Abstract
Although it has been shown that unfractionated bone marrow, hematopoietic stem cells, common myeloid progenitors, and bipotent megakaryocyte/erythrocyte progenitors can give rise to megakaryocyte colonies in culture, monopotent megakaryocyte-committed progenitors (MKP) have never been prospectively isolated from the bone marrow of adult mice. Here, we use a monoclonal antibody to the megakaryocyte-associated surface protein, CD9, to purify MKPs from the c-kit+Sca-1−IL7Rα−Thy1.1−Lin− fraction of adult C57BL/Ka-Thy1.1 bone marrow. The CD9+ fraction contained a subset of CD41+FcγRloCD34+CD38+ cells that represent ≈0.01% of the total nucleated bone marrow cells. They give rise mainly to colony-forming unit–megakaryocytes and occasionally burst-forming unit–megakaryocytes, with a plating efficiency >60% at the single-cell level. In vivo, MKPs do not have spleen colony-forming activity nor do they contribute to long-term multilineage hematopoiesis; they give rise only to platelets for ≈3 weeks. Common myeloid progenitors and megakaryocyte/erythrocyte progenitors can differentiate into MKPs after 72 h in stromal cultures, indicating that MKPs are downstream of these two progenitors. These isolatable MKPs will be very useful for further studies of megakaryopoiesis as well as the elucidation of their gene expression patterns.
Hematopoiesis is a complex developmental process in which a rare population of bone marrow cells called hematopoietic stem cells (HSC) continuously generate all blood cells for the life of an animal. According to the current model of hematopoiesis, HSC give rise to progenies in each lineage through multiple steps of committed progenitors (1, 2). This paradigm is based historically on the results of in vitro clonogenic assays of bone marrow cells in semisolid media (3, 4). Unfractionated mouse bone marrow has been shown to contain multiple types of colony-forming units (CFU) such as (i) multipotent CFU for all myeloid cells, (ii) bipotent CFU for granulocytes and macrophages (CFU-GM), for megakaryocytes and erythrocytes (CFU-MegE), as well as (iii) monopotent CFU for granulocytes (CFU-G), macrophages (CFU-M), erythrocytes (CFU-E), or megakaryocytes (CFU-MK). Accordingly, they are believed to form different compartments of the myeloid progenitors in bone marrow (5–10). By using the same methodology developed for the isolation of HSC (11, 12), we have prospectively identified three different populations of murine myeloid progenitors: common myeloid progenitors (CMP), granulocyte/monocyte progenitors (GMP), and megakaryocyte/erythrocyte progenitors (MEP). CMPs give rise to all myeloid lineages, whereas GMPs and MEPs give rise to cells in the granulocyte/monocyte and megakaryocyte/erythrocyte lineages, respectively. The restricted differentiation capacity and lineage commitment of these cells were clearly demonstrated by both in vitro culture and in vivo transplantation assay (13, 14). Whether monopotent progenitors for each myeloid lineage exist in the same manner is yet to be proven, because they have not been prospectively isolated and tested for the in vitro and in vivo differentiation potentials at the clonal level.
In the megakaryocytic lineage, it is widely believed that CFU-MK are derived from monopotent megakaryocyte-committed progenitors (MKP) in the bone marrow (15–18). However, at the single-cell level, CMPs and MEPs can also give rise to pure megakaryocyte colonies (13). This can be explained by stochastic or deterministic cell fate decisions taken by CMPs or MEPs that could differentiate first into MKPs, which then give rise to CFU-MK. Alternatively, it is also possible that these progenitors may remain in the multipotent or oligopotent stage, but the culture conditions do not allow them to differentiate into cells of the other lineages. Therefore, the presence of CFU-MK in vitro does not necessarily indicate the existence of the MKP population in the bone marrow.
To address this question, we prospectively searched for a population of myeloid progenitors in the bone marrow that can give rise only to megakaryocytes. Because the megakaryocytic and erythroid lineages are closely related and have been shown to share some common precursors, even at the late stages (19), we speculated that, to isolate MKPs successfully, one needs to use cell-surface markers that are differentially expressed in the megakaryocytic lineage but not in the erythroid lineage. CD9 is such a marker and has been shown to be involved in the differentiation of human megakaryocytes (20, 21). In fact, we found CFU-MK activity to be highly enriched in the CD9+CD41+FcγRloc-kit+Sca-1−IL7Rα−Thy1.1−Lin− fraction of bone marrow. These cells can give rise mainly to megakaryocytes and platelets both in vitro and in vivo; thus, they represent the population of MKPs in mouse bone marrow.
Materials and Methods
Mouse Strains.
C57BL/Ka-Thy1.1 mice (6–8 weeks old) were used for the isolation of MKPs and other myeloid progenitors. Competitive repopulation and spleen CFU (CFU-S) assays were performed in the congenic Ly5 antigen system (Ly5.1 vs. Ly5.2), as described (14). β-actin GFP transgenic mice were generated in our laboratory (22) and have been back-crossed to C57BL/Ka-Thy1.1 mice for at least five generations. All animals were maintained on acidified water (pH 2.5) in the Stanford University Laboratory Animal Facility in accordance with Stanford guidelines.
