Abstract
Lymphocytic choriomeningitis virus (LCMV) and Lassa virus can cause hemorrhagic fever and liver disease in primates. The WE strain of LCMV (LCMV-WE) causes a fatal Lassa fever-like disease in rhesus macaques and provides a model for arenavirus pathogenesis in humans. LCMV-WE delivered intravenously or intragastrically to rhesus macaques targets hepatocytes and induces high levels of liver enzymes, interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), and soluble tumor necrosis factor receptors (sTNFRI and -II) in plasma during acute infection. Proinflammatory cytokines TNF-α and IL-1β were not detected in plasma of infected animals, but increased plasma gamma interferon was noted in fatally infected animals. Immunohistochemistry of acute liver biopsies revealed that 25 to 40% of nuclei were positive for proliferation antigen Ki-67. The increases in IL-6, sIL-6R, sTNFR, and proliferation antigen that we observe are similar to the profile of incipient liver regeneration after surgical or toxic injury (N. Fausto, Am. J. Physiol. 277:G917-G921, 1999). Although IL-6 was not directly induced by virus infection in vitro, peripheral blood mononuclear cells from acutely infected monkeys produced higher levels of IL-6 upon lipopolysaccharide stimulation than did healthy controls. Our data confirm that acute infection is associated with weak inflammatory responses in tissues and initiates a program of liver regeneration in primates.
Arenaviruses are zoonotic pathogens that are carried by rodents and occasionally transmitted to humans. Lymphocytic choriomeningitis virus (LCMV), the prototype arenavirus, induces a spectrum of outcomes in humans, ranging from subclinical infection to acute aseptic meningitis (55, 56). LCMV is also a teratogenic and underdiagnosed cause of congenital infection (4). In New World primates, LCMV can cause fatal callitrichid hepatitis. Feeding monkeys neonatal mice that were infected with LCMV caused outbreaks of hepatitis among captive callitrichid monkeys in the United States, the United Kingdom, and Germany (3, 44, 45, 58). Lassa virus, the etiological agent of Lassa fever, may also be transmitted to humans by ingestion of rodents. Lassa virus is carried primarily by the multimammate mouse Mastomys natalensis and causes up to 300,000 infections annually, of which approximately 30% result in disease varying from mild influenza-like illness to lethal hemorrhagic fever (HF) (23, 38, 40, 41). It has been shown that hunting rodents and consuming their meat are major risk factors for rodent-to-human transmission (33, 60). Our experimental studies with LCMV-infected mice (52, 53) and monkeys (reference 35 and this study) indicate that oral inoculation leads to attenuated infections that occasionally cause disease. Thus, oral transmission is biologically important, and therefore here we compare the progression of intravenously (i.v.) and intragastrically (i.g.) initiated disease.
LCMV and Lassa virus belong to the Old World complex of the Arenaviridae (55, 56). Both viruses have extensive strain diversity with remarkable biological variations in lethality and pathogenicity (13, 23, 48, 49). The WE strain of LCMV (LCMV-WE) used in these studies is known for its hepatotropism and virulence in guinea pig and primate models (35, 54). It induces a lethal Lassa fever-like HF in rhesus macaques (22, 35, 48) and serves as a good model for pathogenesis of human disease. In addition to Lassa HF, four New World arenaviral HFs have been described, i.e., Argentine, Bolivian, Venezuelan, and Brazilian HF, caused by the Junin, Machupo, Guanarito, and Sabia viruses, respectively. Fatal arenaviral HFs share one significant postmortem finding in humans: noninflammatory necrosis of hepatocytes (14, 15, 17, 28, 40, 49, 62, 64).
Most information on arenavirus pathogenesis has been obtained from murine infection studies using LCMV. In the mouse, pathology is associated with inflammatory infiltrates of cytotoxic T lymphocytes that occur over a wide dose range (5, 11, 19, 67). Unlike in the mouse, a fatal outcome of Lassa HF in primates depends on high levels of virus in plasma and in tissues (17, 24, 38, 48). Viremia of ≥103.6 50% tissue culture infective doses per ml on admission was associated with a case-fatality rate of 76% (24). In the mouse, LCMV-WE can elicit lethal immunopathology of liver (5), but the disease differs from that observed in primates. Although arenavirus-mediated liver disease in humans was characterized by minimal lymphocytic infiltration (18, 40), LCMV-mediated disease in the mouse was characterized by lymphocytic infiltration that was preventable by treatment with immunosuppressive agents (11, 19).
The major virulence determinants for LCMV-WE and Lassa virus are encoded on the large genomic segment (10, 30, 31, 54). Since this segment carries genes determining the level of virus replication, the replication level is likely to be the primary determinant of virulence (10). We hypothesize that virus must first replicate to a certain level before tropism and other factors begin to affect virulence. The highest level of replication of Lassa virus was found in liver tissues of fatally infected patients, and viremia of >103.2 50% tissue culture infective doses per ml and an aspartate aminotransferase (AST) level in plasma of >150 IU/liter together carried a risk of death of nearly 80% (38, 39).
We used Lassa fever-like HF in rhesus macaques as a model to study pathogenesis of arenavirus-mediated liver injury. In this paper we demonstrate that (i) LCMV-WE delivered i.v. or i.g. targets hepatocytes and the infection significantly affects the liver as judged by aminotransferase levels in plasma; (ii) LCMV induces a proliferation response in livers of rhesus macaques, with up to 25 to 40% of hepatocytes stained on Ki-67 nuclear proliferation-associated antigen; (iii) the hepatocyte activation correlates with interleukin-6 (IL-6)/soluble IL-6 receptor (sIL-6R) and soluble tumor necrosis factor receptor I (sTNFRI) and sTNFRII up-regulation and resembles the cytokine pattern after surgical or toxic injury (16); and (iv) in vitro infection of hepatocytes did not induce IL-6 and TNF-α, suggesting that the hepatocyte activation signals seem to come from other sources, e.g., activated intrahepatic macrophage-like Kupffer cells or peripheral blood mononuclear cells (PBMC).
(This work has been presented in part by I. S. Lukashevich, I. Tikhonov, J. D. Rodas, D. Djavani, J. C. Zapata, and M. Salvato at the International Conference on Emerging Infectious Disease, Atlanta, Ga., 24 to 27 March 2002 [abstr. LB1-59].)
MATERIALS AND METHODS
Virus.
LCMV-WE was received from Peter B. Jahrling (U.S. Army Medical Research Institute for Infectious Diseases, Fort Detrick, Frederick, Md.). The virus was amplified in Vero cells and stored at 107 PFU/ml. Detection of infectious virus in culture media and tissue samples was performed by plaque assay as previously described (12). For virus inactivation, 1-ml aliquots of the virus stock were exposed to UV light in petri dishes for 1 h at 4°C. Inactivation was verified by Vero cell plaque assay.
Rhesus macaques, LCMV-WE inoculations, and blood and tissue collection.
All experimental protocols were approved by the institutional Animal Care and Use Committee. Animals were inoculated by intravenous (i.v.) and intragastric (i.g.) routes as previously described (35). Animal groups, routes of inoculation, and virus doses are summarized in Table 1. Blood samples from infected animals were drawn at weekly or biweekly intervals and submitted to the clinical laboratory for hematology and blood chemistry (20). Most tissue specimens were obtained at necropsy, but occasionally liver biopsies were obtained with a specially designed needle (Microvasive Boston Scientific Co.).
TABLE 1.
Outcome of LCMV/WE infection of rhesus macaques
| Monkey | Inoculation route and dose (PFU) | Outcome | Viremiaa | Virus in liverb | Antibody responsec |
|---|---|---|---|---|---|
| Rh-iv3 | i.v., 103 | Died | 1.0 × 106 | 5.5 × 105 | <2 (E), negative (N) |
| Rh-iv6 | i.v., 106 | Died | 1.2 × 107 | 1.0 × 108 | <2 (E), negative (N) |
| Rh-ig6a | i.g., 106 | Survived | PCR negative | PCR negative | <2 (E), negative (N) |
| Rh-ig6b | i.g., 106 | Survived | PCR negative | PCR negative | <2 (E), negative (N) |
| Rh-ig7a | i.g., 107 | Survived | PCR negative | PCR negative | <2 (E), negative (N) |
| Rh-ig7b | i.g., 107 | Illness and full recovery | 8.8 × 104 | PCR positive and negatived | 7.2 (E), 3.1 (N) |
For Rh-iv3 and Rh-iv6, viremia on day of death is shown (in PFU per milliliter). For Rh-ig7b, viremia was detected on day 28 p.i. and was not detectable by plaque assay and RT-PCR on day 42 and later.
