Abstract
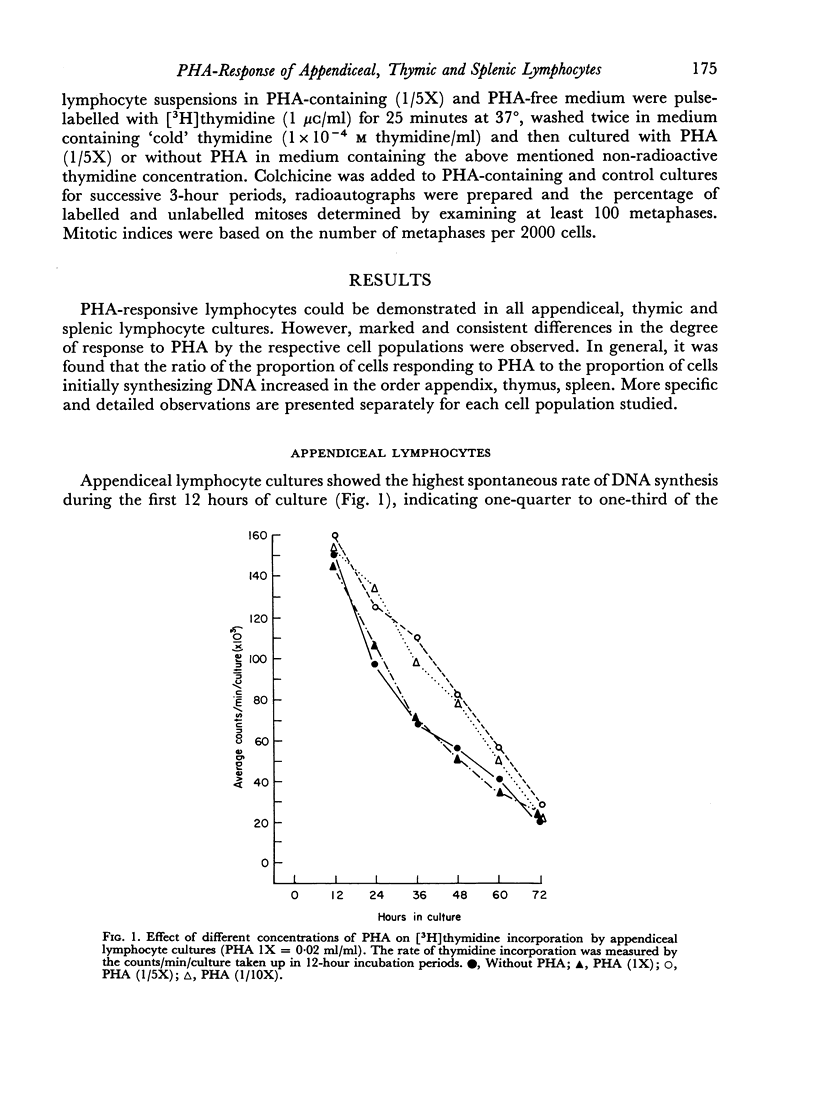
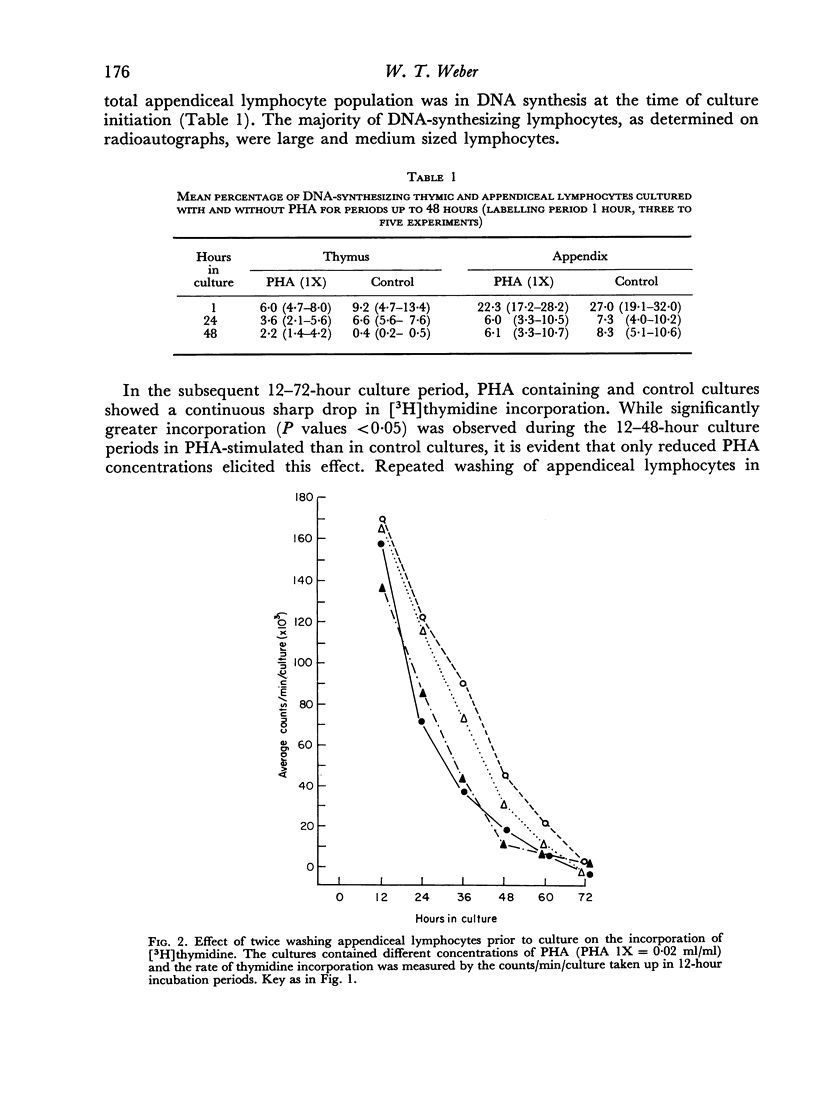
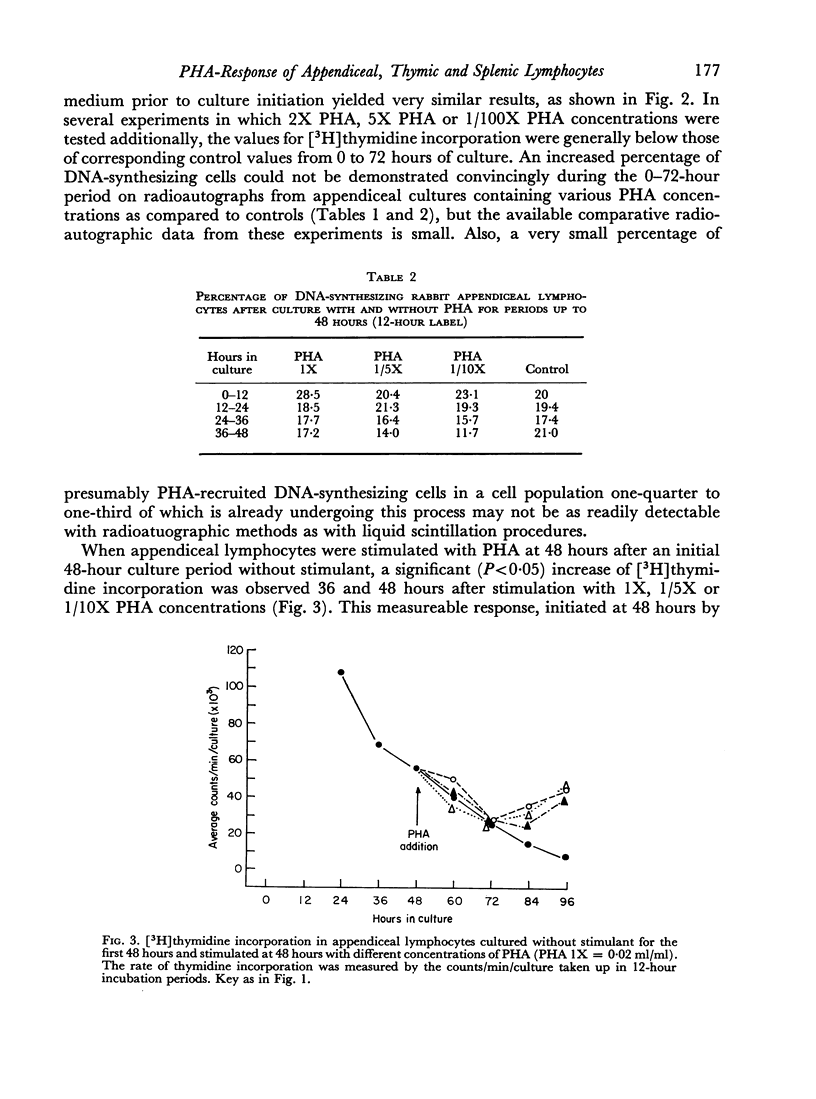
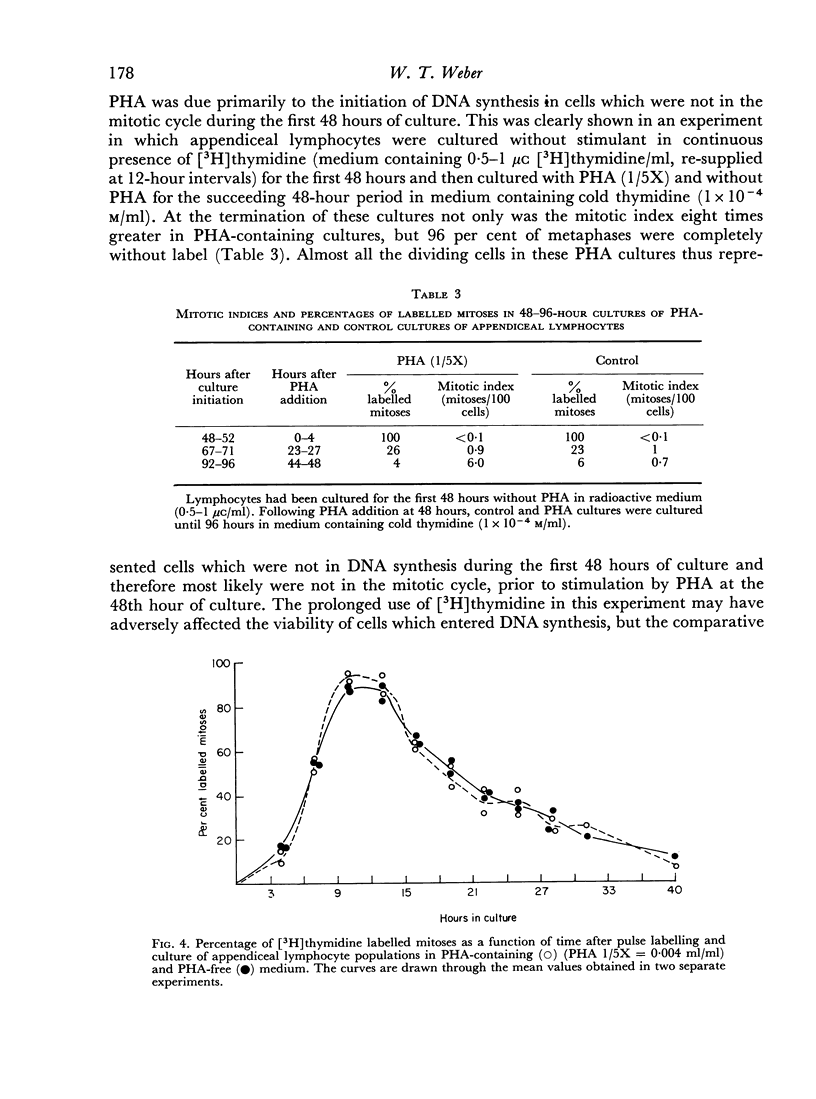
Lymphocytes from the appendix, thymus and spleen of young rabbits were cultured in the presence of varying concentrations of phytohaemagglutinin and their proliferative response during a 72-hour culture period measured with [3H]thymidine uptake. At the time of culture initiation, appendiceal lymphocyte populations showed the highest spontaneous rate of DNA synthesis followed by thymic and splenic lymphocytes in that order. In the rabbit appendix the presence of a small nondividing population of phytohaemagglutinin-responsive small lymphocytes was demonstrated. Additional evidence suggests that low concentrations of PHA accelerated or initiated DNA synthesis during the first 24-hour culture period in a proportion of appendiceal lymphocytes which were in the mitotic cycle at the time of culture initiation. In the thymus, as in the appendix, only a small fraction of the lymphocyte population responded to phytohaemagglutinin during the 72-hour culture period. Splenic