Abstract
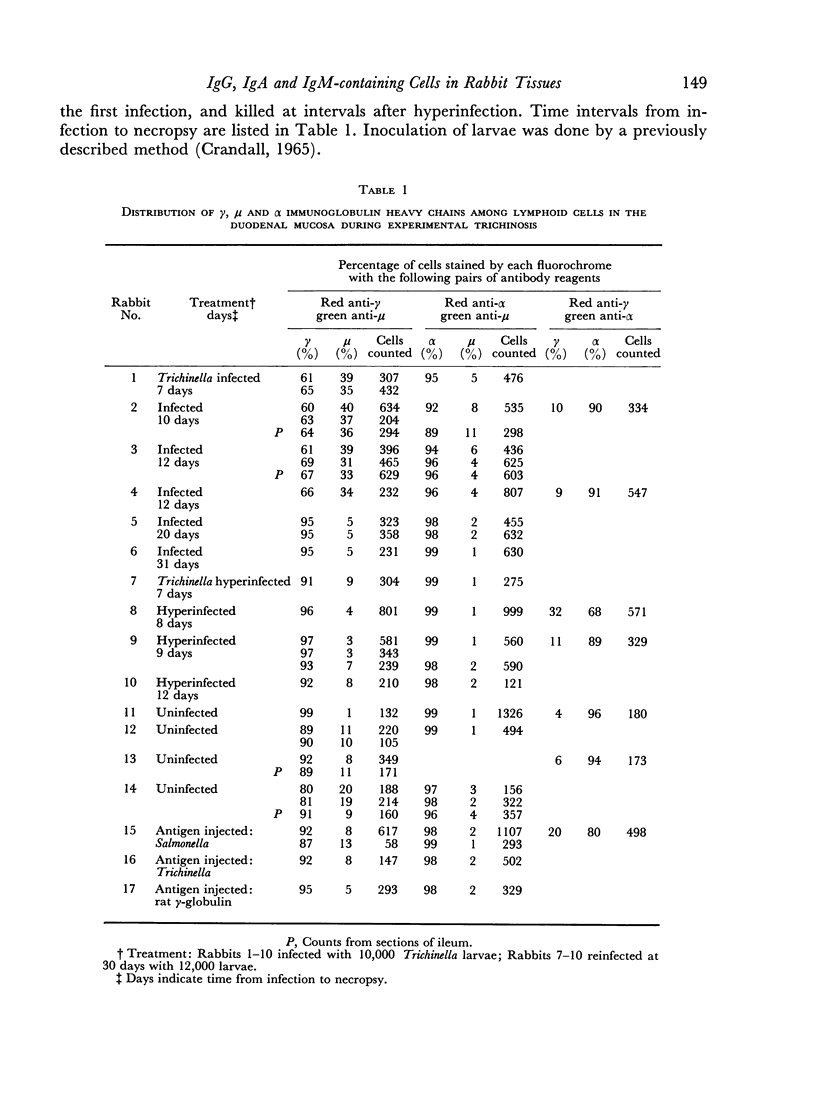
The relative proportions of IgG, IgM and IgA immunoglobulin-containing cells were determined in the intestinal mucosa, spleen, popliteal lymph nodes and diaphragm of rabbits after a single infection and after hyperinfection with Trichinella spiralis. Staining with pairs of immunofluorescent reagents, specifically reactive with γ, μ or α immunoglobulin heavy chains and labelled with contrasting fluorochromes, permitted direct counting of cells containing two different immunoglobulin classes in a single tissue section. By employing two different pairs of reagents on adjacent sections the relative numbers of cells containing IgG, IgM and IgA were calculated.