RT-PCR was performed with RNA samples extracted from livers of Rh-iv3 and Rh-iv6 on the day of death and from Rh-ig6a and Rh-ig6b on day 28, when the animals were sacrificed. For Rh-ig7a and Rh-ig7b, biopsies were performed before the infection and at weeks 4 and 11 p.i. Titers are in PFU per gram of tissue.
Antibody response was evaluated in ELISA (E) and in plaque reduction neutralization assay (N) and expressed as 1/log10 of the final serum dilution (see Materials and Methods for details).
Rh-ig7b was RT-PCR negative for virus before infection and at weeks 2 and 6 after infection and was RT-PCR positive at week 4 after infection.
Cell cultures.
Vero E6 cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) (GIBCO-BRL) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). For virus propagation, DMEM with 2% fetal calf serum (FCS) was used. PBMC were purified from EDTA-treated blood of monkeys on Ficoll-Hypaque and cultivated in 24-well plates in RPMI 1640 with 10% FCS. Human monocytes/macrophages were obtained from PBMC by separation of nonadherent cells in T25 flasks (Falcon) that had been incubated overnight at 37°C in RPMI 1640 with 10% FCS. To stimulate differentiation of monocytic cells into macrophages, adherent cells were cultured in RPMI 1640 with 10% FCS for 6 to 7 days with one or two washes during cultivation. Normal human primary hepatocytes (hNHep cells) were obtained from Clonetics (BioWhittaker, Inc., Walkersville, Md.) and cultivated according to the manufacturer's protocol. HepG2 cells (ATCC HB-8065) derived from human hepatocellular carcinoma were propagated in minimal essential medium with 10% FBS.
Detection of anti-LCMV antibodies.
Antiviral antibodies in the blood of infected animals were detected by enzyme-linked immunosorbent assay (ELISA) and by neutralization assays. Viral antigen for ELISA was prepared from serum-free culture medium of Vero cells. Cells were infected at a multiplicity of infection of 0.01 PFU/ml and cultivated in DMEM with 2% FCS. At 60 h postinfection (p.i.) culture medium was replaced with DMEM without FCS, and virus was harvested at 72 h p.i. Virus-containing culture medium (108 PFU/ml) was diluted 1:1 with carbonate-bicarbonate buffer (pH 9.6) and subjected to three freeze-thaw cycles. One hundred microliters of viral antigen was used to cover wells of microtitration plates and incubated overnight at 4°C. After blocking with 10% FCS in phosphate-buffered saline, 10-fold dilutions of monkey sera were added and incubated for 2 h at room temperature. Peroxidase-labeled goat anti-monkey immunoglobulin G (IgG) (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used at a final dilution of 1:2,000, and substrate solution (Turbo TMB-ELISA; Pierce, Rockford, Ill.) was added for color development. Neutralization antibody titers were measured by plaque reduction neutralization assay with a constant dose of virus, Vero cell monolayers, and serial 1-log-unit dilutions of plasma. Incubation of virus with serum was performed at 37°C for 1 h. As a control, serum collected before infection was used. End points were calculated from the highest serum dilution inducing 50% plaque reduction.
Cytokine ELISA.
TNF-α, IL-1β, and IL-6 in plasma of rhesus macaques or in culture medium of infected cells were measured by ELISA with OptEIA sets from PharMingen (San Diego, Calif.). Monkey gamma interferon (IFN-γ), IL-2, IL-4, and IL-10 were measured with ELISA kits from BioSource International (Camarillo, Calif.). sTNFRI, sTNFRII, IL-6R, and gp130 were measured with ELISA kits from R&D Systems (Minneapolis, Minn.). In some cases IL-6 and IL-8 were measured in extracts of liver and spleen. To prepare extracts, frozen samples were homogenized in T-PER extraction reagent with protease inhibitor cocktail according to the manufacturer's protocol (Pierce). After centrifugation at 8,160 × g for 5 min, supernatants were adjusted for protein concentration and used in ELISA. IL-6 and IL-8 in tissue extracts were measured with OptEIA sets from PharMingen. Polyclonal anti-IL-6 and anti-IL-8 human antibodies from R&D Systems were used to neutralize IL-6 and IL-8 activities in tissue extracts in order to control for specificity.
Histological studies and immunohistochemisty.
For light microscopy, tissue samples were fixed in buffered formalin, dehydrated, embedded in paraffin, sliced into 4- to 6-μm sections, placed on Fisher Plus slides (Fisher Scientific, Pittsburgh, Pa.), and stained with hematoxylin and eosin. To detect proliferating cells in liver sections, immunochemical staining for Ki-67 nuclear antigen was performed with the ResGen IHC kit (Invitrogen Co.) according to the manufacturer's protocol with some modifications. Briefly, sections of paraffin-embedded tissues were placed on Fisher Plus slides treated with Histogrip (Zymed Laboratories, Inc., San Francisco, Calif.). After deparaffinization and rehydration, tissue sections were treated with pepsin. Antigen/epitope recovery was performed by boiling in Retrievit-10 solution (InnoGenex, San Ramon, Calif.) for 15 min. Polyclonal rabbit antibody against a carboxy-terminal segment of human Ki-67, a rabbit primary antibody isotype control, and Ki-67 control slides were purchased from Zymed. Anti-Ki-67 antibody was used at a final dilution of 1:50. After incubation with the secondary biotinylated antibody and blocking of endogenous peroxidase (horseradish peroxidase [HPR]) activity, streptavidin-HPR conjugate was added and the signal was developed with diaminobenzidine. All incubations were done at room temperature for 10 to 60 min. Slides were counterstained with hematoxylin before mounting. To determine the fraction of cells staining for Ki-67 (the cell proliferation index), positively stained liver cell nuclei in each field were counted under 40× objective (Zeiss Plan-Neofluar 40×/0.75 lens) and expressed as a percentage of cells per field and as an average of data from 10 fields. Immunostaining for LCMV-WE antigen used biotin-conjugated anti-LCMV-WE IgG. Immune IgG was isolated from 1 ml of plasma from monkey Rh-ig7b by using protein G columns (Pierce). The immune IgG and IgG from nonimmune monkeys were conjugated with EZ-Link Sulfo-NHS-LC-LC-Biotin (Pierce). Before conjugation, immune IgG had an ELISA titer of 7.2 1/log10, and after conjugation it had a titer of 5.5 1/log10. Immune and nonimmune IgG conjugates were adjusted to a protein concentration of 1 mg/ml and used at a final dilution of 1:200. After incubation with IgG-biotin, slides were treated with streptavidin-HPR conjugate and developed with 3-amino-9-ethylcarbazole.
RT-PCR.
For detection of LCMV-WE RNA in the blood, viral RNA was extracted from 140 μl of plasma by using a QIAamp viral RNA minispin protocol (Qiagen GmbH). RNA from cell cultures and tissues was extracted with an RNeasy mini kit (Qiagen) or Trizol (GIBCO-BRL). To extract RNA from paraffin-embedded liver samples, 20-μm sections were deparaffinized and treated with proteinase K, and RNA was extracted with guanidine thiocyanate and phenol-chloroform by using a Paraffin Block RNA isolation kit from Ambion, Inc. (Houston, Tex.). RNA was converted to cDNA with avian myeloblastosis virus reverse transcriptase (RT) and random primers by using a kit from Roche Molecular Biochemicals (Roche Diagnostic Co., Indianapolis, Ind.) and analyzed by using real-time PCR with SYBR green technology as previously described (34, 35). Primers for LCMV-WE glycoprotein and nucleocapsid protein have been described previously (35). To detect IL-6 mRNA in monkey tissues, primers were designed by using Primer Express software (Applied Biosystems, Foster City, Calif.) according to sequence accession no. L26028, generating a 101-bp fragment (primers mkIL-6-483f [5′AGATGCAATAACCACCCCTGAA] and mkIL-6-583r [5′GGATGAGATGCGTCGTCATGT]). Relative IL-6 message levels were determined as described in detail previously (35). Published Macaca mulatta IL-6 primers (61) did not amplify rhesus macaque IL-6 mRNA in our experiments.
