice were anesthetized by injecting a mixture of 1.5 mg of Ketaminium (Ketasol, E. Gräub, Bern, Switzerland) and 0.35 mg Xylazinium (Rompun, Bayer, Zürich) by 20 g of body weight.
Virus Neutralization Assay.
Serum was complement-depleted by heating for 30 min at 56°C, and different dilutions were mixed with virus as described (13). The amount of MMTV(SW) used was titered to give an increase from 12% to 30% of SAg-reactive T cells 4.5 days after virus injection. After 1 h incubation of sera with MMTV on ice, 10 μl of the mixture was injected into the hind footpad of BALB/c mice, and after 4.5 days the SAg response was determined in the draining popliteal LN by measuring the increase in percentage of SAg-reactive CD4+Vβ6+ T cells by flow cytometry.
Flow Cytometry.
FITC-labeled anti-Vβ6 (44-22-1), phycoerythrin labeled anti-CD45R (RA3-6B2), and CyChrome-labeled anti-CD4 (PharMingen) mAbs were used. Analysis was performed on a FACScan (Becton Dickinson) by using cellquest software for data evaluation. Dead cells were excluded by forward and side scatter characteristics.
PCR Quantification.
Five hundred nanograms of DNA from cells extracted from the different organs was analyzed by semiquantitative PCR. Twenty six cycles were used for linear amplification of reverse-transcribed DNA from nondraining tissues (first cycle: 5 min at 94°C; 26 cycles: 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C) and finally an extension step for 10 min at 72°C. To semiquantify the number of MMTV(SW)-infected cells, DNA from BALB.D2 mice containing two copies of the endogenous provirus Mtv-7 per cell was amplified from 10-fold dilutions of DNA starting with 50 ng of DNA mixed with 450 ng of DNA of BALB/c mice, keeping the total DNA concentration constant. The PCR product was detected by liquid hybridization by using the end-labeled radioactive oligonucleotide 5′-CAA GGA GGT CTA GCT CTG GCG-3′ as described (9). The annealing reaction was performed with one cycle consisting of 5 min at 98°C and one cycle consisting of 15 min at 55°C. PCR products were separated on a 6% denaturing polyacrylamide gel, which was dried and exposed to Kodak X-Omat film.
ELISA.
For ELISA, 96-well plates (F96 Nunc Maxisorp, Life Technologies, Basel) were coated overnight with 200 ng/ml recombinant Env gp52 (kindly provided by F. Baribaud, Institute for Microbiology, University of Lausanne). Dilutions (1/20, 1/40, and 1/160) of sera from Mtv-free mice and Mtv-8- and Mtv-9-expressing littermate mice were incubated overnight at 4°C. A high titer anti-MMTV immune serum of BALB/c mice or purified murine IgG was used as positive controls. Preimmune serum of mice served as negative control. Specifically bound Abs were detected by using the biotin-conjugated goat anti-murine IgG (Amersham Pharmacia) followed by streptavidin-conjugated alkaline phosphatase (Roche Molecular Biochemicals). The absorbance was determined at 405 nm of p-nitrophenyl phosphate (Sigma)-filled wells with a Titertek (Huntsville, AL) Multiskan spectrophotometer.
Results
Loss of Systemic Neutralization After LN Removal.
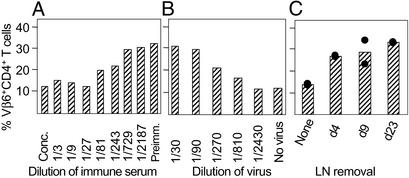
We had shown that MMTV establishes a chronic immune response with GC persisting for >120 days (4) and a neutralizing anti-Env immune response that is able to protect from reinfection with MMTV (13). To study whether the chronic GC response in the draining LN plays a role in maintaining virus-neutralizing Ab responses and/or viral infection we removed the draining popliteal LN after installation of a systemic infection. Day 4 after footpad injection is the peak of the extrafollicular B cell response when infected plasmablast B cells exit the draining LN and home to peripheral tissues (9). GC are initiated by days 9–10, and by day 23 a chronic GC response in the draining LN is established. Sera were taken 90 days after LN removal and the neutralization capacity was determined by mixing MMTV with different dilutions of serum and injected into naive BALB/c mice. The outcome of a neutralization assay with different dilutions of a serum of MMTV-infected or control mice is shown in Fig. 1A. Control sera from uninfected mice did not influence the SAg response 4.5 days later (34% Vβ6). In the presence of neutralizing Abs the SAg response was reduced in a dose-dependent fashion, reaching complete neutralization at a 1/27 dilution (12.8%). Therefore, in this assay, the absence of an increase in SAg-specific T cells after virus injection indicates efficient neutralization. A titration of MMTV(SW) is shown in Fig. 1B. Immune sera (diluted 1/3) from MMTV-infected mock-operated mice neutralized MMTV(SW) and completely prevented Vβ6+CD4+ T cell stimulation (12.5% Vβ6+CD4+ T cells) (Fig. 1C). When the draining popliteal LN was removed before induction of neutralizing Ab responses (d4), 90 days later no neutralizing Abs were produced because Vβ6+ T cells became fully activated to 28% as with uninfected control preimmune serum. Removal of the nondraining popliteal LN did not affect the outcome of the infection, thus excluding an unspecific effect of the surgical procedure on the outcome. By testing sera from day-9 and day-23 LN-operated mice, the already installed neutralizing response dropped by >95% after the kinetics of the natural half-life of IgG1 (2 weeks). These data indicate that the draining LN plays a key role in the maintenance of the neutralizing response after s.c. MMTV(SW) infection.