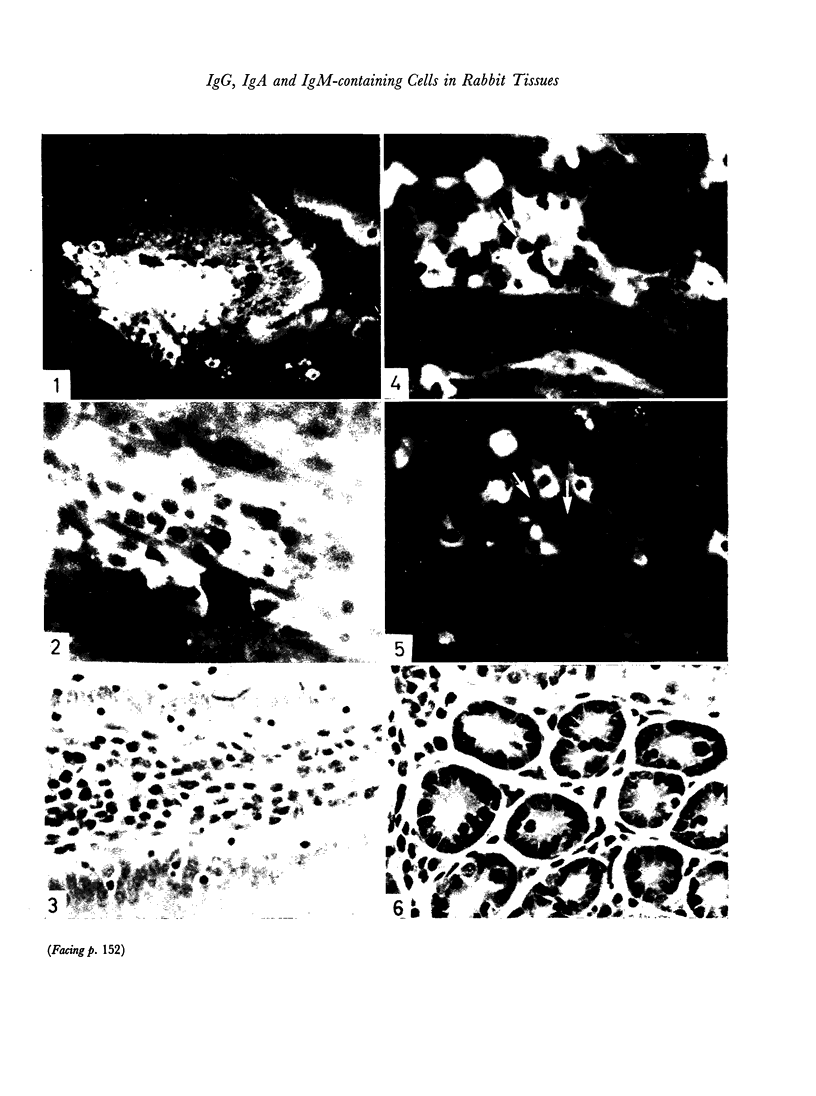
The observed cellular distribution of γ, μ and α heavy chains in the rabbit intestinal mucosa corresponded with the reported distribution in the human intestine. A relative increase in IgM-containing cells in the mucosa was observed after early infection with Trichinella, followed by an apparent increase in cells with IgG late in infection and after hyperinfection. The proportion of cells staining for IgA remained uniformly high in the intestine throughout the course of infection.
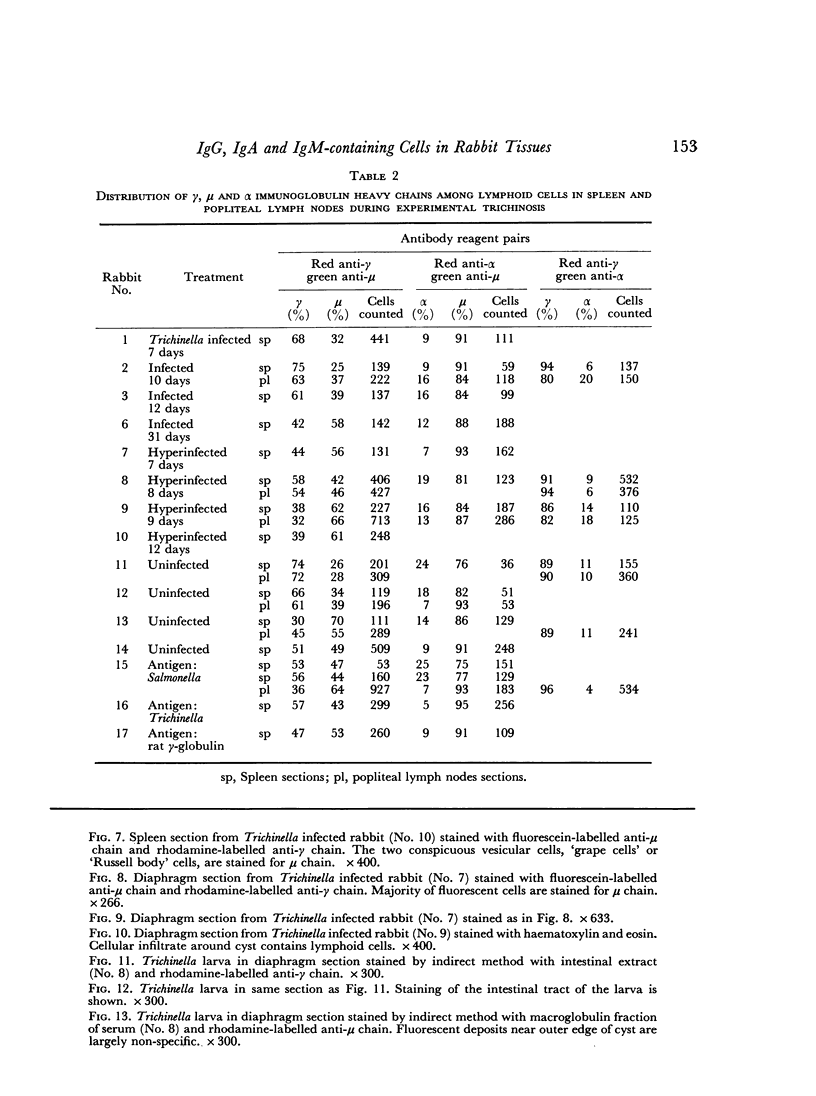
The proportions of cells containing different immunoglobulin classes in the spleen contrasted with those observed in the intestinal mucosa, particularly with respect to cells containing α chain. IgA cells made up 2–10 per cent of the immunoglobulin-containing cells in the spleen as compared to 80–90 per cent in the intestinal mucosa. Most spleen sections showed an increase in IgM cells late in infection with Trichinella and after hyperinfection. The proportions of immunoglobulin-containing cells in the popliteal lymph nodes generally paralleled those observed in the spleen.
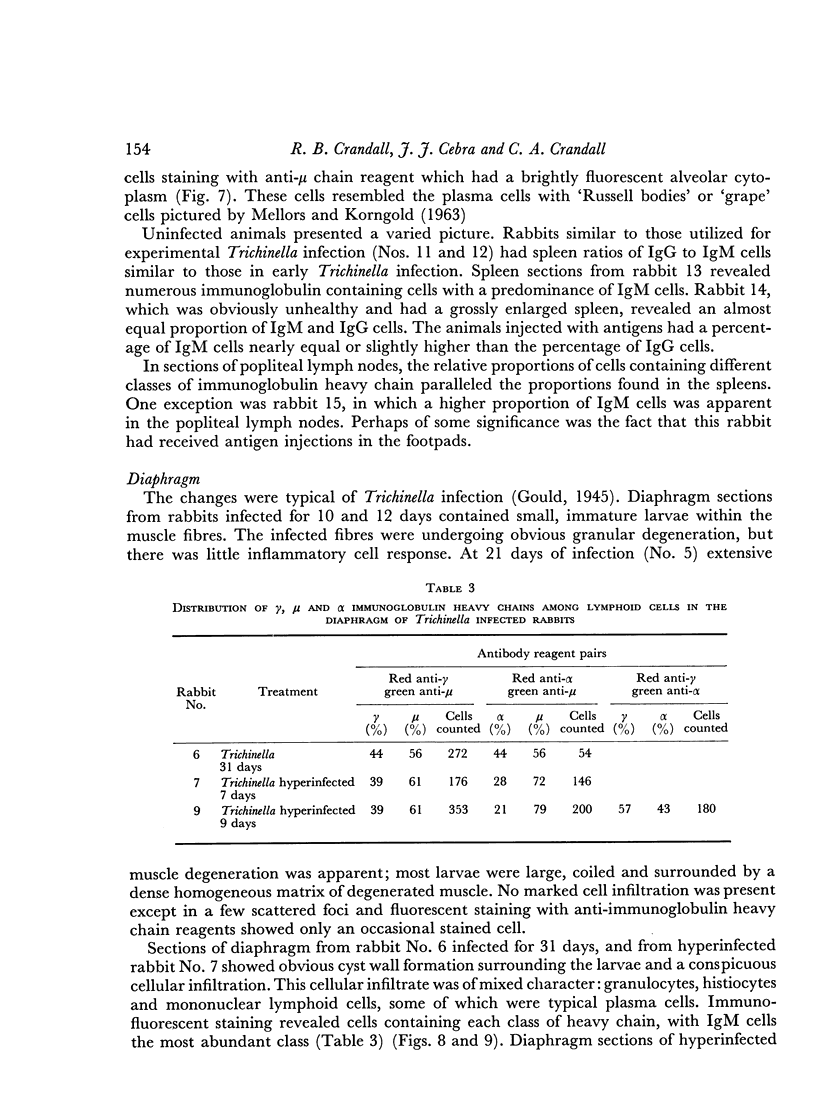
