Abstract
Behavioral reactions to stress are altered in numerous psychiatric and neurodegenerative syndromes, but the corresponding molecular processes and signal transduction pathways are yet unknown. Here, we report that, in mice, the stress-induced splice variant of acetylcholinesterase, AChE-R, interacts intraneuronally with the scaffold protein RACK1 and through it, with its target, protein kinase CβII (PKCβII), which is known to be involved in fear conditioning. In stress-responsive brain regions of normal FVB/N mice, the mild stress of i.p. injection increased AChE and PKCβII levels in a manner suppressible by antisense prevention of AChE-R accumulation. Injection stress also prolonged conflict between escape and hiding in the emergence into an open field test. Moreover, transgenic FVB/N mice overexpressing AChE-R displayed prolonged delay to emerge into another field (fear-induced behavioral inhibition), associated with chronically intensified neuronal colabeling of RACK1 and PKCβII in stress-responsive brain regions. These findings are consistent with the hypothesis that stress-associated changes in cholinergic gene expression regulate neuronal PKCβII functioning, promoting fear-induced conflict behavior after stress.
Behavioral reactions to traumatic events are altered from those of the general population in several psychiatric disorders, e.g., posttraumatic stress disorder (PTSD; ref. 1), depression (2), and Alzheimer's disease (3). Disease-associated changes often intensify “freezing” or “fear-induced behavioral inhibition” characterized by suppression of behavior, as opposed to excitatory “flight” or exploratory behavior (4). Impaired resolution of the conflict between these potential responses to stress thus yields an imbalanced response; however, the mechanisms underlying these changes are yet unknown. Stress responses involve several neural pathways (5) and endocrine systems (6). For example, the cholinergic pathway from the medial septum to the hippocampus suppresses escape reaction in favor of freezing or hiding reaction (7). In the hippocampus, stress initially induces cholinergic excitation followed by feedback inhibition of neural activity (8). This information suggests involvement of cholinergic elements in the conflict among competing behavioral responses to stressful events.
Of the key cholinergic elements, acetylcholinesterase (AChE) possesses both catalytic and neuronal plasticity activities (9). In mice, stress-induced alternative splicing facilitates overproduction of the normally rare AChE-R variant, associated with weeks-long neuronal hypersensitivity (10). In humans, anticholinesterase therapies, which affect behavior, induce AChE-R accumulation in the cerebrospinal fluid of Alzheimer's disease patients (11). At extracellular sites, AChE-R reduces the stress-induced acetylcholine levels (8). However, AChE-R also accumulates in neuronal cell bodies, where the presence of acetylcholine is unlikely. Under no challenge, transgenic mice overexpressing intraneuronal AChE-R (TgR), display reduced levels of stress-associated neuropathologies compared with parental strain (12), suggesting that this protein is functionally effective. However, stress responses had not previously been tested in these mice. Because the core domain, common to all of the AChE variants, is sufficient for acetylcholine hydrolysis (13), the C-terminal domain unique to AChE-R emerged as an attractive candidate for intracellular protein–protein interactions transducing stress-induced signals.
Signal transduction pathways involve specific subtypes of protein kinase C (PKC; ref. 14). These pathways are activated under physiological (15, 16), biochemical (17), and cellular stresses (18). PKC activity enhances peak N-methyl-d-aspartate (NMDA)-evoked currents, compatible with the weeks-long enhancement of glutamatergic activity following stress (10). In addition, however, PKC phosphorylation of the C-terminal peptide of the NR1 subunit of the NMDA receptor depresses steady-state NMDA-evoked currents, through a Ca2+-dependent, Src-signaling independent pathway (19). Together, these effects predict complex PKC-mediated changes in glutamatergic neurotransmission and related behavioral phenotypes under stress. In particular, PKCβII, an alternative splicing product of the PKCβ gene (20), is intimately associated with oxidative (17) and ischemic (21) stresses and is essential for fear conditioning (22).
Exposure to phorbol ester or dopamine β2 agonists activates PKCβII, promoting its subcellular movement in complex with the shuttling protein RACK1 (23). RACK1 belongs to the family of tryptophan/aspartate (WD) proteins, which, by having seven homologous domains arranged like propeller blades around a center, can simultaneously bind different proteins (24). For example, RACK1 interacts with β-integrin (25), cAMP phosphodiesterase (26), phospholipase C-γ1 (27), src kinase (28), or the β-adrenergic receptor (29). However, the ubiquitous nature of RACK1 makes it unlikely to be the primary inducer of PKCβII-mediated stress-related cascades.
By using a yeast two-hybrid screen (30) we discovered that AChE-R forms tight, coimmunoprecipitable triple complexes with RACK1 and PKCβII and facilitates stress-induced, PKCβII accumulation associated with prolonged conflict behavior patterns. Our findings suggest that the formation of neuronal AChE-R/RACK1/PKCβII complexes may tilt the balance of stress-induced behavior toward intensifying conflict behavior.
Materials and Methods
Vectors.
The “bait” plasmid pGBK-ARP51 included an EcoRI/HpaI fragment of human AChE-R cDNA [nucleotides 1796–1865 of hAChE, accession no. M55040, followed by nucleotides 1–111 from the genomic hAChE I4-E5 domain (accession S71129, stop codon in position 86)] cloned into the EcoR1/SmaI sites of pGBK-T7 (CLONTECH). pGARP contains the same fragment cloned into the Bsp120I/XbaI sites of pEGFP-C2 (CLONTECH).