Figure 1.
Loss of neutralization after LN removal. (A) Titration of a serum of MMTV(SW)-infected BALB/c mice obtained 3 months after MMTV injection. Conc., concentrated milk. (B) Titration of MMTV. Different doses of MMTV were injected into the footpad of BALB/c mice, and the SAg response was determined 4.5 days later in the draining popliteal LN. (C) Mice were infected s.c. with MMTV, and 4, 9, or 23 days later the draining popliteal LN was either left in place or removed. Sera were taken 90 days after operation. The amount of neutralizing Abs in the sera was tested by neutralization assay. Two to three individual mice were used for the experiments and the mean (A and B) and individual values (C) are shown.
Normal Systemic Spread of Infected Cells After LN Removal but Increased Infection of Exocrine Tissue.
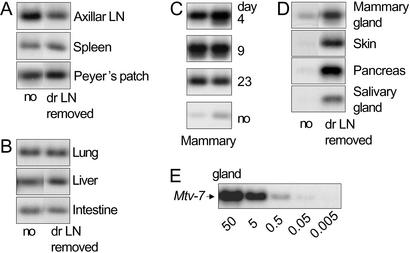
The MMTV SAg response leads to an efficient amplification of infected B cells, peaking on days 5–6 after infection. A proportion of these infected B cells emigrate from the draining LN and is found in peripheral organs. They contain integrated viral DNA copies. To assess whether spread of virus infection is abolished when the draining LN is removed we measured infection levels in lymphoid organs 1 week, 3 weeks, or 5 months after LN removal. Semiquantitative PCR was performed by using serial dilutions of Mtv-7 congenic BALB/c (BALB.D2) DNA mixed with a constant amount of BALB/c DNA (Fig. 2E). We found viral DNA in the axillar LN, spleen, and Peyer's patches at comparable levels regardless of whether or not the draining LN was removed (Fig. 2A). The same was true for lung, liver, and intestine (Fig. 2B). On the contrary, the mammary gland contained up to 100 times more viral DNA when the draining LN was removed at days 4, 9, or 23 (Fig. 2C). Three additional exocrine organs repeatedly gave up to 100 times stronger infection when the LN was removed (skin, pancreas, and salivary gland) (Fig. 2D). Moreover, mammary gland, salivary gland, and skin support viral replication and production of infectious particles. These results suggest that the draining LN controls infection levels in organs that produce and secrete infectious virus but that viral spread to other peripheral tissues does not depend on the presence of the draining LN and the neutralizing Ab response that it initiates.
Figure 2.
Infection levels of peripheral organs after LN removal. After determination of the linear range of the PCR, PCR and liquid hybridization of 500 ng of DNA were repeated three times with DNA from a total of six mice with similar results. The results shown are from operated mice (dr LN-removed) and mock-operated control mice (no) 6 months after operation. (A) Peripheral lymphoid organs of day 23-operated mice. (B) Peripheral nonlymphoid organs of day 23-operated mice. (C) Mammary gland from day 4-, 9-, or 23-operated or mock-operated mice. (D) Exocrine epithelial tissues from day 23-operated and mock-operated mice. (E) Dilution of 50, 5, 0.5, 0.05, and 0.005 ng of DNA isolated from BALB.D2 (Mtv-7+) diluted in a total amount of 500 ng of BALB/c DNA.
Lack of Virus Transmission After a Strong Neutralizing Response.