Cell Staining and Sorting.
Myeloid progenitor cells (CMPs, MEPs, and GMPs) were isolated by using the staining protocol described (13). For the isolation of MKPs, bone marrow cells were stained with antibodies specific for the following lineage markers (Lin): CD3 (KT31.1), CD4 (GK1.5), CD8 (53-6.7), B220 (6B2), Gr-1 (8C5), Mac-1 (M1/70), TER119, Thy1.1 (19XE5) plus IL-7Rα (A7R34) and Sca-1 (E13–161-7). Lin+IL-7Rα+Sca-1+ cells then were removed with sheep anti-rat IgG-conjugated magnetic beads (Dynabeads M-450, Dynal A.S., Oslo), and the remaining cells were stained with Cy5-PE-conjugated goat anti-rat IgG polyclonal antibodies (Caltag, South San Francisco, CA). After incubation with rat IgG (Sigma), cells were stained with PE-conjugated anti-FcγRII/III (2.4G2), FITC-conjugated anti-CD41 (MWReg30; PharMingen), biotinylated anti-CD9 (KMC8; PharMingen), and APC-conjugated anti-c-Kit (2B8) monoclonal antibodies. CD9 was then visualized by Streptavidin-Texas red (Caltag). Cells were sorted or analyzed by using a highly modified triple laser (488-nm argon laser, 599-nm dye laser, and UV laser), FACSVantage (Becton Dickinson). Progenitors were purified by two rounds of sorting to obtain the populations that were essentially pure for the indicated surface marker phenotype. In some assays, the re-sort was performed by using a carefully calibrated automatic cell deposition unit system (ACDU, Becton Dickinson). This system deposited a specific number of purified cells into each well of 24- or 96-well plates.
DNA Staining.
Cells were fixed in ice-cold 80% ethanol for 30 min, washed twice with PBS, and then incubated overnight at 4°C in 250 μl of propidium iodide (PI)/RNase solution (50 μg/ml PI + 10 μl/ml RNase in 0.1% Triton X-100). The analysis was done on the FACScan cytometer (Becton Dickinson) with 488-nm excitation.
In Vitro Differentiation Assays.
The colony-forming assay was performed in methylcellulose medium (MethoCult M3231, StemCell Technologies, Vancouver) supplemented with stem cell factor (SCF), IL-3, IL-11, Flt3-ligand, granulocyte/macrophage–colony stimulating factor (GM-CSF), erythropoietin (Epo), and thrombopoietin (Tpo), as described (13). Colonies were scored at day 10 of the culture with an inverted microscope. The morphology of cells in the individual colonies was confirmed by Giemsa staining. To evaluate the lineage relationships among the progenitors, 10,000 CMPs and MEPs were sorted onto OP9 stromal cell layers in 24-well plates with RPMI medium 1640 containing 10% (vol/vol) FBS (Summit Biotechnology, Fort Collins, CO) and cytokines (SCF, IL-11, and Tpo). Seventy-two hours later, cells were harvested and stained with monoclonal antibodies as described for the MKP isolation. They were later analyzed and sorted by FACSVantage.
In Vivo Differentiation Assays.
The reconstitution assays were done by injecting 1,000 purified MKPs into the retro-orbital venous sinus of lethally irradiated (9.5 Gy delivered in two fractions) Ly5-congenic recipients together with 200,000 host-type unfractionated bone marrow cells. CFU-S assays were performed with 500–1,000 progenitor cells, as described (14). Spleens of the recipients also were subjected to standard histological examination. For the in vivo platelet readouts, progenitors were purified from β-actin GFP transgenic mice and transplanted into sublethally irradiated (4.5 Gy) C57BL/Ka. Mice were bled weekly after transplant for the flow cytometric analysis of GFP+ platelets in the peripheral blood.
Results
MKPs Reside Within the CD9+CD41+FcγRloc-kit+Sca-1−IL-7Rα−Thy1.1−Lin− Fraction of Bone Marrow.
