Abstract
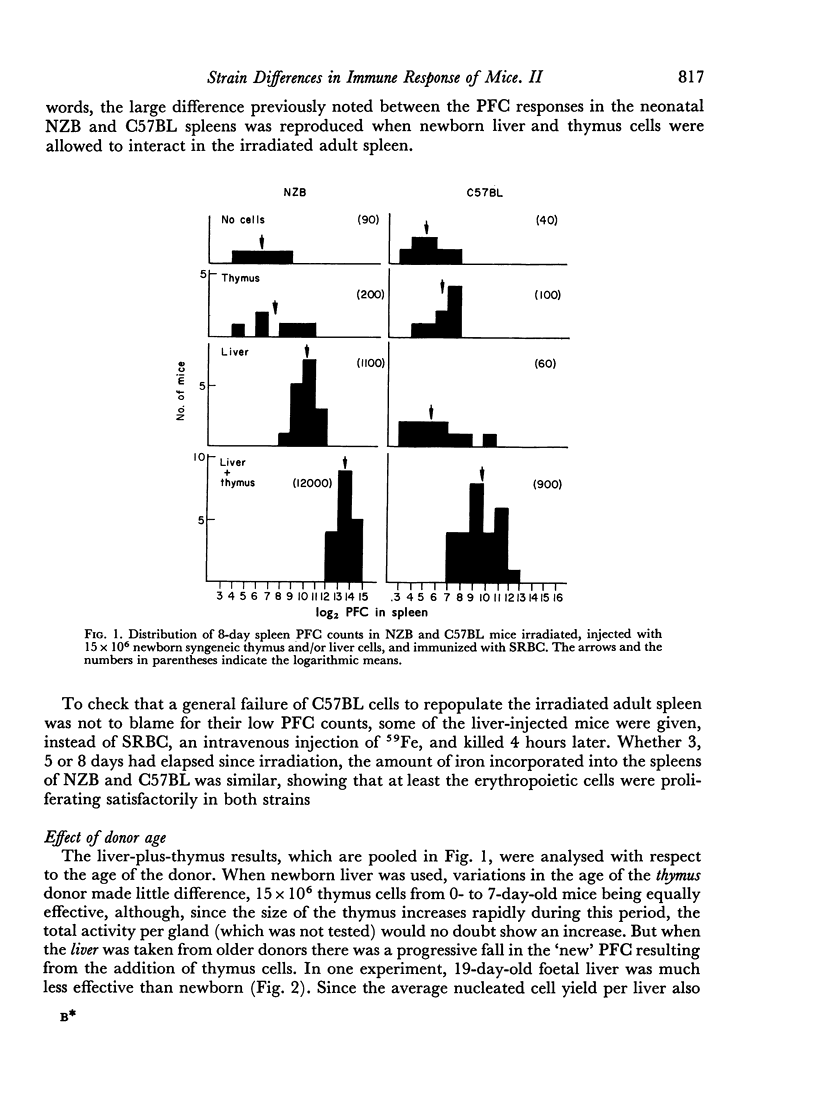
Lethally irradiated NZB and C57BL mice were injected with syngeneic thymus and marrow, or thymus and liver cells, and immunized with sheep red blood cells (SRBC). As in intact neonatal mice, NZB plaque-forming cell (PFC) responses were significantly higher than C57BL.