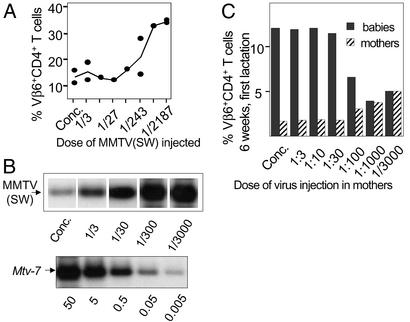
To address the role of stronger neutralization we injected mice with decreasing doses of MMTV(SW) and tested their sera 20 days later in a neutralization assay (serum dilution 1/21). Injection of more virus induced stronger neutralization (Fig. 3A). This effect was mediated by Env-specific Abs because it could be eliminated by preincubation with Env or virus particles. The chronic deletion of SAg-reactive T cells and PCR analysis of peripheral lymphoid organs indicates that higher virus doses induced a slightly stronger systemic infection than lower virus doses (data not shown). However, for infection of the exocrine organs, including the mammary gland, an inverse correlation between virus dose injected and infection was observed (Fig. 3B). To study the correlation between virus neutralization and transmission, we bred female mice injected 90 days earlier with different virus doses and analyzed transmission by monitoring deletion of SAg-reactive T cells in both mothers and offspring. Assessment of CD4+Vβ6+ T cell deletion is a highly sensitive method to determine MMTV(SW) infection. High virus doses (concentrated to 1/30) induce complete deletion of CD4+Vβ6+ T cells in the mothers whereas low virus doses (1/1,000–1/3,000) lead to partial deletion (Fig. 3C). At dilutions of 1/10,000 no deletion was observed. As virus infection and production in the mammary gland is responsible for virus transmission to the offspring, we determined the deletion in the offspring of mothers infected with different virus doses. Strikingly, an inverse correlation between the concentration of virus injected and the transfer of virus to the next generation was found (Fig. 3C). Infection of mothers with high concentrations of virus (concentrated milk to 1/30 diluted milk) completely prevented transmission to the first generation because the percentage of SAg-reactive CD4+Vβ6+ T cells remained at 12%. Low virus doses, however, induced deletion of SAg-reactive T cells. Only after three to five lactations did virus become detectable in the milk as judged by the slow and partial deletion of Vβ6+ T cells in the offspring (data not shown). Because deletion of SAg-reactive T cells is a direct readout for infection, we can conclude that mammary gland infection and hence virus transmission occurred inefficiently in the presence of high titers of neutralizing Abs. Taken together, strong neutralization correlates with decreased mammary gland infection and virus transmission.
Figure 3.
Inverse correlation among virus dose, mammary gland infection, and virus transmission. (A) Mice were injected with serial dilutions of MMTV. Sera of these mice were tested in a neutralization assay 20 days later. Individual mice and mean values (curve) are shown. The experiment was repeated twice. (B) Serial dilutions of MMTV(SW) were injected into adult mice, and the amount of reverse-transcribed retroviral DNA isolated from mammary gland was tested 5 weeks later from 500 ng. As control 50, 5, 0.5, 0.05, and 0.005 ng of DNA from BALB/D2 mice mixed with 500 ng of BALB/c DNA was used. (C) Different virus doses were injected into mice, and 3 months later infection in mothers and their first litters was monitored by deletion of SAg-reactive T cells.
Mammary Tumor Development in the Absence of Neutralization.
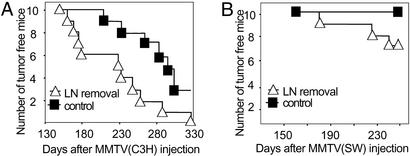
Because the absence of neutralization leads to higher mammary gland infection, we wanted to determine whether, under these conditions, mammary tumor formation was accelerated. A high virus load is thought to increase the risk of activating protooncogenes such as wnt-1, int-2, and others (11). For this purpose the highly tumorigenic MMTV(C3H) was injected at a 1/100 dilution in the highly susceptible BALB/c mouse strain. Using such virus doses led to tumor development after 300 days in ≈50% of mice and most of the remaining mice develop tumors until 12 months of age (Fig. 4A). To determine whether there was any effect on tumor acceleration when neutralization was lost, we removed the draining LN 18 days after virus injection in one group of mice (n = 10); the control group (n = 10) was mock-operated. The mice were force-bred, and the kinetics of tumor development was determined. As shown in Fig. 4A, a striking acceleration of tumor development was observed in the LN-removed mice as compared with LN-intact mice. In LN-removed mice tumors appeared on the average 2 months earlier than in mock-operated mice. A similar experiment was performed with the less oncogenic MMTV(SW) virus. After injection of 1/30 diluted virus, no tumors were observed before 1 year of age (0/10) but in LN-removed mice tumors were observed (3/10 after 245 days) (Fig. 4B). Tumors were adenocarcinomas that were not different from the classical MMTV tumors (data not shown).
Figure 4.
Reduced neutralization results in accelerated cancer development. (A) The number of tumor-free mice after s.c. infection of either control or LN-operated mice (day 18 after virus injection). The highly tumorigenic MMTV(C3H) was used. (B) The number of tumor-free mice after s.c. infection of either control or LN-operated mice (day 18 after virus injection). The low tumorigenic MMTV(SW) was used.
