Abstract
The viral replication rate in patients infected with human immunodeficiency virus type 1 (HIV-1) is controlled in part by regulation of the transcription of viral genes. The rate of transcription is determined by a complex interplay between cellular and viral proteins and the promoter elements found in the long terminal repeats. Protein phosphatase 2A (PP2A) is a phosphoprotein that plays important roles in the regulation of signal transduction and cell growth. In this report, we demonstrate that overexpression of the catalytic subunit of protein phosphatase 2A (PP2Ac) increases the basal activity of the HIV-1 promoter and, especially, enhances the promoter's response to the protein kinase C (PKC) activator 12-O-tetradecanoyl phorbol-13-acetate (PMA). Additionally, ectopic PP2Ac enhances activation of HIV-1 provirus by PMA. Okadaic acid, a potent inhibitor of PP2A, markedly reduces both HIV-1 enhancer and proviral activation. Fostriecin, a PP2A inhibitor which has been used as an antineoplastic agent in clinical trials, is also able to inhibit PMA-stimulated HIV-1 proviral activation. These observations demonstrate a role for the important cellular phosphatase PP2A in HIV-1 transcription and replication and also suggest that PKC can potentiate the activity of PP2A. PP2A is a potential target for therapeutic intervention in patients infected with HIV-1.
The human immunodeficiency virus type 1 (HIV-1) promoter, located in the long terminal repeat (LTR), contains multiple response elements that bind a number of cellular and viral transcription factors (27). The promoter has been shown to respond to a variety of stimulatory agents such as 12-O-tetradecanoyl phorbol-13-acetate (PMA) and tumor necrosis factor alpha (TNF-α), which trigger signal transduction cascades leading to increased kinase activity. PMA is a potent activator of protein kinase C (PKC), and TNF-α signals through the mitogen-associated protein kinase pathway. Additionally, other kinases such as Ras, DNA-dependent protein kinase, and Jun kinase have also been shown to play a role in activating HIV-1 in response to various stimulatory agents (3, 4, 6, 7, 15, 16, 21, 22). Much less well studied has been the role of phosphatases in regulating HIV-1 gene expression. Recently, several reports have been published providing evidence that, in addition to the aforementioned kinases, protein phosphatase 2A (PP2A) might play a role in regulating HIV-1 transcription and virus replication (17, 34, 38, 39). These previous reports have generally suggested that PP2A would have an inhibitory effect on HIV-1 replication, but this was not directly addressed.
PP2A accounts for the majority of the Ser/Thr intracellular phosphatase activity, and its importance is underscored by its roles in the regulation of cell growth, signaling pathways, and mitotic division. PP2A exists intracellularly as an oligomeric protein composed of three subunits, A, B, and C, which together form the holoenzyme or an AC heterodimer known as the core enzyme. The A (65-kDa) subunit acts as a scaffold to which the B (55-kDa) and catalytic C (37-kDa) subunits bind. The PP2A holoenzyme and core enzyme exhibit different substrate specificities and/or intracellular locations. Recently, researchers have begun to investigate the involvement of PP2A in the regulation of HIV-1 transcription and replication. Ruediger et al. (34) demonstrated that increasing the ratio of PP2A core enzyme to holoenzyme by expression of a mutant A subunit that binds the C but not the B subunit results in a decrease in Tat-mediated HIV-1 transcription and virus production. Their experiments imply that PP2A may play a role in regulating HIV-1 replication. Furthermore, the observation that okadaic acid (OKA), a potent inhibitor of PP2A and protein phosphatase 1, induces NF-κB binding and activates the HIV-1 promoter suggested that PP2A might inhibit HIV-1 transcription and, hence, replication (17, 38, 39).