Two-Hybrid Screening.
Yeast AH109 cells (CLONTECH) were transformed with the pGBK-ARP51 plasmid (Yeastmaker transformation system, CLONTECH), including the DNA-binding domain (BD; residues 1–147) of the yeast GAL4 transcriptional activator, then with 10–25 μg of an amplified human fetal brain cDNA library cloned into the GAL4 activation domain vector (30). Screening covered 240,000 independent clones.
Cell Cultures and Transfection Experiments.
Transient transfections of PC12 and COS cells with AChE-R-encoding plasmid (12) and Lipofectamine Plus (Life Technologies, Paisley, Scotland) were followed by lysis 24 h later in 0.1 M phosphate buffer, pH 7.4/1% Triton X-100 and complete mini protease inhibitor mixture (Roche Molecular Biochemicals). Clear supernatants were prepared by centrifugation (12,000 × g, 4°C, 30 min).
Coimmunoprecipitation.
Cell supernatants (200 μl, 1.5 mg protein per ml) were diluted 1:5 in 50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM EDTA/0.25% gelatin/protease inhibitors mixture (Roche Molecular Biochemicals)/0.05% Triton X-100 for overnight rotation at 4°C with goat polyclonal antibodies (SC-6431, Santa Cruz Biotechnology) targeted to the N-terminal domain of hAChE (10 μl, 200 μg/ml). Mixtures with protein G MicroBeads (75 μl, 1 h, Miltenyi Biotec, Auburn, CA) were loaded on MACS columns (Miltenyi Biotec), washed [3 × 200 μl of TBS (0.1 M Tris, pH 7.4/1.7 M NaCl/0.05% Tween-20)], and eluted with gel loading buffer [50 mM Tris, pH 6.8/100 mM DTT/2% (wt/vol) SDS/0.1% bromophenol blue/10% glycerol]. Eluates were separated by denaturing SDS/PAGE (Bio-Rad), blocked [1 h, 3% nonfat dried milk/2% (wt/vol) BSA/0.2% Tween-20 in TBS], and blotted. Immunodetection was done with rabbit anti-N terminus AChE antibodies (N-19, Santa Cruz Biotechnology), dilution 1:500; mouse-anti-PKCβII (P8083, Sigma), dilution 1:8,000, or a mouse monoclonal antibody against RACK1 (R20620, Transduction Laboratories, Lexington, KY), dilution 1:2,500. Secondary antibodies were horseradish-peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse (1:10,000, The Jackson Laboratory). Chemiluminescent detection was with the enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Life Sciences, Uppsala), as instructed.
Laboratory animal experiments were approved by the Hebrew University's Committee for Animal Experimentation. FVB/N and TgR mice, 6–8 weeks old, were used as naive or were injected with saline (0.2 ml, 0.9%, i.p.) or with 500 μg/kg of the antisense agent EN101 (10). These treatments induce mild psychological stress with (EN101) or without (saline) suppression of AChE-R production. Mice were deeply anesthetized with Pental (pentobarbitone sodium, 100 mg/kg, CTS Chemical Industries, Petach Tikva, Israel) and killed 6 or 24 h after injection. For neuroanatomy analyses, anesthetized mice were transcardially perfused with 4% (wt/vol) paraformaldehyde and processed as reported (12). Brain homogenates were prepared from nontransfused mice in 0.01 M Na-phosphate buffer, pH 7.4/1% Triton X-100 (9:1, vol:vol), incubated on ice (1 h), and centrifuged (12,000 × g, 45 min in an Eppendorf centrifuge); supernatants were kept at −20°C until use.
Enzyme activity measurements involved two distinct PKC peptide substrates and were performed essentially as instructed in the corresponding kits (PKC assay kit, Upstate Biotechnology, Lake Placid, NY; PepTag, Promega). Reported PKC activity values were confirmed in both methods. AChE activity was measured as described (12).
Immunohistochemical analyses were performed essentially as described by using rabbit anti-ARP (10, 12) 1:100, rabbit anti-PKCβII (sc-210, Santa Cruz Biotechnology) 1:100, rabbit anti-PKCβII (Sigma) 1:250, and mouse anti-RACK1 (R20620, Transduction Laboratories) 1:200. Sections were incubated with the primary antibody, then with biotin-conjugated donkey anti-rabbit antibody (AP132B, 1 h, room temperature, overnight at 2–8°C, Chemicon). RACK1 staining was preceded by treatment with trypsin (type II, Sigma), 1 μg/ml with 0.001% CaCl2, 0.001% soybean trypsin inhibitor (Sigma) for 2 min at room temperature. Detection was with horseradish peroxidase-conjugated goat anti-mouse antibody (1:100 dilution, Sigma), followed by incubation in 0.0125% diaminobenzidine, 0.05% nickel ammonium sulfate, and 0.00018% hydrogen peroxide. Anti-RACK1 preincubation with 10 μM RACK1 (1 h, room temperature) totally eliminated anti-RACK1 staining, demonstrating specificity.
Emergence into an Open Field.
A 9 × 10 × 11 cm tilt bin box (Arnon Caine, Niles, IL) was placed in the center of a black painted plywood 100 × 100 cm open field with 50 cm walls. A 32 × 14 cm stainless steel cage top (Techniplast, Milan) leaning on this box formed a platform reaching 5.5 cm above the open field floor. Latencies were measured for exit from the tilt-bin box to the platform and for descending from the platform to the open field floor.
