Abstract
The human papillomavirus (HPV) E2 protein plays an important role in viral DNA replication. Many studies with high-risk HPVs have demonstrated that the E2 protein can also repress transcription of the E6 and E7 oncogenes. This conclusion, based on experiments carried out with cervical cancer cells bearing integrated HPV genomes, is currently assumed to be applicable to the normal HPV life cycle, in which the viral genomes are episomal. Here, we have tested experimentally whether this assumption is correct. We made use of a pair of isogenic cell lines, W12 and S12. W12 cells contain episomal HPV16 genomes, whereas S12 cells, which are derived from the W12 line, contain HPV DNA as integrated copies. When we expressed E2 in S12 cells, we observed strong repression of E6 and E7 transcription. In contrast, no effect of E2 on the transcription of these genes was detected in W12 cells. While integration of the viral genome into the host DNA contributes to the difference between W12 and S12 cells, integration by itself is not sufficient to explain this difference. Instead, the chromatin structure in the region of the E6 and E7 promoter (p97), which we show to be very different in these two cell lines, is likely to be the cause of the different responsiveness of p97 to the E2 protein. Experiments with the histone deacetylase inhibitor trichostatin A (TSA) indicated that the episomal HPV16 DNA is in a relatively inaccessible state prior to TSA treatment. Our results, together with those of others, suggest that any effect of the E2 protein on the expression of the E6 and E7 genes during the normal viral life cycle is of secondary importance compared to the function of E2 in replication.
Cervical cancer is one of the leading causes of death from cancer among women worldwide. The principal factor responsible for this cancer is infection of the cervical epithelium by some types of human papillomaviruses (HPVs). The HPV capsid proteins enclose the viral minichromosome, which is composed of approximately 8,000 bp of circular double-stranded DNA, in the form of nucleosomes similar to those of cellular chromatin. The HPV type 16 (HPV16) DNA encodes eight proteins required for viral DNA replication, encapsidation, and virus release. In addition, there is a noncoding region called the long control region (LCR), which contains enhancer elements and a promoter (p97 in HPV16 and p105 in HPV18) from which the viral oncogenes E6 and E7 are transcribed. The E7 protein binds and inactivates the pRb tumor suppressor protein and in some instances targets its destruction (16, 20, 32, 33). This results in the release of the E2F transcription factor and the consequent expression of many S-phase genes whose promoters are responsive to E2F. The E6 protein exhibits many different effects in the cell, of which destruction of the p53 tumor suppressor protein (32, 39, 40) and the activation of telomerase (28, 29) are two that contribute to tumorigenesis. Overexpression of the E6 and E7 proteins is thought to be responsible for initiating and maintaining processes that lead to cervical cancer.
The expression of the E6 and E7 oncogenes can be influenced by another virus-encoded protein, E2. The E2 protein is primarily involved in the replication of the HPV DNA. This 42-kDa nuclear protein physically associates with the viral E1 helicase (25) and tethers the latter to the origin of replication on the viral DNA (9, 18, 30, 42, 52). In transient reporter assays, E2 was also shown to be able to activate or repress transcription of the early genes (1, 3, 23, 45, 50). However, when E2 is ectopically expressed at high levels in cells that harbor integrated HPV genomes, the p97 promoter of HPV16 or the p105 promoter of HPV18 is very effectively repressed, causing a sharp reduction in E6 and E7 transcription (8, 13, 21, 34). This repression is brought about by the binding of the E2 protein to the palindromic sequences ACC(N6)GGT (E2-binding sites) located in close proximity to the promoter region in the LCR. The binding of E2 proteins to these sites is thought to exclude the Sp1 and TFIID transcription factors from the p97 and p105 promoter, hence silencing it (6, 7, 10, 12, 19, 24, 48, 49).
This observation takes on greater significance when coupled to the fact that in most cervical cancers, the HPV DNA is integrated in the cellular genome (2, 35, 41, 53) with preservation of the E6 and E7 genes and loss of the E2 open reading frame (ORF) (31, 37, 41). These are the key observations on which the HPV-induced tumorigenesis model is built. This model posits that overexpression of the E6 and E7 genes initiates events that lead to cervical cancer and that E2 is able to limit this by repressing the transcription of the oncogenes. It follows that disruption of the E2 ORF during HPV DNA integration would confer a growth advantage to the cell due to augmented amounts of E6 and E7 proteins (27, 37). This will set the cell on the journey toward tumorigenesis, a fate that is not compatible with HPV replication. It is assumed that in the normal life cycle of HPV, the E2 protein also represses E6 and E7 transcription from the episomal HPV16 minichromosome. However, the ability of E2 to repress E6 and E7 transcription has been demonstrated only in cells harboring integrated HPV DNA, that is, in cells where the viral life cycle has been disrupted, but not in cells containing viral episomes. While extrapolation of the repressive activity of the E2 protein to the normal life cycle of the virus seems reasonable, it is not supported by in situ hybridization analyses of episomal HPV DNA-containing cervical lesions (4, 15, 22, 46). These analyses did not detect any evidence of repression of E6 and E7 transcription. Rather, they revealed a steady increase of E6 and E7 transcripts from the lower layers of the epithelium to the top while E2 transcripts were readily detected. However, it remains possible that the increase in E6 and E7 transcripts is due to an increased amount of HPV DNA templates formed during replication and a resulting increased rate of E6 and E7 transcription. It could be that, if not for E2's repressive activity, the increase of E6 and E7 transcripts would be even greater, to a level detrimental to the viral life cycle.