Correlation of Natural Resistance to MMTV Infection, Reduced Cancer Susceptibility, and Virus-Specific Neutralizing Ab Responses.
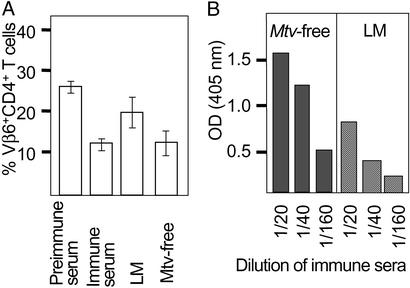
We have observed that Mtv-free mice are more resistant to MMTV infection and cancer development (data not shown). To assess whether mice lacking endogenous MMTV copies induce stronger anti-MMTV responses caused by lack of immune tolerance, we injected infectious MMTV into either Mtv-free or Mtv-8- and Mtv-9-expressing littermate mice and measured the neutralizing activity (Fig. 5A) and titers of virus-specific Ab (Fig. 5B) 3 months later. Significantly, stronger MMTV Env-specific Ab responses were observed in Mtv-free mice. There is a clear correlation between neutralizing activity and virus-specific Abs in the sera. In agreement with these observations, Mtv-free mice showed reduction in mammary gland infection, which was evident by the first generation, and after the fourth generation no further virus transmission was observed as judged by the lack of deletion of the SAg-reactive T cells in the offspring (data not shown). In contrast, infection of BALB/c mice resulted in a continuous transmission of the infection from the mothers to their offspring for >15 generations of breeding. These results suggest that the induction of a stronger neutralizing Ab response partially protected against chronic MMTV infection, leading to viral clearance within a few generations. We had shown that MMTV expressing strong SAg [MMTV(SW) and MMTV(C4)] induce faster deletion of SAg-reactive T cells than MMTV expressing weak SAg [MMTV(C3H) and MMTV(SHN)] (14). In addition, we have shown that strong SAg responses are required to induce efficient neutralization (13). In agreement with the protection from mammary tumor generation by neutralizing Abs noted above, we found a striking correlation between weak SAg and high cancer incidence. Several different MMTV isolates have been described that differ dramatically in their kinetics of cancer induction in BALB/c mice after natural transmission. Virus isolates such as MMTV(C3H) and MMTV(SHN) induce tumors after a short period with high incidence (14). More than half of the mice die within 9 months after neonatal infection. On the contrary MMTV(SW), MMTV(JYG), and MMTV(C4) induce tumors only rarely and it is rare to observe any tumors before 1 year of age after natural virus transmission (10). In summary, we observed a strong correlation between strong virus-specific Ab responses and natural resistance to mammary gland infection and cancer.
Figure 5.
Mice lacking endogenous Env copies induce stronger neutralization titers after MMTV infection. (A) Thirteen Mtv-free or Mtv-expressing littermates were injected with MMTV(SW), and the neutralizing Abs were measured in a neutralization assay 4 weeks later. Immune and preimmune sera were used as controls from both mouse strains. A Student's t test showed P < 0.01 for Mtv-free compared with Mtv-expressing littermates. (B) Mtv-free and littermate (LM) mice were injected with MMTV and the anti-Env IgG Abs were measured 3 months later by ELISA. Dilutions of sera from three animals are shown. Experiments were repeated three times with similar results. Background values of 0.06–0.08 OD were subtracted.
Discussion
Although the role of Abs in preventing virus infection and reinfection is unquestionable, their contribution to the resolution of noncytopathic viral disease is much more controversial. Upon MMTV infection, neutralizing Ab responses are generated that offer protection from reinfection. In this study we demonstrate that removal of the LN draining the site of virus injection led to a striking drop in virus neutralization. Because systemic viral spread or the survival of infected cells in the periphery was not altered, these findings demonstrate the role of chronic neutralizing B cell responses in the control of virus infection and virus-induced tumor development. Induction of stronger neutralization resulted in a block of infection of epithelia and an interruption of the viral life cycle. Overall, our results illustrate the existence of a tight balance between the levels of virus neutralization critical for virus transmission to the next generation and mammary tumor development.
Viral Infection and Tumor Development in the Absence of Neutralization.
Several studies have clearly demonstrated that MMTV is transported to the mammary gland by lymphocytes and that infected B and T cells can release MMTV particles (6–9). Because removal of the draining LN after virus infection abolished the neutralizing Ab responses, we were able to address the viral spread to lymphoid and nonlymphoid compartments in the absence of neutralizing Abs. Whereas most lymphoid and nonlymphoid organs did not show any differences in infection levels in the presence or absence of neutralization, levels of infection in exocrine secretory organs were up to 100-fold higher. Most of these organs have been described as sites of production of infectious virus. Mammary gland is the classical site of virus production but also salivary gland and sebaceous glands produce infectious virus (15–18). Exocrine pancreas shares the secretory function with these other organs.