lymphocytes showed the greatest response to PHA with a peak of DNA synthesis between 48 and 60 hours. The results indicate that varying concentrations of PHA should be employed to detect optimum responses by lymphocyte populations from various lymphoid organs and that different lymphoid organs have different proportions of G0 vs. G1 and PHA-responsive vs. non-PHA-responsive cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER O. K., SUTHERLAND D. E., GOOD R. A. APPENDIX OF THE RABBIT: A HOMOLOGUE OF THE BURSA IN THE CHICKEN? Nature. 1963 Oct 26;200:337–339. doi: 10.1038/200337a0. [DOI] [PubMed] [Google Scholar]
- CHAPMAN N. D., DUTTON R. W. THE STIMULATION OF DNA SYNTHESIS IN CULTURES OF RABBIT LYMPH NODE AND SPLEEN CELL SUSPENSIONS BY HOMOLOGOUS CELLS. J Exp Med. 1965 Jan 1;121:85–100. doi: 10.1084/jem.121.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Human thymus cell cultures-evidence for two functional populations. Proc Soc Exp Biol Med. 1966 Jan;121(1):236–240. doi: 10.3181/00379727-121-30746. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Further studies of the stimulation of DNA synthesis in cultures of spleen cell suspensions by homologous cells in inbred strains of mice and rats. J Exp Med. 1965 Oct 1;122(4):759–770. doi: 10.1084/jem.122.