Local cellular infiltration of the diaphragm occurred at the time of larval encystment. Immunoglobulin-containing cells were often prominent and the cellular distribution of immunoglobulin classes resembled that found in the spleen.
The indirect fluorescent antibody technique was employed to detect anti-Trichinella antibody of the three immunoglobulin classes in sera and extracts of the gut of hyperinfected rabbits. Only IgG antibody was detected in gut extracts although both IgG and IgA were demonstrated to be present by Ouchterlony analysis. Both IgG and IgA were demonstrated to be present by Ouchterlony analysis. Both IgG and IgM antibodies were demonstrated in the sera.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier G. M., Cebra J. J. Frequency distribution of alpha, gamma, kappa and lambda polypeptide chains in human lymphoid tissues. J Immunol. 1965 Aug;95(2):246–253. [PubMed] [Google Scholar]
- CHODIRKER W. B., TOMASI T. B., Jr GAMMA-GLOBULINS: QUANTITATIVE RELATIONSHIPS IN HUMAN SERUM AND NONVASCULAR FLUIDS. Science. 1963 Nov 22;142(3595):1080–1081. doi: 10.1126/science.142.3595.1080. [DOI] [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- CRABBE P. A., CARBONARA A. O., HEREMANS J. F. THE NORMAL HUMAN INTESTINAL MUCOSA AS A MAJOR SOURCE OF PLASMA CELLS CONTAINING GAMMA-A-IMMUNOGLOBULIN. Lab Invest. 1965 Mar;14:235–248. [PubMed] [Google Scholar]
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Cebra J. J., Robbins J. B. Gamma-A-immunoglobulin from rabbit colostrum. J Immunol. 1966 Jul;97(1):12–24. [PubMed] [Google Scholar]
- Crandall R. B. Chemotactic response of polymorphonuclear leukocytes to Trichinella spiralis and Ascaris suum extracts. J Parasitol. 1965 Jun;51(3):397–404. [PubMed] [Google Scholar]
- LARSH J. E., Jr EXPERIMENTAL TRICHINIASIS. Adv Parasitol. 1963;1:213–286. doi: 10.1016/s0065-308x(08)60505-9. [DOI] [PubMed] [Google Scholar]
- LARSH J. E., Jr, RACE G. J. A histopathologic study of the anterior small intestine of immunized and nonimmunized mice infected with Trichinella spiralis. J Infect Dis. 1954 May-Jun;94(3):262–272. doi: 10.1093/infdis/94.3.262. [DOI] [PubMed] [Google Scholar]
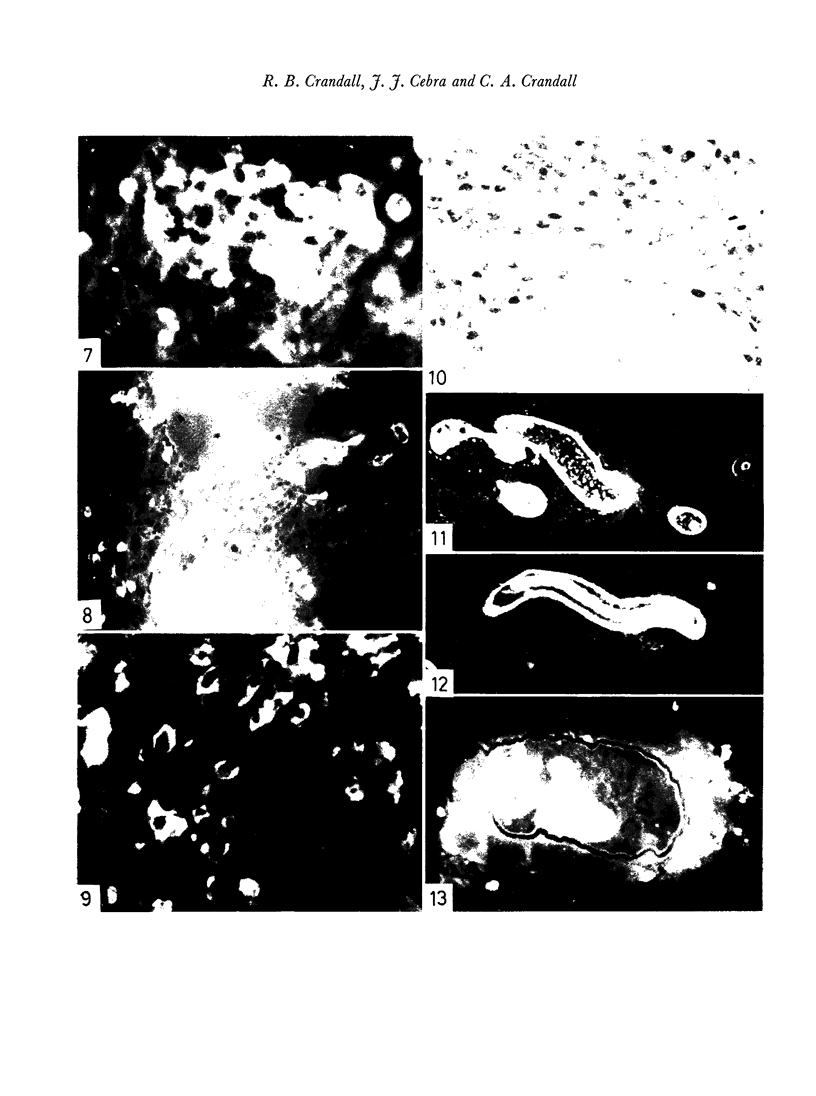