In mice injected with high virus doses, high virus neutralization and weak mammary gland infection were observed despite even higher peripheral chronic infection. This finding can be caused by low mammary gland infection levels due to neutralization and/or to effects of neutralization once mammary gland infection has occurred by blocking interepithelial spread. In support for the second it is generally accepted that higher neutralization titers are required to block direct cell-to-cell spread than to neutralize infection by free virus (for review see ref. 19). An effect of neutralizing Abs on virus replication has been described for measles viruses, sindbis virus, rabies virus, and Friend leukemia virus infection. These responses act either against virus transcription, assembly, or release of infectious particles (20–23).
Tumor development in mice after LN removal was strikingly faster in the absence of neutralization. Mammary tumors are induced by MMTV integration close to protooncogenes (11). Multiple integration sites of MMTV increase the probability of carcinoma development (24). Therefore, it was not surprising that higher infection levels accelerated the onset of cancer development.
The removal of the draining LN could have nonspecific effects on viral spread caused by surgical procedures. This notion was excluded because removal of the nondraining LN did not affect viral spread. Because of the similar spread of infected cells in operated and mock-operated mice it is unlikely that the removal of virus particles or infected cells in the draining LN is responsible for the observed effects. Most likely, removal of the GC in the draining LN inducing loss of helper T cells, centroblasts, centrocytes, resident memory, or plasma cells as well as removal of the antigen deposits on follicular dendritic cells are responsible for the disappearance of neutralization. Alternatively, the immune response in the draining LN is chronic but after systemic spread there is tolerance induction in the periphery. These results clearly demonstrate a role for neutralizing Ab in the later stages of MMTV infection that lead to protection from mammary tumor formation.
A Tight Balance in Neutralization and Virus Transmission.
One can speculate that strong neutralizing responses might represent an evolutionary disadvantage for the virus because it interrupts the viral life cycle. On the other hand, weak neutralization would provoke lethal tumors early in life before transmission to the next generation via milk is granted. In evolutionary terms it seems likely that an intermediate neutralization response is optimal for virus propagation when the host survives until efficient mammary gland infection and transmission of the virus occurs and mammary gland infection is high. The results also explain an earlier observation describing accelerated occurrence of mammary carcinomas after maternal transmission than after injection of very high doses of virus (25). Based on our observations, these differences are not related to differences in mucosal and peripheral immune responses but are rather a consequence of virus dose-dependent induction of neutralizing Abs. We transferred neutralizing sera into LN-operated mice to further prove the role of neutralizing Abs in the reduction of target cell infection (data not shown). These experiments did not allow the maintenance of sufficiently high neutralization titers for the >3 months required to find a significant decrease in mammary gland infection. Further experiments are required.
Natural MMTV Transmission and Resistance.
Natural transmission of MMTV as well as virus injection induce a neutralizing Ab response despite the presence of endogenous Mtv copies (13, 26–28). This response results in weak neutralizing titers (13). Several naturally MMTV-resistant mouse strains have been described. Expression of an endogenous Mtv-SAg in the thymus and periphery leads to deletion of the SAg-reactive T cells and therefore prevents amplification of virus-infected B cells and chronic infection (29, 30). A second genetic mechanism of protection is the lack of expression of I-E molecules because I-A molecules are unable to efficiently present several MMTV SAg (10). We report that Mtv-free mice are also relatively resistant to virus transmission. This finding most likely reflects a third mechanism of resistance: the strength of the neutralizing Ab response. Mtv-free mice induce stronger neutralizing Ab responses, most likely caused by lack of immune tolerance induced by endogenous env genes. Similar to Mtv-free mice, the I/LnJ strain of mice is particularly resistant to MMTV-dependent tumor formation, nevertheless allowing normal peripheral infection (31, 32). It has been described that although vaccination against MMTV Env blocks mammary cancer development it does not inhibit peripheral infection (33). These observations of normal peripheral infection and inhibited transmission to the mammary gland correspond perfectly to the observations made in this study with mice having high neutralization titers. Our study strongly suggests that virus neutralization is one of the parameters conferring resistance to MMTV and protection from transmission to the mammary gland. The results are summarized in Table 1.
Table 1.