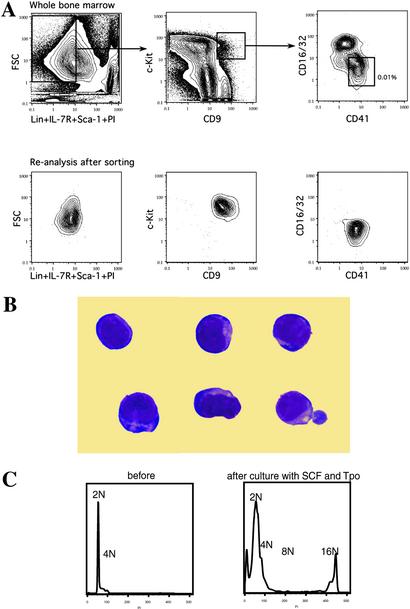
We first checked the expression of CD9 in the bone marrow cells of adult C57BL/Ka-Thy1.1 mice by flow cytometry. Virtually all Gr-1+Mac-1+ granulomonocytic (GM) and CD61+ megakaryocytic cells expressed CD9 at high levels, as reported (23). In contrast, less than 5% of TER119+ erythroid cells were CD9+. Lymphoid cells moderately coexpressed CD9, with 15% positivity found in the B220+ B cells and 7% in the CD3+ T cells (data not shown). In the c-kit+Sca-1−IL-7Rα−Lin− myeloid progenitor compartment, ≈1–2% of cells were also found to be CD9+. They can be further subdivided into two populations according to the expression of CD16/32 (FcγR) and CD41 (Fig. 1A). The CD41loFcγRhi fraction did not have significant myeloid colony-forming activity and was composed mainly of immature GM cells identified by Giemsa staining (data not shown). In contrast, CD41+FcγRloc-kit+Sca-1−IL-7Rα−Thy1.1−Lin− cells appeared to be all myeloblast-like cells (Fig. 1B). Additional phenotypic analysis using antibodies to the other two markers for myeloid progenitors showed the uniformly positive expression pattern of both CD34 and CD38 in this population (not shown). These cells were clearly isolatable by FACS and represented ≈0.008–0.012% of total nucleated bone marrow cells. They initially contained 2–4 N amount of DNA but developed into polyploid (16N) cells with the characteristic features of megakaryocytes when cultured with SCF and Tpo for 5 days (Fig. 1C). This population of cells will hereafter be referred to as MKP in this study.
Figure 1.
Identification of MKPs in mouse bone marrow. (A) Flow cytometric analysis of bone marrow after depleting the lineage-positive cells with magnetic beads. (Upper) The sorting gates for MKPs. The frequency of MKPs is shown as relative to total nucleated bone marrow cells before the negative depletion. Reanalysis after the first round of sorting found MKPs to be cleanly isolatable population (Lower). (B) MKPs were myeloblast-like cells with no characteristic features of megakaryocytes (Giemsa staining, original magnification ×1,000). (C) DNA content analysis showed MKPs to be diploid cells. After a 5-day incubation with SCF and Tpo, polyploid (16N) megakaryocytes were found in the culture.
MKPs Exclusively Form Megakaryocyte Colonies in Vitro.
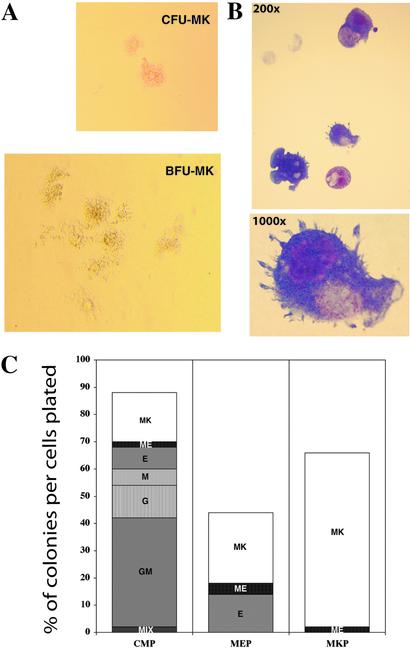
In the methylcellulose culture system that was set up to detect all possible outcomes of myeloid differentiation (13), MKPs gave rise mainly to CFU-MK with <1% CFU-GM, CFU-M, and burst-forming unit–erythrocytes (Table 1). The readouts of these non-megakaryocyte colonies can be totally eliminated by using more restricted sorting gates, indicating that they were derived from the contaminants from the other progenitor pools rather than from MKPs. Interestingly, ≈1–2% of MKP-derived colonies were large colonies that resembled the burst-forming unit–megakaryocyte (BFU-MK) described by Long et al. (24). The morphology of MKP-derived CFU-MK and BFU-MK is shown in Fig. 2A. Giemsa staining of cells in the individual colonies revealed that CFU-MK contained only mature megakaryocytes (Fig. 2B), whereas BFU-MK also frequently contained small numbers of erythroid cells (not shown). Therefore, these colonies should be more accurately defined as BFU-MK/E and probably represent the earlier stage of megakaryocyte progenitors (18).
Table 1.
CFU activity of CD9+CD41+FcγRloc-kit+Sca-1−IL-7Rα−Thy1.1−Lin− cells
| Experiment | CFU-MK | BFU-MK/E | BFU-E | CFU-GM | CFU-M |
|---|---|---|---|---|---|
| 1 | 47.6 ± 6.7 | 1.3 ± 0.6 | 0.3 ± 0.6 | 0 | 0 |
| 2 | 49.3 ± 11.0 | 1.3 ± 1.2 | 0.3 ± 0.6 | 1.0 ± 1.0 | 0 |
| 3 | 46.7 ± 7.2 | 1.3 ± 1.5 | 0 | 0 | 0.3 ± 0.6 |
One hundred cells were sorted directly onto 35-mm dishes containing methylcellulose medium supplemented with SCF, Flt3-ligand, IL-3, IL-11, GM-CSF, Tpo, and Epo. Colonies were counted at day 10. Each experiment was done in triplicate. The data are presented here as the average number of colonies per dish ± SEM. BFU-MK/E, burst-forming unit–megakaryocytes/erythrocytes. BFU-E, burst-forming unit-erythrocytes.