These assumptions need to be tested experimentally. In view of the importance of HPV and cervical cancers, we undertook this task with the aim of verifying the model or, if necessary, revising it based on experimental data. We tested the assumption that the E2 protein represses E6 and E7 transcription from episomal HPV16 minichromosomes, in the same way as it does on integrated HPV DNA. We investigated this by employing a pair of isogenic human cervical keratinocyte cell lines in which one of the isogenic partners harbored HPV16 minichromosomes while the other partner harbored integrated HPV16 DNA. We observed that while the E2 protein can efficiently repress the p97 promoter of integrated HPV16 DNA, it has no effect on this promoter in episomal HPV16 genomes. Our experiments also show that this difference is not due merely to different states of the HPV DNA (episomal or integrated) but is more likely to be associated with the chromatin conformation of the p97 promoter region. We propose that these and other recent observations call for a reassessment of the current model of E2 function in the life cycle of HPV.
MATERIALS AND METHODS
Cell culture.
W12 20863 cells were cultured with lethally irradiated Swiss 3T3 feeder cells. The cells were cultured in S12 medium, which consists of a mix of one-quarter Dulbecco's modified eagle's medium (Gibco) and three-quarters Ham F-12 medium (Gibco) containing 5% fetal calf serum, penicillin, streptomycin, and supplements (all from Sigma) as follows: 8.4 ng of cholera toxin/ml, 5 μg of insulin/ml, 24.3 μg of adenine/ml, 0.5 μg of hydrocortisone/ml, and 10 ng of epithelial growth factor per ml. Cells were split when they reached 80% confluence. S12 cells were obtained by collecting surviving W12 cells cultured without feeder support. S12 and HaCat cells, which are spontaneously immortalized human keratinocytes, are maintained in S12 medium. Both cell lines were infected with recombinant adenovirus strains rAd5 and rAdE2 at a multiplicity of infection (MOI) between 5 and 10. CIN612 9E cells, which derived from a HPV31b-positive CIN I grade lesion, were grown in E medium (26, 36) and antibiotics with lethally irradiated fibroblast feeders. W12 and CIN612 9E cells were infected with rAd5 and rAdE2 at MOI of 5 to 10. HeLa HPV18-transformed human keratinocytes, SiHa, and CasKi cell lines (HPV16-transformed human keratinocytes) were grown in Dulbecco's modified eagle's medium containing 10% fetal calf serum and antibiotics. HeLa, SiHa, and CasKi cells were all infected with rAd5 and rAdE2 at MOI of 1 to 5. All cells used were grown as monolayer cultures at 37°C with 5% CO2.
HPV16 episome extraction.
After having washed the cells with phosphate-buffered saline (PBS) and removed the feeder cells from W12 cells by washing them with 0.1 M EDTA, cells were trypsinized and resuspended in 0.5 ml of PBS at a concentration of 2 × 106 cells/ml. The pellet was lysed by gently adding an equal volume of 2× Hirt buffer (2% sodium dodecyl sulfate, 2 mM EDTA) to the cells. The tubes were left at room temperature for 10 min. NaCl (0.25 ml; 5 M) was added, and the tubes were gently inverted three times and stored overnight at 4°C. The next day, the genomic DNA was pelleted for 30 min at 9,000 × g at 4°C. The supernatant was gently harvested, and DNA was extracted with 1 volume of phenol-chloroform. The tubes were centrifuged 5 min at 9,000 × g at room temperature, and the upper phase was harvested. The DNA was precipitated with 2 volumes of 100% ethyl alcohol and stored overnight at −20°C. The tubes were centrifuged at 9,000 × g at 4°C for 30 min. The DNA pellets were washed with 1 ml of 75% ethyl alcohol. The pellets were recovered after rapid centrifugation (5 min at 9,000 × g) at room temperature and resuspended in Tris-EDTA (pH 8) buffer. Equivalent amounts of DNA were digested with BamHI (Roche) and analyzed by Southern blotting.
Total DNA extraction.
Cells were placed on ice and washed once with PBS. The cell pellets were resuspended in 500 μl of PBS and lysed with 500 μl of 2× Hirt buffer. Proteinase K (100 μg/ml) (Roche) was added to the lysate, which was then incubated 2 h at 65°C. Genomic DNA was sheared for 45 s with a rotor-stator homogenizer (Kinematica AG). Total cellular DNA was recovered after two phenol-chloroform extractions followed by ethanol precipitation and resuspended in Tris-EDTA buffer. Equal amounts of DNA were digested with BamHI or PstI (Roche) according to the manufacturer's instructions or left undigested and observed by Southern blot analysis.
Southern blot analysis.
DNA samples were separated on a 1% agarose gel, transferred to a nylon membrane (Hybond N+), and probed with a 32P-labeled HPV16-specific probe. Hybridizing species were visualized by autoradiography. HPV DNA quantification in W12 and S12 cells was done by exposing the nylon membrane to an imaging plate (Fujifilm). This screen was scanned through a fluorescent spectrophotometer (Raytest) and analyzed with the AIDA software (Fuji).
RNA extraction and Northern blot analysis.
Total cellular RNA was extracted by using the RNeasy minikit (Qiagen) according to the manufacturer's instructions. Equal amounts of RNA samples as determined by UV absorption were separated on a 1% agarose formaldehyde gel and were transferred to a nylon membrane (Hybond N+). 32P-labeled DNA probes comprising the E6-E7 sequence from HPV16 DNA were used for the analysis of W12 and S12 RNAs, and the E6-E7 sequence of HPV31b was used for the analysis of CIN612 9E RNAs. Hybridizing transcripts were visualized by autoradiography.
Nuclear extraction.
Approximately 6 × 106 cells were collected by trypsinization and washed with 2 ml of PBS. Cell pellets were gently resuspended in 300 μl of buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1/1,000 proteinase inhibitor cocktail [Calbiochem]), after which 20 μl of 10% (vol/vol) Nonidet NP-40 (Fluka) was added and the tube was vigorously vortexed for 10 s. The homogenate was centrifuged for 30 s, and the supernatant containing cytoplasm was recovered. The nuclear pellet was resuspended in 50 μl of buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, and 1/1,000 proteinase inhibitor cocktail), and the tube was vigorously rocked at 4°C for 15 min. The suspension was centrifuged for 5 min, and the supernatant containing the nuclear extract was collected.
Immunoblotting.