Summary of the inverse correlation between virus neutralizing and tumor formation
| Condition | Susceptible mice | Susceptible mice dLN removal | Resistant mice |
|---|---|---|---|
| Chronic lymphoid infection | Normal | Normal | Normal or − |
| Chronic neutralization | + | − | +++ |
| Cancer | + | +++ | − |
After MMTV infection both resistant and susceptible mouse strains develop a chronic infection of lymphoid organs. Resistant mice mount a stronger neutralizing Ab response than susceptible mice and draining LN (dLN) removal completely abrogates chronic neutralizing responses. Mammary gland tumor development is strongly enhanced after LN removal in susceptible mice, whereas, in resistant animals, strong neutralizing Ab responses correlate with resistance to cancer development.
Ab-Mediated Control of Noncytopathic and Cytopathic Viruses.
Noncytopathic viruses such as lymphocytic choriomeningitis virus, HIV, and hepatitis B and C are initially controlled by CTL responses and in the control of chronic infection, both CTL and neutralizing Ab play a crucial role (34, 35).
Vesicular stomatitis virus (VSV) is an example of a cytopathic virus that is mostly controlled by neutralizing Abs (36). In the VSV system, IgM and IgG responses are virus dose-dependent and increase with the amount of infectious antigen as in the case of MMTV (37). Moreover, specific T cells were supportive in expansion and maintenance of VSV-specific IgG2a titers. Additionally, virus production in B cell-deficient mice during recurrence of primary murine cytomegalovirus infection is higher than in normal mice (38), indicating a critical role for Abs in limiting dissemination of reactivated virus. The role of virus neutralization is less clear for EBV but neutralizing Abs have been shown to inhibit recurrence of MHV-68 in lung epithelium and to control persistent infection (39, 40). MMTV is a noncytopathic virus that represents a special case because it is controlled by Ab but not by CTL responses.
Different Strategies Leading to Similar Outcomes: MMTV vs. EBV.
EBV and MMTV have adopted very similar strategies to exploit the immune system. They both infect B cells and epithelial cells, exploit the B cell biology, and are tumorigenic. γ-Herpesviruses such as EBV have a large genome allowing the presence of many different genes that play a role in infection. The small MMTV genome only contains a few genes. Both viruses can infect naïve B cells and induce their differentiation into effector and long-lived memory cells (10, 41).
Whereas MMTV preferentially infects naive B cells, EBV can infect naive as well as memory B cells. After EBV infection, a well-orchestrated viral gene expression program initiates responses leading to generation of memory phenotype, latency, avoidance of CTL function, and periodic B cell reactivation for virus production in the salivary gland (41, 42). Expression of viral latent membrane proteins LMP1 and LMP2 in EBV-infected cells allows induction of cell cycle and B cell differentiation. These molecules mimic continuous CD40 and Ig signaling, respectively (43–45). Two major scenarios for the fixation of infection in resting memory B cells have been proposed: first, infection of naïve B cells followed by induction of cell cycle and development of all stages of GC B cells and finally resting memory B cells (46, 47); second, infection of memory B cells followed by development of resting memory cells (48). In the first scenario it is not clear whether the B cell has to pass through GC or can achieve differentiation without formation of GC caused by expression of viral genes (45, 49). Whereas EBV is controlled by cytotoxic T cell responses, no such response is detectable against MMTV (5, 50). We have argued that the initial SAg response is induced by very few infected dendritic cells that are saturated with SAg-reactive CD4+ T cells and hence cannot prime an efficient CTL response. At later stages the systemic spread of infected B cells leads to tolerance induction (5).
γ-Herpesviruses such as EBV and murine γ-herpesvirus 68 (MHV-68) show a SAg-like activity: EBV activates a host SAg on infection (51). MHV-68 shows a Vβ4-specific MHC unrestricted oligoclonal CD8 T cell stimulation (52).
It should be noted that in patients with Ab deficiencies severe viral infections have been documented that were markedly reduced by high-dose i.v. IgG therapy (53–56). Especially in the case of neurotropic viruses, Abs have been shown to limit or prevent virus spread to the CNS system or restrict virus expression (57–60). Other viruses infecting epithelia such as pulmonary influenza virus can be cleared by application of Abs after establishment of infection (61).
In conclusion, we have described (i) the role of neutralizing Ab responses in controlling retroviral infection of secretory organs; (ii) the contribution of the draining LN in maintenance of neutralizing Abs; and (iii) an important mechanism for controlling virus-induced cancer development. These data emphasize the importance of clinical studies to investigate the ability of Ab-mediated therapy in providing protection during an ongoing viral disease.
Acknowledgments
We thank Werner Held and Roger Voyle for stimulating discussions and critical reading of the manuscript. H.A.-O. is supported by the Giorgi-Cavalieri Foundation and the Swiss National Science Foundation (Grant 31-59165.99).