- MELLORS R. C., KORNGOLD L. THE CELLULAR ORIGIN OF HUMAN IMMUNOGLOBULINS (GAMMA-2, GAMMA-1M, GAMMA-1A). J Exp Med. 1963 Sep 1;118:387–396. doi: 10.1084/jem.118.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey E. M., Fahey J. L. Immunoglobulin changes in disease: quantitation on the basis of heavy polypeptide chains, IgG (gammaG), IgA (gammaA), and IgM (gammaM), and of light polypeptide chains, type K (I) and type L (II). J Clin Invest. 1965 Nov;44(11):1778–1787. doi: 10.1172/JCI105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN W., FAUCI A. S., MARVIN S. F., SLEISENGER M. H., JEFRIES G. H. IMMUNOFLUORESCENT STUDIES IN ADULT CELIAC DISEASE. J Clin Invest. 1965 Mar;44:475–485. doi: 10.1172/JCI105161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADUN E. H., ANDERSON R. I., WILLIAMS J. S. Fluorescent antibody test for the serological diagnosis of trichinosis. Exp Parasitol. 1962 Dec;12:423–433. doi: 10.1016/0014-4894(62)90078-4. [DOI] [PubMed] [Google Scholar]
- TORRIGIANI G., ROITT I. M. THE ENHANCEMENT OF 19S ANTIBODY PRODUCTION BY PARTICULATE ANTIGEN. J Exp Med. 1965 Jul 1;122:181–193. doi: 10.1084/jem.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963 Mar 1;117:457–477. doi: 10.1084/jem.117.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]