Cell pellets were washed and resuspended in PBS and lysed in reporter lysis buffer (Promega) containing the protease inhibitor cocktail. Equal amounts of total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell) by electrophoresis. Western blot analyses were performed by use of the ECL chemiluminescent detection kit (Amersham) as recommended by the manufacturer. The p53 protein was detected with the mouse monoclonal DO1 antibody (a gift from R. Iggo and E. Saller). The pRb antibody was obtained from PharMingen, while the E2 antibody was the mouse monoclonal TVG 261 (gift from M. H. Hibma).
Integration of the HPV16 promoter into cellular DNA.
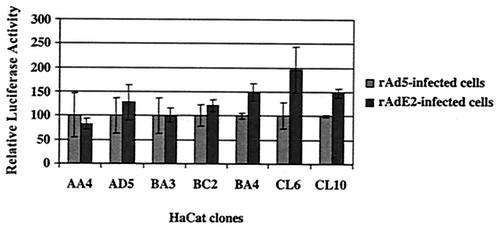
The pGl3b 822.4 plasmid contains the HPV16 LCR that controls expression of a luciferase reporter gene. Fifteen micrograms of the plasmid was cotransfected with the pBabe-puro plasmid at a ratio of 1 to 10 into HaCat cells with the Dotap transfection kit (Roche). The cells were selected with puromycin (Sigma) and subcloned by flow cytometry (Becton Dickinson FACScan). Isolated clones were selected for their stable luciferase expression with the luciferase assay system (Promega). These cells were seeded at 5 × 106 cells per 10-cm-diameter plate and were infected with rAdE2 or rAd5. Cells were harvested 48 h later, and luciferase expression levels were again analyzed. The relative luciferase expression values given correspond to the luciferase units per microgram of protein. The values obtained with the different clones were between 8,000 and 45,000 luciferase light units, with background values between 1 and 10 units.
Trichostatin A treatment.
W12 and S12 cells were treated with the histone deacetylase inhibitor trichostatin A (TSA) (Sigma) at a concentration of 200 or 400 nmol in the culture medium for 4 h. Cells were harvested, and RNA was extracted and analyzed by Northern blotting.
RESULTS
Generation of an isogenic pair of human cervical keratinocyte lines containing either episomal or integrated HPV16 DNA.
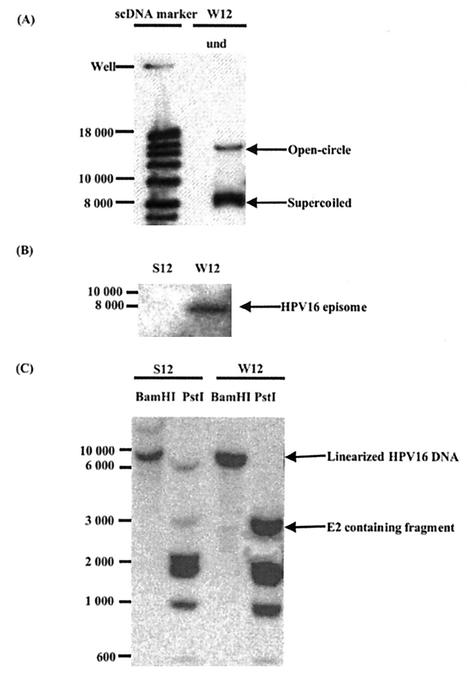
The W12 cell line has been previously characterized as containing multiple copies of the HPV16 genome in a monomeric episomal form (27, 44). This cell line was derived from a low-grade lesion of the cervix and is normally grown in the presence of 3T3 feeder cells in culture. By removing this feeder support from W12 cultures, we observed that the cells grew poorly at first but after further culture adapted to growth in the absence of feeders and proliferated well. These cells, which we called S12, grew faster than the W12 cells. Southern blot analysis of undigested W12 cell total DNA (Fig. 1A) confirms that W12 cells do not contain integrated copies but only the episomal form of the HPV16 DNA. To check whether HPV16 episomes are present in S12 cells, DNA was isolated from them and from W12 cells by Hirt extraction and analyzed by electrophoresis and hybridization with a 32P-labeled HPV16-specific DNA probe. HPV16 episomes were detected in extracts of W12 cells but not in those of S12 cells (Fig. 1B). In parallel, total DNA was also extracted from similar numbers of S12 and W12 cells and digested with BamHI and PstI enzymes. The digestion pattern of DNA from W12 cells correlated with the expected pattern of HPV16 DNA in the episomal form; one band for BamHI digestion corresponding to the linearized DNA and a series of bands for the PstI digestion (Fig. 1C). By comparing the digestion pattern of the HPV16 DNA of W12 cells with that of S12 cells, it is clear that they are markedly different. After BamHI digestion, S12 cell DNA presented two bands including one of around 8,000 bp. This and the absence of the HPV episome in Hirt extracts suggest that the HPV16 DNA in S12 cells is integrated. By phosphorimager quantification, the W12 cell line was estimated to contain approximately 850 episomes per cell, while the S12 cells were estimated to have about 350 integrated copies of HPV DNA per cell. Hence, W12 and S12 cells are isogenic cell lines that contain different forms of the HPV16 genome.
FIG. 1.
(A) Southern blot analysis of undigested HPV16 DNA from W12 cells after total DNA extraction. W12 cell DNA was compared to a supercoiled DNA marker (scDNA marker). (B) Southern blot analysis of HPV16 episomes isolated by the Hirt extraction method from W12 and S12 cells and digested with BamHI. (C) Southern blot analysis of HPV16 DNA in W12 and S12 cells. DNA was extracted from 6 × 106 cells, and equal amounts of total DNA were digested with BamHI or PstI and subjected to electrophoresis and hybridization using a 32P-labeled HPV16 specific DNA probe.