Abbreviations
- EBV
Epstein–Barr virus
- SAg
superantigen
- MMTV
mouse mammary tumor virus
- GC
germinal center
- LN
lymph nodes
- CTL
cytotoxic T lymphocytes
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vacheron S, Luther S A, Acha-Orbea H. J Immunol. 2002;168:3470–3476. doi: 10.4049/jimmunol.168.7.3470. [DOI] [PubMed] [Google Scholar]
- 2.Held W, Shakhov A N, Izui S, Waanders G A, Scarpellino L, MacDonald H R, Acha-Orbea H. J Exp Med. 1993;177:359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutner U, Kraus E, Kitamura D, Rajewsky K, Huber B T. J Exp Med. 1994;179:1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luther S A, Gulbranson-Judge A, Acha-Orbea H, MacLennan I C. J Exp Med. 1997;185:551–562. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acha-Orbea H, Finke D, Attinger A, Schmid S, Wehrli N, Vacheron S, Xenarios I, Scarpellino L, Toellner K M, MacLennan I C, Luther S A. Immunol Rev. 1999;168:287–303. doi: 10.1111/j.1600-065x.1999.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsubura A, Inaba M, Imai S, Murakami A, Oyaizu N, Yasumizu R, Ohnishi Y, Tanaka H, Morii S, Ikehara S. Cancer Res. 1988;48:6555–6559. [PubMed] [Google Scholar]
- 7.Waanders G A, Shakhov A N, Held W, Karapetian O, Acha-Orbea H, MacDonald H R. J Exp Med. 1993;177:1359–1366. doi: 10.1084/jem.177.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzuris J L, Golovkina T V, Ross S R. J Virol. 1997;71:6044–6048. doi: 10.1128/jvi.71.8.6044-6048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finke D, Baribaud F, Diggelmann H, Acha-Orbea H. J Immunol. 2001;166:6266–6275. doi: 10.4049/jimmunol.166.10.6266. [DOI] [PubMed] [Google Scholar]
- 10.Luther S A, Acha-Orbea H. Adv Immunol. 1997;65:139–243. [PubMed] [Google Scholar]
- 11.Nusse R, Varmus H E. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 12.Braun M Y, Jouvin-Marche E, Marche P N, MacDonald H R, Acha-Orbea H. Eur J Immunol. 1995;25:857–862. doi: 10.1002/eji.1830250334. [DOI] [PubMed] [Google Scholar]
- 13.Luther S A, Maillard I, Luthi F, Scarpellino L, Diggelmann H, Acha-Orbea H. J Immunol. 1997;159:2807–2814. [PubMed] [Google Scholar]
- 14.Luther S, Shakhov A N, Xenarios I, Haga S, Imai S, Acha-Orbea H. Eur J Immunol. 1994;24:1757–1764. doi: 10.1002/eji.1830240806. [DOI] [PubMed] [Google Scholar]
- 15.Rongey R W, Abtin A H, Estes J D, Gardner M B. J Natl Cancer Inst. 1975;54:1149–1156. doi: 10.1093/jnci/54.5.1149. [DOI] [PubMed] [Google Scholar]
- 16.Kozma S, Osterrieth P M, François C, Calberg-Bacq C M. J Gen Virol. 1980;51:327–339. doi: 10.1099/0022-1317-51-2-327. [DOI] [PubMed] [Google Scholar]
- 17.Imai S, Morimoto J, Tsubura Y, Iwai Y, Okumoto M, Takamori Y, Tsubura A, Hilgers J. Eur J Cancer Clin Oncol. 1983;19:1011–1019. doi: 10.1016/0277-5379(83)90071-8. [DOI] [PubMed] [Google Scholar]
- 18.Wajjwalku W, Takahashi M, Miyaishi O, Lu J, Sakata K, Yokoi T, Saga S, Imai M, Matsuyama M, Hoshino M. Jpn J Cancer Res. 1991;82:1413–1420. doi: 10.1111/j.1349-7006.1991.tb01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parren P W H I, Burton D R. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujinami R S, Oldstone M B. Nature. 1979;279:529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- 21.Levine B, Hardwick J, Trapp B D, Crawford T O, Bolliger R C, Griffins D E. FASEB J. 1991;5:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 22.Dietzschold B, Kao M, Zheng Y M, Chen Z Y, Maul G, Fu Z F, Rupprecht C E, Koprowski H. Proc Natl Acad Sci USA. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesbro B, Wehrly K, Doig D, Nishio J. Proc Natl Acad Sci USA. 1979;76:5784–5788. doi: 10.1073/pnas.76.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters G, Lee A E, Dickson C. Nature. 1986;320:628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- 25.Papiernik M, Wache A C, Pontoux C, Nabarra B. Eur J Immunol. 1997;27:2145–2151. doi: 10.1002/eji.1830270905. [DOI] [PubMed] [Google Scholar]
- 26.Arthur L O, Fine D L. Int J Cancer. 1978;22:734–740. doi: 10.1002/ijc.2910220616. [DOI] [PubMed] [Google Scholar]
- 27.Altrock B W, Cardiff R D. J Natl Cancer Inst. 1979;63:1075–1080. [PubMed] [Google Scholar]
- 28.Altrock B W, Cardiff R D, Blair P B. J Natl Cancer Inst. 1981;67:163–168. [PubMed] [Google Scholar]
