Abstract
Spumaviruses, commonly called foamy viruses, are complex retroviruses that establish life-long persistent infections in the absence of accompanying pathology. Depending upon cell type, infection of cells in tissue culture cells can result in either lytic replication, persistence, or latency. The cellular factors that mediate foamy virus (FV) latency are poorly understood. In this study we show that the only known inhibitor of FV replication, the promyelocytic leukemia protein (PML), which binds the FV transactivator (Tas), does not play an important role in FV latency in vitro. We found no significant differences in PML levels in cells that supported lytic replication compared to those that were latently infected. Furthermore, endogenous PML levels did not change following exposure to phorbol myristate acetate (PMA), which induces FV replication. We demonstrated that FV replication proceeded in the presence of substantial levels of PML, both in fully permissive cells and during reactivation of latent FV. Endogenous PML did not efficiently colocalize with Tas, even after upregulation by alpha interferon (IFN-α) treatment. IFN-α did, however, partially suppress the reactivation of latent FV by PMA. Finally, depletion of endogenous PML by small interfering RNA did not promote activation of FV in cells that responded to PMA treatment. Taken together, these data indicate that endogenous PML does not play an important role in mediating FV latency.
A hallmark of natural, accidental, and experimental infection by foamy viruses is the establishment of life-long latency in the absence of detectable virus replication. Foamy viruses (FVs) are members of the Spumavirus family of the genus Retroviridae and are unique among the retroviruses in many ways (18). FV replication is regulated transcriptionally through the use of two promoters, the long terminal repeat promoter found in all retroviruses, which directs transcription of the gag, pol, and env genes, and a second promoter unique to FVs, the internal promoter, which directs expression of the accessory genes tas and bet. Tas is a DNA binding protein that functions to transactivate both its own promoter and the FV long terminal repeat (7, 12, 19). The two promoters are temporally regulated. The internal promoter has a higher affinity for Tas and is activated first; only after sufficient Tas is produced from the internal promoter can long terminal repeat-mediated transcription proceed (12, 20). Although limited transcription is a prerequisite of FV latency, the factors that regulate this process are poorly understood.
Recently, the promyelocytic leukemia protein (PML) was shown to inhibit replication of the prototypic FV (27). PML was first identified as a fusion with the retinoic acid receptor alpha gene in patients with acute promyelocytic leukemia (4, 11, 15). Overexpression of PML suppresses cell growth, resulting in arrest or apoptosis, depending upon cell type (reviewed in reference 26). In contrast, ablation of the PML gene in transgenic mice resulted in an increase in cell growth and susceptibility to a variety of tumors (34). PML has a punctate, speckled appearance and is an integral component of nuclear bodies (3, 5, 35). The function of nuclear bodies is largely unknown, but in addition to PML, they also contain a number of transcription factors, tumor suppressors, and interferon (IFN)-regulated genes (reviewed in reference 22).
The PML promoter contains an IFN-α/β-stimulated response element and an IFN-γ activation site, resulting in transcriptional induction of PML following exposure to interferons (33). interferons have potent antiviral activity on a wide variety of viruses. The link between PML expression and antiviral activity has been demonstrated for a number of RNA viruses, including influenza virus and vesicular stomatitis virus (2). Interestingly, a wide variety of DNA viruses express proteins that are targeted to and/or disrupt nuclear bodies (reviewed in reference 8). In many cases, the genomes of DNA viruses are found in association with nuclear bodies, but the importance of this association and of the disruption of nuclear bodies by these viruses remains unclear.
Since PML is an IFN-regulated gene (2) and foamy viruses are sensitive to the inhibitory effects of interferon (9, 14, 27, 28, 30), it is possible that the interplay between IFN and PML could be important in regulation of FV expression, although FV infection does not induce the production of interferon (10, 26-28). Previous work has shown that through its ring finger domain, PML binds to the amino terminus of Tas, thus sequestering Tas from the FV internal promoter and inhibiting FV replication (27). Using cells from PML knockout mice, Regad et al. demonstrated that the sensitivity of FV to interferon requires PML (27). The inhibitory effect of PML overexpression on FV replication is evident in cell types that are fully permissive for FV replication (27).