It appears that during the integration process of HPV episomes into the S12 cellular genome, an HPV DNA fragment containing the E2 and E4 ORFs was disrupted. This is clearly seen in the PstI digestion pattern of S12 cell DNA, in which a 3-kb band corresponding to the DNA fragment that contains the E1, E2, and E4 ORFs is greatly reduced. Interestingly, the disappearance of the E2-containing fragment observed here is reminiscent of that reported for cell lines such as HeLa, CasKi, or SiHa, which were derived from cervical cancers. In addition, a 6-kb DNA band, not present in PstI digests of W12 cell DNA, appears in the S12 sample.
The E2 protein is present in W12 cells but not in S12 cells.
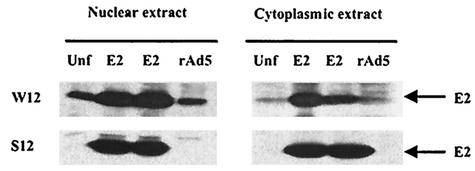
To determine the consequence of the E2 ORF disruption in S12 cells at the protein level, nuclear and cytoplasmic extracts were prepared from W12 and S12 cells. To serve as controls, similar types of extracts were prepared from W12 and S12 cells that were infected with recombinant adenovirus expressing the E2 protein (rAdE2) or recombinant adenovirus bearing just the back-bone vector (rAd5). As seen in Fig. 2, the TVG261 anti-E2 antibody showed the E2 protein to be present in rAdE2-infected cells, as expected. Endogenous E2 protein was detected in the nuclear fraction of the uninfected W12 cells but not in that of the S12 cells. Not surprisingly, little or no endogenous E2 was detected in the cytoplasmic fraction, E2 being a nuclear protein. However, a fraction of the E2 protein expressed by rAdE2 was also detected in the cytoplasmic extracts, presumably due to the high levels of E2 made in the cells. The absence of E2 protein in S12 cells is consistent with the disruption of the E2 ORF in these cells.
FIG. 2.
HPV16 E2 protein levels in W12 and S12 cells after nuclear and cytoplasmic extraction analyzed by Western blotting. Noninfected and rAd5-infected cell extracts were compared to rAdE2-infected cell extracts used as positive control for the presence of the E2 protein at 48 h postinfection.
HPV16 E2 protein represses transcription of the E6 and E7 genes in S12 cells but not in W12 cells.
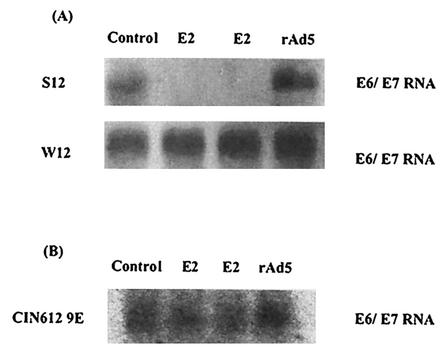
To study the effect of the E2 protein on the HPV16 p97 promoter in S12 and W12 cells, rAd5 and rAdE2 viruses were used to infect these cells. Transcription of the E6 and E7 genes was monitored by Northern blot analyses of RNA extracted 48 h after infection (Fig. 3A). The expression of E2 in S12 cells resulted in a strong repression of transcription of the E6 and E7 genes as previously reported for HeLa cells (8, 13, 20, 33). In contrast, no effect on the transcription of these genes was detected in the rAdE2-infected W12 cells. To determine if the lack of repression by E2 on the transcription of E6 and E7 is general for episomal HPV genomes, we tested the effect of E2 on the transcription of the E6 and E7 genes in another cell line that harbors the HPV DNA as episomes, the CIN612 9E line. This cell line was derived from a low-grade cervical lesion and contains multiple episomes of the HPV31b genome (26, 36). As shown in Fig. 3B, transcription of E6 and E7 in CIN612 9E cells, as in W12 cells, was not significantly altered by the E2 protein.
FIG. 3.
Effects of expression of E2 on the E6 and E7 transcription in S12 and W12 cells (A) and CIN612 9E cells (B). At 48 h postinfection, RNA was isolated and equal amounts were analyzed by Northern blotting with a 32P-labeled HPV31 E6-E7-specific DNA probe.
HPV16 E2 protein increases the amount of pRb in S12 cells but not in W12 cells; E2 does not affect the p53 protein levels in either cell line.
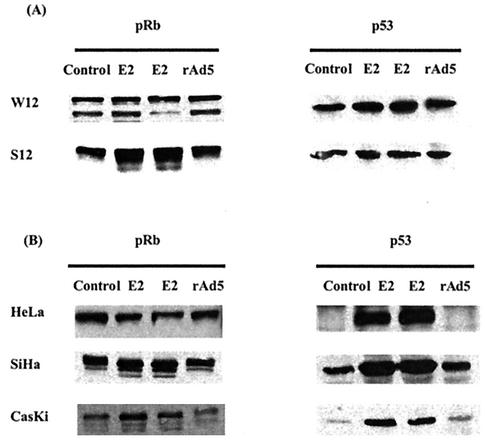
The E6 protein targets the p53 protein for destruction, while the E7 protein binds the pRb protein, and in doing so, inactivates it. In addition, in some cells E7 can also induce the destruction of the pRb protein. Hence, it follows that changes in the level of E6 and E7 expression would have consequences for the p53 and possibly also the pRb proteins. Western blot analysis in Fig. 4A shows that the E2 protein increases the pRb protein levels in S12 cells but not in W12 cells. This is consistent with the reduction of E6 and E7 transcripts caused by E2 in S12 cells but not in W12 cells. However, p53 protein amounts did not increase as expected in S12 cells or W12 cells. Although initially surprising, this observation is consistent with the failure to detect potential full-length E6-encoding transcripts in these cells (11) and the readily detectable levels of p53 in suggesting that the E6 protein is not expressed in these cells. The p53 in these cells was tested and found to be of the wild type (data not shown), supporting the previous observation that inactivation of p53 by E6 is not a prerequisite for cellular immortalization but maintenance of telomere length is (14, 51). We tested W12 cells to determine if telomerase activity was present, and we found this to be the case (data not shown). This observation was not surprising, since E6 protein activity is not the only way to activate telomerase. Importantly, the E2 protein was correctly expressed by rAdE2 viruses and able to induce an increase of p53 protein levels in HeLa, SiHa, and CasKi cells (Fig. 4B), all of which contain either integrated HPV18 or HPV16 DNA. The effect of E2 on the protein level of pRb varies in the different cell lines. This is in keeping with the observation that in some cells, E7 can induce the destruction of pRb. This appears to be the case at least for CasKi cells.