Data analysis.
Statistical analyses and graphing were performed with the Origin 6.0 package (Micrococcal Software, Inc., Northampton, Mass.).
RESULTS
LCMV-WE targets hepatocytes and affects liver function in rhesus macaques after i.v. or i.g. inoculation.
Six rhesus macaques were inoculated with a virulent isolate of the LCMV-WE strain. Although four of these animals were described previously (35), we reiterate some of their data for comparison with two additional animals, one of which recovered from the disease. As shown in Table 1, two i.v.-inoculated animals died within 2 weeks after inoculation with doses of 103 or 106 PFU. Before death the animals developed fever, lymphopenia, thrombocytopenia, and petecchial hemorrhaging as described previously (35). Three of four animals given an i.g. dose of 106 or 107 PFU showed no immediate disease signs, but one developed fever and became ill at 21 days after infection. The disease lasted for 2 weeks, followed by recovery to healthy status. In the i.v.-inoculated animals, the highest viral titers were found in liver, blood, and spleen, in that order. Significantly, monkey Rh-iv6 had 108 PFU/g in liver and 107 PFU/ml in blood upon necropsy. In situ hybridizations (35) and immunostaining (Fig. 1B) revealed viral nucleic acids and antigens in hepatocytes. For monkey Rh-ig7b, transient viremia was detected by plaque assay and RT-PCR at week 4 and was not detectable at week 6 and later. Liver biopsy samples taken at week 4 were negative for LCMV antigens by immunohistochemistry but gave strong positive signals after RT-PCR with primers to the LCMV glycoprotein gene (not shown). Among the i.g.-inoculated monkeys, an antibody response was detectable only in the transiently ill monkey Rh-ig7b. In this animal, anti-LCMV antibodies were found at week 4 p.i. and neutralizing antibodies appeared at week 6 p.i. On week 10, anti-LCMV ELISA and neutralizing antibody titers reached 7.2 and 3.1 1/log, respectively, at a time when the animal was well on the way to recovery.
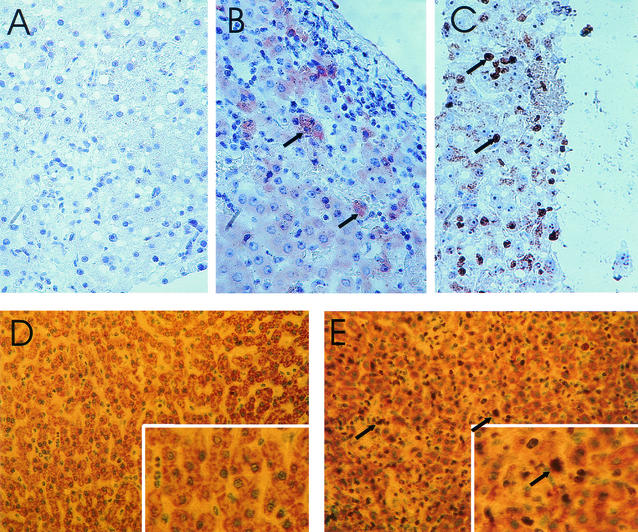
FIG. 1.
Liver sections from rhesus macaques infected with LCMV-WE. (A and B) Immunoperoxidase staining of necropsy samples from healthy monkey Rh-ig6a (A) and lethally infected monkey Rh-iv6 (B). LCMV viral antigen (red) in hepatocytes of Rh-iv6 is indicated with arrows. Magnification, ×200. (C) Biopsy sample, taken 4 weeks after infection, from transiently ill monkey Rh-ig7b during the onset of illness. Brown staining of hepatocyte nuclei (arrows) indicates positivity for proliferation antigen Ki-67. Liver biopsies taken before infection and stained with Ki-67 looked like the picture seen in panel A. Magnification, ×300. (D and E) Ki-67 staining of necropsy liver tissue in healthy monkey Rh-ig6a (D) and in fatally infected monkey Rh-iv6 (E). Note brown staining of Ki-67-positive nuclei (arrows) in panel E. Magnifications, ×100 (main panels) and ×300 (insets).
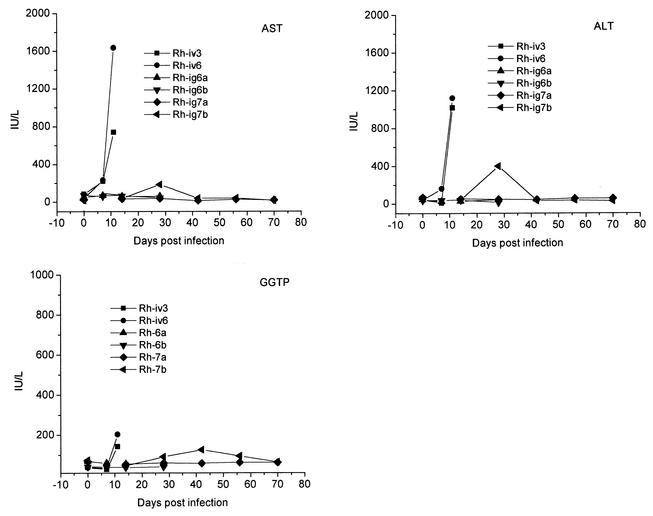
LCMV-WE infection caused significant liver damage, including abnormally high aminotransferase levels in plasma from i.v.-inoculated monkeys (Fig. 2). AST was elevated 3- and 6-fold by day 7 p.i. and increased 9- and 42-fold upon death for Rh-iv3 and Rh-iv6, respectively, indicating that liver dysfunction is an early sign of disease. Alanine aminotransferase (ALT) was 4-fold elevated at day 7 for Rh-iv6 and 23- and 28-fold increased upon death for Rh-iv3 and Rh-iv6. For the i.g.-inoculated monkeys without apparent disease, AST and ALT levels were within the normal ranges. In Rh-ig7b, AST and ALT levels were transiently elevated at week 4 p.i. and later dropped to the normal ranges.
FIG. 2.
Liver enzymes in plasma of LCMV-WE-infected monkeys. Reference ranges are 18 to 82 IU/liter for AST, 22 to 87 IU/liter for ALT, and 28 to 86 IU/liter for GGTP.
Gamma-glutamyltransferase (GGTP) is a very sensitive test for liver damage associated with obstruction of bile ducts. Although none of the LCMV-infected monkeys became jaundiced, GGTP levels were raised 1.5 to 2.5 times in i.v.-inoculated monkeys by the day of death and remained within the normal range for monkeys showing no clinical disease. In Rh-ig7b (ill from week 4 to 6), GGTP was marginally abnormal (90 IU/liter) on week 4, reached 125 IU/liter on week 6, and gradually returned to normal (28 to 86 IU/liter) thereafter (Fig. 2).
Ki-67 staining revealed hepatocyte proliferation in LCMV-WE-infected monkeys.
Despite the substantial liver dysfunction marked by serum transaminase levels, there were few indications of microscopic pathology in livers from LCMV-WE-infected monkeys (35). Mild to moderate multifocal necrosis with minimal inflammatory infiltrate was observed in liver sections from lethally infected animals (35). Only necropsy liver samples were obtained for Rh-iv3, Rh-iv6, Rh-ig6a, and Rh-ig6b, whereas both biopsy and necropsy samples were obtained for Rh-ig7a and Rh-ig7b. Biopsy samples from monkey Rh-ig7a before infection and on week 4 p.i. were not distinguishable after hematoxylin and eosin staining (not shown). Liver biopsy and necropsy samples were also stained for Ki-67 antigen, a nuclear marker of cell proliferation that is more accurate than PCNA protein for determining the fraction of proliferating cells in situ (57). Liver biopsy samples taken from the transiently ill monkey, Rh-ig7b, 4 weeks after infection were positive for the Ki-67 antigen (Fig. 1C). Control biopsy samples taken before infection looked like the sample in Fig. 1A. Necropsy liver samples from fatally infected monkeys were strongly positive for Ki-67 antigen (Fig. 1E), in contrast to samples taken from monkeys without apparent disease (Fig. 1D). Even though all samples were equally treated to block endogenous HRP, liver samples prepared from necropsy tissue routinely had high HPR (yellow) backgrounds in comparison with biopsy samples. Even so, brown-stained Ki-67-positive nuclei are clearly distinguishable from light green, nonstained nuclei (Fig. 1C to E). By counting Ki-67-stained nuclei in multiple fields, we demonstrated that the percentage of positively stained nuclei reached 25 to 40% in biopsy or necropsy samples from infected, diseased monkeys Rh-ig7b, Rh-iv3, and Rh-iv6 and was less than 1% in samples from infected, healthy monkeys Rh-ig6a, Rh-ig6b, and Rh-ig7a.
