Abstract
Human immunodeficiency virus (HIV) protein R (Vpr) induces G2 arrest, and prolonged G2 arrest leads to apoptosis. We find that in HeLa cells the cell cycle regulatory kinase, Wee-1, is depleted following prolonged G2 arrest induced by Vpr. Of note, small interfering RNAs directed to Wee-1 triggered apoptosis, suggesting a direct role for Wee-1 in apoptosis. In support of this hypothesis, overexpression of Wee-1 suppressed Vpr-mediated apoptosis. Importantly, similar results were observed with cells induced to undergo apoptosis gamma irradiation. Thus, Wee-1 may serve as a key regulator of both HIV type 1 Vpr- and gamma irradiation-mediated apoptosis and possibly serve as a general regulator linking the cell cycle to some pathways of apoptosis.
Human immunodeficiency virus type 1 (HIV-1) protein R (Vpr) is a 14-kDa, 96-amino-acid, virion-associated protein that is highly conserved in HIV-1, HIV-2, and simian immunodeficiency virus type 1 isolates (6, 22, 36). Vpr plays an important role in facilitating infection of nondividing cells, such as macrophages (3, 10, 31, 32). We and others have shown that in various human cell lines Vpr causes G2 arrest which is associated with Cdc2 inactivation and resembles the G2 checkpoint induced by DNA damage (8, 13). Vpr also induces apoptosis following G2 arrest, the mechanism of which is unclear (2, 11, 12, 29, 30). We found that Vpr induces apoptosis through activation of caspase-3 (30), and recently Muthumani et al. reported that caspase-9 is also activated (20, 21). Jacotot et al. found that Vpr induces apoptosis through a direct effect on the mitochondria permeability transition pore (12). Therefore, Vpr may utilize some general pathways of apoptosis. Given that Vpr likely contributes to HIV-mediated immune destruction by killing target T cells, we sought to further explore the mechanism of Vpr-induced apoptosis.
The mechanisms governing progression of the cell cycle are highly conserved. The transition from G2 to M phase is regulated by Cdc2-cyclin B complex. The activity of Cdc2 kinase is controlled by the opposing effect of kinase Wee-1 and Myt1 and the phosphatase Cdc25C. Wee-1 inhibits Cdc2 activity through tyrosine phosphorylation of Cdc2, which is required to prevent premature activation of Cdc2 and maintain the timing of cell division (23). Normally, Wee-1 protein levels and activity increase in S and G2 phases but decrease during M phase as a result of phosphorylation and degradation (17, 33). Dysfunction of Wee-1 can result in inappropriate activation of Cdc2 and premature mitosis. In fission yeast, this leads to mitotic catastrophe, an aberrant mitotic process that resembles apoptosis (26).
In this study, we demonstrate that in HeLa cells apoptosis induced by both Vpr and gamma irradiation following cell cycle arrest in G2 requires a reduction in levels of Wee-1 kinase.
MATERIALS AND METHODS
siRNA.
A 21-nucleotide (nt) RNA duplex with symmetric 2-nt 3′ (2′-deoxy)thymidine overhangs corresponding to human Wee-1, encoding nt 954 to 972, was synthesized and purified (Dharmacon Research, Inc.). RNA sequences were as follows: sense, 5′ CUG GAC UUC CAG AAG AAC ATT; and antisense, 5′ UGU UCU UCU GGA AGU CCA GTT. Lamin A/C small interfering RNA (siRNA) was synthesized as described previously (5). HeLa cells (1.5 × 105 cells per well of a six-well plate) were transfected using Oligofectamine (Invitrogen) as described elsewhere (5).
Cell culture and gamma irradiation.
HeLa cells and 293T cells were maintained in Dulbecco's modified Eagle's medium with 10% bovine calf serum and penicillin-streptomycin. Aliquots of 1.5 × 105 HeLa cells per well in a six-well plate (Falcon) were seeded 1 day before exposure to 40 Gy of gamma irradiation. Medium was changed 3 h after irradiation and cells were collected and analyzed at the indicated times.
Preparation of viral stocks.