In this study, we investigated whether endogenous PML expression is important in restricting FV replication in cells that support latent FV infection. We found no significant differences in the levels of endogenous PML expression in cells with different responses to FV infection, and FV replication occurred even in the presence of high levels of endogenous PML. We also demonstrate that Tas and proteins containing the putative PML binding domain of Tas do not efficiently colocalize with endogenous PML even after IFN-α treatment. However, IFN-α treatment can attenuate the inductive effect of PMA on latent FV. Finally, depletion of endogenous PML by small interfering RNA (siRNA) does not promote FV replication in 293T cells. Although PML may inhibit FV replication under antiviral conditions, such as exposure to IFN, these data indicate that factors other than PML are important in restricting FV expression in cell types that support latent infection.
MATERIALS AND METHODS
Cells and viruses.
Virus titers were determined with the FAB indicator cell line (36). 293T (ATCC 293tsA1609neo), HT1080 (ATCC CCL-121), and FAB cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and antibiotics. Jurkat cells (ATCC TIB-152) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. Jurkat cells were infected by coculture as previously described (23).
Plasmids.
The molecular clone pHFV13 (21) was the source for all constructs used. For brevity, pHFV13 will be abbreviated to pFV and FV will be used to describe viruses derived from pFV. The vector pLTasSD was generated by cloning the region from nucleotide 9201 to nucleotide 10441, encompassing the tas open reading frame, into BclI- and HindIII-digested LXSN. The Bet splice donor at nucleotide 9700 was mutated (italic) with oligonucleotide-directed mutagenesis (Stratagene) with the oligonucleotides TasSDF (5′-CCACACCAGAGGAGATGTCAAAGTCACTCTGTAAAAG-3′) andTasSDR (5′-CTTTTACAGAGTGACTTTGACATCTCCTCTGGTGTGG-3′).
The vector pLTasNgfp was constructed by ligating a PCR fragment encoding enhanced green fluorescent protein (EGFP)-1 (Clontech) into BamHI- and HindIII-digested pLTasSD. pLTasΔDBD, in which amino acids 96 to 98 have been changed from RPR to DLG (13), was constructed with the mutagenic oligonucleotides TasDBDF (5′-GAAGGTCCAAAACCAGACCTGGGCCACGATCCTGTCC-3′) and TasDBDR (5′-GGACAGGATCGTGGCCCAGGTCTGGTTTTGGACCTTC-3′). The nucleotides mutated are in italics. The retroviral vectors pLNCZ, which contains the lacZ gene under control of the cytomegalovirus immediate-early promoter, and pLN were obtained from Dusty Miller, Fred Hutchinson Cancer Research Center.
Western blotting.
Western blot analysis was performed essentially as previously described (23). Briefly, 293T cells transfected with pFV in 12-well plates were harvested and pelleted by low-speed centrifugation. Lysates were prepared in 250 μl of Ab buffer (20 mM Tris-Cl [pH 7.5], 50 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium dodecyl sulfate, 0.5% deoxycholate, and 0.5% aprotinin), and genomic DNA was sheared by passing it through a 23-gauge needle. Lysates were cleared by high-speed centrifugation before running on sodium dodecyl sulfate-10% polyacrylamide gels. FV-specific proteins were detected with polyclonal rabbit anti-Gag antiserum, polyclonal anti-Bel1 antiserum (detects Tas and Bet), or FV-infected rabbit serum and visualization with the ECL kit (Amersham). All sera were used at a 1:2,000 dilution.
Microscopy.
HT1080 cells were grown directly on coverslips in 12-well plates. Jurkat and 293T cells were adhered to coverslips by cytocentrifugation. All coverslips were fixed for 10 min in freshly prepared 4% paraformaldehyde in phosphate-buffered saline, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline, and then blocked in phosphate-buffered saline containing 1% bovine serum albumin and 20% fetal bovine serum. Coverslips were incubated with primary antibodies for 1 h at room temperature in phosphate-buffered saline containing 1% bovine serum albumin. Rabbit anti-Gag polyclonal antiserum was used at 1:2,500, rabbit anti-Bel1 polyclonal antiserum was used at 1:2,500, and mouse anti-PML monoclonal antibody (PG-M3; Santa Cruz) was used at 1:200. Coverslips were incubated with secondary antibodies (Molecular Probes) for 45 min at a 1:500 dilution.