FIG. 4.
pRb and p53 proteins in rAd5- and rAdE2-infected cells analyzed by Western blotting at 48 h postinfection.
Integration of the HPV DNA into the cellular genome does not by itself affect the p97 promoter's sensitivity to the E2 protein.
It is clear that the E2 protein expressed from rAdE2 was functioning, and yet it was unable to exert an effect on the level of E6 and E7 transcription in W12 cells, as it so efficiently does in the isogenic S12 cells. Since the HPV16 DNA in these two cells is in different forms, we asked whether merely by being integrated into the cellular genome the HPV16 p97 promoter becomes responsive to the E2 protein. We generated clones of HaCaT cells that harbor complete integrated HPV16 LCR sequences in their genome. To do so, we cotransfected the pGL3b822.4 plasmid, which contains the complete HPV16 LCR followed by a luciferase gene, together with the pBabe-puro plasmid, which contains the puromycin resistance gene. Transfectants were selected with puromycin, and clones were isolated and cultured individually. Of the 48 clones that were analyzed for luciferase activity, 7 clones expressed luciferase. Integration of the entire LCR was confirmed for each clone by PCR (data not shown). These clones were infected with rAdE2 or rAd5, and the results were compared (Fig. 5). Four clones of seven were refractive to E2, while the remaining three clones exhibited a 1.5- to 2-fold increase in activation. This experiment demonstrates that just being integrated into the host genome does not make the p97 promoter repressible by the E2 protein. Hence, the insensitivity to E2 of the p97 promoter in W12 cells, compared with its sensitivity in the S12 cells, must be attributed to some other cause.
FIG. 5.
Effects of HPV16 E2 protein on the integrated p97 promoter. Different HaCat clones containing stably integrated pGl3b 822.4 plasmids were infected with rAd5 and rAdE2. Activity of the HPV16 p97 promoter was determined by luciferase assay and normalized according to the control samples infected with rAd5.
Chromatin structure proximal to the p97 promoter affects its activity.
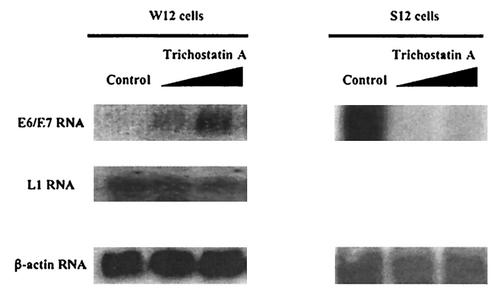
We asked whether the HPV DNA in S12 cells, like the viral DNA in HeLa, SiHa, and CasKi cells, was integrated in chromosomal regions that were open, hence allowing not only expression of the E6 and E7 genes but also access of the E2 protein to the p97 promoter. If so, this would infer that in W12 cells the chromatin structure around the p97 promoter of the HPV16 episomes is more closed, obstructing the access of the E2 protein. To test this, W12 cells were treated with the histone deacetylase inhibitor TSA (Fig. 6). Within 4 h an increase in the levels of E6 and E7 transcripts was observed. The increase was proportional to the amount of TSA used. It is noteworthy that no effect on the transcription of the L1 gene was observed, indicating that TSA does not induce the activation of all promoters indiscriminately.
FIG. 6.
E6 and E7 RNA levels in W12 and S12 cells and L1 RNA levels in W12 cells after treatment with increasing amounts of TSA. RNA was harvested, and equal amounts were analyzed by Northern blotting with a 32P-labeled HPV16 E6- and E7-specific probe or a 32P-labeled HPV16 L1-specific DNA probe. A 32P-labeled β-actin DNA sequence was used as control.
On the other hand, S12 cells treated the same way with TSA did not show an increase of E6 and E7 transcription. Instead, the E6 and E7 transcript level, which was initially high, was reduced. This reduction seems unexpected but has been observed previously (17). This demonstrates that the chromatin structure in the region of the p97 promoter in the episomal form or integrated form is indeed very different and may explain their different responsiveness to the E2 protein in W12 and S12 cells.
DISCUSSION
W12 cells were derived from a low-grade cervical lesion by M. A. Stanley (44). The initial isolate emerged from a mixture of several keratinocyte colonies that grew on the tissue culture plate. Analyses of the HPV DNA in these cells showed that they contained, on average, 100 copies of HPV16 DNA as episomes per cell. However, continuous passaging of W12 cells resulted in the steady reduction of the copy number of HPV16 episomes per cell. This could be due either to gradual loss of the episomes or to the presence of a mixed population of cells, among which only some harbored HPV16 DNA as episomes. These cells might have a slower growth rate and subsequently be outgrown by cells bearing integrated HPV16 DNA. The later possibility was substantiated when Jeon et al. (27) isolated single-cell clones from W12 and showed that some clones contained up to 1,000 copies of the episome while others contained only integrated copies of the viral genome. Furthermore, the HPV16 episome-containing clones maintained the copy number of the viral genomes after at least 30 passages.