Figure 2.
Clonogenic megakaryocyte colony formation. (A) Morphology of typical CFU-MK and BFU-MK derived from MKP at day 10 of culture (phase contrast; original magnification ×100 for CFU-MK, ×40 for BCU-MK). (B) Mature megakaryocytes from a single colony (Giemsa; original magnification ×200 and ×1,000). (C) Day-10 colony readouts of single progenitors in methylcellulose-containing SCF, Flt3-ligand, IL-3, IL-11, GM-CSF, Epo, and Tpo. A total of 270 wells deposited with single progenitors were scored. CMPs gave rise to all types of myeloid colonies with plating efficiency >80%. MEPs gave rise to both megakaryocyte and erythroid colonies, whereas MKPs formed megakaryocyte colonies exclusively.
To compare the clonogenic colony formation among the myeloid progenitor populations, single progenitors were sorted into each well of 96-well plates containing methylcellulose medium and cytokines. Of 150 wells, 99 (i.e., 66%) deposited with single MKP developed CFU-MK at day 10 of the culture (Fig. 2C). The average number of megakaryocytes in each colony was 3.9. In the same condition, 16.7% and 21.6% of CMPs and MEPs, respectively, gave rise to CFU-MK with the average number of 8.9 and 8.7 megakaryocytes per colony, respectively. Large colonies including BFU-MK/E were more frequently derived from CMPs or MEPs than from MKPs. These results suggest that MKPs are committed only to the megakaryocytic lineage and are at least one step downstream of CMPs and MEPs in the developmental hierarchy of hematopoiesis.
Thrombopoietin Is Required for CFU-MK Activity of MKPs.
Next, we tested the cytokine requirement for the development of megakaryocytes from MKPs. As shown in Table 2, Tpo is the most important cytokine for CFU-MK formation. The plating efficiency of MKP dropped dramatically to <10% in the absence of Tpo. No colony was observed when MKPs were cultured with only early acting cytokines such as SCF and IL-11. With the addition of Tpo alone, ≈50–60% of the potential CFU-MK were rescued. Other cytokines had little, if any, effect on the CFU-MK development from MKPs. However, the synergy between GM-directed cytokines (GM-CSF and IL-3) and ME-directed cytokines (Epo and Tpo) was also observed. In fact, large CFU-MK including BFU-MK/E could be found only when all cytokines were present in the culture.
Table 2.
Effects of cytokines on the colony-forming activity of MKPs
| Cytokines added | Number of day-10 colonies per 100 MKPs
|
|||
|---|---|---|---|---|
| CFU-MK | BFU-MK/E | BFU-E | CFU-GM | |
| SCF + IL-11 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | |
| SCF + IL-11 + Tpo | 28 | 0 | 0 | 0 |
| 31 | 0 | 0 | 0 | |
| SCF + IL-11 + Epo | 3 | 0 | 1 | 0 |
| 2 | 0 | 0 | 0 | |
| SCF + IL-11 + IL-3 + GM-CSF | 5 | 0 | 0 | 0 |
| 6 | 0 | 0 | 1 | |
| 4 | 0 | 0 | 0 | |
| 8 | 0 | 0 | 0 | |
| SCF + IL-11 + Tpo + Epo | 29 | 0 | 0 | 0 |
| 34 | 0 | 0 | 0 | |
| 42 | 0 | 1 | 0 | |
| 37 | 0 | 0 | 0 | |
| SCF + IL-11 + IL-3 + GM-CSF + Tpo + Epo | 61 | 0 | 1 | 1 |
| 46 | 2 | 0 | 0 | |
| 55 | 1 | 0 | 0 | |
| 42 | 3 | 0 | 0 | |
BFU-E, burst-forming unit–erythrocytes.
In Vivo Differentiation of MKPs.