In this report, we demonstrate that, surprisingly, the catalytic subunit of PP2A (PP2Ac) is able to increase the basal activity of the HIV-1 promoter and markedly enhances the promoter's response to PMA. Additionally, we show that ectopic PP2Ac and PMA act together to increase the level of proviral activation above the levels seen with either alone. OKA, a potent inhibitor of PP2A, is able to reduce the phorbol-induced activation of the HIV-1 enhancer and markedly decrease HIV-1 proviral activation. Furthermore, fostriecin (FST), a PP2A inhibitor which has been reasonably well tolerated by cancer patients in clinical trials, can similarly inhibit PMA-induced HIV-1 induction. These observations directly demonstrate a role for PP2A in driving HIV-1 transcription and proviral induction and suggest that PP2A can potentiate the activity of PKC. These findings also point to PP2A as a target for therapeutic intervention against AIDS.
It has recently been demonstrated that the promoter of HIV type 2 (HIV-2) is activated in monocytic cells by PP2A (14). Several recent reports have suggested that PP2A activity may be altered in differentiating monocytes. Differentiation of HL-60 cells into granulocytes by use of retinoic acid is augmented by the PP2A inhibitor OKA (24). Methylprednisolone-induced differentiation of leukemic HL-60 cells results in an increase in PP2A regulatory subunits and catalytic activity (1). Since infected monocytes serve as a reservoir for HIV in vivo (9, 18, 37), we decided to utilize the promyelocytic cell line U937 in our experiments to examine the effect of overexpression of PP2Ac on HIV-1 transcription. U937 cells were grown as previously described (14) and were transfected by using an Invitrogen Electroporator II at a setting of 300 V, 1,000 μF, and infinite resistance with an input voltage of approximately 325 V. We cotransfected U937 cells with a plasmid in which expression of the chloramphenicol acetyltransferase (CAT) reporter gene is under the control of the HIV-1 promoter (33) and with various amounts of a PP2Ac expression vector (Fig. 1A). The transfected U937 cells were also stimulated with the phorbol ester PMA, an agent that has been shown to activate the HIV-1 promoter and promote monocytic differentiation of U937 cells. As shown in Fig. 1A, ectopic PP2Ac activated the HIV-1 promoter (P = 0.04 when the value obtained with the control is compared with that obtained with 5 μg of PP2Ac expression vector by Student's t test). Increasing the amount of the PP2A expression vector beyond 5 μg led to a somewhat further increased but variable stimulation (Fig. 1A). PP2A also markedly enhanced the response of the HIV-1 promoter to PMA stimulation (Fig. 1B). To confirm that the effects we observed were indeed due to overexpression of the C subunit, we performed assays using whole-cell lysates from cells transfected with the PP2Ac expression plasmid with or without PMA stimulation to detect any increases in intracellular protein phosphatase activity (Fig. 1C). Ectopic expression of the C subunit led to a 20% increase in activity in the absence of phorbol stimulation and up to a 50% increase following PMA treatment of the transfected U937 cells, thus confirming functional overexpression of the C subunit. This increase in phosphatase activity is similar to that measured by use of a different assay in a previous study (14).
FIG. 1.
(A) PP2A activates the HIV-1 promoter. U937 cells were cotransfected with 5 μg of the HIV-1 CAT reporter plasmid and 2.5, 5, 10, or 20 μg of pCMV5 PP2Ac, in which the catalytic domain of PP2A is under the control of the CMV promoter. CAT assays (36) to determine reporter gene transcription levels were performed 18 to 24 h later. PP2Ac produced a fourfold increase in HIV-1 promoter activity at the highest levels of transfected expression vector used. Data shown are from one experiment that is representative of 10 separate experiments. (B) PP2A augments the response of the HIV-1 promoter to PMA. U937 cells were cotransfected with 5 μg of the HIV-1 CAT reporter plasmid and 2.5 or 5 μg of pCMV5 PP2Ac. At 12 h posttransfection, cells received either no treatment (open bars) or 32 nM PMA (solid bars). CAT activity levels were determined 18 to 24 h posttreatment. Data shown are from one experiment that is representative of 10 separate experiments. (C) Ectopic PP2Ac overexpression increases intracellular phosphatase activity. Phosphatase assays were performed using whole-cell lysates from U937 cells transfected with increasing amounts of PP2Ac expression plasmid, followed by no treatment (open bars) or treatment with 50 nM PMA (solid bars). PP2A activity was determined using a protein phosphatase assay system from Promega. Ectopic PP2Ac expression resulted in a 20 or 50% increase in phosphatase activity in the absence or presence of PMA, respectively.