HR′Thy, HR′Vpr, and HR′Thy (Vpr+) were produced in 293T cells by transient transfection as previously described (13, 30). pHR′Wee1 was generated by cloning the full-length cDNA with a C-terminal hemagglutinin (HA) tag into a lentiviral vector derived from pHR′CMV-EGFP (1). HR′Wee1 virus was produced by cotransfection of 293T cells with 12.5 μg of pHR′Wee1, 12.5 μg of pCMVΔR8.2ΔVpr, and 5 μg of pCMVVSV-G and collected as described previously (13, 30).
Infections and flow cytometry.
Cells were infected and analyzed for both cell cycle and apoptosis as previously described (13, 30). Apoptosis was measured by double staining with Annexin V-fluorescein isothiocyanate (FITC) and 7-amino-actinomycin D (7-AAD) (Biosource). Cell cycles were measured by DNA staining with propidium iodide (PI; Sigma). All stained cells were acquired ona FACScan II (Becton Dickinson) and analyzed with the Cell Quest software package. A total of 5,000 events were collected and analyzed in each sample.
Western blotting and Cdc2 kinase assay.
Cells were lysed with lysis buffer containing 1% NP-40, 137 mM sodium chloride, 20 mM Tris, pH 7.4, 1 mM dithiothreitol, 10% glycerol, 10 mM sodium fluoride, 1 mM pyrophosphate, 2 mM sodium vanadate, and protease inhibitor cocktail (Sigma). Protein concentrations were measured using a Bradford assay (Bio-Rad). For Western blotting, cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted to nitrocellulose membrane. Blots were incubated with antibodies specific for human Wee-1 (SC-5285), Cdc2 (SC-54), and β-actin (SC-1616) (Santa Cruz Biotech). For the Cdc2 kinase assay, cell lysates containing 150 μg of total protein were precleared with 30 μl of protein A/G-Sepharose beads (Santa Cruz Biotech) for 30 min at 4°C and then incubated with 2 μl of anti-Cdc2 monoclonal antibody and 30 μl of protein A/G-Sepharose beads for 3 h at 4°C with agitation. The immunocomplexes were pelleted and washed three times with cold lysis buffer in the absence of protease and phosphatase inhibitors and twice with cold kinase buffer containing 50 mM Tris, pH 7.4, 10 mM magnesium chloride, and 1 mM dithiothreitol. The kinase reactions were performed for 30 min at 30°C in the presence of 5 μCi of [γ-32P]ATP, 33 μM ATP, and 2 μg of histone H1 as substrate (Boehringer-Mannheim). The reaction was terminated by the addition of 4× SDS sample buffer. The samples were denatured and subjected to SDS-15%-PAGE. The results were visualized and quantified using a PhosphorImager (Molecular Dynamics).
Northern blotting.
Ten micrograms of total RNA isolated using an RNeasy kit (Qiagen) was transferred to nitrocellulose membrane and hybridized with human Wee-1 (nt 648 to 1938) and β-actin cDNAs in ExpressHyb hybridization solution (Clontech) according to the manufacturer's instructions.
RESULTS
Wee-1 is depleted in HIV protein R-induced apoptotic cells.
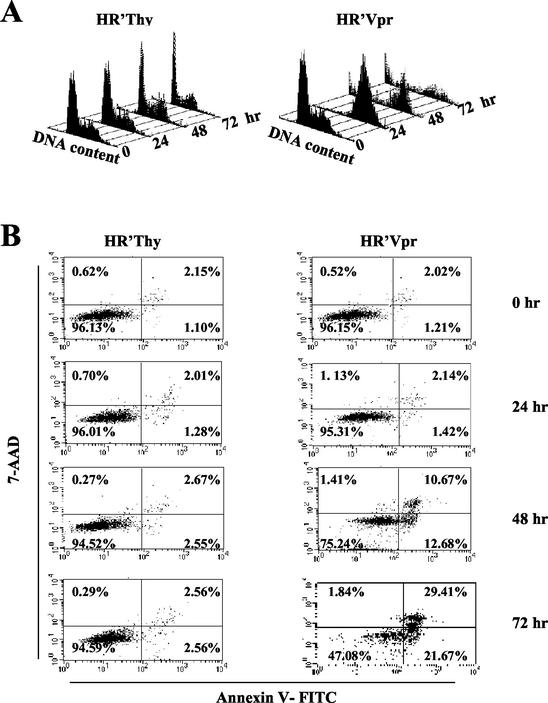
Vpr was expressed in HeLa cells with a lentiviral vector (pHR′Vpr) (30). In agreement with our previous observations, the majority (>95%) of cells were arrested in G2 within 24 h. The cells remained in G2/M and gradually died of apoptosis as evidenced by a sub-G1 fraction of cells and Annexin V staining (Fig. 1A and B). Cells undergoing apoptosis first appeared at 48 h postinfection (12.7%) and the percentage increased over time, reaching a maximum at 72 h (21.7%) following infection. The proportion of Annexin V-positive cells, representing both apoptotic and dead cells, was 51% at 72 h following infection.