For this work, we used the W12 clone 20863, obtained from P. Lambert, that harbors approximately 1,000 HPV16 episomes and does not contain integrated HPV DNA (Fig. 1). The growth of these cells is dependent on the presence of 3T3 feeder cells, and removal of feeder cells resulted in severe growth retardation. However, continued passing of these cells at high density (but never complete confluence) permitted the maintenance of cell growth. After several passages, the cells were clearly adapted to grow without feeder support. These cells initially possessed episomal HPV16 DNA (data not shown), but serial passing of the cells resulted in the gradual loss of the episomes. Above passage 50, episomes were no longer detected. Instead, the HPV DNA was found to be integrated into the cellular DNA. These cells were then called S12 cells. The integration was accompanied by the loss of the E2 ORF-containing PstI fragment (Fig. 1C). Quantification of HPV DNA in S12 cells showed that they contain 2.5 times fewer HPV16 copies than do W12 cells. Although the HPV DNA was integrated, the majority had a length that was close to the unit length of the HPV episome observed after BamHI digestion of S12 DNA. This pattern of integration, as revealed by PstI and BamHI restriction digestion, suggests the HPV DNA to have integrated predominantly in a tandem fashion. At this stage it also became clear that the S12 cells proliferated much faster than the parental W12 cells. The association between accelerated cell growth and loss of the E2 ORF-containing fragment is not surprising, since E2 is reported to repress expression of the viral oncogenes E6 and E7. The consequence of the loss of the E2 ORF-containing fragment is reflected in Western blot analysis, which failed to detect E2 protein in S12 cells, while clearly detecting it in W12 cells. To our knowledge, this is the first report of the detection of endogenously expressed E2 protein in a cell line.
The availability of S12 cells, together with their isogenic partner, W12 cells, enabled us to compare the effect of the E2 protein on the p97 promoter of HPV DNA in an integrated state with its effect in the episomal state. This we thought to be important, because hitherto the effect of E2 on p97 in a natural context has been studied exclusively with cells that bore integrated HPV DNA (8, 13, 21, 34). In our studies, we observed that while E2 represses transcription of the E6 and E7 genes in S12 cells, it does not do so in W12 cells. If the repression of the p97 promoter by E2 is solely dependent on the levels of the E2 protein, as is currently assumed (1, 3, 22, 44, 49), then the p97 promoter in W12 cells would be expected to be more readily repressed since these cells have been shown to contain already a significant amount of endogenous E2 protein. According to our results, this is clearly not the case. These results show that while the E2 protein can indeed repress the p97 promoter in S12 cells, the quantity of E2 in the cell is clearly not the sole determining factor. Instead, it appears that the conformation of the HPV chromatin itself exerts a significant influence on whether the E2 protein can repress the p97 promoter. This is further supported by the observation that E2 was also not able to repress E6 and E7 transcription in CIN612 9E cells, which contain high-risk HPV31b DNA as episomes, while being able to do so in HeLa, SiHa, and CasKi cells, all of which harbor integrated viral genomes. These results are unexpected in view of the fact that earlier studies have demonstrated E2 to repress the p97 promoter from reporter plasmids. This discrepancy is likely to be due to the difference in DNA conformation between reporter plasmids and the HPV minichromosome.
Although the conformation of the viral genomes seems to be the cause of the differential sensitivity of the p97 promoter to E2's repressive activity, integration of the HPV DNA into cellular DNA is not, by itself, sufficient to render the p97 viral promoter repressible by the E2 protein. This is demonstrated in Fig. 5, which shows that the E2 protein was unable to repress the p97 promoter in cellular clones that contain an integrated luciferase reporter construct controlled by the p97 promoter. While integration of the viral genome per se cannot expose the p97 promoter to E2, the site on the host genome in which the HPV DNA integrates may do so, if the region in question is an open or transcriptionally active locus of the chromosome. To analyze the conformation of the HPV16 minichromosome in the region of the promoter, W12 cells were treated with TSA, a drug that inhibits deacetylation of histones. The transcription of the E6 and E7 genes was greatly augmented within 4 h, indicating that the HPV16 minichromosomes were in a relatively closed state prior to treatment with TSA. Treatment with TSA did not influence the transcription of the L1 gene, demonstrating that not all promoters are indiscriminately affected by TSA. Significantly, this observation echoes that reported by del Mar Pena and Laimins (5) with CIN612 9E cells. They also showed that TSA was unable to activate late gene expression in undifferentiated cells while it increased transcription of early genes from episomal HPV31b DNA. In contrast, S12 cells treated with TSA did not increase E6 and E7 transcription. Instead, for reasons not known to us, a repression was observed. This downregulation of viral E6 and E7 expression was also detected by Finzer and colleagues (17) in HeLa cells treated with sodium butyrate or TSA under conditions similar to ours. Taken together these results support the hypothesis that the region of the p97 promoter of HPV DNA in W12 cells exists in a relatively closed conformation, while that of the S12 cells exists in a relatively open conformation. This difference could affect the access of the E2 protein to the promoter, resulting in the p97 promoter in S12 cells being sensitive to E2 while the same promoter in W12 cells is refractive to E2. If this were the case, it is not clear how E2 can be involved in HPV DNA replication in W12 cells and yet not affect transcription of E6 and E7 from the p97 promoter. An alternative and perhaps more likely hypothesis is therefore that access of E2 to the E2-binding sites is not inhibited but that the promoter region proximal to E2-binding sites is itself in a closed conformation in W12 cells. This would suggest that the p97 promoter in W12 cells is already relatively repressed (in comparison to that in S12 cells). Hence, the binding of E2 would have a minimal repressive effect on it, while still permitting E2-mediated replication of the viral DNA.
In cells that are immortalized or transformed by natural HPV infection, the viral genomes are frequently found to be integrated in loci called matrix attachment regions (MARS) (43). These regions are transcriptionally active or relatively accessible sites on the genome. Hence, integration of the HPV DNA in these regions would favor E6 and E7 expression and confer a selective growth advantage to these cells as opposed to cells in which the HPV DNA was either not integrated or integrated in non-MARS loci. Such integration would inadvertently also render the HPV promoter sensitive to the E2 protein. Therefore, it follows that a possible consequence of HPV integration in MARS is the exposure of the p97 promoter to the E2 protein, explaining the ability of E2 to repress this promoter in HeLa, SiHa, CasKi, and S12, but not in W12 and CIN612 9E cells.