Statistical Analyses.
Two-factor analysis of variance subjected to post hoc analyses involved transgenic overexpression (TgR vs. FVB/N) and stress (saline or EN101 injection vs. no injection).
Results
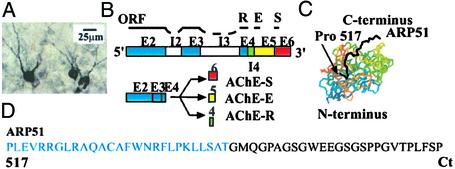
Despite its secretory nature, AChE-R accumulates within neurons (e.g., the cell body and processes of hippocampal basket cells in the granule cell layer of the dentate gyrus; Fig. 1A). We used the Gal4 two-hybrid system to search for its intracellular partner(s).
Figure 1.
The ARP51 C-terminal domain of AChE. (A) Dentate gyrus neurons from TgR mice expressing human (h)AChE-R under cytomegalovirus (CMV) control. Staining was done with anti-AChE-R antibodies. (B) Schematic diagram of the human ACHE gene (E, exon; I, intron; ORF, open reading frame) with its synaptic (AChE-S), erythrocyte (AChE-E), and “readthrough” (AChE-R) mRNA 3′ alternative splicing products, giving rise to protein variants with different C termini. (C) Three-dimensional-simulated structure of mouse AChE (www.rcsb.org/pdb/index.html, PDB ID code 1C2B). Residues 1–517 but not the C-terminal 51 residues (ARP51) are required for acetylcholine hydrolysis. (D) The ARP51 sequence. The residues in black are unique to the hAChE-R variant.
Two-Hybrid Screening for Protein Partners of AChE-R.
As bait, we used the human AChE-R readthrough peptide ARP51, composed of the C-terminal 51 amino acid residues of AChE-R, 25 of which are tightly conserved in evolution (Fig. 1 B–D, and Esther database, www.ensam.inra.fr/cgi-bin/ace/index).
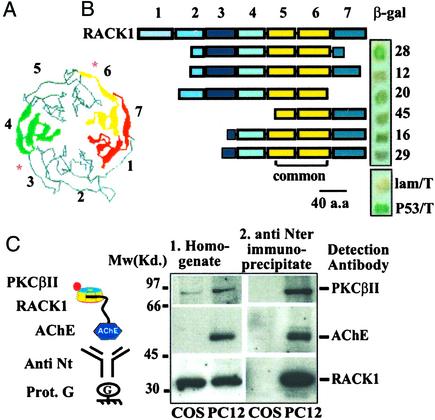
Several potential partner proteins from human fetal brain enabled survival in the screening procedure of yeast clones expressing the ARP51 peptide. Of these, only the WD protein RACK1 appeared in six cDNA fragments of different lengths (Fig. 2A and sequence data not shown). Individual ARP51/RACK1 expressing yeast colonies displayed variable β-galactosidase (β-gal) activity in the same range as that induced by p53–T antigen interactions (dissociation constant = 2 × 108 M−1; Fig. 2B Inset; ref. 31). RACK1 is homologous to the propeller part of the G protein β2-subunit, a prototypic member of the WD domain family (24). The sequence domain which conferred binding to ARP51 in the two-hybrid system included large parts of blades 5 and 6 of the seven domains in the wheel-like propeller structure of the RACK1 protein. The binding site to PKCβII on blade 6 of RACK1 (Fig. 2A; ref. 29), but not the PKCβII binding site on blade 3, emerged as apparently masked under ARP51 interaction.
Figure 2.
Two-hybrid positive RACK1 clones. (A) Schematic structure of the G protein β-subunit (http://pdb.ccdc.cam.ac.uk/pdb/, PDB ID code 1gp2). The concentric numbered blades of WD proteins are highlighted in color; each blade is composed of four antiparallel strands (ref. 24; RACK1 sequence blast identity = 26%, similarity = 44%; accession no. AAH04186). Asterisks denote PKCβII-binding sites in RACK1. (B) Schematic alignment of RACK1 clones. Scaled drawings of the seven WD domains (boxes numbered above) in RACK1 (top, accession no. P25388) and the six viable RACK1 clones of human fetal brain origin, in the two-hybrid screen. The common sequence included in all clones (within yellow WD domains 5 and 6) is underlined. ARP51–RACK1 interactions in representative colonies are shown by β-gal staining (blue spots). Positive and negative controls were p53–T-antigen (p53/T) and lamin C–T antigen (lam/T) interactions in yeast. (C) RACK1 and PKCβII coimmunoprecipitate with anti-AChE antibodies. Drawing shows the experimental design. 1, Homogenate, immunodetected PKCβII and RACK1 but not AChE appear in total homogenates of COS cells. PC12 cell homogenates display PKCβII, RACK1, and AChE. 2, Anti-Nter immunoprecipitates, dissolved immunoprecipitates with antibodies to the N terminus of AChE display all three partner proteins in PC12 cells but no signals in COS cells. Note intensified PKCβII and RACK1 signals as compared with those observed in homogenates.
Detecting AChE-R–RACK1–PKCβII Interaction in Cell Homogenates.