Rabbit primary antibodies were detected with goat anti-rabbit immunoglobulin-Alexa 478, and mouse primary antibodies were detected with goat anti-mouse immunoglobulin-Alexa 592 (Molecular Probes). Coverslips were mounted in Vectashield (Molecular Probes) and visualized by Deltavision microscopy. Deconvolved 0.25-μm z-sections were used to determine colocalization of proteins after normalization of signal levels (SoftWoRx; Applied Precision). To determine total protein expression, sequential deconvolved 0.25-μm z-sections were flattened with the quick-projection algorithm (SoftWoRx; Applied Precision).
RNA interference.
RNA duplexes 21 nucleotides long with symmetric two-nucleotide 3′(2′-deoxy)thymidine overhangs corresponding to PML coding nucleotides 563 to 583 were synthesized and annealed (Dharmacon Research). RNA interference assays were performed with Oligofectamine reagent (Invitrogen) according to procedures described previously (6, 10). Depletion of PML protein levels in 293T cells was determined by indirect immunofluorescence with anti-PML monoclonal antibody (PG-M3; Santa Cruz). At 24 h after transfection of the siRNA, pFV was transfected, and 72 h later, FV replication was assessed by Western blotting against FV Gag and FV Bet proteins. In duplicate wells, cells were treated with 50 ng of phorbol myristate acetate (PMA) per ml.
RESULTS
Expression of PML-binding domain of Tas does not induce FV replication in 293T cells.
As PML has been implicated as a repressor of FV transcription (27), we were interested in investigating the role of PML in cells that support latent FV infection. PML has been shown to be involved in restricting FV expression through direct binding to the amino terminus of Tas (27). One testable model of FV latency is that the Tas protein is bound by an inhibitor, possibly PML, which prevents Tas from transactivating the internal promoter. We hypothesized that if the sequestration of Tas by PML is important for establishment of latency, then overexpression of the amino-terminal PML-binding region of Tas might saturate the Tas binding sites on PML and permit endogenous Tas to transactivate the internal promoter.
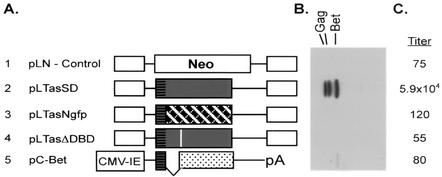
Many cell types that support latent FV infection, such as Jurkat cells, are difficult to transfect, limiting their utility in investigation of the mechanism of latency. Since we found that 293T cells are readily transfectable and can be latently infected, we tested the ability of constructs containing the amino terminus of Tas to induce replication in pFV-transfected 293T cells. We have recently shown that small amounts of Tas can activate replication of latent FV in Jurkat cells (24). Transfection of the pFV vector with a neomycin-expressing control vector resulted in no detectable FV protein expression and a titer of 75 IU/ml (Fig. 1, row 1).
FIG. 1.
Activation of cotransfected FV by Tas in 293T cells. Viral gene expression and infectivity were measured 72 h posttransfection of 293T cells with pFV and the constructs shown. (A) Structures of the cotransfected plasmids. In plasmids A1 to A4, expression of each downstream gene is from the murine leukemia virus long terminal repeat. The gray boxes indicate the Tas open reading frame. Horizontal stripes at the amino terminus of Tas represent the putative PML binding domain. Row 1, pLN, control vector expressing the neomycin resistance gene; row 2, pLTasSD, FV tas containing a mutation at the bet splice donor site; row 3, pLTasNgfp, amino-terminal 88 amino acids from Tas fused to GFP, cross-hatched area; row 4, FV tas containing a mutation in the DNA binding domain, denoted by a white bar; row 5, FV bet expressed from the cytomegalovirus (CMV) immediate-early (IE) promoter. (B) Western blot analysis of FV Gag and Bet at 72 h posttransfection with rabbit-anti FV serum. (C) FV titers 72 h posttransfection, assayed on FAB cells, expressed as infectious units per milliliter.