Cytokine markers of hepatocyte proliferation are elevated in plasma of monkeys infected with LCMV-WE.
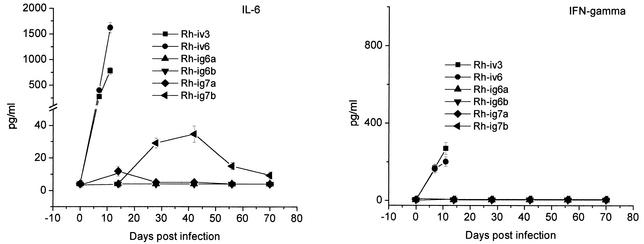
Proinflammatory cytokines are involved in liver regeneration after surgery or toxic injuries (9, 42, 59). In the next experiments we evaluated levels of TNF-α, IL-1β, IL-6, and IFN-γ in rhesus macaques infected with LCMV-WE. In addition, we looked at IL-8 expression in tissues of infected animals, because our previous in vitro studies and data on patients with fatal Lassa fever implicated IL-8 in reducing neutrophil infiltration into the liver and other tissues (34-36, 62). As seen in Fig. 3, IL-1β, TNF-α, IL-6, and IFN-γ were not detected in the plasma of clinically healthy animals Rh-ig6a, and Rh-ig6b. In Rh-ig7a only marginal elevation of IL-6 was detected on day 14 after infection. In fatally infected macaques Rh-iv3 and Rh-iv6 and in recovered monkey Rh-ig7b, levels of IL-1β and TNF-α in plasma were below detection limits, whereas IL-6 was significantly elevated in these animals. On day 7, plasma IL-6 for monkeys Rh-iv3 and Rh-iv6 was 270 to 400 pg/ml, and it increased three- to fourfold by the day of death, reaching more than 1.6 ng/ml for Rh-iv6.
FIG. 3.
IL-6 and IFN-γ in plasma of LCMV-infected monkeys. Monkey plasma samples were assayed in triplicate, and cytokine concentrations were expressed as mean values. The error bars represent one standard deviation from the mean value. The minimum detectable doses of IL-6 and IFN-γ were <5 and 4 pg/ml, respectively. Levels of IL-β and TNF-α were below detectable limits (4 pg/ml) in all samples.
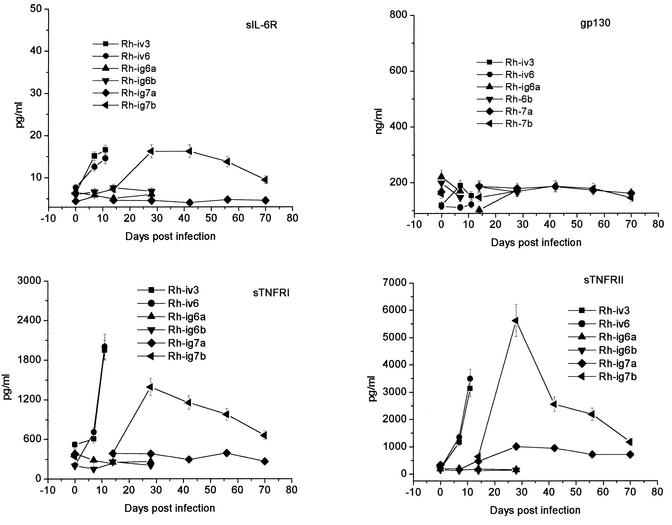
In addition to cytokines, we measured levels of their soluble receptors since those are known to modulate cytokine activity. IL-6 activity is initiated by binding to IL-6R (80 kDa) and formation of a complex with high affinity for the signal transducer, gp130 (130 kDa) (21). For all diseased monkeys, levels of gp130 were not changed, but the levels of sIL-6R after infection increased by 1.5- to 2.5-fold. In contrast to the moderate elevation of sIL-6R levels, sTNFR and sTNFRII levels were substantially elevated in the plasma of diseased macaques. In the plasma of healthy animals, sTNFRI and -II levels varied in ranges of 150 to 400 and 130 to 330 pg/ml, respectively. In Rh-iv3 the plasma TNFRI level was slightly higher (for unknown reasons). Nevertheless, at the day of death, sTNFRI levels were elevated more than three- to fourfold for Rh-iv3 and Rh-iv6. In the recovered monkey Rh-ig7b, sTNFRI peaked on day 28 p.i. (fourfold in comparison with the level before infection) and gradually decreased to twofold by day 70 after infection. The sTNFRII was strongly elevated (6-fold) at day 7 after infection in Rv-iv6 and reached 15-fold on the day of death for both i.v.-inoculated animals (Fig. 4). Interestingly, in monkey Rh-ig7a with no signs of disease (except marginal elevation of IL-6 in plasma on day 14 p.i.), sTNFRII levels were moderately elevated as of day 28 p.i. In monkey Rh-ig7b, sTNFRII was clearly elevated at the early stage of the disease, before any clinical manifestations, and peaked by day 28 after infection, reaching 5.6 ng/ml (19-fold over preinfection levels). By 4 weeks p.i. the level of this receptor gradually dropped. However, even after 10 weeks p.i. it had not come down to preinfection levels.
FIG. 4.
Soluble TNF and IL-6 receptors in plasma of infected monkeys. Monkey plasma samples were assayed in triplicate and expressed as mean values. The minimum detectable doses were 3.0, 6.5, 1.0, and 80 pg/ml for sTNFR1, sTNFRII, sIL-6R, and gp130, respectively. Error bars indicate standard deviations.
IL-6 and IL-8 expression in tissues of infected monkeys.
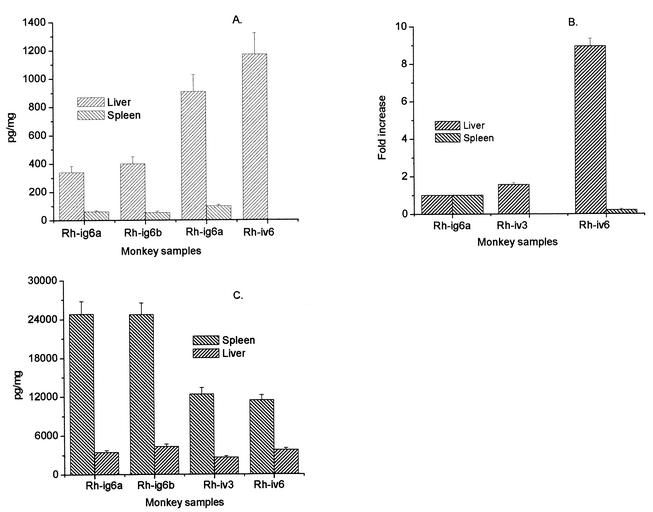
Tissue extracts were prepared from liver and spleen to measure IL-6 and IL-8 concentrations by antigen capture ELISA. In addition, IL-6 mRNA expression in tissues was measured by quantitative PCR. As seen in Fig. 5, IL-6 concentrations in liver were increased by 2.5- to 3.0-fold in Rh-iv3 and Rh-iv6 in comparison with clinically healthy monkeys. Relative levels of IL-6 mRNA in liver were also elevated by 1.5- and 9-fold for Rh-iv3 and Rh-iv6, respectively. In contrast, IL-6 concentrations in spleen did not change significantly, and IL-6 mRNA expression was depressed in spleen tissue from fatally infected Rh-iv6. In clinically healthy monkeys (Rh-ig6a and Rh-ig6b), the IL-8 levels in spleen were 10-fold higher than levels in liver. As seen in Fig. 5C, LCMV-WE infection of rhesus macaques was associated with low concentrations of IL-8 in spleen and liver in comparison with IL-8 levels in the tissues of healthy monkeys. Levels of IL-8 in plasma were very variable, even in healthy monkeys. Before or on the day of infection for Rh-iv3 and Rh-iv6, the level of plasma IL-8 was 650 to 970 pg/ml, and it dropped to 300 to 320 pg/ml on day 7 and 110 to 180 pg/ml on day 11 (day of death). We attempted to measure TNF-α, IL-1β, and lymphotactin in tissues, but since measurements of these cytokines were not blocked by specific antibodies, we did not consider the results specific enough to report.
