Abstract
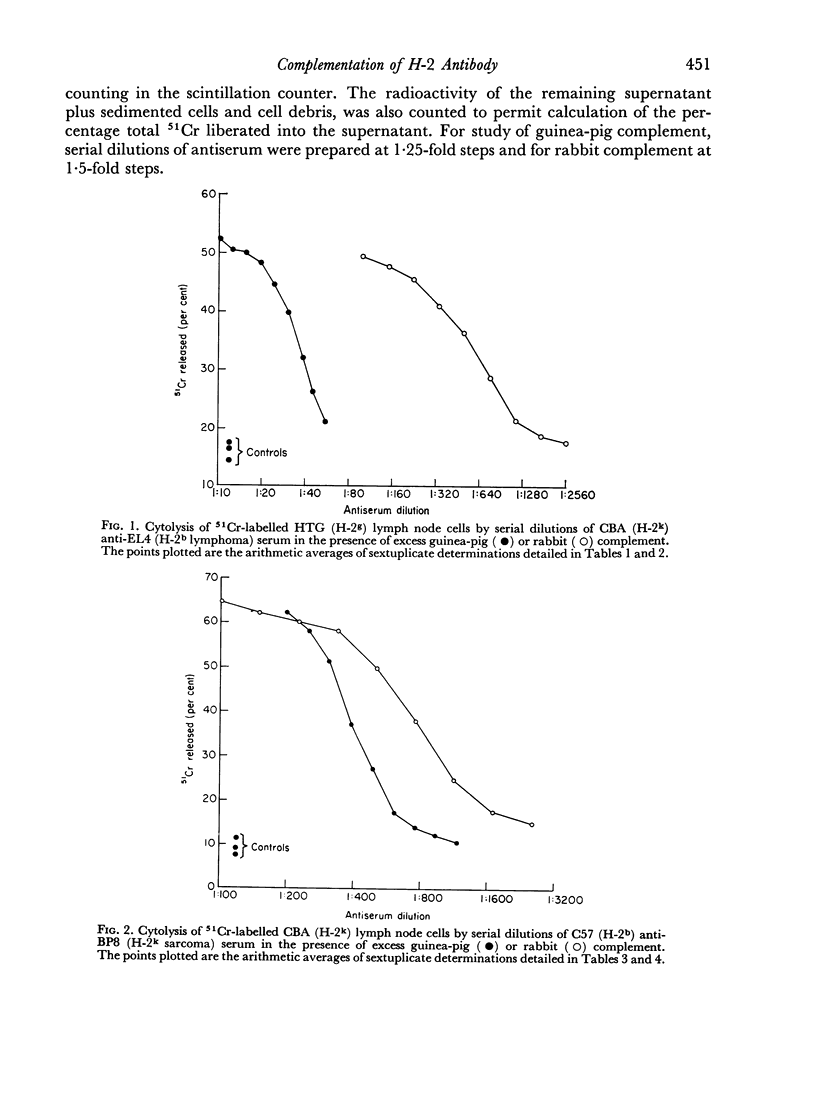
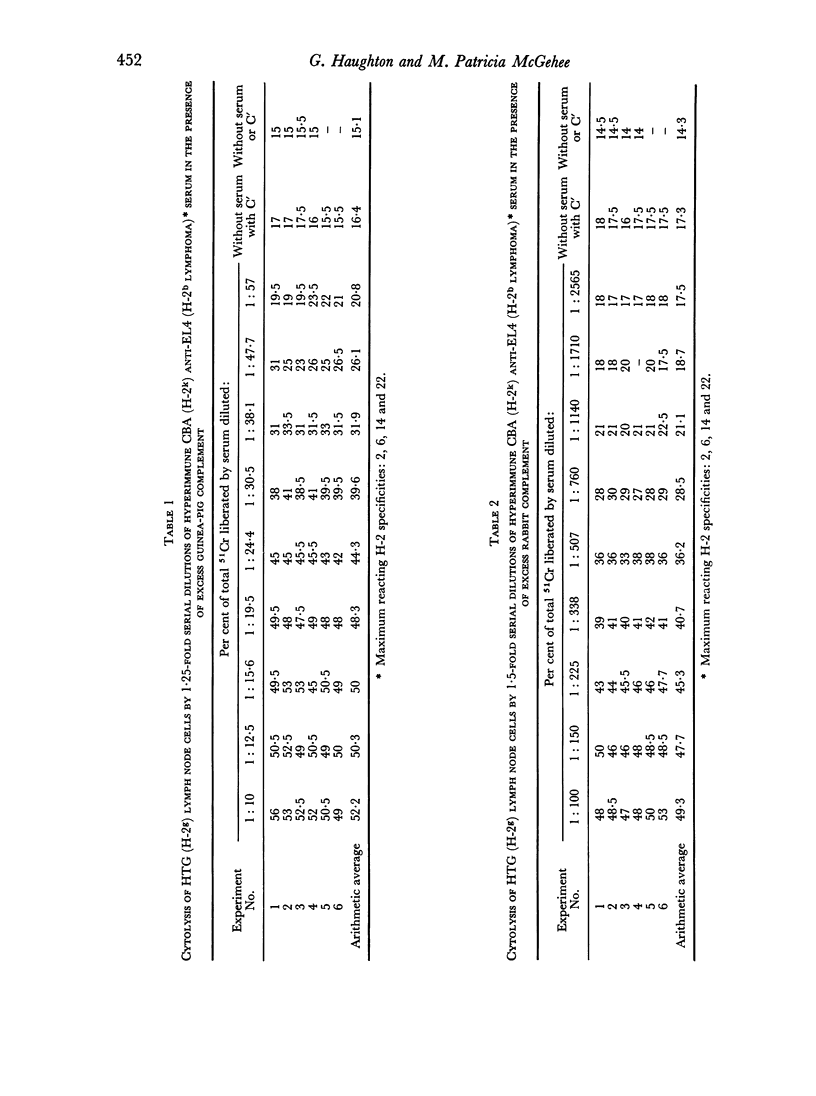
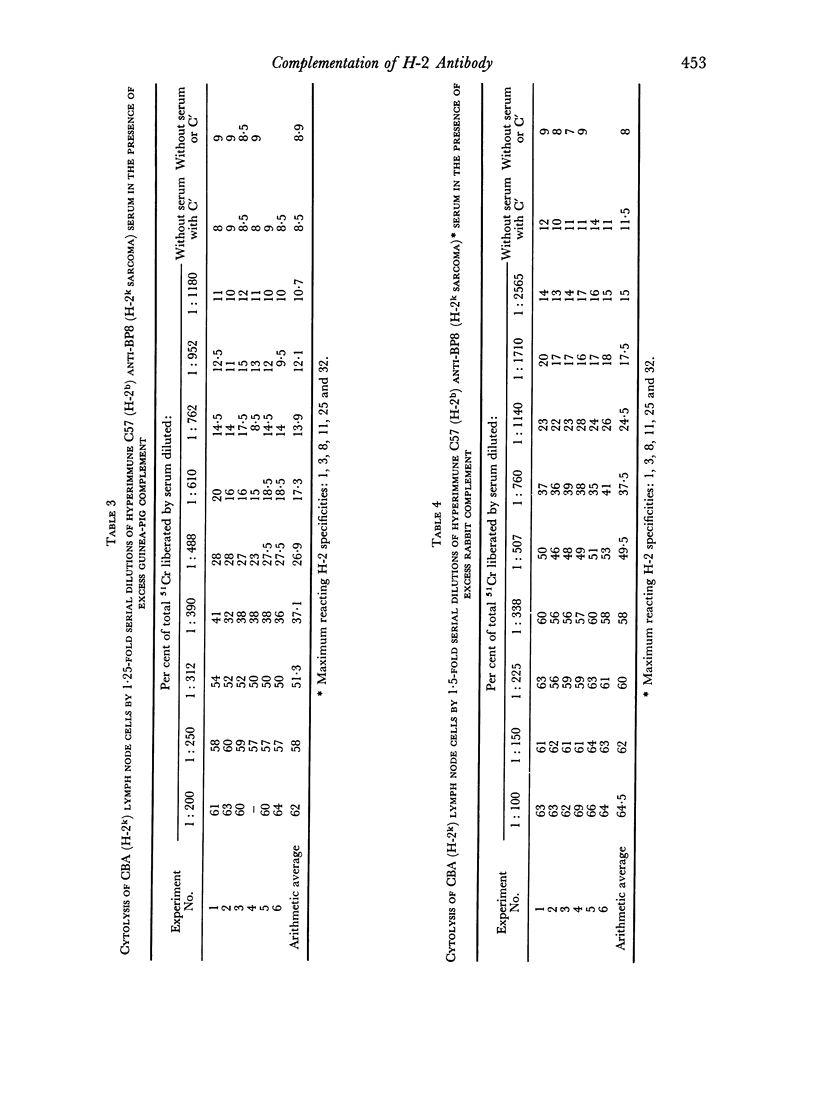
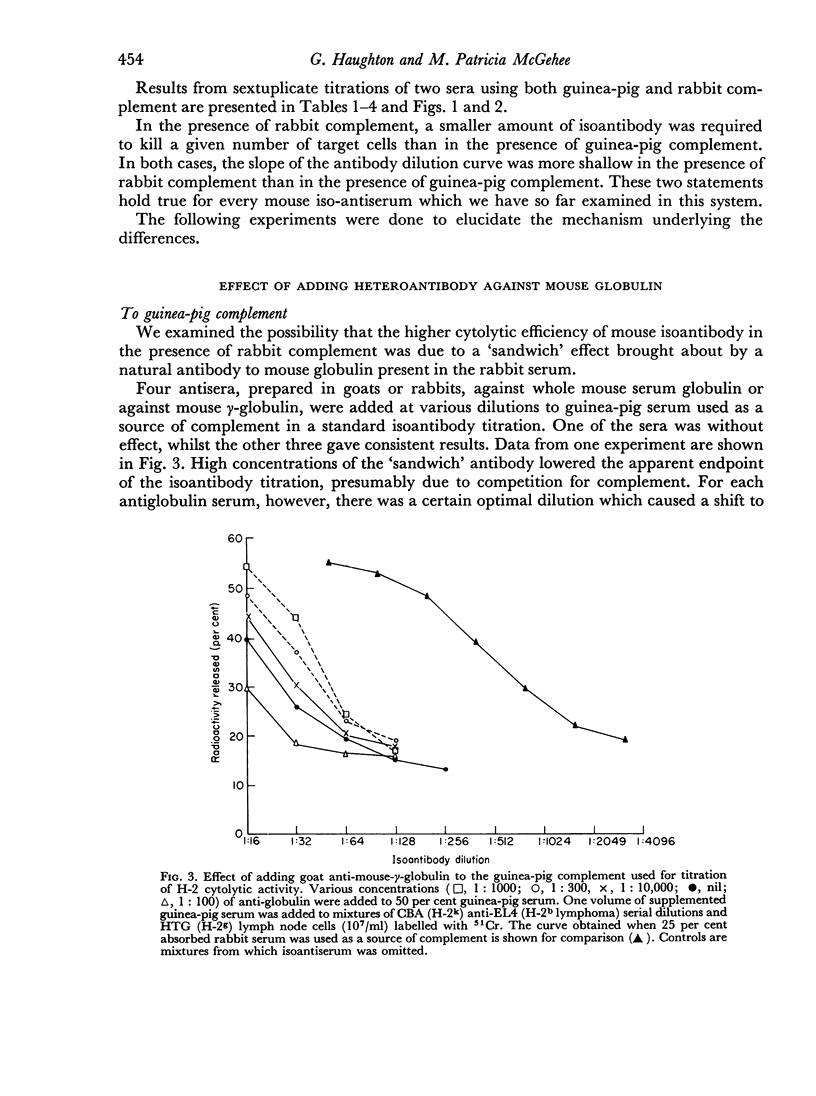
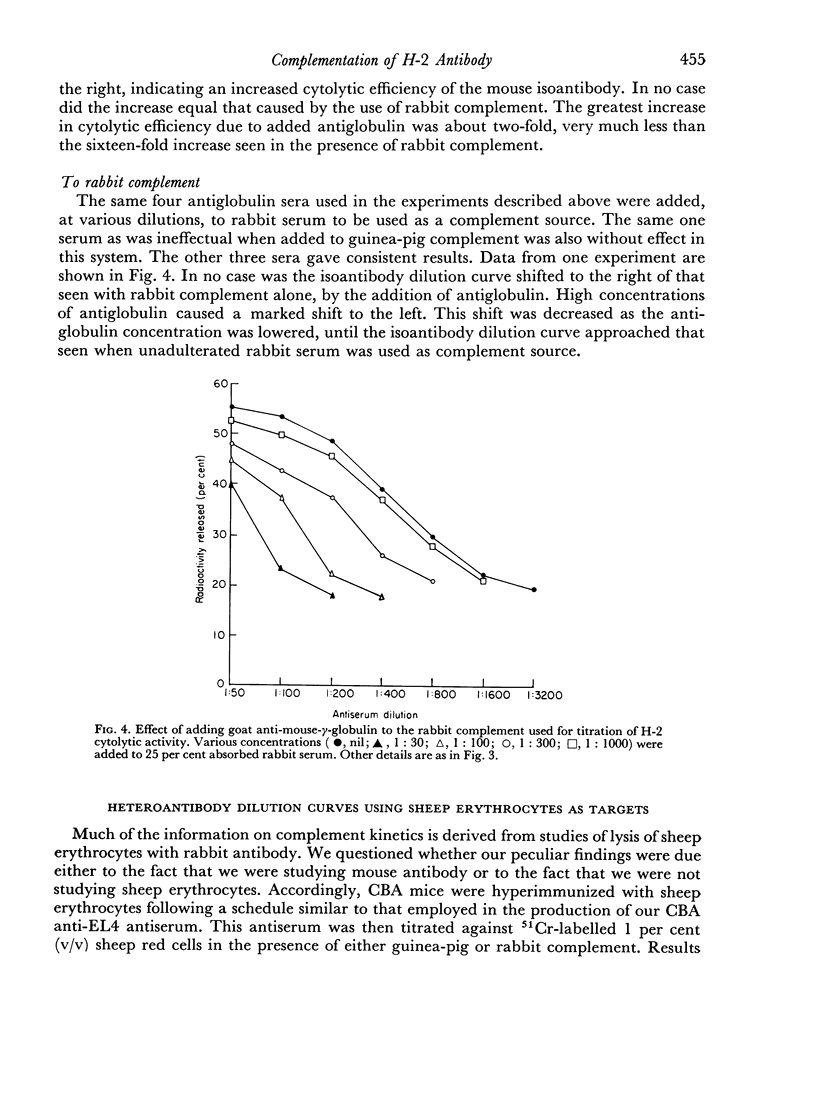
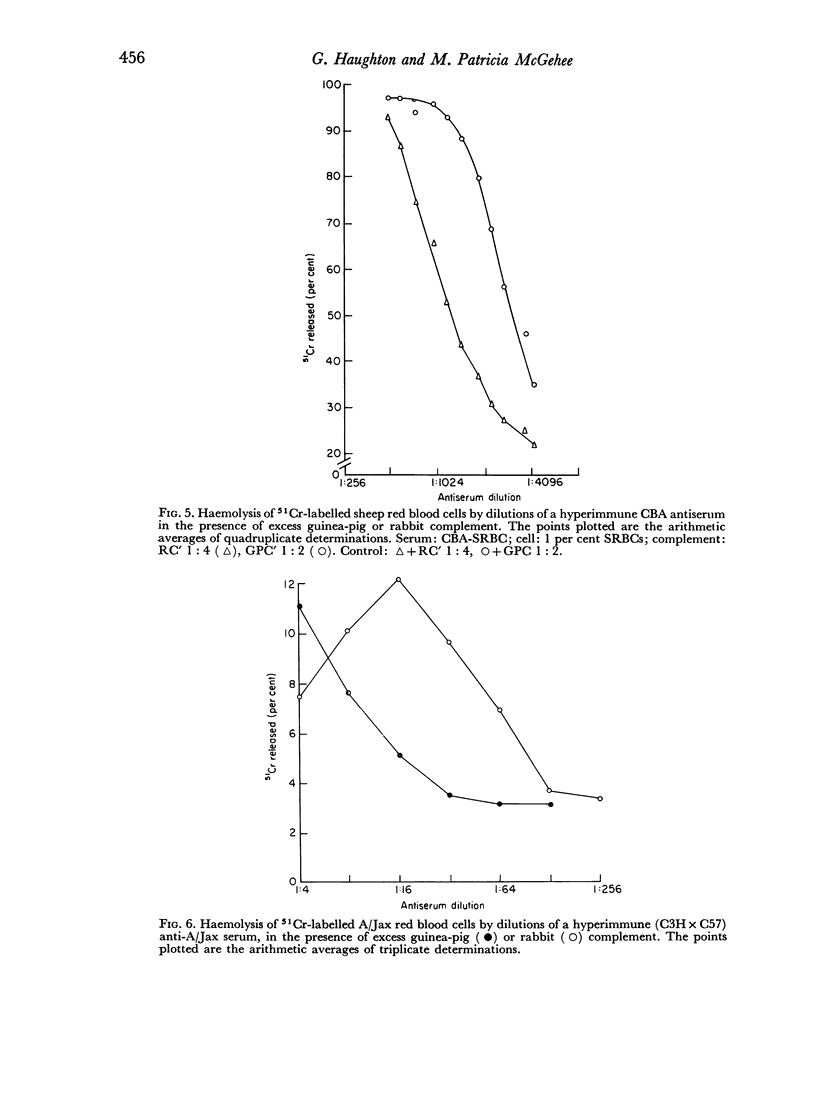
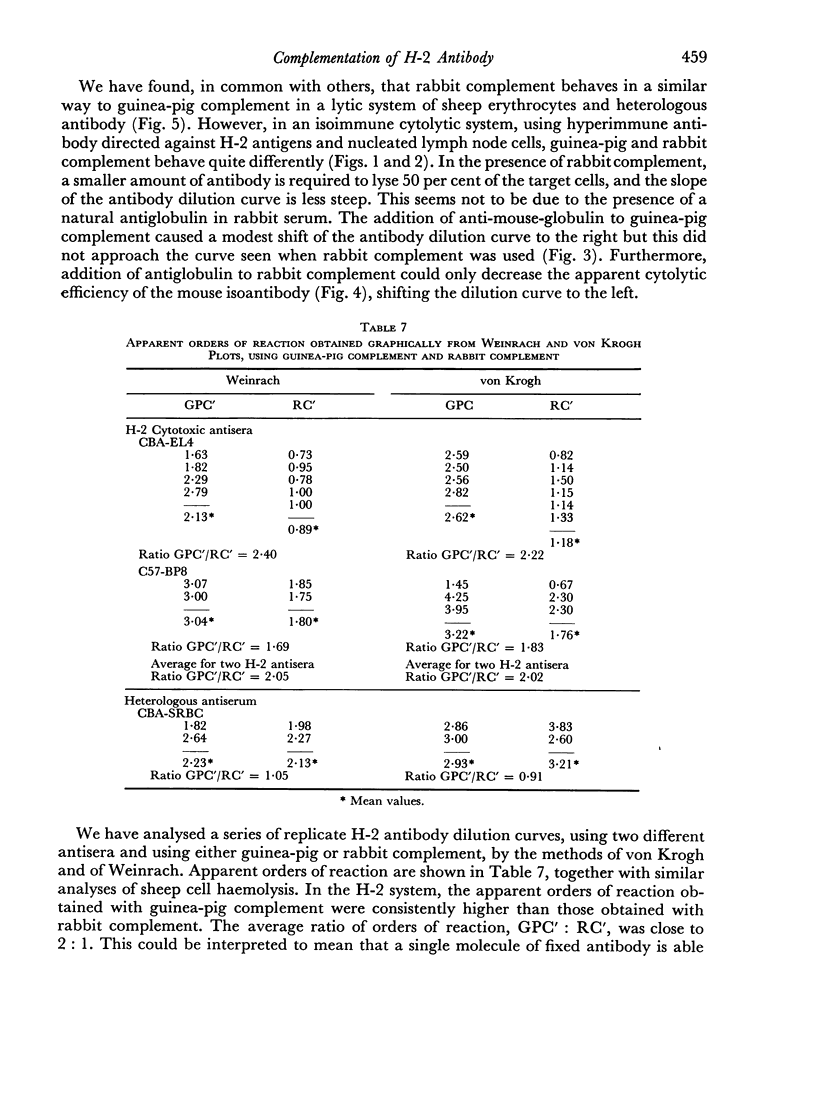
Rabbit serum obtained from selected animals, and absorbed with mouse liver and spleen, was used as a source of complement in the 51Cr monitored cytolysis of mouse lymph node cells by H-2 alloantibody. Antibody was more efficiently cytolytic in the presence of rabbit complement (RC′) than in the presence of guinea-pig complement (GPC′). Addition of rabbit or goat anti-mouse globulin to GPC′ caused a modest increase in the cytolytic efficiency of the alloantibody. Addition of antiglobulin to RC′ reduced the cytolytic efficiency of alloantibody. The slope of the dilution curve of H-2 antibody was less steep in the presence of RC′ than in the presence of GPC′. The apparent order of reaction with respect to antibody was lower with RC′ than with GPC′. Mouse heteroantibody against sheep erythrocytes was slightly more haemolytically efficient in the presence of GPC′ than in the presence of RC′. The dilution curves of heteroantibody in the presence of RC′ or GPC′ had the same slope, and the apparent orders of reaction with respect to antibody were similar. A greater concentration of GPC was required to complement the alloantibody than the heteroantibody whereas a similar concentration of RC′ sufficed in the two systems. Possible interpretations of these results are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYSE E. A., OLD L. J., STOCKERT E. Some further data on cytotoxic isoantibodies in the mouse. Ann N Y Acad Sci. 1962 Oct 24;99:574–587. doi: 10.1111/j.1749-6632.1962.tb45339.x. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Hemolysin titration based on fixation of the activated first component of complement: evidence that one molecule of hemolysin suffices to sensitize an erythrocyte. J Immunol. 1965 Sep;95(3):559–566. [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. The action of antibody and complement on mammalian cells. Ann N Y Acad Sci. 1960 May 31;87:352–362. doi: 10.1111/j.1749-6632.1960.tb23205.x. [DOI] [PubMed] [Google Scholar]
- HAUGHTON G. MOLONEY VIRUS-INDUCED LEUKEMIAS OF MICE: MEASUREMENT IN VITRO OF SPECIFIC ANTIGEN. Science. 1965 Jan 29;147(3657):506–507. doi: 10.1126/science.147.3657.506. [DOI] [PubMed] [Google Scholar]
- HILDEMANN W. H. A method for detecting hemolysins in mouse isoimmune serums. Transplant Bull. 1957 Oct;4(4):148–149. [PubMed] [Google Scholar]
- Nathenson S. G., Davies D. A. Solubilization and partial purification of mouse histocompatibility antigens from a membranous lipoprotein fraction. Proc Natl Acad Sci U S A. 1966 Aug;56(2):476–483. doi: 10.1073/pnas.56.