FIG. 1.
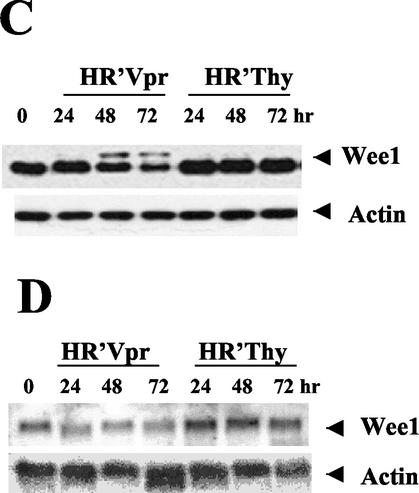
Wee-1 levels decrease in Vpr-induced apoptotic cells. HeLa cells were infected and analyzed for both cell cycle and apoptosis as previously described (9, 12). All stained cells were analyzed on a FACScan II (Becton Dickinson) and acquired by the Cell Quest software package. (A) Cell cycle profile of HeLa cells in response to HR′Vpr infection. Virus stock was produced and titrated to ensure that more than 95% of HeLa cells were arrested in G2 phase at 24 h postinfection (the equivalent of 1.2 μg of viral p24 was used for 2 × 105 HeLa cells per well of a six-well plate [Falcon]). Equivalent amounts of HR′Thy virus were used as negative control. Cells were stained with PI, and DNA profiles of 5,000 cells were analyzed at the indicated times. (B) Kinetics of apoptosis after virus infection. HeLa cells were stained with Annexin V-FITC and 7-AAD at the indicated times. Percentages of positive cells within a quadrant are indicated. The lower right quadrant represents apoptotic cells. The upper right quadrant represents dead cells. (C) Protein immunoblot of Wee-1. Twenty micrograms of cell lysate was loaded in each lane and separated by SDS-10% PAGE, followed by immunoblotting with antibodies to Wee-1 or β-actin. (D) Northern blot analysis of Wee-1 RNA. Ten micrograms of total RNA was transferred to a nitrocellulose membrane and hybridized with human Wee-1 (nt 648 to 1938) and β-actin cDNAs in ExpressHyb hybridization solution.
In cells arrested at G2/M by Vpr, Wee-1 protein levels remained high initially, consistent with the cell cycle-inhibitory role of Wee-1 at a G2/M checkpoint. However, Wee-1 levels decreased after 48 and 72 h. This decrease paralleled the increase in apoptosis over the same time period (Fig. 1C). We also observed the appearance of a slower-migrating species. This species of Wee-1 observed in apoptotic cells comigrates with that induced following nocadazole treatment of cells and is likely to represent a phosphorylated form of Wee-1 (17, 33). Similar results were observed using HR′Thy(Vpr+) virus where Vpr was introduced only as protein packaged in virions and not expressed de novo (24) (data not shown).
We analyzed Wee-1 mRNA levels over the same period of time as above and found that Wee-1 mRNA levels began to decrease at 24 h in the presence of Vpr, prior to the onset of apoptosis, but protein levels did not show a decrease until 48 h. No change was observed in control cells (Fig. 1D). Utilizing a Wee-1 promoter construct with luciferase as a reporter gene (14), we found that expression from the Wee-1 promoter is down-regulated about twofold in cells cotransfected with Vpr (data not shown).
Depletion of Wee-1 leads to apoptosis.