FIG. 5.
IL-6 and IL-8 in tissues of LCMV-infected monkeys. Pieces of liver and spleen (100 mg) were taken from four anatomically different parts of organs and protein extracts, and RNA samples were prepared as described in Materials and Methods. (A) IL-6 protein per milligram of tissue (liver or spleen). (B) IL-6 mRNA in liver and spleen. cDNAs were amplified with IL-6 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers by using the GeneAmp 5700 System with SYBR Green I chemistry (34). IL-6 CT values were normalized to GAPDH amplicons, and samples from monkey Rh-ig6a were referred to as 1.0. (C) IL-8 in tissue extracts. IL-8 concentrations were measured by ELISA and expressed per milligram of tissue. Error bars indicate standard deviations.
LCMV-WE infection of hepatocytes and monocytes/macrophages in vitro does not induce IL-6 and TNF-α.
Hepatoma-derived cell lines are permissive for arenavirus replication (29). To determine whether LCMV infection of cultured hepatocytes induced IL-6 or TNF-α, we infected HepG2 and primary hNHep cells with LCMV-WE and measured these cytokines in the culture medium by a sensitive ELISA. As expected, LCMV replicated moderately well in hepatocytes. At a multiplicity of infection of ≥1 PFU/cell, the virus titer was 1.0 × 106 to 1.5 × 106 PFU/ml at 24 h p.i. (Table 2). In contrast to our prediction, the infection did not induce IL-6 in HepG2 cells. Maintenance of primary hNHep cells in culture was accompanied by IL-6 production, but the IL-6 production by mock- and LCMV-WE-infected cells was practically identical (Table 2). TNF-α was not detected in culture medium of mock-, WE-, or WE-UV-infected cells, and the in vitro infection did not enhance LPS-inducible IL-6 and TNF-α production in hepatocyte cultures.
TABLE 2.
IL-6 and TNF-α in culture media of LCMV/WE-infected cells
| Cells | Infectiona | Virus titer, 106 PFU/ml | IL-6, pg/ml (mean ± SD) | TNF-α, pg/ml (mean ± SD) |
|---|---|---|---|---|
| HepG2 | Mock | NDc | <4 | <5 |
| LPS | ND | <4 | <5 | |
| WE | 1.1 | <4 | <5 | |
| WE-UV | ND | <4 | <5 | |
| WE + LPS | 1.3 | <4 | <5 | |
| hNHepsb | Mock | ND | 414 ± 39 | <5 |
| WE | 1.2 | 397 ± 56 | <5 | |
| WE-UV | ND | 430 ± 39 | <5 | |
| WE + LPS | 1.5 | 427 ± 49 | <5 | |
| Human MDM | Mock | ND | <4 | <5 |
| LPS | ND | 7,241 ± 340 | 38,080 ± 2,365 | |
| WE | 0.1 | <4 | <5 | |
| WE-UV | ND | <4 | <5 | |
| WE + LPS | 0.08 | 7,086 ± 296 | 40,980 ± 2,963 |
PHA (GIBCO-BRL) and LPS from Escherichia coli 055:B55 (Sigma, St. Louis, Mo.) gave very similar results and were added to maintenance culture medium after virus adsorption to final concentrations of 5 μg/ml and 100 ng/ml, respectively. LCMV-WE was inactivated by UV light (WE-UV) as described in Materials and Methods.
Results with hNHep were very similar to results with human PBMC.
ND, not determined.
Kupffer cells, the intrasinusoidal hepatic macrophages, play an important role in secreting soluble mediators of liver injury and regeneration (9, 16, 42, 43). Therefore, we investigated whether LCMV infection of monocyte-derived macrophages (MDM) in vitro may induce IL-6 and TNF-α. As seen in Table 2, LCMV replicated poorly in MDM and did not induce inflammatory cytokines. This result resembled our previous results from Lassa virus-MDM infection (34). Also, LCMV infection did not affect lipopolysaccharide (LPS)-inducible IL-6 or TNF-α responses in MDM.
Cultured PBMC from LCMV-infected monkeys are highly inducible for IL-6 production.
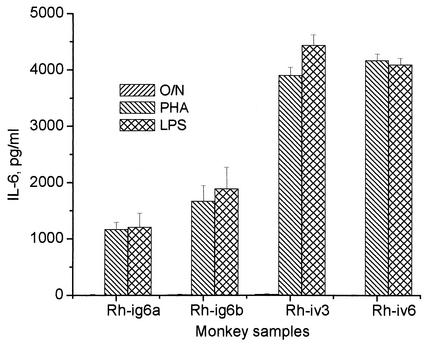
Circulating mononuclear cells are another possible source of IL-6. PBMC were isolated from both acute and asymptomatic monkeys and cultivated with phytohemagglutinin (PHA) or LPS. Stimulated PBMC from all monkeys produced high levels of Th1 cytokines IFN-γ and IL-2 but not IL-4 and IL-10 (not shown). A difference was observed between the two groups of animals with respect to IL-6 production: LPS or PHA treatment of PBMC from Rh-iv3 and Rh-iv6 induced higher levels of IL-6 than did treatment of PBMC from healthy animals (Fig. 6).
FIG. 6.
IL-6 in culture medium of stimulated PBMC. PBMC from LCMV-infected monkeys were plated on 12-well plates (106 cells/well) and incubated overnight with PHA (5 μg/ml), with LPS (100 ng/ml), or without stimulants (O/N). Results with IL-6 in culture medium were obtained by ELISA. Error bars indicate standard deviations.
DISCUSSION
Virus infections causing acute liver failure can be separated into two groups: (i) primary hepatitis (e.g., hepatitis A and B virus infection) and (ii) hepatitis occurring as part of systemic infection (e.g., yellow fever virus, adenovirus, and cytomegalovirus infection). Viral infections causing primary hepatitis usually result in clinical jaundice, accompanied by striking abnormalities of liver enzymes due to massive hepatic necrosis. Postmortem histological studies of Lassa HF patients and Lassa virus-infected rhesus macaques indicated that the liver is one of the most affected organs participating in a systemic breakdown. Jaundice is rarely observed even in fatal Lassa fever cases, and the extent of hepatic necrosis is not consistent with hepatic failure being the cause of death (17, 22, 40, 48, 49, 62). In contrast, jaundice was a consistent finding in callitrichid hepatitis, where hepatocellular swelling and extensive degeneration, necrosis, and inflammation of the liver were observed (3, 44, 45).
In this study we demonstrate that (i) LCMV-WE delivered i.v. or i.g. can cause hepatitis, (ii) infection induces a strong proliferative response in liver, (iii) up-regulation of IL-6/sIL-6R and sTNFRI and -II resembles the cytokine pattern seen after a partial hepatectomy, and (iv) in vitro infection of hepatocytes with LCMV-WE did not induce IL-6 and TNF-α, suggesting that the hepatocyte activation signals come from other sources, e.g., intrahepatic Kupffer cells or PBMC. Taken together, these data suggest that rhesus macaques can be a valuable model to study liver pathogenesis in Lassa fever. On the other hand, guinea pigs are more appropriate model in studying lung pathology and pulmonary edema (17, 22, 48).
Rhesus macaques i.v. inoculated with LCMV-WE rapidly develop fatal infection with Lassa fever-like manifestations (22, 35, 48). Only one of four monkeys inoculated by the i.g. route developed disease and viremia. The diseased monkey, Rh-ig7b, was unique in developing neutralizing antibodies that possibly contributed to its full recovery. The i.g. dose that this animal received (107 PFU) was within the range of the lethal inoculum ingested by captive South American marmosets and tamarins in an outbreak of callitrichid hepatitis (45). They were given a single feeding of neonatal mice, each of which can harbor virus titers of up to 108 PFU/g of liver. In our previous experimental studies with LCMV-inoculated mice, mucosal inoculations required higher doses than i.v. inoculations to achieve the same levels of viremia and immunity (52, 53). In our primate studies, i.g. inoculation with high-dose LCMV-WE usually leads to subclinical infection, occasionally leads to disease, and rarely leads to death (I. S. Lukashevich et al., unpublished data). Taken together with the studies implicating consumption of rodents as a risk factor for infection, it is reasonable to propose that the mucosal route (via airways or the gastrointestinal tract) is a natural mode of virus transmission that could account for high levels of seropositive survivors in areas where Lassa virus is endemic (32, 33, 60).