To find out whether ARP51 and full-length AChE-R interact with RACK1 to form triple complexes with PKCβII, we used gel-overlay assays (see Figs. 5 and 6, which are published as supporting information on the PNAS web site, www.pnas.org, and corresponding text) and immunoprecipitation experiments from cultured cell homogenates. Both COS and PC12 cells express RACK1 and PKCβII, whereas only PC12 cells express AChE-R. Neither the anti-RACK1 nor the anti-PKCβII antibody was capable of coprecipitating triple complexes. However, antibodies targeted to the N terminus of AChE coimmunoprecipitated both PKCβII and RACK1 from the soluble fraction of PC12 cell homogenates but not the control COS cells (Fig. 2C). This finding supported the notion of tight AChE-R/RACK1/PKCβII linkage in PC12 cells.
Coaccumulation of AChE-R and PKCβII in Stress-Responding Brain Regions.
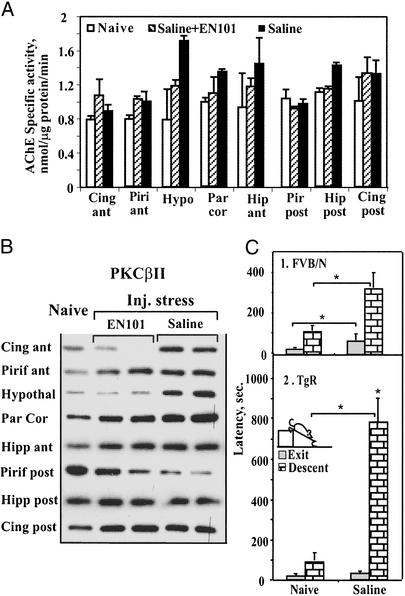
In the brain of naive mice, total catalytic AChE activities ranged from 0.8 ± 0.03 nmol substrate hydrolyzed per min per μg protein (average ± SEM) in the anterior cingulate cortex to 1.00 ± 0.04 in the parietal cortex. Then, we measured AChE activities 6 h after i.p. saline injection, which activates similar stress-responsive brain regions to those activated by immobilization, footshock, or forced swim stresses (32).
In FVB/N mice, injection stress induced significant (P < 0.01) increases in the cumulative total AChE hydrolytic activities in several stress-responding regions, indicating specificity (e.g., >2-fold injection-induced increases in hypothalamic AChE activity, Fig. 3A). Parallel increases in the closely related enzyme butyrylcholinesterase (BuChE) in these brain regions were significant but limited to 50% (P < 0.05), attesting to the intensity of these AChE effects. An exception was the posterior piriform cortex, which showed no increase (Fig. 3A). EN101, a 2′-oxymethylated antisense oligonucleotide, which successfully suppresses stress-induced increases in locomotor activity (33, 34), was administered i.p. and served to study the physiological significance of AChE-R–RACK1–PKCβII interactions. 2′-Oxymethylation of oligonucleotides promotes their brain penetrance (35). Yet, EN101 is well tolerated, with no detrimental effects on behavior. In the mouse brain, EN101 has been shown to reduce the levels of AChE-R but not of the synaptic AChE-S protein under conditions where an irrelevant oligonucleotide targeted to the homologous BuChE gene has no effect (34). In this study, EN101 reduced the injection-induced increases in AChE hydrolytic activity in several stress-responsive brain regions (P < 0.05, Fig. 3A). BuChE increases were not affected, attesting to the specificity of this suppression. Other regions, e.g., posterior cortex, showed neither increase nor EN101 effect (Fig. 3A).
Figure 3.
PKCβII/AChE-R coaccumulation parallels delayed reaction to mild stress. (A) AChE accumulation under mild stress of saline injection. Shown are average total AChE-specific activities ± SEM in brain regions from FVB/N naive mice or mice killed 6 h after i.p. injection of saline or EN101. AChE-R may amount to 30% of total AChE activity in the homogenates from stressed brain (A. Salmon and H.S., unpublished data). Data shown are from one of three experiments. (B) Immunopositive PKCβII accumulation under injection stress. Shown are immunolabeling intensities of PKCβII in brain region homogenates from individual mice treated as in A. PKCβII signals are shown in the cingulate anterior cortex (Cing ant), piriform anterior cortex (Piri ant), Hypothalamus (Hypothal), Parietal cortex (Par Cor), anterior hippocampus (Hipp ant), posterior piriform (Piri post) cortex, posterior hippocamus (Hipp post), and posterior cingulate cortex (Cing post). Note that EN101 injection ablates much of the injection stress-induced increases in PKCβII. (C) Poststress exit and descent latencies into an open field. Latency times (sec) of exit from a box after contextual habituation time and descent over a tilted cage cover into an open field are shown for (1) FVB/N mice (n = 6 naive and 6 saline-injected) and (2) TgR mice. Asterisks note significant differences from naive or saline-injected FVB/N mice, respectively. (Inset) Scheme of the emergence test.
Injection-induced and EN101-preventable increases were also observed in immunoreactive PKCβII in the cingulate, parietal, and piriform cortices, hypothalamus, and anterior hippocampus (Fig. 3B and Fig. 7, which is published as supporting information on the PNAS web site). In the posterior piriform cortex, both EN101 and injection stress reduced PKCβII levels, reflecting a region-specific response. The posterior hippocampus sample included CA1, CA3, and dentate gyrus, with both stress-excitatory and stress-inhibitory neurons. The anterior hippocampus sample, however, was mostly composed of the CA3 region, enriched with stress-excitatory neurons. These differences were compatible with the high basal PKC activity in the posterior sample and the stress-induced PKCβII increases in the anterior one (Fig. 8, which is published as supporting information on the PNAS web site).
Delayed Emergence into an Open Field.