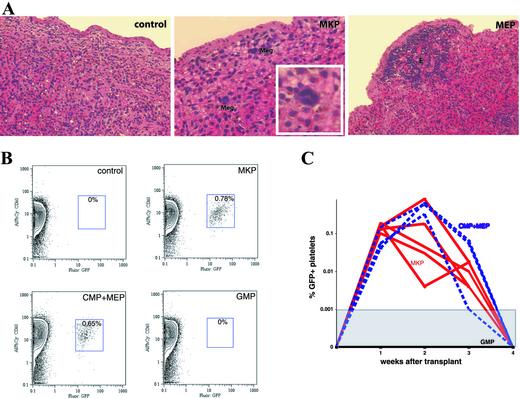
One thousand MKPs did not give rise to any surface colonies in the spleens of recipients at either day 8 or day 12. This finding was in contrast with the results of our previous experiments using the other myeloid progenitor populations, in which we found MEPs to be the major source of day-8 CFU-S (14). However, histological examination of the day-8 spleens revealed some microscopic foci of megakaryocytes in mice receiving MKPs (Fig. 3A). At the same time point, CMPs and MEPs formed mainly erythroid colonies (Fig. 3A and ref. 14), suggesting that MKPs are devoid of erythroid differentiation potential in vivo. In the reconstitution assays, no donor-derived Gr-1+, Mac-1+, CD3+, B220+, or Ter119+ cells were detected in blood, bone marrow, or spleen of mice receiving 1,000 MKPs together with helper bone marrow cells (data not shown). Because platelets do not express the Ly5 antigen, engraftment in the megakaryocytic lineage cannot be evaluated in this congenic system. To measure platelet generation directly in vivo, we purified CD9+FcγRloc-kit+Sca-1−IL-7Rα−Lin− MKPs from the bone marrow of β-actin GFP mice and transplanted them into sublethally irradiated recipients. Similarly to what was observed in the congenic transplant system, MKPs did not give rise to any GFP+ GM, erythroid, or lymphoid cells at any time point of analysis. However, they produced detectable levels of platelets in these mice (Fig. 3B). The peak of platelet engraftment occurred around day 14 after transplant, with the percentages of GFP+ platelets in the peripheral blood ranging from 0.005% to 1%. No GFP+ platelets were detected beyond 3 weeks after transplant (Fig. 3C). In the same experiment, 10,000 CD9−FcγRloc-kit+Sca-1−IL-7Rα−Lin− cells (CMPs + MEPs) exhibited a similar pattern of platelet engraftment, whereas 10,000 CD9−FcγRhic-kit+Sca-1−IL-7Rα−Lin− cells (GMPs) failed to generate any detectable platelets in the peripheral blood. This result confirms our previous observation that GMPs can only give rise to GM cells in vivo (14). More importantly, it excludes the possibility that GFP signals observed in the platelets may have been generated nonspecifically from other transplanted GFP+ cells. Taken together, these data strongly suggest that MKPs do not have significant in vivo self-renewal capacity or multipotent differentiation potentials, but transiently give rise only to megakaryocytes and platelets.
Figure 3.
In vivo differentiation of MKPs. (A) Histology of the spleen at day 8 (H&E, original magnification: control and MEP, ×200; MKP, ×400; Inset, ×1,000). Mice were lethally irradiated and then injected with no cell (control), 1,000 MKPs, or 500 MEPs. MKPs gave rise to microscopic foci of megakaryocytes (Meg), whereas MEPs formed large colonies composed of only erythroid cells (E). No erythroid or megakaryocyte colonies were observed in the control animals. (B) Representative FACS plots of platelets in the peripheral blood of recipient mice 14 days after receiving the purified progenitors from β-actin GFP transgenic donors. Blood cells were first gated on the forward scatter and side scatter. Percentages of GFP+CD61+ cells in the platelet gate are shown in the boxes. (C) MKPs generated platelets in vivo for 3 weeks. The kinetics of platelet engraftment of 3,000 MKPs (red lines) was similar to 10,000 CMPs and MEPs (blue dashed lines). In contrast, 10,000 GMPs did not generate detectable platelets at any time point of analysis (black line). The threshold for detecting GFP+ platelets in the whole population was 1 in 100,000.
CMPs and MEPs Give Rise to MKPs in Vitro.
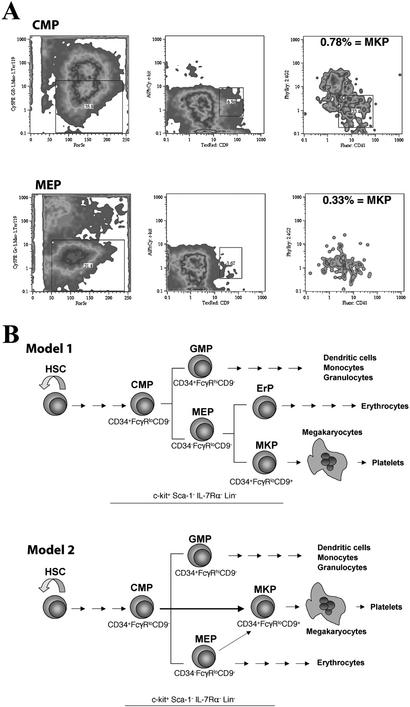
To examine the relationships among the myeloid progenitors, we cultured CMPs and MEPs (CD9−) on OP9 stromal layers in the presence of SCF, IL-11, and Tpo for 72 h. Both CMPs and MEPs can give rise to cells with the phenotype of MKPs as found in freshly isolated bone marrow (Fig. 4A). These CD9+CD41+FcγRloc-kit+Lin− cells represented ≈0.78% and 0.33% of total cells in the CMP and MEP culture, respectively. They also formed only CFU-MK in methylcellulose culture (data not shown). In the same culture condition, MKPs never gave rise to CMPs or MEPs but differentiated into multinucleated megakaryocytes within 5 days. In combination with the results of single-progenitor colony formation assays, these findings lead us to conclude that MKPs are the committed progenitors downstream of CMPs and MEPs.