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Plocinik B. A. Application of the 51Cr cytotoxicity technique to the analysis of human lymphocyte isoantigens. Transplantation. 1967 Sep 5;5(5):1323–1333. doi: 10.1097/00007890-196709000-00010. [DOI] [PubMed] [Google Scholar]
- Sanderson A. R. Quantitative titration, kinetic behaviour, and inhibition of cytotoxic mouse isoantisera. Immunology. 1965 Sep;9(3):287–300. [PMC free article] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963 Mar 1;117:457–477. doi: 10.1084/jem.117.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINRACH R. S., LAI M., TALMAGE D. W. The relation between hemolysin concentration and hemolytic rate as measured with chromium 51 labeled cells. J Infect Dis. 1958 Jan-Feb;102(1):60–73. doi: 10.1093/infdis/102.1.60. [DOI] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Wigzell H. Antibody synthesis at the cellular level. Antibody-induced suppression of 7S antibody synthesis. J Exp Med. 1966 Nov 1;124(5):953–969. doi: 10.1084/jem.124.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Möller G., Andersson B. Studies at the cellular level of the 19S immune response. Acta Pathol Microbiol Scand. 1966;66(4):530–540. doi: 10.1111/apm.1966.66.4.530. [DOI] [PubMed] [Google Scholar]