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S., Ling N. R., Sell S., Oxnard C. E. The transformation in vitro of peripheral lymphocytes of some laboratory animals. Immunology. 1965 Dec;9(6):565–574. [PMC free article] [PubMed] [Google Scholar]
- Metcalf W. K., Osmond D. G. A radioautographic investigation of the identity of phytohaemagglutinin responsive cells in the lymphoid tissues of the rat. Exp Cell Res. 1966 Mar;41(3):669–672. doi: 10.1016/s0014-4827(66)80118-0. [DOI] [PubMed] [Google Scholar]
- Schwarz M. R., Rieke W. O. The effect of phytohemagglutinin on rat thymus cells in vitro. Anat Rec. 1966 Aug;155(4):493–501. doi: 10.1002/ar.1091550402. [DOI] [PubMed] [Google Scholar]
- Sell S., Rowe D. S., Gell P. G. Studies on rabbit lymphocytes in vitro. 3. Proteins, RNA, and DNA synthesis by lymphocyte cultures after stimulation with phytohaemagglutinin, with staphylococcal filtrate, with antiallotype serum, and with heterologous antiserum to rabbit whole serum. J Exp Med. 1965 Oct 1;122(4):823–839. doi: 10.1084/jem.122.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER N. L. THE IMMUNOLOGICAL ROLE OF DIFFERENT LYMPHOID ORGANS IN THE CHICKEN. IV. FUNCTIONAL DIFFERENCES BETWEEN THYMIC AND BURSAL CELLS. Aust J Exp Biol Med Sci. 1965 Jul;43:439–450. doi: 10.1038/icb.1965.78. [DOI] [PubMed] [Google Scholar]
- Weber W. T. Difference between medullary and cortical thymic lymphocytes of the pig in their response to phytohemagglutinin. J Cell Physiol. 1966 Oct;68(2):117–125. doi: 10.1002/jcp.1040680206. [DOI] [PubMed] [Google Scholar]
- Weber W. T. The in vitro response of thymic lymphocytes of the pig to phytohemagglutinin. J Cell Physiol. 1966 Apr;67(2):285–299. doi: 10.1002/jcp.1040670209. [DOI] [PubMed] [Google Scholar]
- Weber W. T. The response to phytohemagglutinin by lymphocytes from the spleen, thymus and bursa of Fabricius of chickens. Exp Cell Res. 1967 May;46(2):464–466. doi: 10.1016/0014-4827(67)90084-5. [DOI] [PubMed] [Google Scholar]
- Winkelstein A., Craddock C. G. Comparative response of normal human thymus and lymph node cells to phytohemagglutinin in culture. Blood. 1967 Apr;29(4 Suppl):594–607. [PubMed] [Google Scholar]