Phorbol esters mimic the actions of diacylglycerol by activating PKC and triggering a cascade of intracellular signaling events, including activation of the MAP kinase pathway and NF-κB (5, 23, 29, 38). To ascertain whether the observed cooperative effect between the PKC activator PMA and ectopic PP2Ac was specific, we stimulated U937 cells with 50 ng of TNF-α/ml (which does not signal through the PKC pathway) or 50 nM PMA after cotransfection with the PP2Ac expression vector and a plasmid in which the HIV-1 promoter regulates expression of the luciferase reporter gene (Fig. 2). This reporter plasmid was generated by cloning the HIV-1 promoter from the HIV-1 CAT vector upstream of the luciferase gene in the pGL3 basic vector (Promega). Stimulation of cells with the cytokine TNF-α (or inhibition of PP2A by OKA [see below]) activates the HIV-1 promoter by inducing NF-κB nuclear translocation and binding to its cognate sites (37, 38). As anticipated on the basis of prior reports (38, 39), both PMA and TNF-α activated transcription of the wild-type HIV-1 promoter (Fig. 2A). Cotransfection of the PP2Ac expression vector further augmented the activation seen with PMA but had no significant effect on TNF-α-mediated HIV-1 promoter activation.
FIG. 2.
(A) Ectopic PP2Ac enhances the response of the HIV-1 promoter to PMA but not to TNF-α. U937 cells were cotransfected with 5 μg of an HIV-1 luciferase reporter plasmid and 10 μg of pCMV5 PP2Ac or a control vector. The cells were left untreated or treated 12 h later with 50 nM PMA or 50 ng of TNF-α/ml. Luciferase activity was measured at 18 to 24 h posttreatment by using a luciferase assay system from Promega, and the values were normalized relative to the total amount of protein. Data shown are from one experiment that is representative of three separate experiments. (B) OKA inhibits activation of the HIV-1 promoter by PMA but not TNF-α. U937 cells were cotransfected with 5 μg of an HIV-1 luciferase reporter and left untreated or treated 24 h later with 50 nM PMA, 50 ng of TNF-α/ml, 100 nM OKA, 50 nM PMA plus 100 nM OKA, or 50 ng of TNF-α/ml plus 100 nM OKA. At 12 to 14 h after treatment, luciferase assays were performed and the values were normalized. Data are from one experiment that is representative of three experiments. RLU, relative light units.
Since PMA and PP2Ac cooperatively enhance HIV-1 promoter activation (Fig. 1B and 2A), we sought to determine whether inhibition of endogenous PP2A by OKA would have an effect on the response of the promoter to PMA. Treatment of U937 cells with OKA prior to PMA stimulation (Fig. 2B) suppressed the response of the HIV-1 promoter to PMA (by threefold). OKA pretreatment, however, had no suppressive effects on the activation of the HIV-1 promoter by TNF-α. Since OKA treatment of cells induces both Sp1 and NF-κB binding (38, 39), 100 nM OKA alone activated the HIV-1 promoter (Fig. 2B), as predicted on the basis of previous work (38, 39).