Lassa virus in humans and in rhesus macaques elevates hepatic aminotransferase levels that seem to correlate with liver failure. An AST level of ≥150 IU/liter upon admission was strongly associated with fatal outcome in Lassa HF. A high AST/ALT ration (>10) is another well-documented phenomenon in Lassa HF patients, indicating that AST is not totally hepatic in origin (17, 18, 40, 41). In our experiments with i.v.- and i.g.-inoculated rhesus macaques, LCMV-WE disease elevated both AST and ALT enzymes similarly, suggesting that more hepatic damage is observed in LCMV-infected monkeys than is normally seen in Lassa HF. The monkey that recovered from disease, Rh-ig7b, had delayed onset and transiently elevated aminotransferases during the peak of disease. Interestingly, an animal that experienced no disease, Rh-ig6a, had a transiently elevated plasma AST level on the seventh day after infection without viremia. Another measure of liver disease, GGTP, which was not mentioned in publications about Lassa fever, showed moderate elevation in the LCMV-infected monkeys during fulminant disease. In contrast, callitrichid hepatitis in New World monkeys was associated with extremely high levels of GGTP, high plasma bilirubin, and consistent jaundice (3, 44, 45, 58).
An elevated plasma TNF receptor (sTNFRII) level is an early and sensitive disease marker in infected monkeys. It is not clear whether elevated levels of sTNFR reflect overexpression or enhanced shedding of these receptors or both. In hepatitis C virus patients, levels of sTNFR do not correlate with TNF-α but do correlate well with aminotransferase levels (68). Mahanty et al. (36) found that the levels of soluble TNF receptors were significantly higher in patients with fatal Lassa fever than in those with less severe disease. A possible explanation is that IFN-γ can up-regulate both sTNFRI and -II (1); however, in monkey Rh-7b we detected significant concentrations of plasma sTNFR without detecting IFN-γ.
Histopathological changes in livers of LCMV-WE-infected monkeys were minimal, although aminotransferase levels indicated substantial injury (reference 35 and this study). Patients with severe Lassa HF also have high AST in plasma and only minor, noninflammatory, alterations in liver (40). It may be that we are observing arenavirus-mediated altering of cellular functions without affecting morphology or cell death, a well-known phenomenon in the LCMV-mouse model (46, 47). It may also be that we are observing the results of apoptosis, as described for Pirital virus, a New World arenavirus that induces a fatal HF in hamsters (64). In hamsters, liver histopathological changes varied from single-cell death by apoptosis to coagulative necrosis of hepatocytes. Acidophilic bodies resembling apoptotic (Councilman-like) inclusions were also observed in monkeys that died from callitrichid hepatitis, indicating the possible involvement of apoptosis in that disease (3). Apoptotic cell death attracts fewer inflammatory infiltrates than necrotic cell death (63) and may account for the lack of infiltrates seen in the LCMV-WE model for hemorrhagic fever.
One goal in initiating this research was to pursue the hypothesis that virulent infection suppresses the inflammatory response, thereby eliminating host defense mechanisms. We showed that Lassa virus, and not its nonvirulent relative Mopeia virus, could suppress IL-8 and TNF-α production in cell culture (34). This finding was extended and corroborated by studies of Lassa fever patients in which fatal infections had lower levels of IL-8 and a related CXC chemokine, IP-10, than nonfatal infections (36). In this study we observed decreased levels of IL-8 in plasma and spleen when comparing healthy, infected monkeys with diseased monkeys. We found high concentrations of soluble TNF receptors in plasma of diseased monkeys, possibly serving to bind and counteract soluble TNF. The proinflammatory cytokines IL-1β and TNF-α were not detectable in plasma of LCMV-infected monkeys, and IFN-γ levels were moderately elevated in lethally infected animals. Similarly, Mahanty et al., (36) did not see elevated TNF-α levels in sera of patients with fatal or nonfatal Lassa HF. Although we found some evidence of reduced inflammatory responses in the monkey model, the chain of events leading to this state and its contribution to pathogenesis remain unclear.
McCormick et al. (40) made the first observation that liver cell proliferation was characteristic of Lassa HF. We observed substantial hepatocyte proliferation in rhesus macaques inoculated both i.v. and i.g. with LCMV. During peak disease, at least 25 to 40% of hepatocyte nuclei in liver biopsy or necropsy samples stained positively for proliferation antigen Ki-67 (Fig. 1). Biopsy samples from the monkey that recovered, Rh-ig7b, were positive for Ki-67 but negative for viral antigen, suggesting that hepatocyte activation can be detected before viral antigens are detectable. In addition to cell proliferation, we observed cytokine profiles typical of those described for initiation of liver regeneration following surgical or toxic injury (16). A large body of evidence, primarily from murine studies, indicates that IL-6, IL-6R, and TNF-R1 are released after liver injury and are essential for regeneration. IL-6 knockout mice fail to regenerate after surgical or toxic liver injury but can recover after treatment with recombinant IL-6 (2, 8, 26-28, 65, 66). sIL-6R enhances the mitogenic effects of IL-6 on hepatocytes (37, 50, 51), and treatment of hepatechtomized mice with IL-6/sIL6R fusion protein, but not with IL-6 alone, accelerates liver regeneration (51). The IL-6 binds to sIL-6R circulating in blood, leading to signal transduction via gp130, bearing all the information required for activation of the intracellular Jak-STAT pathway (21). sIL-6R shows agonistic activity for IL-6, and the soluble form of gp130 has an antagonistic effect on the IL-6/sIL-6R complex (21). We have shown that IL-6 expression was up-regulated in livers of LCMV-WE-infected monkeys and that levels of IL-6 and sIL-6R were elevated in the circulating blood of infected rhesus macaques. In contrast, plasma gp130, serving as a ubiquitous signal transducer for IL-6 and other cytokines, was in the normal range in all monkeys.
The mechanisms up-regulating IL-6 after liver injury are not clearly understood. It has been hypothesized that resident macrophages, or Kupffer cells, are sources of IL-6 and other cytokines involved in liver regeneration. Exposure of Kupffer cells to gut-derived bacterial products (e.g., LPS) can induce cytokines (e.g., TNF-α) involved in liver injury and repair. TNF-α signaling through TNFRI is responsible for NF-κB and STAT3 activation leading to IL-6 overexpression (16, 25, 59, 66). Replacement of Kupffer cells in IL-6−/− mice with IL-6+/+ bone marrow successfully restored STAT3 activation and hepatocyte DNA replication after hepatectomy (2). Depletion of Kupffer cells significantly delayed liver regeneration and abolished the hepatic IL-6 mRNA synthesis in hepatechtomized rats (42). Finally, in germfree, athymic, and LPS-resistant mice, liver regeneration was significantly depressed (7). Although the involvement of Kupffer cells and TNFRI has been described, there are cytokine-independent pathways of liver regeneration as well (16, 43). We showed that LCMV-WE infection of primary hepatocytes and HepG2 cells did not induce IL-6 or TNF-α expression and that cell culture infection did not stimulate LPS-inducible expression of these cytokines. Human and monkey monocytes/macrophages cultured in vitro and infected with LCMV-WE also did not induce IL-6 and TNF-α, and, similar to the case for hepatocytes, their infection did not potentiate LPS-inducible IL-6 or TNF-α. We showed previously that Lassa virus down-regulated LPS-stimulated TNF-α mRNA synthesis in human monocytes/macrophages (34), so the failure of LCMV-WE to induce TNF-α was expected. In a surprising new development, we found that stimulation of PBMC from lethally infected monkeys (Rh-iv3 and Rh-iv-6) induced a higher IL-6 response than stimulation of PBMC from healthy animals. This suggests that PBMC from LCMV-infected animals are more sensitive to stimulation.