Taken together, our results show that the effect of E2 on the transcription of E6 and E7 is not solely dependent on the level of the E2 protein in the cell. Instead, the conformation of the chromatin structure in the region of the p97 promoter is also a significant contributing factor. Hence, the simple model of E2's repressive effect on the p97 promoter should be reevaluated and be applied to situations in which the HPV DNA is integrated but not to situations in which the viral genome exists extrachromosomally.
Although these results lead us to conclude that E2 does not affect the transcription of E6 and E7 from episomal HPV genomes in proliferating keratinocytes (W12 and CIN612 9E), it is conceivable that E2 might affect E6 and E7 transcription from HPV episomes in differentiating keratinocytes or during other phases of the viral life cycle. This possibility can be tested by using organotypic cultures. Stubenrauch et al. (47) carried out such experiments by using cells containing HPV31 episomes coding for E2 proteins that were defective for either the transactivation or the replication function (38). They observed that in the absence of the replication activity of E2, the HPV genome was rapidly integrated into the cellular genome. The HPV DNA containing E2 defective in the transactivating function, on the other hand, was maintained as an episome and amplified in the differentiated layers, as was observed for the wild-type HPV31 episome. By comparing the transcription of the early genes in differentiated cellular layers, the authors observed that the amount of transcripts initiated at the p97 promoter was comparable in cells harboring either wild-type HPV31 episomes or episomes bearing a mutation in the transactivation region of the E2 protein. Their study demonstrates that the transactivation ability of the E2 protein is not essential for the expression of the early or late genes in HPVs. Collectively, these results argue for a revision of the current model of the HPV life cycle, specifically in relation to E2 function. We propose that the major function of the E2 protein is to replicate the viral genome and that any role that E2 might have in influencing viral transcription is likely to be minor. The E2 protein can indeed repress E6 and E7 transcription, but only from HPV DNA which has integrated into regions of the cellular genome that permit the E2 protein to act on the p97 promoter. It will be of interest to determine the purpose of the transcriptional regulatory functions of E2 in the life cycle of the virus. They could be related to E2's role in viral DNA replication, since it is known that interaction between transcription and replication factors can occur. Alternatively, E2 could have a function in deregulating cellular gene expression.
Acknowledgments
This work was supported by the Fonds National Suisse de la Recherche Scientifique and Recherche Suisse Contre le Cancer.
We thank Bernhard Hirt for support, helpful discussions, and critical reading of the manuscript. We are very grateful to Beatrice Bentele for cell culture and Patrick Reichenbach for the nuclear extraction. We also thank Roland Sahli for the rAdE2 and rAd5 recombinant viruses, Laimonis Laimins for providing the CIN612 9E cell line, Richard Iggo and Elisabeth Saller for the DO1 p53 antibody, and Merilyn Hibma for the TVG261 E2 antibody.
REFERENCES
- 1.Bernard, B. A., C. Bailly, M. C. Lenoir, M. Darmon, F. Thierry, and M. Yaniv. 1989. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J. Virol. 63:4317-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshart, M., L. Gissmann, H. Ikenberg, A. Kleinheinz, W. Scheurlen, and H. zur Hausen. 1984. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 3:1151-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvard, V., A. Storey, D. Pim, and L. Banks. 1994. Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J. 13:5451-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crum, C. P., M. Symbula, and B. E. Ward. 1989. Topography of early HPV 16 transcription in high-grade genital precancers. Am. J. Pathol. 134:1183-1188. [PMC free article] [PubMed] [Google Scholar]
- 5.del Mar Pena, L. M., and L. A. Laimins. 2001. Differentiation-dependent chromatin rearrangement coincides with activation of human papillomavirus type 31 late gene expression. J. Virol. 75:10005-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeret, C., C. Desaintes, M. Yaniv, and F. Thierry. 1997. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J. Virol. 71:9343-9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demeret, C., M. Yaniv, and F. Thierry. 1994. The E2 transcriptional repressor can compensate for Sp1 activation of the human papillomavirus type 18 early promoter. J. Virol. 68:7075-7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desaintes, C., C. Demeret, S. Goyat, M. Yaniv, and F. Thierry. 1997. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 16:504-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon, E. P., G. L. Pahel, W. J. Rocque, J. A. Barnes, D. C. Lobe, M. H. Hanlon, K. A. Alexander, S. F. Chao, K. Lindley, and W. C. Phelps. 2000. The E1 helicase of human papillomavirus type 11 binds to the origin of replication with low sequence specificity. Virology 270:345-357. [DOI] [PubMed] [Google Scholar]
- 10.Dong, G., T. R. Broker, and L. T. Chow. 1994. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J. Virol. 68:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doorbar, J., A. Parton, K. Hartley, L. Banks, T. Crook, M. Stanley, and L. Crawford. 1990. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology 178:254-262. [DOI] [PubMed] [Google Scholar]
- 12.Dostatni, N., P. F. Lambert, R. Sousa, J. Ham, P. M. Howley, and M. Yaniv. 1991. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 5:1657-1671. [DOI] [PubMed] [Google Scholar]
- 13.Dowhanick, J. J., A. A. McBride, and P. M. Howley. 1995. Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol. 69:7791-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drayton, S., and G. Peters. 2002. Immortalisation and transformation revisited. Curr. Opin. Genet. Dev. 12:98-104. [DOI] [PubMed] [Google Scholar]
- 15.Durst, M., D. Glitz, A. Schneider, and H. zur Hausen. 1992. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridization. Virology 189:132-140. [DOI] [PubMed] [Google Scholar]
- 16.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 17.Finzer, P., C. Kuntzen, U. Soto, H. zur Hausen, and F. Rosl. 2001. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene 20:4768-4776. [DOI] [PubMed] [Google Scholar]