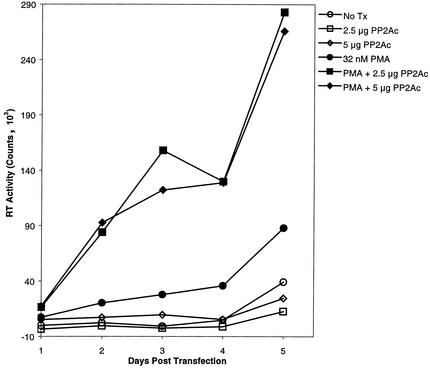
To determine whether the observed effects of ectopic PP2Ac on HIV-1 promoter activity translated into an increase in viral replication, we cotransfected U937 cells with the HIV-1 infectious clone HXB3 (30) and increasing amounts of a PP2Ac expression vector. PP2A overexpression resulted in a small increase in the HIV-1 baseline replication rate and significantly augmented the PMA-induced increase in replication, as determined by reverse transcriptase (RT) assay (Fig. 3). The observed effects of combined PP2A overexpression and PMA stimulation on viral replication in U937 cells correlate with the changes in transcriptional activity seen in the reporter gene studies (Fig. 1A and B). As further evidence that PP2A participates in the activation of HIV-1, OKA administered to the chronically infected U937 cell line U1 diminished the PMA-induced increase in viral induction in a dose-dependent fashion (Fig. 4). This reduction in PMA-induced viral activation by OKA fits with the suppressive effect of OKA on transcriptional activation by PMA (Fig. 2B). At the maximal dose of OKA utilized (100 nM), there was a significant decrease (five- to sevenfold) in PMA-induced viral induction by days 2 to 3. Cellular toxicity, as measured by the MTT assay, was not seen in these experiments, as OKA was added for only 4 h and then washed away prior to PMA stimulation.
FIG. 3.
PMA-induced HIV-1 replication is stimulated by ectopic PP2A. (A) U937 cells were transfected with the HIV-1 HXB3 infectious clone or HIV-1 HXB3 plus 2.5 or 5 μg of pCMV5 PP2Ac. Cells were left untreated or were treated with 50 nM PMA (solid symbols) for 24 h. The average RT assay values (28) from four separate experiments are shown. No Tx, no treatment.
FIG. 4.
Induction of HIV-1 provirus by PMA is blocked by OKA. U1 (HIV-1 chronically infected U937 cells) were pretreated with 25, 50, or 100 nM OKA for 4 h, washed, and then treated with 50 nM PMA (indicated by the solid symbols). RT assays were performed on supernatants harvested daily over a period of 3 days. The average RT assay values from four separate experiments are shown. No Tx, no treatment.
We next investigated the effect of another inhibitor of PP2A, FST, on HIV-1 replication. Using the U1 cell system, we first investigated whether FST treatment would alter cellular viability by using the MTT assay. Cellular viability was not negatively affected by FST treatment (data not shown). U1 cells were treated with PMA for 72 h prior to and during treatment with FST. HIV-1 levels in the supernatants were determined by using a p24 enzyme-linked immunosorbent assay (ELISA). FST at doses between 0.2 and 1 μM suppressed the stimulatory effect of PMA on viral induction (Fig. 5), similar to the effect seen with OKA. Concentrations of FST that are less than or equal to 1 μM have been shown to very specifically block PP2A activity in cell culture (40; R. Honkanen, personal communication), so these findings confirm that it is the specific inhibition of PP2A which decreases HIV-1 activation. Similar results were obtained when U1 cells were treated with PMA for 24 h prior to FST treatment or were treated with FST for 1 or 24 h prior to PMA treatment (data not shown). The results from the FST experiments thus further confirm the role of PP2A in stimulating HIV-1 and attest to the specificity of this interaction. In addition, as FST has been used to treat cancer patients without undue toxicity (10-13, 20, 32, 40), these findings suggest that PP2A inhibition might prove to be a useful therapeutic strategy for the treatment of HIV-infected patients.
FIG. 5.
Induction of HIV-1 provirus is blocked by the PP2A inhibitor FST. U1 cells were pretreated with PMA for 72 h, washed, and then treated with PMA plus 0, 0.2, or 1 μM FST. Viral replication was determined by using a p24 ELISA assay. Assays were performed on supernatants over a period of 4 days. The average p24 ELISA values are shown; error bars indicate the standard deviation for results from three separate cultures. Data are from one experiment that is representative of three independent experiments. No Tx, no treatment.
