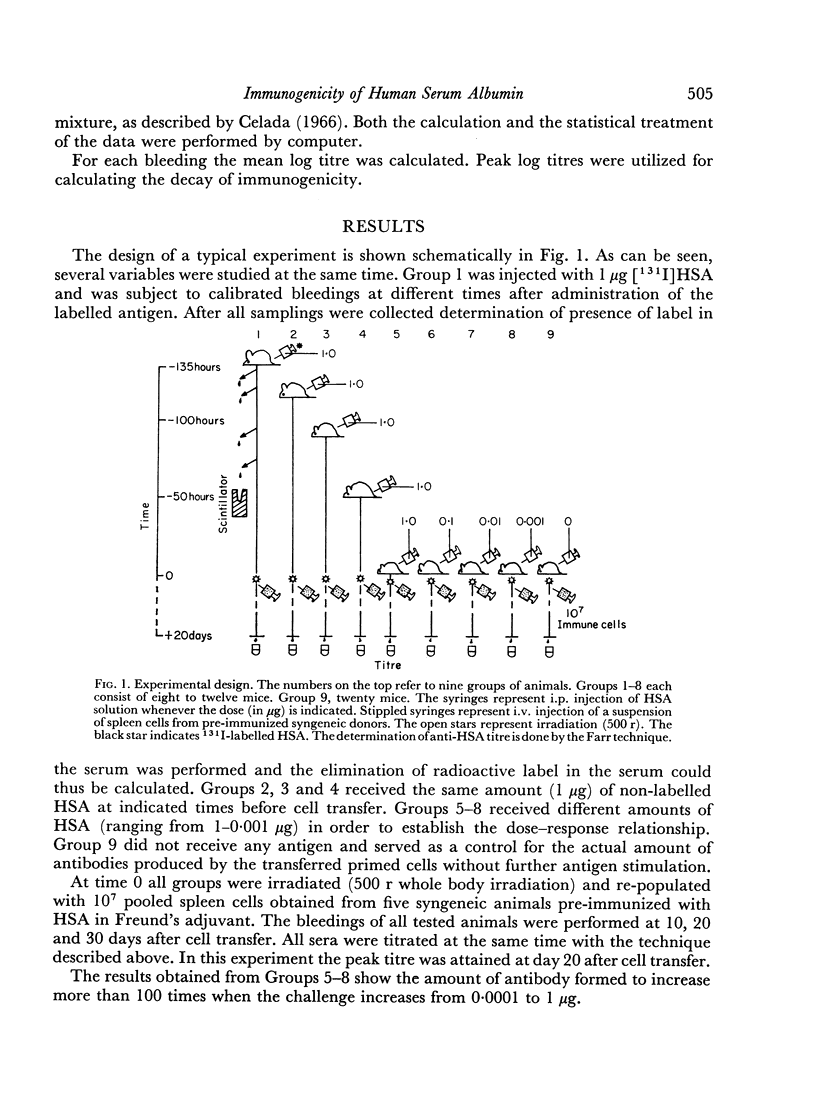
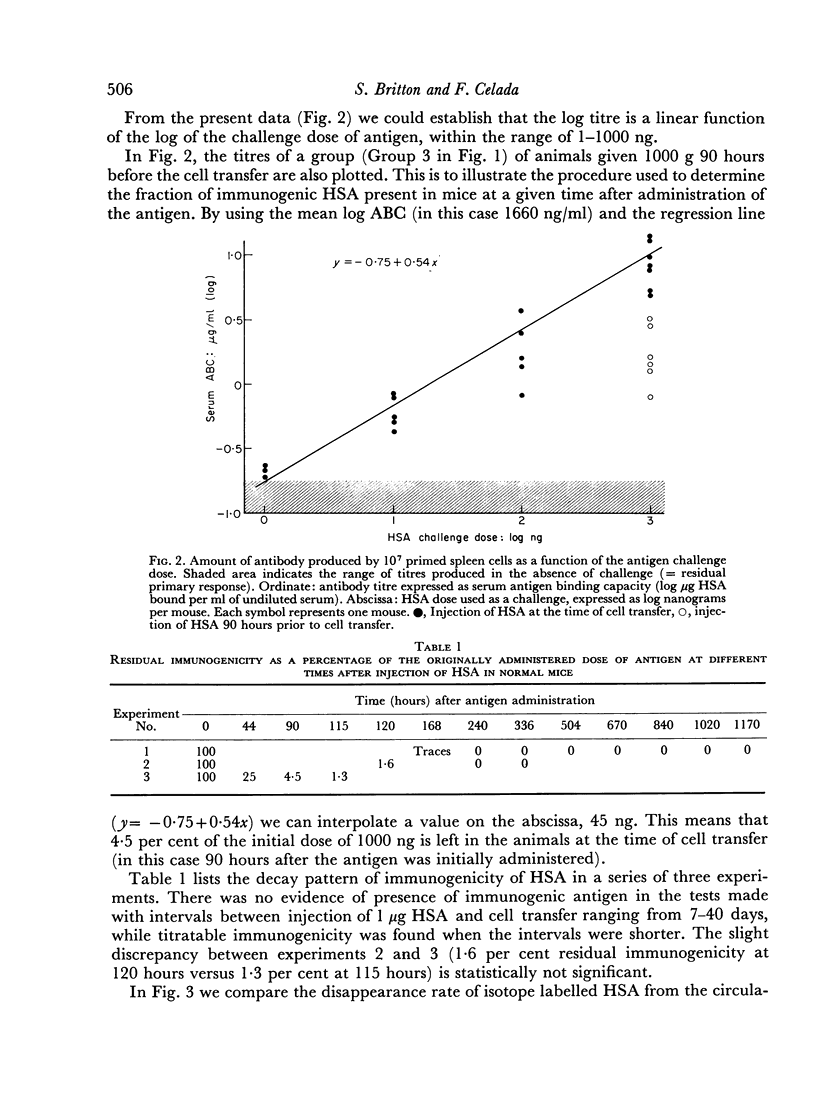
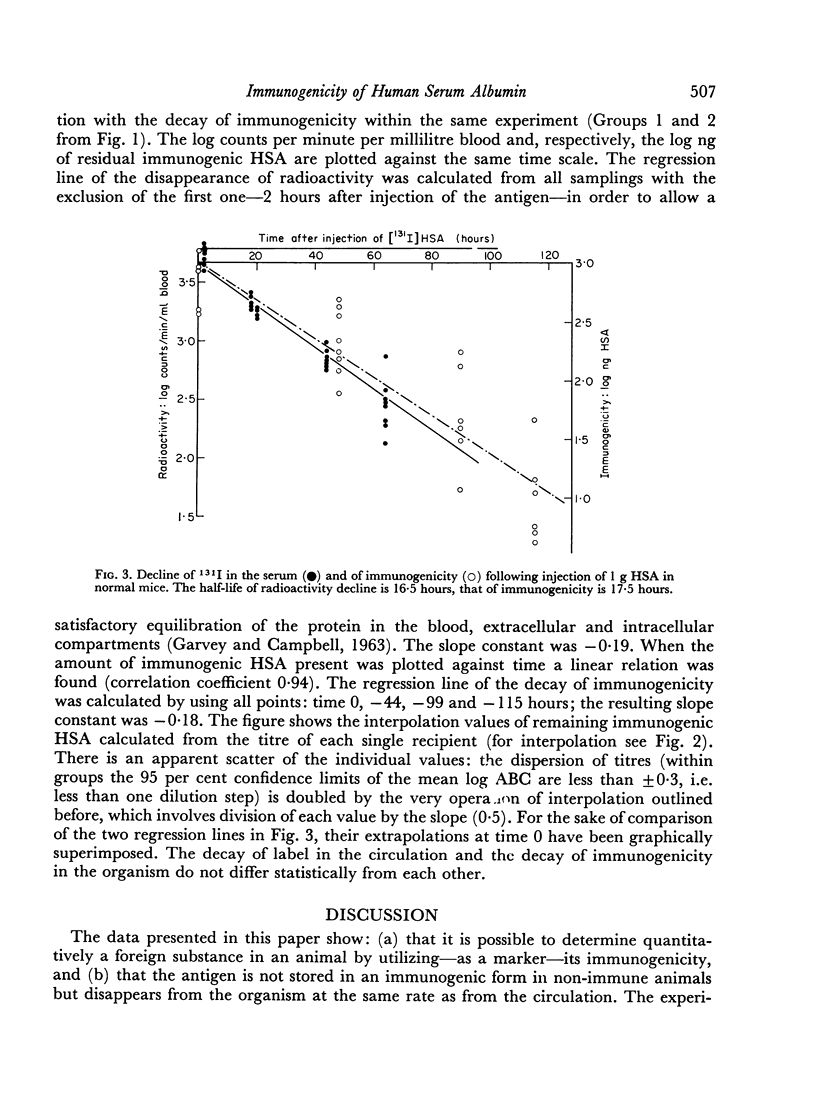
Abstract
The disappearance of immunogenicity of human serum albumin (HSA) was measured by injection of 1 μg of this protein in normal mice and by transferring to them, at intervals, a fixed number of HSA-sensitized syngeneic spleen cells: subsequently the antigen binding capacity of the serum was determined. This system served to reveal the residual amount of immunogen present in the animal, since under these conditions the adoptive secondary response is proportional to the HSA available, and no antibody is formed by the host. The half-life of HSA immunogenicity was 17½ hours. A similar rate of decline (T = 16½ hours) was found by following 131I coupled to the HSA in the circulation of the same mice. This demonstrates that the albumin antigen is not stored in an active form in non-pre-immunized mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton S., Wepsic T., Möller G. Persistence of immunogenicity of two complex antigens retained in vivo. Immunology. 1968 Apr;14(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F. Quantitative studies of the adoptive immunological memory in mice. II. Linear transmission of cellular memory. J Exp Med. 1967 Feb 1;125(2):199–211. doi: 10.1084/jem.125.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETRICH F. M., WEIGLE W. O. Induction of tolerance to heterologous proteins and their catabolism in C57BL/6 mice. J Exp Med. 1963 Apr 1;117:621–631. doi: 10.1084/jem.117.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Askonas B. A., Humphrey J. H., Schechter I., Sela M. The localization of antigen in relation to specific antibody-producing cells. I. Use of a synthetic polypeptide [(T,G)-A--L] labelled with iodine-125. Immunology. 1966 Oct;11(4):337–351. [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. Recovery from immunological paralysis in relation to age and residual antigen. Immunology. 1965 Aug;9(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä O., Mitchison N. A. The effect of antigen dosage on the response of adoptively transferred cells. Immunology. 1965 Jun;8(6):549–556. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Ada G. L., Austin C. M., Pye J. Antigens in immunity. 8. Localization of 125-I-labelled antigens in the secondary response. Immunology. 1965 Oct;9(4):349–357. [PMC free article] [PubMed] [Google Scholar]
- PERKINS E. H., MAKINODAN T. THE SUPPRESSIVE ROLE OF MOUSE PERITONEAL PHAGOCYTES IN AGGLUTININ RESPONSE. J Immunol. 1965 May;94:765–777. [PubMed] [Google Scholar]