Figure 4.
Lineage relationships among myeloid progenitors. (A) Flow cytometric analysis for cells with the MKP phenotype in the culture of CMPs and MEPs. Note more MKPs present in the CMP culture. (B) Two models for the MKP development. The first model suggests that commitment to the megakaryocytic lineage occurs at the level of MEP where the progenitor has to make a choice to become either MKP or erythrocyte progenitor (ErP; yet to be prospectively identified). In the second model, the commitment occurs earlier, at the stage of CMP. Although MEPs are bipotent, they differentiate mainly into the erythroid lineage. The majority of MKPs are the direct descendants of CMPs.
Discussion
The concept of megakaryocyte-committed progenitors was introduced in the 1970s after the discovery of megakaryocyte colonies from the culture of mouse bone marrow cells in semisolid media (9, 10). Since then, several groups of investigators have developed culture systems to detect different types of megakaryocyte colonies in both humans and mice (reviewed in refs. 25 and 26). Of those megakaryocyte-containing colonies described in the literature, large colonies that additionally contain cells in the other lineages are largely attributed to the multipotent progenitors (27, 28), whereas pure megakaryocyte colonies are considered to represent the true monopotent megakaryocyte-committed progenitors (15, 16, 18). However, this assumption may not be entirely correct, since many pure colonies were later found to be bi- or oligo-potent when the assays were done in different conditions. Moreover, in vivo experiments were rarely performed to confirm lineage restriction of these cells (29, 30). Recently we reported that both MEPs and CMPs frequently give rise to CFU-MK and yet can also differentiate into cells of other lineages when injected into lethally irradiated mice (13, 14). These findings raised the possibility that an isolatable precursor downstream of MEPs might be the origin of these megakaryocyte colonies. In this study, we prospectively searched for cells that could give rise only to megakaryocytes and extensively tested their differentiation potentials in several in vitro and in vivo assays. The MKPs isolated here meet all criteria for megakaryocyte-committed progenitors and, therefore, provide a definitive proof of the existence of these monopotent progenitors in mouse bone marrow.
Previous attempts to isolate megakaryocyte progenitors have included the use of various methods such as physical separation by sedimentation velocity (9, 16), density gradient (31), cytochemical staining (17), in vivo enrichment with the chemotherapeutic agent 5-fluorouracil (29, 32), and, recently, flow cytometry (33–35). Although the CFU-MK can be recovered in some isolated populations, a significant number of other CFU activities were always observed. One of the major limitations in the search for MKPs is the paucity of specific surface markers in the megakaryocytic lineage. In the human system, platelet glycoproteins (GP), such as GPIIb (CD41) or GPIIIa (CD61), have been widely used for the enrichment of megakaryocyte progenitors (36). Hodohara et al. (35) have also shown recently that the CD41+c-kit+ bone marrow cells from Tpo-treated mice were highly enriched for CFU-MK activity. In our culture system, these cells gave rise to significant numbers of CFU-GM and BFU-MK/E in addition to CFU-MK. Furthermore, we found that the level of CD41 expression was not different among the three populations of myeloid progenitors (not shown). Our observation is consistent with the studies in human (37, 38) and chicken (39), in which multipotent progenitors were reported to be CD41+. Therefore, we believe that the CD41+c−kit+ fraction of mouse bone marrow contains other myeloid progenitor populations in addition to the MKPs.
CD9 belongs to the tetraspanin superfamily of cell-surface proteins that are generally involved in many cellular processes such as cell adhesion, motility, proliferation, and differentiation, as well as in signal transduction (reviewed in ref. 40). CD9 is expressed in various tissues, including bone marrow, where the highest expression can be observed in platelets, megakaryocytes, granulomonocytic, and stromal cells (23). Although the function of CD9 is still unknown, anti-CD9 antibody has been shown to inhibit myeloid differentiation of primitive progenitors in stromal cultures, presumably through the interaction with stromal cells, because it had no direct effect in the CFU assay (23, 41). The fact that CD9 is expressed in the megakaryocytic lineage but not in the erythroid lineage prompted us to use this antibody to isolate MKPs in the c-kit+Sca-1−IL-7Rα−Thy1.1−Lin− fraction of bone marrow, which we previously found to contain almost all myeloerythoid colony-forming activities outside the HSC compartment (13). Our study indicates that CD9 is a useful differentiation marker to define the commitment into the megakaryocytic lineage. Interestingly, CD9+FcγRhic-kit+Sca-1−IL-7Rα−Lin− cells do not have significant colony-forming activity despite the high expression of CD9 in GM cells. This result suggests that, unlike in the megakaryocytic lineage, CD9 expression in the GM lineage occurs relatively late in development. This possibility is confirmed by the fact that GMPs, the earliest GM-committed progenitors identified in bone marrow, only express CD9 at a low level, and CD9 expression gradually increases along with the differentiation of GMP toward mature GM cells (not shown). Recently, mice lacking CD9 protein have been generated by two independent groups and are reported to have female infertility caused by a defect in sperm–oocyte fusion (42, 43). Surprisingly, no abnormality in the hematopoietic system was observed. The results from our data suggest that detailed analysis of the myeloid progenitors, particularly of the megakaryocytic lineage, should be done in these mice to address the role of CD9 in hematopoiesis.