Latencies were measured for exit from a sheltered box to a higher platform and then for descent from this platform to an unfamiliar and, therefore, threatening open field floor. Conflict was manifested in repeated episodes of approach to the edge of the platform and retreat back to the box.
In FVB/N mice, and yet more so in transgenic mice expressing human AChE-R under the cytomegalovirus (CMV) promoter (TgR; ref. 12), saline injection increased latencies to exit and to descend from the platform to the open field floor (Fig. 3C). Video monitoring of free movement showed that this response pattern did not reflect neuromuscular impairment in TgR mice. Saline injection itself exerted a main effect on exit latency [F (1, 33) = 7.74; P < 0.001], but had no interaction with AChE-R overexpression. Within a 25-min limit, all FVB/N mice descended, but 2 of the 12 TgR mice did not descend at all to the open field floor (Fig. 3C).
Saline injection induced a significant main effect on exit latency of TgR mice as well [F (1, 33) = 11.36, P < 0.002] and on their descent latency [F (1, 34) = 51.8, P < 0.0001; Fig. 3C]. Transgenic overexpression did not affect exit latency but increased descent latency after saline injection [F (1, 34) = 7.03, P < 0.02; Fig. 3C], revealing significant interaction of transgenic overexpression and injection stress [F (1, 34) = 4.69, P < 0.04], with a greater latency in saline-injected TgR mice compared with saline-injected FVB/N mice (P < 0.005). TgR mice also displayed increased conflict behavior after a stressful event, in the elevated plus maze (EPM) test (see Fig. 9, which is published as supporting information on the PNAS web site, and corresponding text).
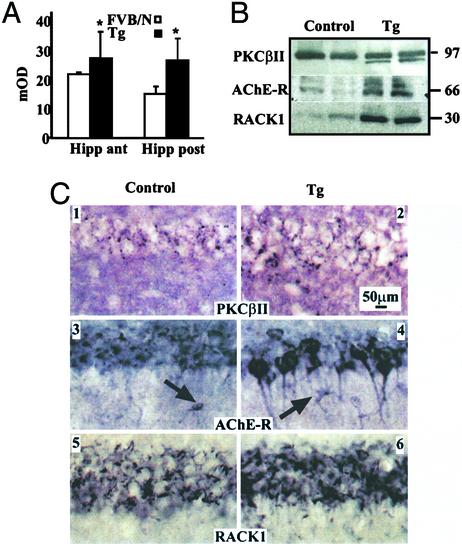
Transgenic AChE-R Overexpression Intensifies Neuronal PKCβII Clusters.
To test whether the extended conflict behavior under chronic AChE-R overproduction is associated with modulated levels, properties, and/or neuronal localization of RACK1 and PKCβII, we used hippocampal homogenates and sections from TgR and control FVB/N mice. Total enzymatic PKC activities were distinctly elevated in hippocampal homogenates from TgR mice, producing AChE-R under the cytomegalovirus (CMV) promoter, as compared with the parent FVB/N strain (Fig. 4A Left). This elevation was accompanied by increases in immunopositive neuronal AChE-R and RACK1 and a faster migrating PKCβII band, only faintly detected in the FVB/N hippocampus (Fig. 4B Right).
Figure 4.
Transgenic AChE-R overexpression accompanies increased neuronal RACK1 and PKCβII labeling in hippocampal CA1 neurons. (A) Total PKC activity in TgR mice. PKC activity in pooled anterior or posterior hippocampal homogenates from two TgR and two FVB/N mice. Asterisks note statistically significant differences (Student's t test, P < 0.03) for all tested samples. (B) RACK1 and PKCβII modulations in TgR mice. Immunoblot signals for PKCβII, AChE-R, and RACK1 after gel electrophoresis of clear hippocampal homogenates from two individual FVB/N controls and two sex- and age-matched TgR mice (Tg). Shown are representative results from five reproducible experiments. (C) Neuronal labeling. Shown are hippocampal CA1 immunohistochemistry analyses from representative control and TgR mice stained with the noted antibodies.
Colocalization studies demonstrated wide brain region distribution for neuronally clustered RACK1, PKCβII, and AChE-R in the TgR brain (Fig. 4C and Supporting Text, which is published as supporting information on the PNAS web site). Punctiform PKCβII staining (Fig. 4C, 1–4) appeared in the cell bodies and proximal processes of a specific subset of CA1 neurons. Both in FVB/N and TgR mice, AChE-R overexpression in hippocampal and cortical neurons was accompanied by positively labeled cells, with morphology reminiscent of microglia (Fig. 4C, 3 and 4). In TgR mice, intensified RACK1 labeling of perikaria and closely proximal neurites of CA1 pyramidal neurons and cortical neurons was also observed (Fig. 4C, 5 and 6, and supporting information).
Diffuse and punctiform PKCβII staining displayed essentially similar distributions in TgR mice and in the FVB/N strain. Yet, punctiform PKCβII staining was intensified within several stress-responding brain areas in some, but not all, of the AChE-R accumulating neurons in TgR mice. These included neurons in upper cortical layers, hippocampal CA1 and CA3, lateral septum, and basolateral amygdala. AChE-R-accumulating neurons in the lateral and ventro-medial hypothalamus, central nucleus of the amygdala, the hippocampal dentate gyrus, ventro-lateral thalamus, and Edinger-Westphal nucleus of TgR mice showed no PKCβII punctiform staining (see supporting information). In other brain regions of TgR mice (globus pallidus, substantia nigra, superior culliculus, medial septum, and diagonal band), AChE-R-accumulating neurons were rare, with little or no PKCβII punctuation, thus demonstrating selectivity of neuronal AChE-R/PKCβII coexistence.