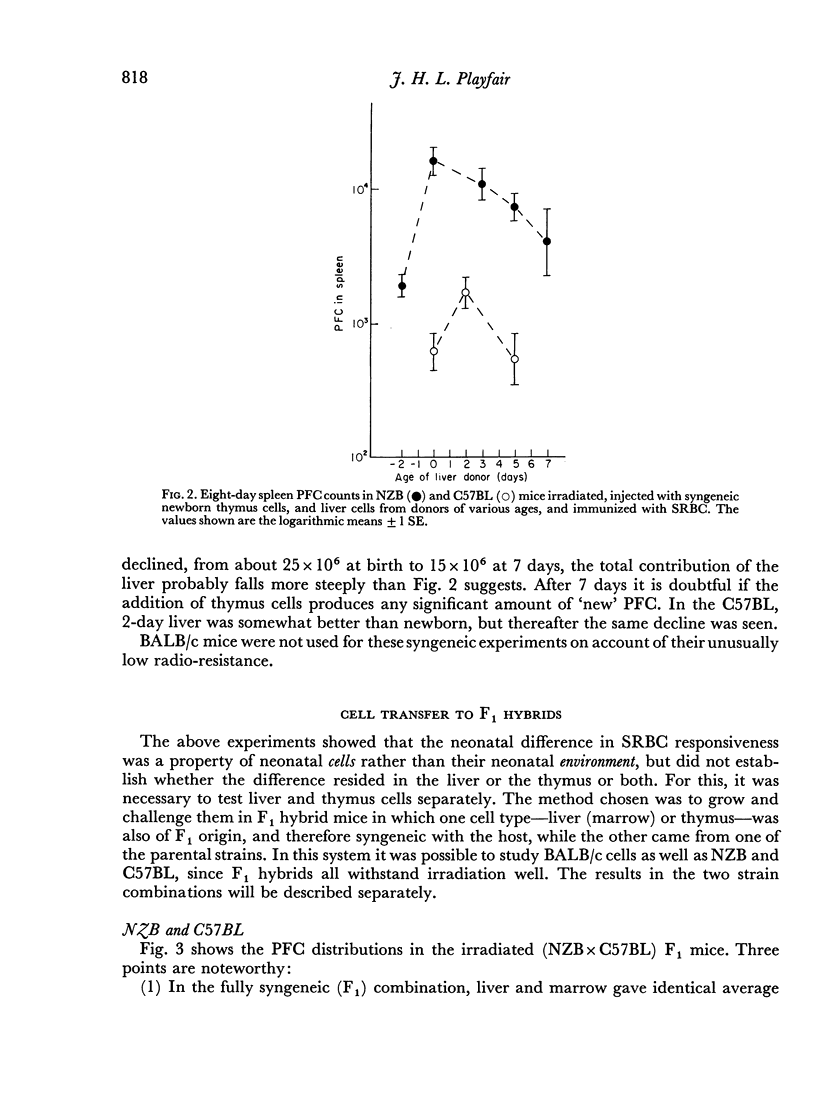
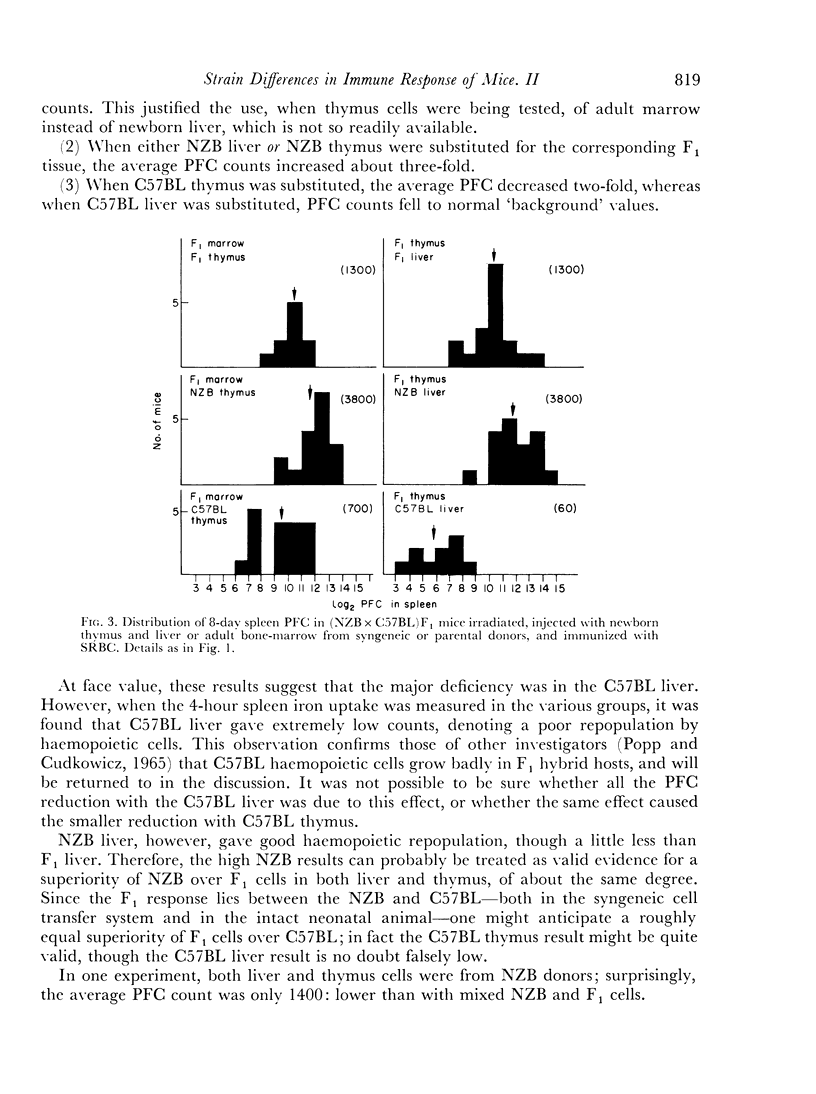
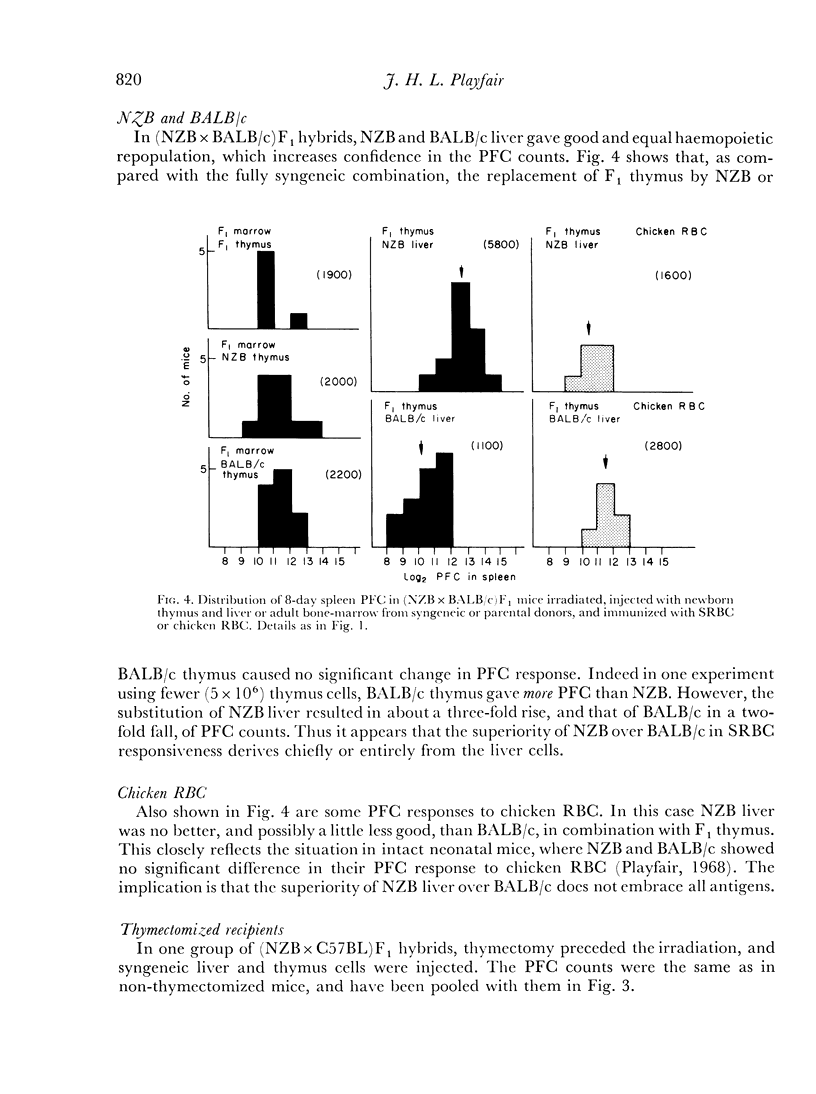
Irradiated (NZB×C57BL)F1 and (NZB×BALB/c)F1 hybrid mice were given mixtures of parental and syngeneic cells, and it was shown that the high NZB response to SRBC was characteristic of the newborn liver rather than of the thymus. NZB liver gave rise to more PFC against SRBC than BALB/c liver, but not more against chicken RBC. It is argued that liver cells contain genetic information regarding antibody specificity.
C57BL cells grew poorly in F1 hosts, but the low SRBC response appeared to be characteristic of both liver and thymus.