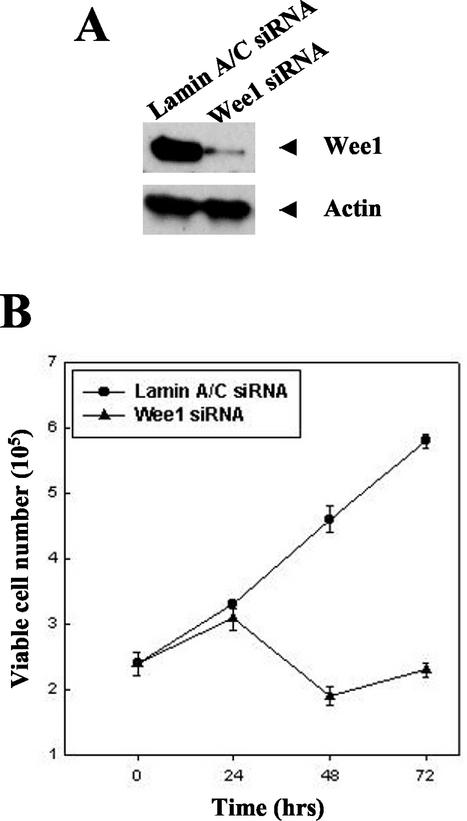
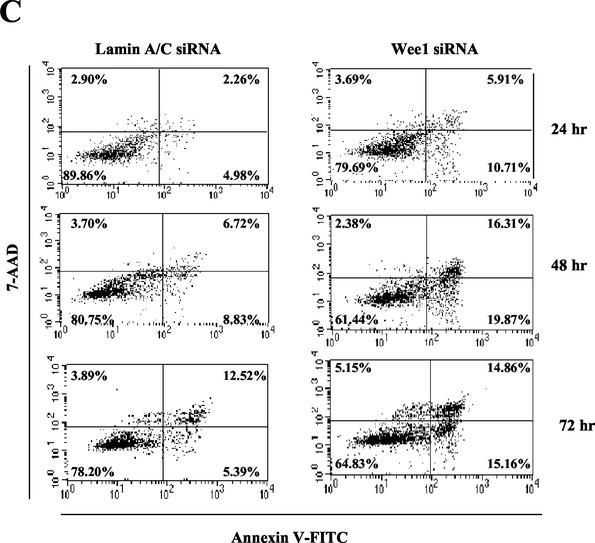
The key role of Wee-1 in the cell cycle transition at G2/M suggested a potential regulatory role in cell cycle arrest at the G2/M checkpoint and/or apoptosis that occurs following prolonged cell cycle arrest. The inverse relationship between the increasing levels of apoptosis and the decreasing levels of Wee-1 over time suggested the hypothesis that depletion of Wee-1 may be responsible for the onset of apoptosis. We tested this hypothesis by utilizing siRNA directed against Wee-1 mRNA. siRNA transfection led to decreases in Wee-1 protein levels, whereas control cells transfected with siRNA directed against Lamin A/C (5) or luciferase (data not shown) showed normal levels of Wee-1 (Fig. 2A). Of note, significant cell death was observed within 48 h following transfection with Wee-1 siRNA (Fig. 2B). Annexin V staining indicated that the cells were dying from apoptosis (Fig. 2C). Thus, specific depletion of Wee-1 induces apoptosis.
FIG. 2.
Depletion of Wee-1 by siRNA transfection leads to apoptosis. (A) Immunoblot of Wee-1. HeLa cells (1.5 × 105 cells per well of a six-well plate) were transfected with 21-nt siRNAs targeting Wee-1 or Lamin A/C as described in the text. Immunoblotting of cell lysates prepared at 24 h after transfection was performed with antibodies against Wee-1 or β-actin. Twenty micrograms of cell lysate was loaded in each lane and separated by SDS-10% PAGE, followed by immunoblotting with antibodies to Wee-1 or β-actin. (B) Cell proliferation after siRNA transfection. Cells were stained with 0.4% trypan blue and were counted under a microscope at different time points. (C) Apoptosis occurred following Wee-1 siRNA transfection. Apoptosis was measured by flow cytometric analysis of Annexin V-FITC- and 7-AAD-stained cells.
Cdc2 is activated in both Vpr and Wee-1 siRNA transfection-induced apoptotic cells.
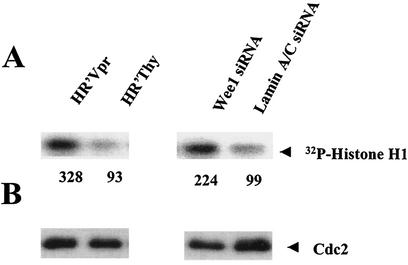
Wee-1 regulates Cdc2 kinase through phosphorylation of Tyr-15, thereby inhibiting Cdc2 kinase activity. Concomitant with decreases in Wee-1 levels, we would expect to observe increases in Cdc2 kinase activity. Histone H1 kinase assays indicated that Cdc2 kinase activity was up-regulated approximately threefold in cells rendered apoptotic both by Vpr and by siRNA directed against Wee-1 (Fig. 3).