The relationships of MKPs with the other two myeloid progenitors were also investigated in this study. The results of in vitro cultures clearly demonstrated that both CMPs and MEPs can give rise to MKPs. At the single-cell level, they also generated larger CFU-MK, with at least twice the numbers of megakaryocytes compared with the MKP-derived colonies. These results indicate that MKPs are indeed downstream of CMPs and MEPs. Because MEPs have been shown to be directly derived from CMPs (13), it is logical to place MKP one step downstream of MEPs in the hematopoietic tree (Fig. 4B, Model 1). This model is consistent with the current dogma of lineage commitment in which progenitors commit to a particular lineage by progressive restriction of their differentiation potentials (2, 3). However, we cannot exclude the alternative hypothesis of MKPs being the direct progeny of CMPs (Fig. 4B, Model 2). In fact, CMPs gave rise to MKPs more efficiently than did MEPs (Fig. 4A). This high proportion of MKPs generated in the culture cannot be explained by the succession model of commitment. We have also examined these lineage relationships in vivo, but the results were inconclusive largely because of the very low frequency of the outcomes. Direct comparison of the gene expression patterns between each progenitor population may allow us to resolve this issue in the future.
In conclusion, we have prospectively isolated clonogenic monopotent megakaryocyte-committed progenitors that uniformly generate CFU-MK in methylcellulose culture. They are downstream of CMPs and MEPs, although the precise lineage relationship is still to be determined. MKPs do not show the properties of multipotent or oligopotent progenitors but are committed exclusively to the megakaryocytic lineage and give rise to platelets in vivo for only 3 weeks. Further studies are needed to elucidate the cellular and molecular mechanisms that determine the commitment of MKPs to the megakaryocytic lineage.
Acknowledgments
We thank S.-I. Nishikawa for anti-IL-7Rα antibody, Julie Christensen and Amy Wagers for technical advice, Susan Prohaska and Motonari Kondo for critical evaluation of the manuscript, Libuse Jerabek for excellent laboratory management and assistance with animal procedures, Stephanie Smith for antibody preparation, the Stanford Shared FACS facility for flow cytometer maintenance, and Lucino Hidalgo, Diosdado Escoto, and Bert Lavarro for animal care. This research was supported by U.S. Public Health Service Grant CA42551 and the Leukemia Society de Villier's International Achievement Award (to I.L.W.). T.N.N. was supported by the Anandamahidol Foundation under the royal patronage of His Majesty the King of Thailand.
Abbreviations
- HSC
hematopoietic stem cells
- CFU
colony-forming unit
- CFU-G
CFU for granulocytes
- CFU-M
CFU for macrophages
- CFU-GM
CFU for granulocytes and macrophages
- CFU-MK
CFU–megakaryocytes
- CMP
common myeloid progenitors
- GMP
granulocyte/monocyte progenitors
- MEP
megakaryocyte/erythrocyte progenitors
- MKP
megakaryocyte progenitors
- SCF
stem cell factor
- Epo
erythropoietin
- Tpo
thrombopoietin
- GM
granulomonocytic
- BFU-MK/E
burst-forming unit–megakaryocytes/erythrocytes
References
- 1.Weissman I L, Anderson D J, Gage F. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf D. Ann NY Acad Sci. 1999;872:289–303. doi: 10.1111/j.1749-6632.1999.tb08473.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradley T R, Metcalf D. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 4.Eaves C J. In: Williams Hematology. Beutler E, Lichtman M A, Coller B S, Kipps T J, editors. New York: McGraw–Hill; 1995. pp. L22–L26. [Google Scholar]
- 5.Ichikawa Y, Pluznik D H, Sachs L. Proc Natl Acad Sci USA. 1966;56:488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metcalf D, Johnson G R, Mandel T E. J Cell Physiol. 1979;98:401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- 7.Johnson G R, Metcalf D. Proc Natl Acad Sci USA. 1977;74:3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson J R, Axelrad A A, McLeod D L, Shreeve M M. Proc Natl Acad Sci USA. 1971;68:1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf D, MacDonald H R, Odartchenko N, Sordat B. Proc Natl Acad Sci USA. 1975;72:1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod D L, Shreve M M, Axelrad A A. Nature. 1976;261:492–494. doi: 10.1038/261492a0. [DOI] [PubMed] [Google Scholar]