The nomenclature of the participating cells, and their role in the development of immunological responsiveness, are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATCHELOR J. R., HOWARD J. G. SYNERGIC AND ANTAGONISTIC EFFECTS OF ISOANTIBODY UPON GRAFT-VERSUS-HOST DISEASE. Transplantation. 1965 Mar;3:161–169. doi: 10.1097/00007890-196503000-00004. [DOI] [PubMed] [Google Scholar]
- Barnes D. W., Loutit J. F. Haemopoietic stem cells in the peripheral blood. Lancet. 1967 Nov 25;2(7526):1138–1141. doi: 10.1016/s0140-6736(67)90636-8. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. Evolution of the immune process in vertebrates. Nature. 1968 May 4;218(5140):426–430. doi: 10.1038/218426a0. [DOI] [PubMed] [Google Scholar]
- CUDKOWICZ G., STIMPFLING J. H. DEFICIENT GROWTH OF C57BL MARROW CELLS TRANSPLANTED IN F1 HYBRID MICE. ASSOCIATION WITH THE HISTOCOMPATIBILITY-2 LOCUS. Immunology. 1964 May;7:291–306. [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Perey D. Y., McKneally M. F., Gabrielsen A. E., Sutherland D. E., Good R. A. A mammalian equivalent of the avian bursa of Fabricius. Lancet. 1966 Jun 25;1(7452):1388–1391. doi: 10.1016/s0140-6736(66)90300-x. [DOI] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. THE THYMUS AND AUTOIMMUNE DISEASE. Lancet. 1963 Nov 16;2(7316):1026–1029. doi: 10.1016/s0140-6736(63)90003-5. [DOI] [PubMed] [Google Scholar]
- Isaković K., Smith S. B., Waksman B. H. Role of the thymus in tolerance. I. Tolerance to bovine gamma globulin in thymectomized, irradiated rats grafted with thymus from tolerant donors. J Exp Med. 1965 Dec 1;122(6):1103–1123. doi: 10.1084/jem.122.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Siminovitch L., Till J. E., McCulloch E. A. A transplantation assay for mouse cells responsive to antigenic stimulation by sheep erythrocytes. Proc Soc Exp Biol Med. 1965 Dec;120(3):868–873. doi: 10.3181/00379727-120-30678. [DOI] [PubMed] [Google Scholar]
- Lawrence W., Jr, Simonsen M. The property of "strength" of histocompatibility antigens, and their ability to produce antigenic competition. Transplantation. 1967 Sep 5;5(5):1304–1322. doi: 10.1097/00007890-196709000-00009. [DOI] [PubMed] [Google Scholar]
- MOELLER E. CONTACT-INDUCED CYTOTOXICITY BY LYMPHOID CELLS CONTAINING FOREIGN ISOANTIGENS. Science. 1965 Feb 19;147(3660):873–879. doi: 10.1126/science.147.3660.873. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPP R. A., CUDKOWICZ G. INDEPENDENCE OF DEFICIENT EARLY GROWTH AND LATER REGRESSION OF (C57BL X 101)F2 MARROW GRAFTS IN (C57BL X 101)F1 HYBRID MICE. Transplantation. 1965 Mar;3:155–160. doi: 10.1097/00007890-196503000-00003. [DOI] [PubMed] [Google Scholar]
- POPP R. A. Regression of grafted bone marrow in homologous irradiated mouse chimeras. J Natl Cancer Inst. 1961 Mar;26:629–640. [PubMed] [Google Scholar]
- Playfair J. H., Papermaster B. W., Cole L. J. Focal antibody production by transferred spleen cells in irradiated mice. Science. 1965 Aug 27;149(3687):998–1000. doi: 10.1126/science.149.3687.998. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune response of mice. I. The neonatal response to sheep red cells. Immunology. 1968 Jul;15(1):35–50. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. A., Marbrook J., Diener E. Primary stimulation and measurement of antibody production to sheep red blood cells in vitro. J Exp Med. 1967 Aug 1;126(2):347–356. doi: 10.1084/jem.126.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyan M. L., Cole L. J., Herzenberg L. A. Fetal liver: a source of immunoglobulin producing cells in the mouse. Proc Soc Exp Biol Med. 1967 Apr;124(4):1161–1163. doi: 10.3181/00379727-124-31951. [DOI] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell migration. J Exp Med. 1967 Aug 1;126(2):291–304. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]