In this report, we demonstrate first that PP2Ac is able to increase HIV-1 promoter activity and, especially, enhance the response of the promoter to the PKC-stimulating agent PMA. Second, ectopic PP2Ac and PMA increase the level of viral induction above the levels seen with either alone. Third, OKA, a potent inhibitor of PP2A, is able to markedly reduce PMA-mediated HIV-1 enhancer activation and viral induction. Finally, FST at concentrations highly specific for PP2A inhibition decreases PMA-induced HIV-1 stimulation. These findings all point to an important role for PP2A in the enhancement of HIV-1 replication, especially in combination with PMA stimulation.
Previous studies with the PP2A inhibitor OKA indirectly implicated PP2A in the regulation of HIV-1 promoter activity. OKA inhibition of PP2A has been shown to result in the activation of the HIV-1 promoter via the NF-κB and Sp1 response elements (38, 39). These previous experiments suggested that PP2A possibly played a repressive role in the regulation of HIV-1 transcription, as its inhibition increased transcription. However, OKA at low concentrations also inhibits the PP2A-related phosphatases PP4 and PP5 (20, 36). This may account for the different effects on identical cellular processes, such as growth, transformation, and signaling, observed with OKA treatment in different cell lines (36).
To more accurately assess the role of PP2A in HIV-1 replication, overexpression of the phosphatase is preferable, but this approach has the potential to be complicated by the translational and posttranslational autoregulatory mechanisms, seen in various cell lines, that serve to maintain fairly constant levels of PP2A (2, 36). To more directly assess the role of PP2A in Tat-mediated HIV-1 transactivation and replication, Ruediger et al. performed experiments using COS, HeLa, and Jurkat T cells and an N-terminal deletion mutant of the A subunit, which lacked the ability to bind the B subunit (34). This led to an increase in the ratio of core enzyme (AC) to holoenzyme (ABC), with the subsequent effect of decreasing Tat stimulation of viral replication and transcription. Since the PP2A core enzyme possesses phosphatase activity and may interact with targets different from those of the holoenzyme, these results imply that the intracellular levels of various forms of PP2A can potentially have differing effects that may, in part, be cell line dependent.
We have been able to achieve overexpression of PP2A, as determined by enzymatic assays, by using a cytomegalovirus-driven expression vector in U937 monocytic cells (Fig. 1C). This has enabled us to directly determine the effect of global increases in the overall PP2A phosphatase activity on HIV-1 transcription and proviral induction. Several recently published reports have used similar expression vectors to successfully achieve PP2A overexpression in a number of cell lines (2, 8, 25, 26). Ectopic PP2A alone somewhat enhances HIV-1 promoter-driven gene transcription (Fig. 1A) and greatly potentiates the response to PKC stimulation by PMA but not to the cytokine TNF-α (Fig. 1B and 2A). The absence of a demonstrable effect of ectopic PP2A on the response of the HIV-1 promoter to TNF-α would suggest that NF-κB is not involved, since TNF-α acts more specifically via the κB response elements than does PMA (19). This was supported by the minimal contribution of the κB elements to the PP2A response seen upon direct testing (data not shown).
This study demonstrates that the stimulation of HIV-1 by PP2A is much more marked in the presence of PMA, a stimulator of PKC. This is consistent with previous findings with the HIV-2 promoter, where PP2A and PKC act together through the pets enhancer element and other sites to activate HIV-2 transcription (14). PP2A is known to exist in complexes with various kinases, allowing for rapid modifications of the phosphorylation state of the kinases or substrates (35). The alpha isoform of PKC (PKCα) phosphorylates PP2Ac in vitro and is dephosphorylated and inactivated by PP2A (31). Our findings here and in an earlier report (14) suggest that at least some forms of PP2A and PKC can function synergistically rather than as antagonists, as might have been predicted on the basis of previous studies.