FIG.3.
Cdc2 kinase activity is increased in HR′Vpr and Wee-1 siRNA-transfected HeLa cells. Cells were infected as described in the legend for Fig. 1 and transfected in parallel with those shown in Fig. 2. Cells were collected at 72 h post-virus infection or 24 h post-siRNA transfection. (A) Kinase assay of Cdc2. The assay was performed as described in Materials and Methods. The results were visualized and quantified using a PhosphorImager (Molecular Dynamics), and the numbers represent the relative density of the 32P-labeled histone H1. (B) Cdc2 protein level the kinase assay shown in panel A. Five micrograms of cell lysates was fractionated by SDS-12% PAGE and immunoblotted with Cdc2 antibody as described in the legend for Fig. 1. Relative Cdc2 activities were determined after normalization with the Cdc2 protein amount.
Ectopic expression of Wee-1 attenuates Vpr-induced apoptosis.
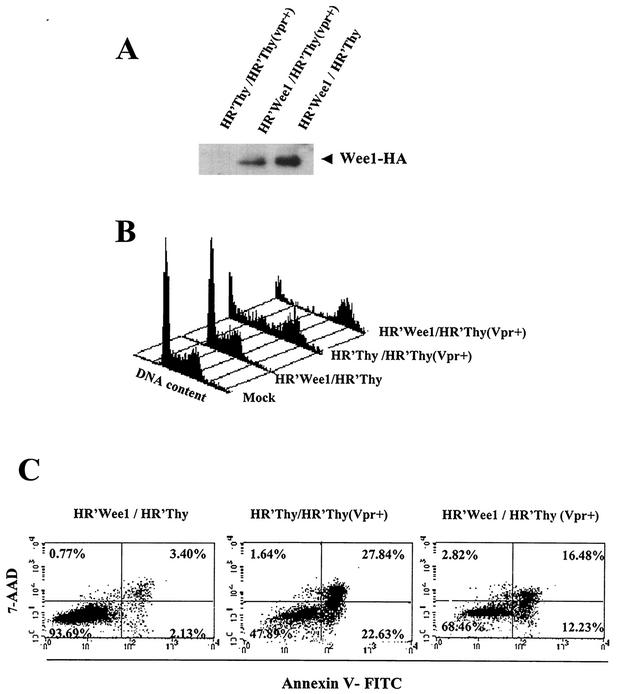
The above results show that Wee-1 levels decrease in cells where Vpr induces prolonged G2 arrest and that specific depletion of Wee-1 results in apoptosis of normal cells. To further test the hypothesis that depletion of Wee-1 is responsible for Vpr-mediated apoptosis, we introduced HA epitope-tagged Wee-1 ectopically via the lentiviral vector pHR′Wee-1. Wee-1 expression was confirmed by anti-HA immunoblotting (Fig. 4A). Introduction of exogenous Wee-1 into mock-treated cells had no effect upon either the cell cycle profile or cell death (Fig. 4B). As expected, HR′Thy (Vpr+) infection induced G2 cell cycle arrest and was followed by massive apoptosis and cell death on day 3. However, overexpression of Wee-1 resulted in a significantly reduced level of apoptosis and cell death (28.7 versus 50.4%) as measured by Annexin V staining at 72 h post-HR′Thy (Vpr+) infection (Fig. 4C). Thus, these results demonstrate that expression of Wee-1 is capable of protecting some cells from apoptosis induced by Vpr. We did not observe any changes in the extent of Vpr-mediated G2 cell cycle arrest in the presence of ectopically expressed Wee-1 (Fig. 4B); however, consistent with the Annexin V staining results, we observed significantly less sub-G1 staining in the presence of Vpr plus ectopic Wee-1 (Fig. 4B).
FIG. 4.