Discussion
Our findings are consistent with the hypothesis that stress-induced neuronal signal transduction involves AChE-R overproduction, which may tilt the balance of poststress conflict behavior away from the excitatory tendency to escape toward the inhibitory freezing behavioral pattern. The two-hybrid screen findings further attribute this modified behavior pattern to the intracellular formation and translocation of triple complexes of AChE-R/RACK1/PKCβII. Although much in this hypothesis awaits further exploration, it points to RACK1 as a hitherto unrecognized component in the molecular cascade controlling physiological stress responses and provides a putative mechanism for the long-known behavioral effects of neuroactive agents that modulate cholinergic neurotransmission.
Intracellular Accumulation of the Stress-Induced AChE-R Variant.
The immunocytochemically observed intracellular localization of AChE-R, an essential prerequisite for AChE's interaction with RACK1 and PKCβII, was rather unexpected because of the existence of a signal peptide in the nascent AChE-R polypeptide and the generally secretory nature of this protein (8). However, nascent polypeptides targeted for ubiquitin-dependent processing and destined for secretion may be released from the rough endoplasmic reticulum into the cytoplasm (36). This finding suggests that the signal peptide by itself does not ensure fool-proof secretion of polypeptides including it and calls for studying the possibility that, similar to alternative splicing, the intracellular compartmentalization of secretory proteins is altered under stress.
Robust Response to Very Mild Signals.
We have previously shown extensive, long-lasting accumulation of AChE-R after multiple stresses (e.g., anticholinesterase exposure, confined swim or circadian switch; refs. 8, 10, and 33). That the same phenomenon also occurs after a single i.p. saline injection and that it affects the fine tuning of conflict behavior came as a second surprise. Conflict behavior is known to depend, to a large extent, on previous experiences, e.g., traumatic events (37) combined with inherited factors (38). That mild injection stress induced significant increases in AChE activity and promoted escape behavior in FVB/N females is compatible with the characteristic female preference for escape in conflict situations that follow a stressful event (39). Combined with the antisense data, our current findings are compatible with the theory that a relatively mild stressful event in an uneventful background (animal colony routine), is sufficient to activate a similar cascade of signal transduction to that induced by intense stresses. This information may be relevant to the posttraumatic stress disorder (PTSD) phenomena in which the severity of the stressor was found to be less important than the change induced in the victim's perception of threat to survival (1).
AChE-R/RACK1/PKCβII Complexes as Modulators of Conflict Behavior.
In TgR mice that present increased PKC activity and modified neuronal distribution of PKCβII, contextual fear responses exceeded those of their parent strain FVB/N, both in the EPM and in the exit-emergence test. In TgR females, the chronic excess of AChE-R, RACK1, and PKCβII supports this interpretation of AChE-R interactions exerting a behavioral inhibitory effect that likely reflects the combined contribution of strain- and sex-specific properties (40, 41).
The RACK1 Scaffold Protein Performs Multiple Shuttling Functions.
The RACK1 region supporting AChE-R interaction consists of ≈30% of the RACK1 perimeter, including two antiparallel four-strand “blades,” with many conserved WD domain residues. Conformational changes in RACK1 likely expose it to the binding of other proteins. RACK1–AChE-R interactions and PKC activators, which modulate RACK1–PKCβII interactions (29), would therefore compete with other RACK1 associations, changing the subcellular balance between the different RACK1-containing complexes under stress.
The AChE-R effect on conflict behavior is consistent with the role of acetylcholine as a modulator of various stimulus-associated neurotransmission pathways (42). For example, RACK1–PKCβII interactions suppress the phosphorylation of the NR2B-subunit of the excitatory NMDA receptor (43), which may interfere with the excitatory serotonergic control over the inhibitory GABA-ergic input toward behavioral responses (15).
Based on the above arguments, the dissociation between RACK1 and PKCβII under ethanol exposure (44) may contribute to the anticonflict and disinhibitory effects of alcohol (45). Also, AChE-R and/or PKCβII accumulation may be relevant for impairments in other processes where fear conditioning is intimately involved, and in which PKCβII plays a major role, e.g., panic attacks (46), posttraumatic stress disorder (PTSD) (1), or poststroke phenomena.
AChE-R-filled neurons also appeared in cell populations devoid of PKCβII punctiform staining. This finding suggests that, in addition, AChE-R is involved in physiological mechanisms other than those that engage PKCβII. Likewise, PKCβII-positive neurons that are not AChE-R-enriched may participate in physiological mechanisms that depend on other, AChE-R-independent PKCβII activities.
Combinatorial Aspects of Stress-Induced Proteins.
PKCβII and RACK1 are readily available in many neurons. However, their previously unrecognized mobilization into densely packed clusters under stress, compatible with the known intracellular mobility of PKCβII, may rapidly change their function.
PKCβII/AChE-R-filled neurons, likely belong to neural circuits with particular combinations of transcription and splicing factors, the functioning of which depends on intracellular PKCβII-mediated signal transduction. AChE-R/RACK1/PKCβII complex formation may, therefore, affect the neuronal distribution of PKCβII in brain development (47) and neurodegeneration (18), which involve considerable modulations in AChE-R levels. AChE-R is further present in other RACK1/PKCβII producing tissues, including epithelial, muscle, and germ cells (reviewed in ref. 33) where its capacity to induce PKCβII-mediated changes should be examined. In hematopoietic progenitor cells, for example, AChE-R exerts myeloid proliferative and growth factor activities, compatible with the role of PKCβII in myeloid differentiation (48).