Although virus infection initiates a program of liver regeneration, it may interfere downstream with the repair process. After initiation, the next stage of liver regeneration involves induction of growth factors (hepatocyte growth factor and transforming growth factor α) with cyclin D1 as an indicator of proliferation (16). We already know that LCMV expression can down-regulate cell cycle factors and differentiated cell functions (6, 46, 47) that could be involved in repair. It will be important in the future to note the effects of virus on regenerative processes, since blocks to repair are likely to be key sources of damage in HF.
Acknowledgments
This work was supported by grant RO1-RR13980 (to I.S.L.) from the National Institutes of Health and by start-up funds to M. Salvato from the Institute of Human Virology.
We are grateful to C. David Pauza for reading and discussing the manuscript.
REFERENCES
- 1.Aggarwal, B. B., T. E. Eessalu, and P. E. Hass. 1985. Characterization of receptors for human tumor necrosis factor and their regulation by gamma-interferon. Nature 318:665-667. [DOI] [PubMed] [Google Scholar]
- 2.Aldeguer, X., F. Debonera, A. Shaked, A. M. Krasinkas, A. E. Gelman, X. Que, G. A. Zamir, S. Hiroyasu, K. K. Kovalovich, R. Taub, and K. M. Olthoff. 2000. Interleukin-6 from intrahepatic cells of bone marrow origin is required for normal murine liver regeneration. Hepatology 35:40-48. [DOI] [PubMed] [Google Scholar]
- 3.Asper, M., P. Hofmann, C. Osmann, J. Funk, C. Metzger, M. Bruns, F.-J. Kaup, H. Schmitz, and S. Gunther. 2001. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology 284:203-213. [DOI] [PubMed] [Google Scholar]
- 4.Barton, L. L., and M. B. Mets. 2001. Congenital lymphocytic choriomeningitis virus infection: decade of rediscovery. Clin. Infect. Dis. 33:370-374. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla, W. V., D. D. Pinschewer, P. Klenerman, V. Rousson, M. Gaboli, P. P. Pandolfi, R. M. Zinkernagel, M. S. Salvato, and H. Hengartner. 2001. A critical role for PML in control of RNA virus infections in vivo. J. Virol. 76:3810-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell Dwyer, E. J., H. K. Lai, R. C. MacDonald, M. S. Salvato, and K. L. B. Borden. 1999. The lymphocytic choriomeningitis virus RING protein Z binds to eukaryotic initiation factor 4E and represses translation in a RING-dependent manner. J. Virol 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornell, R., B. L. Liljequist, and K. Bartizal. 1990. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology 11:916-922. [DOI] [PubMed] [Google Scholar]
- 8.Cressman, D. E., L. E. Greenbaum, R. A. DeAngelis, G. Ciliberto, E. E. Furth, V. Poli, and R. Traub. 1996. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379-1383. [DOI] [PubMed] [Google Scholar]
- 9.Diel, A. M., and R. Rai. 1996. Regulation of liver regeneration by pro-inflammatory cytokines. J. Gastoenterol. Hepatol. 11:466-479. [DOI] [PubMed] [Google Scholar]
- 10.Djavani, M., I. S. Lukashevich, and M. S. Salvato. 1998. Sequence comparison of the large genomic RNA segments of two strains of lymphocytic choriomeningitis virus differing in pathogenic potential for guinea pigs. Virus Genes 17:151-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty, P. C., and R. M. Zinkernagel. 1974. T-cell mediated immunopathology in viral infections. Transplant. Rev. 19:89-120. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, M. V., and M. B. A. Oldstone. 1987. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J. Immunol. 121:1262-1269. [PubMed] [Google Scholar]
- 13.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variations among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689-1698. [DOI] [PubMed] [Google Scholar]
- 14.Edington, G., and H. White. 1972. The pathology of Lassa fever. Trans. R. Soc. Med. Hyg. 66:381-389. [DOI] [PubMed] [Google Scholar]
- 15.Elsner, B., E. R. Schwarz, O. G. Mando, J. I. Maiztegui, and A. Vilches. 1973. Pathology of 12 fatal cases of Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 22:229-236. [PubMed] [Google Scholar]
- 16.Fausto, N. 1999. Lessons from genetically engineered animal models. V. Knocking out genes to study liver regeneration: present and future. Am. J. Physiol. 277:G917-G921. [DOI] [PubMed]
- 17.Fisher-Hoch, S. P. 1993. Arenavirus pathophysiology, p. 299-323. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
- 18.Fisher-Hoch, S. P., and J. B. McCormick. 1987. Pathophysiology and treatment of Lassa fever. Curr. Top. Microbiol. Immunol. 134:231-239. [DOI] [PubMed] [Google Scholar]
- 19.Gilden, D. H., G. A. Cole, A. A. Monjan, and N. Nathanson. 1972. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. I. Cyclophosphamide-mediated induction of virus-carrier state in adult mice. J. Exp. Med. 135:860-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinds, P. M., C. Yin, M. S. Salvato, and C. D. Pauza. 1997. Pertussis toxin-induced leukocytosis. J. Med. Primatol. 25:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Inhle, J. N. 1996. STATs: signal transducers and activators of transcription. Cell 84:331-334. [DOI] [PubMed] [Google Scholar]
- 22.Jahrling, P. B., R. A. Hesse, G. A. Eddy, K. M. Johnson, R. T. Callis, and E. L. Stephen. 1980. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 141:580-589. [DOI] [PubMed] [Google Scholar]
- 23.Jahrling, P. B., J. D. Frame, S. B. Smith, and M. H. Monson. 1985. Endemic Lassa fever in Liberia. III. Characterization of Lassa virus isolates. Trans. R. Soc. Trop. Med. Hyg. 79:374-379. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, K. M., J. B. McCormick, P. A. Webb, E. S. Smith, L. H. Ellliot, and I. J. King. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:134-145. [DOI] [PubMed] [Google Scholar]
- 25.Kirillova, I., M. Chaisson, and N. Fausto. 1999. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor κB activation. Cell Growth Differ. 10:819-828. [PubMed] [Google Scholar]
- 26.Kovalovich, K., R. DeAngelis, W. Li, E. Furth, G. Gilberto, and R. Taub. 2000. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology 31:149-159. [DOI] [PubMed] [Google Scholar]
- 27.Li, W., X. Liang, J. I. Leu, K. Kovalovich, G. Ciliberto, and R. Taub. 2001. Global changes in interleukin-6-dependent gene expression patterns in mouse livers after partial hepatectomy. Hepatology 33:1377-1386. [DOI] [PubMed] [Google Scholar]
- 28.Lucia, H. L., D. H. Coppenhaver, R. L. Harrison, and S. Baron. 1990. The effect of an arenavirus infection on liver morphology and function. Am. J. Trop. Med. Hyg. 43:93-98. [DOI] [PubMed] [Google Scholar]
- 29.Lukashevich, I. S., R. F. Maryankova, and F. M. Fidarov. 1983. Reproduction of Lassa virus in different cell cultures. Acta Virol. 27:282-285. [PubMed] [Google Scholar]
- 30.Lukashevich, I. S., A. D. Vasiuchkov, T. A. Stel'makh, E. P. Scheslenok, and A. G. Shabanov. 1991. The isolation and characteristics of reassortants between the Lassa and Mopeia arenaviruses. Vopr. Virusol. 36:146-150. [PubMed] [Google Scholar]
- 31.Lukashevich, I. S. 1992. Generation of reassortants between African arenaviruses. Virology 188:600-605. [DOI] [PubMed] [Google Scholar]
- 32.Lukashevich, I. S., V. D. Lukashevich, A. S. Vladyko, M. S. Diallo, M. A. Ba, and A. Silla. 1993. Serological evidence of Lassa virus circulation in the Republic of Guinea. Vopr. Virusol. 38:24-28. [PubMed] [Google Scholar]
- 33.Lukashevich, I. S., J. C. Clegg, and K. Sidibe. 1993. Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J. Med. Virol. 40:210-217. [DOI] [PubMed] [Google Scholar]