Ectopic expression of Wee-1 inhibits Vpr-induced apoptosis. Wee-1 with a C-terminal HA tag was expressed with a lentiviral vector derived from pHR′CMV-EGFP (1). HR′Wee1 and HR′Thy (Vpr+) viruses were made as described in the text. HR′Thy (Vpr+) packages Vpr but does not express Vpr de novo (30). HR′Thy (Vpr+) was titrated as described in the legend for Fig. 1. A virus inoculum equivalent to 2.5 μg of p24 was needed to achieve more than 95% G2 arrest in 2 × 105 HeLa cells. Cells were infected by HR′Wee-1 or HR′Thy (control) 1 day before HR′Thy (Vpr+) or HR′Thy (control) infection. Viruses with an equivalent amount of p24 were used in each infection. Cells were stained either with Annexin V and 7-AAD or with PI or were lysed for Western blotting 72 h postinfection. (A) Expression of HA-tagged Wee-1 in the coinfected HeLa cells. Cell lysates were fractionated by SDS-10% PAGE and immunoblotted with HA antibody (Convence) as described in the legend for Fig. 1. (B) Cell cycle analysis of coinfected HeLa cells. (C) Apoptosis of HeLa cells coinfected with viruses as indicated.
Wee-1 depletion correlates with gamma irradiation-induced apoptosis.
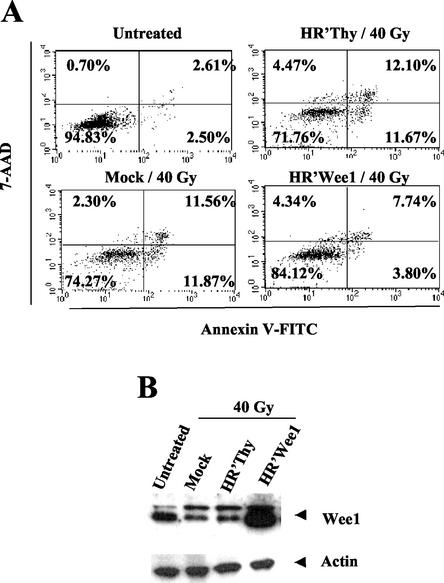
Gamma irradiation can induce cell cycle arrest and apoptosis at different stages of the cell cycle. The mechanisms are complex and appear to involve multiple pathways (25). In some cell types, including HeLa cells, DNA damage induced by gamma irradiation can induce cell cycle arrest in G2/M that phenotypically resembles Vpr-induced cell cycle arrest. If the DNA remains unrepaired, subsequent apoptosis results (7). Given these similarities in phenotype to HIV-1 Vpr, we determined whether gamma irradiation also induced a reduction in Wee-1 protein levels. Fifty hours following gamma irradiation, Wee-1 protein levels decreased. At the same time point, apoptotic cells comprised about 12% of the population (Fig. 5). Ectopic expression of Wee-1 resulted in a reduction to 3.8% apoptotic cells. Thus, similar to the above experiments with HIV-1 Vpr, overexpression of Wee-1 resulted in alleviation of apoptosis mediated by gamma irradiation. These results demonstrate that apoptosis induced by both HIV-1 Vpr and at least some forms of gamma irradiation-mediated apoptosis are affected similarly by Wee-1 levels.
FIG. 5.
Expression of exogenous Wee-1 suppresses gamma irradiation-induced apoptosis. A total of 2 × 105 HeLa cells were infected with HR′Wee-1 or HR′Thy 1 day before exposure to 40 Gy of gamma irradiation. Cells were harvested and divided equally for assessment of apoptosis and Western blotting at 50 h after irradiation, as described in the legend for Fig. 1. (A) HR′Wee-1 infection alleviated gamma irradiation-induced apoptosis. (B) Wee-1 protein levels in the samples shown in panel A.
DISCUSSION
Previous reports have conflicted regarding the correlation of Wee-1 levels and apoptosis. Some studies indicate that decreased Wee-1 levels correlate with apoptosis, including p53- and Fas-induced apoptosis (15, 38) and mitotic catastrophe in fission yeast. However, opposing results were reported in Xenopus laevis oocytes (28). Our results provide evidence that in human HeLa cells the level of Wee-1 is involved in determining the extent of apoptosis. In normal cycling cells, experimental suppression of Wee-1 leads to apoptosis. Overexpression of Wee-1 alleviates apoptosis in cells arrested in G2 by HIV-1 Vpr and gamma irradiation. We note that the alleviation of apoptosis is not complete. One possibility is that not all cells in the population express Wee-1 at sufficient levels. Alternatively, there may be other pathways for apoptosis that do not involve Wee-1.