Many previous studies have focused on the role of kinases in the regulation of HIV transcription and replication. Our report demonstrates that phosphatases such as PP2A play an important role in regulating HIV-1 promoter-mediated gene expression and viral induction. The PP2A inhibitor FST, which has been used in clinical trials for the treatment of cancer, has not proved overly toxic in patients despite the importance of PP2A to cellular function (10-13, 20, 32, 40, 41). Thus, our demonstration that PP2A stimulates HIV-1 induction and that inhibition of PP2A activity by FST diminishes viral growth suggests that PP2A is an interesting new target for the therapy of HIV-1 infection.
Acknowledgments
This work was supported by grants to D.M.M. from the National Institute of Allergy and Infectious Diseases (grant 36685) and the American Cancer Society. Additional support for N.E.F. was obtained through a Rackham Merit Fellowship from the University of Michigan. N.E.F., B.R.L., and P.J.B. were supported by the Medical Scientist Training Program of the University of Michigan (National Institutes of Health grant NIGMS T32 GM07863). B.R.L. received additional funds from a Molecular Mechanisms of Microbial Pathogenesis training grant (NIH grant AI 07528) and the Harvey Fellows Program.
We thank Richard Honkanen for helpful advice, Marc Mumby for supplying the PP2Ac expression vectors, and Gloria Wanty for manuscript preparation.
REFERENCES
- 1.Aydin, H. H., N. Selvi, G. Saydam, M. Tobu, S. Uzunoglu, R. Uslu, F. Buyukkececi, and S. B. Omay. 2000. Up-regulation of serine/threonine protein phosphatase type 2A regulatory subunits during methylprednisolone-induced differentiation of leukaemic HL-60 cells. Clin. Lab. Haematol. 22:271-274. [DOI] [PubMed] [Google Scholar]
- 2.Baharians, Z., and A. H. Schonthal. 1998. Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 273:19019-19024. [DOI] [PubMed] [Google Scholar]
- 3.Baldari, C. T., G. Macchia, A. Massone, and J. L. Telford. 1992. p21ras contributes to HIV-1 activation in T-cells. FEBS Lett. 304:261-264. [DOI] [PubMed] [Google Scholar]
- 4.Bell, B., and I. Sadowski. 1996. Ras-responsiveness of the HIV-1 LTR requires RBF-1 and RBF-2 binding sites. Oncogene 13:2687-2697. [PubMed] [Google Scholar]
- 5.Chang, Y. Y., S. J. Kim, T. K. Park, S. S. Kang, M. J. Ha, J. F. Mushinski, and J. S. Chun. 1998. Modulation of MAP kinase signaling and growth characteristics by the overexpression of protein kinase C in NIH3T3 cells. Biochem. Mol. Biol. Int. 45:1139-1148. [DOI] [PubMed] [Google Scholar]
- 6.Chen, P., E. Flory, A. Avots, B. W. Jordan, F. Kirchhoff, S. Ludwig, and U. R. Rapp. 2000. Transactivation of naturally occurring HIV-1 long terminal repeats by the JNK signaling pathway. The most frequent naturally occurring length polymorphism sequence introduces a novel binding site for AP-1 factors. J. Biol. Chem. 275:20382-20390. [DOI] [PubMed] [Google Scholar]
- 7.Chun, R. F., O. J. Semmes, C. Neuveut, and K. T. Jeang. 1998. Modulation of Sp1 phosphorylation by human immunodeficiency virus type 1 Tat. J. Virol. 72:2615-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, H., and D. L. Brautigan. 1999. Protein phosphatase 2A suppresses MAP kinase signalling and ectopic protein expression. Cell. Signal. 11:575-580. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, S. 1995. Role of macrophages in the pathogenesis of human immunodeficiency virus (HIV) infection. Aust. N. Z. J. Med. 25:777-783. [DOI] [PubMed] [Google Scholar]
- 10.de Jong, R. S., E. G. de Vries, S. Meijer, P. E. de Jong, and N. H. Mulder. 1998. Renal toxicity of the anticancer drug fostriecin. Cancer Chemother. Pharmacol. 42:160-164. [DOI] [PubMed] [Google Scholar]
- 11.de Jong, R. S., E. G. de Vries, and N. H. Mulder. 1997. Fostriecin: a review of the preclinical data. Anticancer Drugs 8:413-418. [PubMed] [Google Scholar]