The signaling cascades involving AChE-R/RACK1/PKCβII complexes may well be just one thread in a complex network of many pathways transducing stress responses. Thus, AChE-R is one of three AChE isoforms, each with its own C-terminal peptide and possibly different interactions. Likewise, other PKC isoforms and splice variants may interact with different shuttling proteins, and RACK1 operates as a shuttling vector to many other proteins. The increase in RACK1 may reflect changes at the transcriptional, posttranscriptional, or stability levels; further screening efforts should shed more light on the potential participants and processes involved in these interactions.
Further Implications.
Of particular interest are the implications of our findings for drug responses and exposure to chemicals that modulate cholinergic neurotransmission. In essence, any agent that induces an increase in acetylcholine levels should promote the feedback response leading to AChE-R mRNA accumulation and dendritic translocation (8, 10). Such agents include insecticidal (e.g., chlorpyrifos; ref. 49) and therapeutic anticholinesterases, e.g., all of the currently approved Alzheimer's disease drugs. Recent studies demonstrate that numerous antidepressants inhibit human AChE (e.g., fluoxine, Sertraline, and amitriptyline; ref. 50), and that various antipsychotic drugs induce ACh release (e.g., chloropromazine, clozapine, haloperidol, risperidone, and Ziprisidone; ref. 51). Thus, it is tempting to speculate that at least some of the physiological effectiveness (2), therapeutic value, and/or attractivity to the caretaker of these agents is due to their capacity to shift the balance of conflict behavior from the excitatory flight pattern to the inhibitory freeze-like phenotype. In a reciprocal extension of this concept, it would be intriguing to test whether patients with posttraumatic stress disorder (PTSD), with reported tendency toward exaggerated inhibitory behavior (1), display higher blood AChE-R levels than those observed in individuals that successfully recover from similarly acute traumatic experiences.
In conclusion, the intracellular interaction of the stress-induced AChE-R variant with RACK1 and PKCβII adds a new dimension to our understanding of the interrelationship between cholinergic gene expression and the complex phenotype of conflict behavior.
Supplementary Material
Acknowledgments
We thank Drs. Daria Mochly-Rosen, Stanford University, and Eugenia Kovalev, Herzog Memorial Hospital, for assistance. This study was supported by U.S. Army Medical Research and Materiel Command Grant DAMD 17-99-1-9547 (to H.S.), Israel Science Fund Grants 618/02 (to H.S.) and 484/02 (to S.S.), U.S.–Israel Binational Scientific Foundation Grant 1999/115 (to H.S.), and Ester Neuroscience (Tel-Aviv). K.R.B. was a European Molecular Biology Organization Postdoctoral Fellow in Jerusalem.
Abbreviations
- AChE
acetylcholinesterase
- WD
tryptophan/aspartate motif
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Newport D J, Nemeroff C B. Curr Opin Neurobiol. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 2.Duman R S, Malberg J, Thome J. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 3.Cummings J L, Back C. Am J Geriatr Psychiatry. 1998;6:S64–S78. doi: 10.1097/00019442-199821001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gray J A. The Neuropsycology of Anxiety: An Inquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford Univ. Press; 2000. [Google Scholar]
- 5.Herman J P, Cullinan W E. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 6.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban P, Bock R, Klein R, Schutz G. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 7.Giovannini M G, Rakovska A, Benton R S, Pazzagli M, Bianchi L, Pepeu G. Neuroscience. 2001;106:43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 8.Kaufer D, Friedman A, Seidman S, Soreq H. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 9.Behra M, Cousin X, Bertrand C, Vonesch J L, Biellmann D, Chatonnet A, Strahle U. Nat Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- 10.Meshorer E, Erb C, Gazit R, Pavlovsky L, Kaufer D, Friedman A, Glick D, Ben-Arie N, Soreq H. Science. 2002;295:508–512. doi: 10.1126/science.1066752. [DOI] [PubMed] [Google Scholar]
- 11.Darreh-Shori T, Almkvist O, Guan Z, Garlined A, Strandberg B, Svensson A-L, Soreq H, Hellstrom-Lindahl E, Nordberg A. Neurology. 2002;59:563–572. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]
- 12.Sternfeld M, Shoham S, Klein O, Flores-Flores C, Evron T, Idelson G H, Kitsberg D, Patrick J W, Soreq H. Proc Natl Acad Sci USA. 2000;97:8647–8652. doi: 10.1073/pnas.140004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval N, Massoulie J, Bon S. J Cell Biol. 1992;118:641–653. doi: 10.1083/jcb.118.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens L, Parker P J, Rhee L, Yang-Feng T L, Chen E, Waterfield M D, Francke U, Ullrich A. Science. 1986;233:859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Cai X, Zhao J, Yan Z. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G Y, Hvalby O, Walaas S I, Albert K A, Skjeflo P, Andersen P, Greengard P. Nature. 1987;328:426–429. doi: 10.1038/328426a0. [DOI] [PubMed] [Google Scholar]