The decrease in Wee-1 levels that parallel the onset of apoptosis is likely to be mediated by several complementary effects. Wee-1 mRNA levels decrease, likely reflecting transcriptional regulation. Wee-1 protein has also been reported to be a target for ubiquitination in Xenopus oocytes (19) and caspase-3 cleavage in human Jurkat cells (37). Vpr has been reported to result in nuclear herniations resulting in transient cytoplasmic release of Wee-1-green fluorescent protein fusion proteins (4). It is possible that this loss of subcellular compartmentalization reduces the stability and/or activity of Wee-1. We also observed a slower-migrating species of Wee-1 in apoptotic cells, likely to be a phosphorylated form of Wee-1 that has been reported to have reduced kinase activity (17, 33).
These results suggest several possibilities for mechanisms of Vpr-induced apoptosis. The consequence of the above effects on Wee-1 is a reduction of Wee-1 kinase activity within the cell. The observed enhancement of Cdc2 kinase activity is consistent with loss of the inhibitory effect of Wee-1 via Tyr-15 phosphorylation of Cdc2. The effect of Cdc2 activation is complex and has been variously reported to enhance, to inhibit, or to not be required for apoptosis, depending on the experimental system (18, 27, 35). In both fission yeast and BHK cells, inappropriate activation of Cdc2 leads to cell death with phenotypically aberrant mitotic features, a process known as mitotic catastrophe (9, 16). Consistent with our observed activation of Cdc2, cells arrested for prolonged periods of time by Vpr can enter M phase but arrest in pro-metaphase with abnormal centrosome duplication followed by apoptosis (34). Thus, one mechanism for Vpr-induced apoptosis may be through activation of aberrant mitotic processes resulting in mitotic catastrophe. Alternatively, activation of Cdc2 may also play other roles in apoptosis independent of its activities important for the initiation of mitosis. It is also possible that targets of Wee-1 other than Cdc2 may play a role in apoptosis.
Our results support the hypothesis that regulation of Wee-1 levels may be one general determinant of whether cells undergo apoptosis. Because Wee-1 is highly conserved throughout evolution from yeast to humans and plays a central role in the regulation of cell cycle progression, it is interesting to consider whether Wee-1 may be a key link between cell cycle pathways and apoptotic pathways and may serve as a general regulator of many forms of apoptosis in cells. Further investigation of the mechanism by which Wee-1 induces apoptosis may provide insight into means to inhibit HIV-1-induced cell death.
Acknowledgments
We thank T. Hunter for human Wee-1 cDNA and S. Shiozawa for the human Wee-1 promoter-controlled luciferase reporter gene construct.
This work was supported by National Institutes of Health grants CA70018, AI43190, and AI39975.
REFERENCES
- 1.An, D. S., R. P. Wersto, B. Agricola, M. Metzger, S. Lu, R. G. Amado, I. S. Y. Chen, and R. E. Donahue. 2000. Marking and gene expression by a lentiviral vector in transplanted human and nonhuman primate CD34+ cells. J. Virol. 74:1286-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyavoo, V., A. Mahboubi, S. Mahalingam, R. Ramalingam, S. Kudchodkar, W. V. Williams, D. R. Green, and D. B. Weiner. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 3:1117-1123. [DOI] [PubMed] [Google Scholar]
- 3.Balotta, C., P. Lusso, R. Crowley, R. C. Gallo, and G. Franchini. 1993. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J. Virol. 67:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 6.Emerman, M. 1996. HIV-1, Vpr and the cell cycle. Curr. Biol. 6:1096-1103. [DOI] [PubMed] [Google Scholar]
- 7.Enoch, T., and C. Norbury. 1995. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem. Sci. 20:426-430. [DOI] [PubMed] [Google Scholar]
- 8.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heald, R., M. McLoughlin, and F. McKeon. 1993. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell 74:463-474. [DOI] [PubMed] [Google Scholar]