- 34.Lukashevich, I. S., R. Maryankova, A. S. Vladyko, N. Nashkevich, S. Koleda, M. Djavani, D. Horejsh, N. Voitenok, and M. Salvato. 1999. Lassa and Mopeia replication in human monocyte/macrophages and in endothelial cells: different effects on IL-8 and TNF-α gene expression. J. Med. Virol. 59:552-560. [PMC free article] [PubMed] [Google Scholar]
- 35.Lukashevich, I. S., M. Djavani, J. D. Rodas, J. C. Zapata, A. Usborne, C. Emerson, J. Mitchen, P. B. Jahrling, and M. S. Salvato. 2002. Hemorrhagic fever occurs after intravenous, but not after intragastric, inoculation of rhesus macaques with lymphocytic choriomeningitis virus. J. Med. Virol. 67:171-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahanty, S., D. G. Bausch, R. L. Thomas, A. Goba, A. Bah, C. J. Peters, and P. E. Rollin. 2001. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J. Infect. Dis. 183:1713-1721. [DOI] [PubMed] [Google Scholar]
- 37.Maione, D., E. Di Carlo, W. Li, P. Musiani, A. Modesti, M. Peters, S. Rose-John, C. Della Rocca, M. Tripodi, D. Lazzaro, R. Taub, R. Savino, and G. Ciliberto. 1998. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 17:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Med. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 39.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Crave, and K. M. Johnson. 1986. Lassa fever: effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 40.McCormick, J. B., D. H. Walker, I. J. King, P. A. Webb, L. H. Elliott, S. G. Whitfield, and K. M. Johnson. 1986. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am. J. Trop. Med. Hyg. 35:401-407. [DOI] [PubMed] [Google Scholar]
- 41.McCormick, J. B., P. A. Webb, J. V. Krebs, K. M. Johnson, and E. S. Smith. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437-444. [DOI] [PubMed] [Google Scholar]
- 42.Meijer, C., M. J. Wiezer, A. M. Diehl, H. J. Schouten, H. J. Schouten, S. Meijer, N. van Rooijen, A. A. van Lambalgen, C. D. Dijkstra, and P. A. van Leeuwen. 2000. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 20:66-77. [DOI] [PubMed] [Google Scholar]
- 43.Michalopoulos, G. K., and M. C. DeFrances. 1997. Liver regeneration. Science 276:60-66. [DOI] [PubMed] [Google Scholar]
- 44.Montali, R. J., B. M. Connolly, D. L. Armstrong, C. A. Scanga, and K. V. Holmes. 1995. Pathology and immunohistochemistry of callitrichid hepatitis, an emerging disease of captive New World primates caused by lymphocytic choriomeningitis virus. Am. J. Pathol. 147:1441-1449. [PMC free article] [PubMed] [Google Scholar]
- 45.Montali, R. J., C. A. Scanga, D. Pernikoff, D. R. Wessner, R. Ward, and K. V. Holmes. 1993. A common-source outbreak of callitrichid hepatitis in captive tamarins and marmosets. J. Infect. Dis. 167:946-950. [DOI] [PubMed] [Google Scholar]
- 46.Oldstone, M. B., M. Rodriguez, W. H., Daughaday, and P. W. Lampert. 1984. Viral perturbation of endocrine function: disordered cell function leads to disturbed homeostasis and disease. Nature 307:278-281. [DOI] [PubMed] [Google Scholar]
- 47.Oldstone, M. B., R. Ahmed, M. J. Buchmeier, P. Blount, and A. Tishon. 1985. Perturbation of differentiated functions during viral infection in vivo. I. Relationship of lymphocytic choriomeningitis virus and host strains to growth hormone deficiency. Virology 142:158-174. [DOI] [PubMed] [Google Scholar]
- 48.Peters, C. J., P. B. Jahrling, C. T. Lui, R. H. Kenyon, K. T. McKee, and J. G. Barrera Oro. 1987. Experimental studies of arenaviral hemorrhagic fevers. Curr. Top. Microbiol. Immunol. 134:5-68. [DOI] [PubMed] [Google Scholar]
- 49.Peters, C. J. 1995. Arenavirus diseases, p. 227-246. In J. Portfield (ed.), Exotic viral infections. Chapman & Hall Medical, London, United Kingdom.
- 50.Peters, M., G. Blinn, T. Jostock, P. Schirmacher, K. H. Meyer zum Buschenfelde, P. R. Galle, and S. Rose-John. 2000. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology 119:1663-1671. [DOI] [PubMed] [Google Scholar]
- 51.Peters, M., F. Solem, J. Goldschmidt, P. Schirmacher, and S. Rose-John. 2001. Interleukin-6 and the soluble interleukin-6 receptor induce stem cell factor and Flt-3L expression in vivo and in vitro. Exp. Hematol. 29:146-155. [DOI] [PubMed] [Google Scholar]
- 52.Rai, S. K., M. Wu, D. Cheung, T. Warner, and M. S. Salvato. 1996. Murine infection with lymphocytic choriomeningitis virus following gastric inoculation. J. Virol. 70:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rai, S. K., B. K. Micales, M. S. Wu, D. S. Cheung, T. D. Pugh, G. E. Lyons, and M. S. Salvato. 1997. Timed appearance of lymphocytic choriomeningitis virus after gastric inoculation in mice. Am. J. Pathol. 151:633-639. [PMC free article] [PubMed] [Google Scholar]
- 54.Riviere, Y., R. Ahmed, P. J. Southern, M. J. Buchmeier, and M. B. A. Oldstone. 1985. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J. Virol. 55:704-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvato, M. S., and I. S. Lukashevich. 2001. Arenavirus, p. 165-173. In C. A. Tidona and G. Darai (ed.), The Springer index of viruses. Springer, Berlin, Germany
- 56.Salvato, M. S., and S. K. Rai. 1998. Arenaviruses, p. 629-650. In B. Mahy and L. Collier (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed. Arnold Publishing, London, United Kingdom.
- 57.Scholzen, T., E. Endl, C. Wohlenberg, S. van der Sar, I. G. Cowell, J. Gerdes, and P. B. Singh. 2002. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: a potential role in the regulation of higher-order chromatin structure. J. Pathol. 196:135-144. [DOI] [PubMed] [Google Scholar]
- 58.Stephensen, C. B., J. Y. Park, and S. R. Blount. 1995. cDNA sequence analysis confirms that the etiologic agent of callitrichid hepatitis is lymphocytic choriomeningitis virus. J. Virol. 69:1349-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streetz, K. L., T. Wustefeld, C. Klein, M. P. Manns, and C. Trautwein. 2001. Mediators of inflammation and acute phase response in the liver. Cell. Mol. Biol. 47:661-673. [PubMed] [Google Scholar]
- 60.ter Meulen, J., I. S. Lukashevich, K. Sidibe, A. P. Inapogui, M. Marx, A. Dorlemann, M. L. Yansane, K. Koulemou, J. Chang-Claude, and H. Schmitz. 1996. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 55:661-666. [DOI] [PubMed] [Google Scholar]
- 61.Villinger, F., S. S. Brar, A. Mayne, N. Chikkala, and A. A. Ansari. 1995. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J. Immunol. 155:3946-3954. [PubMed] [Google Scholar]
- 62.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]
- 63.Wyllie, A. H., and Morris, R. G. 1982. Hormone induced cell death. Purification and properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am. J. Pathol. 109:78-87. [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao, S.-Y., H. Zhang, Y. Yang, and R. B. Tesh. 2001. Pirital virus (Arenaviridae) infection in the syrian golden hamster, Mesocricetus Auratus: a new animal model for arenaviral hemorrhagic fevers. Am. J. Trop. Med. Hyg. 64:111-118. [DOI] [PubMed] [Google Scholar]
- 65.Yamada, Y., I. Kirillova, J. J. Peschon, and N. Fausto. 1997. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA 94:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamada, Y., E. M. Webber, I. Kirillova, J. J. Peschon, and N. Fausto. 1998. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology 28:959-970. [DOI] [PubMed] [Google Scholar]
- 67.Zinkernagel, R. M., and P. C. Doherty. 1974. Restriction of in vitro T cell-mediated cytotoxicity in LCMV with syngenic or semiallogenic system. Nature 248:701-702. [DOI] [PubMed] [Google Scholar]
- 68.Zylberberg, H., A. C. Rimaniol, S. Pol, A. Masson, D. De Groote, P. Berthelot, J. F. Bach, C. Brechot, and F. Zavala. 1999. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J. Hepatol. 30:185-191. [DOI] [PubMed] [Google Scholar]