Abstract
Homologous or heterologous anti-sheep erythrocyte serum given passively to normal rats markedly suppressed their spleen plaque-forming cell and serum antibody response to sheep erythrocytes. Passive immunization against bovine γ-globulin prevented `sensitization' by a first injection of the antigen. The suppressive effect of passive antibody was prevented or partially prevented by adjuvants, B. pertussis vaccine, S. typhi endotoxin or Freund's complete adjuvant. Passive antibody or adjuvants had relatively little effect on the primary response when given more than 24 hours after antigen or on the secondary response when given with antigen.
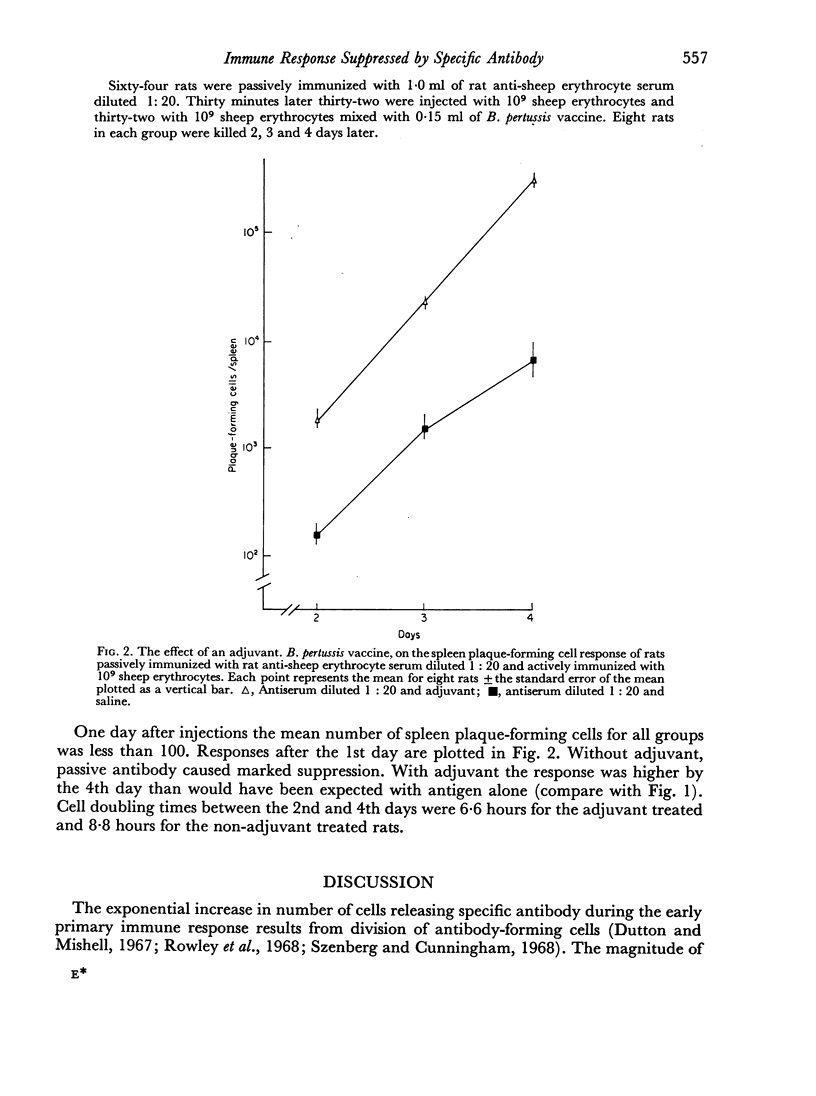
The kinetics of the early spleen plaque-forming cell response were measured using sheep erythrocytes as antigen, homologous anti-sheep erythrocyte serum for passive immunization and B. pertussis vaccine as adjuvant. With a constant antigen dose, larger amounts of passive antibody caused increased suppression. Suppression apparently resulted from a decrease in the number of cells initially responding; the rate of proliferation of cells that did respond was not affected by passive antibody. If the amount of passive antibody was kept constant, an increase in antigen dose or addition of adjuvant to the antigen increased the rate of proliferation of the cells that did respond; an effect sufficient to completely mask suppression produced by smaller amounts of passive antibody. These findings can be accounted for by assuming that passive antibody and higher antigen doses or adjuvant affect different interactions required for the antibody response. Thus, the magnitude of the antibody response is dependent not only on the amounts of antigen and passively given antibody but also on the amount and activity of any intentionally or unintentionally introduced factor having adjuvant activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad M. A. Suppression of delayed hypersensitivity by antigen and antibody. Is a common precursor cell responsible for both delayed hypersensitivity and antibody formation? Immunology. 1968 Aug;15(2):159–171. [PMC free article] [PubMed] [Google Scholar]
- Axelrad M., Rowley D. A. Hypersensitivity: specific immunologic suppression of the delayed type. Science. 1968 Jun 28;160(3835):1465–1467. doi: 10.1126/science.160.3835.1465. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freda V. J., Gorman J. G., Pollack W. Rh factor: prevention of isoimmunization and clinical trial on mothers. Science. 1966 Feb 18;151(3712):828–830. doi: 10.1126/science.151.3712.828. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A., CHUTKOW J., ATTIG C. Severe active cutaneous hypersensitivity in the rat produced by Hemophilus pertussis vaccine. J Exp Med. 1959 Nov 1;110:751–769. doi: 10.1084/jem.110.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. A., Fitch F. W., Mosier D. E., Solliday S., Coppleson L. W., Brown B. W. The rate of division of antibody-forming cells during the early primary immune response. J Exp Med. 1968 May 1;127(5):983–1002. doi: 10.1084/jem.127.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- Stuart F. P., Saitoh T., Fitch F. W. Rejection of renal allografts: specific immunologic suppression. Science. 1968 Jun 28;160(3835):1463–1465. doi: 10.1126/science.160.3835.1463. [DOI] [PubMed] [Google Scholar]
- Szenberg A., Cunningham A. J. DNA synthesis in the development of antibody-forming cells during the early stages of the immune response. Nature. 1968 Feb 24;217(5130):747–748. doi: 10.1038/217747a0. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]