- 12.de Jong, R. S., N. H. Mulder, D. R. Ugles, D. T. Sleijfer, F. J. Hoppener, H. J. Groen, P. H. Willemse, W. T. van der Graaf, and E. G. de Vries. 1999. Phase I and pharmacokinetic study of the topoisomerase II catalytic inhibitor fostriecin. Br. J. Cancer 79:882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong, S., J. G. Zijlstra, N. H. Mulder, and E. G. de Vries. 1991. Lack of cross-resistance to fostriecin in a human small-cell lung carcinoma cell line showing topoisomerase II-related drug resistance. Cancer Chemother. Pharmacol. 28:461-464. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner, N. E., J. M. Hilfinger, and D. M. Markovitz. 2001. Protein phosphatase 2A (PP2A) activates the HIV-2 promoter through enhancer elements that include the pets site. J. Biol. Chem. 276:25804-25812. [DOI] [PubMed] [Google Scholar]
- 15.Flory, E., C. K. Weber, P. Chen, A. Hoffmeyer, C. Jassoy, and U. R. Rapp. 1998. Plasma membrane-targeted Raf kinase activates NF-κB and human immunodeficiency virus type 1 replication in T lymphocytes. J. Virol. 72:2788-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folgueira, L., A. Algeciras, W. S. MacMorran, G. D. Bren, and C. V. Paya. 1996. The Ras-Raf pathway is activated in human immunodeficiency virus-infected monocytes and participates in the activation of NF-κB. J. Virol. 70:2332-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, A., S. Cereghini, and E. Sontag. 2000. Protein phosphatase 2A and phosphatidylinositol 3-kinase regulate the activity of Sp1-responsive promoters. J. Biol. Chem. 275:9385-9389. [DOI] [PubMed] [Google Scholar]
- 18.Gendelman, H. E., and M. S. Meltzer. 1989. Mononuclear phagocytes and the human immunodeficiency virus. Curr. Opin. Immunol. 2:414-419. [DOI] [PubMed] [Google Scholar]
- 19.Hannibal, M. C., D. M. Markovitz, N. Clark, and G. J. Nabel. 1993. Differential activation of human immunodeficiency virus type 1 and 2 transcription by specific T-cell activation signals. J. Virol. 67:5035-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastie, C. J., and P. T. Cohen. 1998. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 431:357-361. [DOI] [PubMed] [Google Scholar]
- 21.Kagnoff, M. F., and K. A. Roebuck. 1999. Human immunodeficiency virus type 1 (HIV-1) infection and expression in intestinal epithelial cells: role of protein kinase A and C pathways in HIV-1 transcription. J Infect. Dis. 179(Suppl. 3):S444-S447. [DOI] [PubMed] [Google Scholar]
- 22.Lembo, D., A. Angeretti, M. Gamboli, R. Cavallo, and S. Landolfo. 1995. Modulation of HIV-LTR activity by ras oncogenes. New Microbiol. 18:111-116. [PubMed] [Google Scholar]
- 23.Marquardt, B., D. Frith, and S. Stabel. 1994. Signalling from TPA to MAP kinase requires protein kinase C, raf and MEK: reconstitution of the signalling pathway in vitro. Oncogene 9:3213-3218. [PubMed] [Google Scholar]
- 24.Morita, K., M. Nishikawa, K. Kobayashi, K. Deguchi, M. Ito, T. Nakano, H. Shima, M. Nagao, T. Kuno, C. Tanaka, et al. 1992. Augmentation of retinoic acid-induced granulocytic differentiation in HL-60 leukemia cells by serine/threonine protein phosphatase inhibitors. FEBS Lett. 314:340-344. [DOI] [PubMed] [Google Scholar]
- 25.Ogris, E., X. Du, K. C. Nelson, E. K. Mak, X. X. Yu, W. S. Lane, and D. C. Pallas. 1999. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J. Biol. Chem. 274:14382-14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogris, E., I. Mudrak, E. Mak, D. Gibson, and D. C. Pallas. 1999. Catalytically inactive protein phosphatase 2A can bind to polyomavirus middle tumor antigen and support complex formation with pp60c-src. J. Virol. 73:7390-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira, L. A., K. Bentley, A. Peeters, M. J. Churchill, and N. J. Deacon. 2000. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res. 28:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potts, B. 1990. In A. Aldovini and B. D. Walker (ed.), Techniques in HIV research. Stockton Press, New York, N.Y.