- 18.Frattini, M. G., and L. A. Laimins. 1994. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc. Natl. Acad. Sci. USA 91:12398-12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Carranca, A., F. Thierry, and M. Yaniv. 1988. Interplay of viral and cellular proteins along the long control region of human papillomavirus type 18. J. Virol. 62:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giarre, M., S. Caldeira, I. Malanchi, F. Ciccolini, M. J. Leao, and M. Tommasino. 2001. Induction of pRb degradation by the human papillomavirus type 16 E7 protein is essential to efficiently overcome p16INK4a-imposed G1 cell cycle arrest. J. Virol. 75:4705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin, E. C., and D. DiMaio. 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 97:12513-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, G. D., D. M. Uzelin, G. E. Phillips, P. McEvoy, R. Marin, and C. J. Burrell. 1992. Transcription patterns of human papillomavirus type 16 in genital intraepithelial neoplasia: evidence for promoter usage within the E7 open reading frame during epithelial differentiation. J. Gen. Virol. 73:2047-2057. [DOI] [PubMed] [Google Scholar]
- 23.Hirochika, H., T. R. Broker, and L. T. Chow. 1987. Enhancers and trans-acting E2 transcriptional factors of papillomaviruses. J. Virol. 61:2599-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou, S. Y., S. Y. Wu, T. Zhou, M. C. Thomas, and C. M. Chiang. 2000. Alleviation of human papillomavirus E2-mediated transcriptional repression via formation of a TATA binding protein (or TFIID)-TFIIB-RNA polymerase II-TFIIF preinitiation complex. Mol. Cell. Biol. 20:113-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes, F. J., and M. A. Romanos. 1993. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 21:5817-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel, M., J. B. Hudson, and L. A. Laimins. 1992. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J. Virol. 66:6070-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon, S., B. L. Allen-Hoffmann, and P. F. Lambert. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 69:2989-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingelhutz, A. J., S. A. Barber, P. P. Smith, K. Dyer, and J. K. McDougall. 1994. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol. Cell. Biol. 14:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 30.Lu, J. Z., Y. N. Sun, R. C. Rose, W. Bonnez, and D. J. McCance. 1993. Two E2 binding sites (E2BS) alone or one E2BS plus an A/T-rich region are minimal requirements for the replication of the human papillomavirus type 11 origin. J. Virol. 67:7131-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsukura, T., T. Kanda, A. Furuno, H. Yoshikawa, T. Kawana, and K. Yoshiike. 1986. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J. Virol. 58:979-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munger, K., M. Scheffner, J. M. Huibregtse, and P. M. Howley. 1992. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 12:197-217. [PubMed] [Google Scholar]
- 33.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura, A., T. Ono, A. Ishimoto, J. J. Dowhanick, M. A. Frizzell, P. M. Howley, and H. Sakai. 2000. Mechanisms of human papillomavirus E2-mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol. 74:3752-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pater, M. M., and A. Pater. 1985. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology 145:313-318. [DOI] [PubMed] [Google Scholar]
- 36.Rader, J. S., T. R. Golub, J. B. Hudson, D. Patel, M. A. Bedell, and L. A. Laimins. 1990. In vitro differentiation of epithelial cells from cervical neoplasias resembles in vivo lesions. Oncogene 5:571-576. [PubMed] [Google Scholar]
- 37.Romanczuk, H., and P. M. Howley. 1992. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl. Acad. Sci. USA 89:3159-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai, H., T. Yasugi, J. D. Benson, J. J. Dowhanick, and P. M. Howley. 1996. Targeted mutagenesis of the human papillomavirus type 16 E2 transactivation domain reveals separable transcriptional activation and DNA replication functions. J. Virol. 70:1602-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 40.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz, E., U. K. Freese, L. Gissmann, W. Mayer, B. Roggenbuck, A. Stremlau, and H. zur Hausen. 1985. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314:111-114. [DOI] [PubMed] [Google Scholar]
- 42.Sedman, J., and A. Stenlund. 1995. Co-operative interaction between the initiator E1 and the transcriptional activator E2 is required for replicator specific DNA replication of bovine papillomavirus in vivo and in vitro. EMBO J. 14:6218-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shera, K. A., C. A. Shera, and J. K. McDougall. 2001. Small tumor virus genomes are integrated near nuclear matrix attachment regions in transformed cells. J. Virol. 75:12339-12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley, M. A., H. M. Browne, M. Appleby, and A. C. Minson. 1989. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int. J. Cancer. 43:672-676. [DOI] [PubMed] [Google Scholar]
- 45.Steger, G., and S. Corbach. 1997. Dose-dependent regulation of the early promoter of human papillomavirus type 18 by the viral E2 protein. J. Virol. 71:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoler, M. H., S. M. Wolinsky, A. Whitbeck, T. R. Broker, and L. T. Chow. 1989. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology 172:331-340. [DOI] [PubMed] [Google Scholar]
- 47.Stubenrauch, F., A. M. Colbert, and L. A. Laimins. 1998. Transactivation by the E2 protein of oncogenic human papillomavirus type 31 is not essential for early and late viral functions. J. Virol. 72:8115-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan, S. H., B. Gloss, and H. U. Bernard. 1992. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res. 20:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan, S. H., L. E. Leong, P. A. Walker, and H. U. Bernard. 1994. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J. Virol. 68:6411-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thierry, F., and P. M. Howley. 1991. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 3:90-100. [PubMed] [Google Scholar]
- 51.Whitaker, N. J., T. M. Bryan, P. Bonnefin, A. C. Chang, E. A. Musgrove, A. W. Braithwaite, and R. R. Reddel. 1995. Involvement of RB-1, p53, p16INK4 and telomerase in immortalisation of human cells. Oncogene 11:971-976. [PubMed] [Google Scholar]
- 52.Yasugi, T., J. D. Benson, H. Sakai, M. Vidal, and P. M. Howley. 1997. Mapping and characterization of the interaction domains of human papillomavirus type 16 E1 and E2 proteins. J. Virol. 71:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yee, C., I. Krishnan-Hewlett, C. C. Baker, R. Schlegel, and P. M. Howley. 1985. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 119:361-366. [PMC free article] [PubMed] [Google Scholar]