- 10.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki, H., K. Komai, Z. Ouyang, M. Murata, M. Hikasa, M. Ohgiri, and S. Shiozawa. 2001. c-Fos/activator protein-1 transactivates wee1 kinase at G1/S to inhibit premature mitosis in antigen-specific Th1 cells. EMBO J. 20:4618-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach, S. D., C. D. Scatena, C. J. Keefer, H. A. Goodman, S. Y. Song, L. Yang, and J. A. Pietenpol. 1998. Negative regulation of Wee1 expression and Cdc2 phosphorylation during p53-mediated growth arrest and apoptosis. Cancer Res. 58:3231-3236. [PubMed] [Google Scholar]
- 16.Lundgren, K., N. Walworth, R. Booher, M. Dembski, M. Kirschner, and D. Beach. 1991. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64:1111-1122. [DOI] [PubMed] [Google Scholar]
- 17.McGowan, C. H., and P. Russell. 1995. Cell cycle regulation of human Wee1. EMBO J. 14:2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meikrantz, W., and R. Schlegel. 1996. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J. Biol. Chem. 271:10205-10209. [DOI] [PubMed] [Google Scholar]
- 19.Michael, W. M., and J. Newport. 1998. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science 282:1886-1889. [DOI] [PubMed] [Google Scholar]
- 20.Muthumani, K., D. S. Hwang, B. M. Desai, D. Zhang, N. S. Dayes, D. R. Green, and D. B. Weiner. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277:37820-37831. [DOI] [PubMed] [Google Scholar]
- 21.Muthumani, K., D. Zhang, D. S. Hwang, S. Kudehodkar, N. S. Dayes, B. M. Desai, A. S. Malik, J. S. Yang, M. A. Chattergoon, H. C. Maguire, Jr., and D. B. Weiner. 2002. Adenovirus encoding HIV-1 Vpr activates caspase 9 and induces apoptotic cell death in both p53 positive and negative human tumor cell lines. Oncogene 30:4613-4625. [DOI] [PubMed] [Google Scholar]
- 22.Myers, G., B. H. Hahn, J. W. Mellors, L. E. Henderson, B. Korber, K.-T. Jeang, F. E. McCutchan, and G. N. Pavlakis. 1995. Human retroviruses and AIDS. Theoretical Biology and Biophysics, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 23.Nurse, P. 1997. Checkpoint pathways come of age. Cell 91:865-867. [DOI] [PubMed] [Google Scholar]
- 24.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Y. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 25.Rich, T., R. L. Allen, and A. H. Wyllie. 2000. Defying death after DNA damage. Nature 407:777-783. [DOI] [PubMed] [Google Scholar]
- 26.Russell, P., and P. Nurse. 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49:559-567. [DOI] [PubMed] [Google Scholar]
- 27.Shi, L., W. K. Nishioka, J. Th'ng, E. M. Bradbury, D. W. Litchfield, and A. H. Greenberg. 1994. Premature p34cdc2 activation required for apoptosis. Science 263:1143-1145. [DOI] [PubMed] [Google Scholar]
- 28.Smith, J. J., E. K. Evans, M. Murakami, M. B. Moyer, M. A. Moseley, G. V. Woude, and S. Kornbluth. 2000. Wee1-regulated apoptosis mediated by the crk adaptor protein in Xenopus egg extracts. J. Cell Biol. 151:1391-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Y. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbramanian, R. A., A. Kessous-Elbaz, R. Lodge, J. Forget, X. J. Yao, D. Bergeron, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med. 187:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe, N., T. Yamaguchi, Y. Akimoto, J. B. Rattner, H. Hirano, and H. Nakauchi. 2000. Induction of M-phase arrest and apoptosis after HIV-1 Vpr expression through uncoupling of nuclear and centrosomal cycle in HeLa cells. Exp. Cell Res. 258:261-269. [DOI] [PubMed] [Google Scholar]
- 35.Yao, S. L., K. A. McKenna, S. J. Sharkis, and A. Bedi. 1996. Requirement of p34cdc2 kinase for apoptosis mediated by the Fas/APO-1 receptor and interleukin 1β-converting enzyme-related proteases. Cancer Res. 56:4551-4555. [PubMed] [Google Scholar]
- 36.Yu, X. F., M. Matsuda, M. Essex, and T. H. Lee. 1990. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J. Virol. 64:5688-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, B. B., H. Li, J. Yuan, and M. W. Kirschner. 1998. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 95:6785-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou, Y., Y. Lu, and L. Ratner. 1998. Arginine residues in the C terminus of HIV-1 Vpr are important for nuclear localization and cell cycle arrest. Virology 242:414-424. [DOI] [PubMed] [Google Scholar]