- 29.Qiu, Z. H., and C. C. Leslie. 1994. Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2. J. Biol. Chem. 269:19480-19487. [PubMed] [Google Scholar]
- 30.Ratner, L., A. Fisher, L. L. Jagodzinski, H. Mitsuya, R. S. Liou, R. C. Gallo, and F. Wong-Staal. 1987. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res. Hum. Retrovir. 3:57-69. [DOI] [PubMed] [Google Scholar]
- 31.Ricciarelli, R., and A. Azzi. 1998. Regulation of recombinant PKC alpha activity by protein phosphatase 1 and protein phosphatase 2A. Arch. Biochem. Biophys. 355:197-200. [DOI] [PubMed] [Google Scholar]
- 32.Roberge, M., C. Tudan, S. M. Hung, K. W. Harder, F. R. Jirik, and H. Anderson. 1994. Antitumor drug fostriecin inhibits the mitotic entry checkpoint and protein phosphatases 1 and 2A. Cancer Res. 54:6115-6121. [PubMed] [Google Scholar]
- 33.Rosen, C. A., J. G. Sodroski, and W. A. Haseltine. 1985. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell 41:813-823. [DOI] [PubMed] [Google Scholar]
- 34.Ruediger, R., N. Brewis, K. Ohst, and G. Walter. 1997. Increasing the ratio of PP2A core enzyme to holoenzyme inhibits Tat-stimulated HIV-1 transcription and virus production. Virology 238:432-443. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schonthal, A. H. 1998. Role of PP2A in intracellular signal transduction pathways. Front. Biosci. 3:D1262-D1273. [DOI] [PubMed] [Google Scholar]
- 37.Seligmann, M. 1990. Immunological features of human immunodeficiency virus disease. Bailliere's Clin. Haematol. 3:37-63. [DOI] [PubMed] [Google Scholar]
- 38.Thevenin, C., S. J. Kim, P. Rieckmann, H. Fujiki, M. A. Norcross, M. B. Sporn, A. S. Fauci, and J. H. Kehrl. 1990. Induction of nuclear factor-kappa B and the human immunodeficiency virus long terminal repeat by okadaic acid, a specific inhibitor of phosphatases 1 and 2A. New Biol. 2:793-800. [PubMed] [Google Scholar]
- 39.Vlach, J., A. Garcia, J. M. Jacque, M. S. Rodrigues, S. Michelson, and J. L. Virelizier. 1995. Induction of Sp1 phosphorylation and NF-κB-independent HIV promoter domain activity in T lymphocytes stimulated by okadaic acid. Virology 208:753-761. [DOI] [PubMed] [Google Scholar]
- 40.Walsh, A. H., A. Cheng, and R. E. Honkanen. 1997. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 416:230-234. [DOI] [PubMed] [Google Scholar]
- 41.Weinbrenner, C., C. P. Baines, G. S. Liu, S. C. Armstrong, C. E. Ganote, A. H. Walsh, R. E. Honkanen, M. V. Cohen, and J. M. Downey. 1998. Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia. Circulation 98:899-905. [DOI] [PubMed] [Google Scholar]