- 11.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Akashi K, Traver D, Miyamoto T, Weissman I L. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 14.Na Nakorn T, Traver D T, Weissman I L, Akashi K. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein S A, Adamson J W, Thorning D, Harker L A. Blood. 1979;54:169–179. [PubMed] [Google Scholar]
- 16.Long M W, Williams N, McDonald T P. J Cell Physiol. 1982;112:339–344. doi: 10.1002/jcp.1041120305. [DOI] [PubMed] [Google Scholar]
- 17.Long M W, Smolen J E, Szczepanski P, Boxer L A. J Clin Invest. 1984;74:1686–1692. doi: 10.1172/JCI111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulus J M, Prenant M, Deschamps J F, Henry-Amar M. Proc Natl Acad Sci USA. 1982;79:4410–4414. doi: 10.1073/pnas.79.14.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanucchi A M, Paoletti F, Linari S, Cellai C, Caporale R, Ferrini P R, Sanchez M, Magliaccio G, Magliaccio A R. Blood. 2000;95:2559–2568. [PubMed] [Google Scholar]
- 20.Le Naour F, Francastel C, Prenant M, Lantz O, Boucheix C, Rubinstein E. Leukemia. 1997;11:1290–1297. doi: 10.1038/sj.leu.2400721. [DOI] [PubMed] [Google Scholar]
- 21.Clay D, Rubinstein E, Mishal Z, Anjo A, Prenant M, Jasmin C, Boucheix C, Le Bousee-Kerdiles M C. Blood. 2001;97:1982–1989. doi: 10.1182/blood.v97.7.1982. [DOI] [PubMed] [Google Scholar]
- 22.Wright D E, Cheshier S H, Wagers A J, Randall T D, Christensen J L, Weissman I L. Blood. 2001;97:2278–2285. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- 23.Oritani K, Wu X, Medina K, Hudson J, Miyake K, Gimble J M, Burstein S A, Kincade P W. Blood. 1996;87:2252–2261. [PubMed] [Google Scholar]
- 24.Long M W, Gragowski L L, Heffner C H, Boxer L A. J Clin Invest. 1985;76:431–438. doi: 10.1172/JCI111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long M W. Semin Hematol. 1998;35:192–199. [PubMed] [Google Scholar]
- 26.Bruno E, Hoffman R. Semin Hematol. 1998;35:183–191. [PubMed] [Google Scholar]
- 27.Jackson H, Williams N, Bertoncello I, Green R. Exp Hematol. 1994;22:954–958. [PubMed] [Google Scholar]
- 28.Lowry P A, Deacon D M, Whitefield P, Rao S, Quesenberry M, Quesenberry P J. Exp Hematol. 1995;23:1135–1140. [PubMed] [Google Scholar]
- 29.Thean L E, Hodgson G S, Bertoncello I, Radley J M. Blood. 1983;62:896–901. [PubMed] [Google Scholar]
- 30.Humphries R K, Jacky P B, Dill F J, Eaves A C, Eaves C J. Nature. 1979;279:718–720. doi: 10.1038/279718a0. [DOI] [PubMed] [Google Scholar]
- 31.Chatelain C, De Bast M, Symann M. Blood. 1988;72:1187–1192. [PubMed] [Google Scholar]
- 32.Radley J M, Hodgson G S, Thean L E, Zangheri O, Levin J. Exp Hematol. 1980;8:1129–1138. [PubMed] [Google Scholar]
- 33.Pallavicini M G, Levin J, Summers L, Levin F. Exp Hematol. 1987;15:704–709. [PubMed] [Google Scholar]
- 34.Bauman J G J, Chen M G. Exp Hematol. 1987;15:1074–1079. [PubMed] [Google Scholar]
- 35.Hodohara K, Fujii N, Yamamoto N, Kaushansky K. Blood. 2000;95:769–775. [PubMed] [Google Scholar]
- 36.Debili N, Issaad C, Masse J M, Guichard J, Katz A, Breton-Gorius J, Vainchenker W. Blood. 1992;80:3022–3035. [PubMed] [Google Scholar]
- 37.Murray L J, Mandich D, Bruno E, DiGiusto R K, Fu W C, Sutherland D R, Hoffman R, Tsukamoto A. Exp Hematol. 1996;24:236–245. [PubMed] [Google Scholar]
- 38.Debili N, Robin C, Schiavon V, Letestu R, Pflumio F, Mitjavila-Garcia M T, Coulombel L, Vainchenker W. Blood. 2001;97:2023–2030. doi: 10.1182/blood.v97.7.2023. [DOI] [PubMed] [Google Scholar]
- 39.Ody C, Vaigot P, Quere P, Imhof B A, Corbel C. Blood. 1999;93:2898–2906. [PubMed] [Google Scholar]
- 40.Maecker H T, Todd S C, Levy S. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 41.Aoyama K, Oritani K, Yokota T, Ishikawa J, Nishiura T, Miyake K, Kanakura Y, Tomiyama Y, Kincade P W, Matsuzawa Y. Blood. 1999;93:2586–2594. [PubMed] [Google Scholar]
- 42.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 43.Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]