- 17.Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, Zaccheo D, Odetti P, Strocchi P, Marinari U M, et al. Biochem Biophys Res Commun. 2000;268:642–646. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- 18.McNamara R K, Wees E A, Lenox R H. J Neurochem. 1999;72:1735–1743. doi: 10.1046/j.1471-4159.1999.721735.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu W Y, Jackson M F, Bai D, Orser B A, MacDonald J F. J Neurosci. 2000;20:4452–4461. doi: 10.1523/JNEUROSCI.20-12-04452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono Y, Kikkawa U, Ogita K, Fujii T, Kurokawa T, Asaoka Y, Sekiguchi K, Ase K, Igarashi K, Nishizuka Y. Science. 1987;236:1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- 21.Cardell M, Wieloch T. J Neurochem. 1993;61:1308–1314. doi: 10.1111/j.1471-4159.1993.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 22.Weeber E J, Atkins C M, Selcher J C, Varga A W, Mirnikjoo B, Paylor R, Leitges M, Sweatt J D. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. J Biol Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- 24.Smith T F, Gaitatzes C, Saxena K, Neer E J. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 25.Liliental J, Chang D D. J Biol Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- 26.Yarwood S J, Steele M R, Scotland G, Houslay M D, Bolger G B. J Biol Chem. 1999;274:14909–14917. doi: 10.1074/jbc.274.21.14909. [DOI] [PubMed] [Google Scholar]
- 27.Disatnik M H, Hernandez-Sotomayor S M, Jones G, Carpenter G, Mochly-Rosen D. Proc Natl Acad Sci USA. 1994;91:559–563. doi: 10.1073/pnas.91.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang B Y, Chiang M, Cartwright C A. J Biol Chem. 2001;276:20346–20356. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez M M, Ron D, Touhara K, Chen C H, Mochly-Rosen D. Biochemistry. 1999;38:13787–13794. doi: 10.1021/bi991055k. [DOI] [PubMed] [Google Scholar]
- 30.Chien C T, Bartel P L, Sternglanz R, Fields S. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn C, Muller F, Melle C, Nasheuer H P, Janus F, Deppert W, Grosse F. Oncogene. 1999;18:769–774. doi: 10.1038/sj.onc.1202327. [DOI] [PubMed] [Google Scholar]
- 32.Ryabinin A E, Wang Y M, Finn D A. Pharmacol Biochem Behav. 1999;63:143–151. doi: 10.1016/s0091-3057(98)00239-1. [DOI] [PubMed] [Google Scholar]
- 33.Soreq H, Seidman S. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 34.Cohen O, Erb C, Ginzberg D, Pollak Y, Seidman S, Shoham S, Yirmiya R, Soreq H. Mol Psychiatry. 2002;7:874–885. doi: 10.1038/sj.mp.4001103. [DOI] [PubMed] [Google Scholar]
- 35.Tavitian B, Terrazzino S, Kuhnast B, Marzabal S, Stettler O, Dolle F, Deverre J R, Jobert A, Hinnen F, Bendriem B, et al. Nat Med. 1998;4:467–471. doi: 10.1038/nm0498-467. [DOI] [PubMed] [Google Scholar]
- 36.Ye Y, Meyer H, Rapoport T. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 37.Martijena I D, Calvo N, Volosin M, Molina V A. Brain Res. 1997;752:136–142. doi: 10.1016/s0006-8993(96)01465-5. [DOI] [PubMed] [Google Scholar]
- 38.Bolivar V J, Pooler O, Flaherty L. Mamm Genome. 2001;12:651–656. doi: 10.1007/s003350020039. [DOI] [PubMed] [Google Scholar]
- 39.Steenbergen H L, Heinsbroek R P, Van Hest A, Van de Poll N E. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- 40.Trullas R, Skolnick P. Psychopharmacology. 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- 41.Ohno M, Frankland P W, Chen A P, Costa R M, Silva A J. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- 42.Changeux J P, Dehaene S. Int J Psychophysiol. 2000;35:179–187. doi: 10.1016/s0167-8760(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 43.Yaka R, Thornton C, Vagts A, Phamluong K, Bonci A, Ron D. Proc Natl Acad Sci USA. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ron D, Vagts A J, Dohrman D P, Yaka R, Jiang Z, Yao L, Crabbe J, Grisel J E, Diamond I. FASEB J. 2000;14:2303–2314. doi: 10.1096/fj.00-0143com. [DOI] [PubMed] [Google Scholar]
- 45.Lapin I P. Pharmacol Biochem Behav. 1993;44:241–243. doi: 10.1016/0091-3057(93)90305-d. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia M. Mol Psychiatry. 2002;7:239–246. doi: 10.1038/sj.mp.4000997. [DOI] [PubMed] [Google Scholar]
- 47.Gallicano G I, Yousef M C, Capco D G. BioEssays. 1997;19:29–36. doi: 10.1002/bies.950190107. [DOI] [PubMed] [Google Scholar]
- 48.Kaneki M, Kharbanda S, Pandey P, Yoshida K, Takekawa M, Liou J, Stone R, Kufe D. Mol Cell Biol. 1999;19:461–470. doi: 10.1128/mcb.19.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Amate M, Flores P, Sanchez-Santed F. Behav Pharmacol. 2001;12:285–292. doi: 10.1097/00008877-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Muller T C, Rocha J B, Morsch V M, Neis R T, Schetinger M R. Biochim Biophys Acta. 2002;1587:92–98. doi: 10.1016/s0925-4439(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 51.Ichikawa J, Dai J, O'Laughlin I, Fowler W, Meltzer H. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.