- 29.Golovkina T V, Chervonsky A, Dudley J P, Ross S R. Cell. 1992;69:637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- 30.Held W, Waanders G A, Shakhov A N, Scarpellino L, Acha-Orbea H, MacDonald H R. Cell. 1993;74:529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 31.Andervont H B. J Natl Cancer Inst. 1964;32:1189–1198. [PubMed] [Google Scholar]
- 32.Golovkina T V. J Virol. 2000;74:2752–2759. doi: 10.1128/jvi.74.6.2752-2759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astori M, Karapetian O. J Gen Virol. 1997;78:1935–1939. doi: 10.1099/0022-1317-78-8-1935. [DOI] [PubMed] [Google Scholar]
- 34.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Nature. 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 35.Kalams S A, Walker B D. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochsenbein A F, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel R M. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 37.Freer G, Burkhart C, Ciernik I, Bachmann M F, Hengartner H, Zinkernagel R M. J Virol. 1994;68:3650–3655. doi: 10.1128/jvi.68.6.3650-3655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski U H. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nash A A, Dutia B M, Stewart J P, Davison A J. Philos Trans R Soc London B. 2001;356:569–574. doi: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim I J, Flano E, Woodland D L, Blackman M A. J Immunol. 2002;168:3958–3964. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
- 41.Thorley-Lawson D A. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 42.Rickinson A B, Lane P J. Curr Biol. 2000;10:R120–R123. doi: 10.1016/s0960-9822(00)00308-0. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell R G, Wilson J B, Anderson S J, Longnecker R. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 44.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 46.Babcock G J, Decker L L, Freeman R B, Thorley-Lawson D A. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babcock G J, Thorley-Lawson D A. Proc Natl Acad Sci USA. 2000;97:12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurth J, Spieker T, Wustrow J, Strickler G J, Hansmann L M, Rajewsky K, Kuppers R. Immunity. 2000;13:485–495. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 49.Araujo I, Foss H D, Hummel M, Anagnostopoulos I, Barbosa H S, Bittencourt A, Stein H. J Pathol. 1999;187:326–330. doi: 10.1002/(SICI)1096-9896(199902)187:3<326::AID-PATH242>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O'Callaghan C A, Steven N, McMichael A J, Rickinson A B. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutkowski N, Conrad B, Thorley-Lawson D A, Huber B T. Immunity. 2001;15:579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- 52.Hardy C L, Silins S L, Woodland D L, Blackman M A. Int Immunol. 2000;12:1193–1204. doi: 10.1093/intimm/12.8.1193. [DOI] [PubMed] [Google Scholar]
- 53.Sanna P P, Burton D R. J Virol. 2000;74:9813–9817. doi: 10.1128/jvi.74.21.9813-9817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heslop H E, Rooney C M. Immunol Rev. 1997;157:217–222. doi: 10.1111/j.1600-065x.1997.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 55.Green M, Reyes J, Webber S, Rowe D. Transplant Infect Dis. 2001;3:97–103. doi: 10.1034/j.1399-3062.2001.003002097.x. [DOI] [PubMed] [Google Scholar]
- 56.Schaar C G, van der Pijl J W, van Hoek B, de Fijter J W, Veenendaal R A, Kluin P M, van Krieken J H, Hekman A, Terpstra W E, Willemze R, Kluin-Nelemans H C. Transplantation. 2001;71:47–52. doi: 10.1097/00007890-200101150-00008. [DOI] [PubMed] [Google Scholar]
- 57.Tyler K L, Mann M A, Fields B N, Virgin H W. J Virol. 1993;67:3446–3453. doi: 10.1128/jvi.67.6.3446-3453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramakrishna C, Stohlman S A, Atkinson R D, Shlomchik M J, Bergmann C C. J Immunol. 2002;168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- 59.Tschen S I, Bergmann C C, Ramakrishna C, Morales S, Atkinson R, Stohlman S A. J Immunol. 2002;168:2922–2929. doi: 10.4049/jimmunol.168.6.2922. [DOI] [PubMed] [Google Scholar]
- 60.Keller M A, Stiehm E R. Clin Microbiol Rev. 2000;13:602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palladino G, Mozdzanowska K, Washko G, Gerhard W. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]