Cotransfection of the pFV vector and pLTasSD, which expresses a fully functional Tas protein, resulted in a marked increase in FV protein expression and a dramatic increase in titer to 5.9 × 104 IU/ml (Fig. 1, row 2). Transfection of pLTasNgfp, which expresses the amino-terminal 88 amino acids of Tas fused to GFP, failed to activate cotransfected pFV vector (Fig. 1, row 3). Transfection of pLTasΔDBD, which contains mutations in the DNA binding domain of Tas that render it nonfunctional for transactivation, or pC-Bet, which also expresses the amino-terminal 88 amino acids of Tas, also failed to induce cotransfected pFV vector (Fig. 1, rows 4 and 5). Thus, similar to the situation in foamy virus-infected Jurkat cells (25), FV replication can be dramatically induced in 293T cells by overexpression of functional Tas. However, cotransfection of pFV vector with pLTasNgfp, pLTasΔDBD, or pC-Bet did not induce replication. This indicates that the transactivation capacity of Tas is required for FV activation in 293T cells and that expression of the amino terminus of Tas is not sufficient to induce viral replication.
Endogenous PML expression levels do not correlate with latent FV infection.
In order to address a possible role of PML in FV latency, we used indirect immunofluorescence analysis to determine the expression levels of endogenous PML in cell types that support different levels of viral replication. HT1080 human fibrosarcoma cells support lytic replication upon FV infection or transfection with pFV. As previously reported, Jurkat cells support latent infection that can be induced by PMA (25, 37) as well as inducers of the mitogen-activated protein kinase pathway, such as phytohemagglutinin (data not shown). Similarly, although 293T cells do not support FV replication after infection, transfected pFV can be induced by treatment with PMA (data not shown and Fig. 6B).
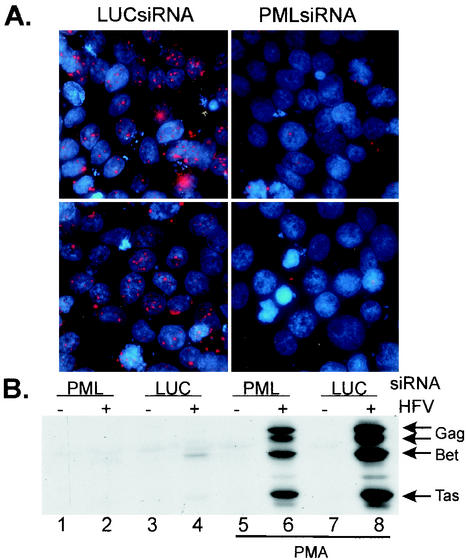
FIG. 6.
Depletion of endogenous PML by RNA interference does not induce virus replication. Depletion of PML was performed with a 21-nucleotide double-stranded RNA, PMLsiRNA, corresponding to nucleotides 563 to 583 of the PML mRNA. A control 21-nucleotide double-stranded RNA targeting firefly luciferase was used as a control. At 24 h following transfection of the siRNAs, pFV was transfected, and replication of FV was assessed by Western blot analysis. (A) Indirect immunofluorescence analysis of PMLsiRNA- and LUCsiRNA-treated 293T cells. Two random fields were selected, and micrographs were taken by Deltavision microscopy. 2′,6′-diamidino-2-phenylindole was used to visualize nuclei. (B) Expression of FV proteins following transfection with pFV and siRNAs. Lane 1, PMLsiRNA only; lane 2, FV and PMLsiRNA; lane 3, LUCsiRNA only; lane 4, FV and LUCsiRNA. Lanes 5 to 8, transfections treated with 50 ng of PMA per ml for 48 h (lane 5, PMLsiRNA only; lane 6, FV and PMLsiRNA lane 7, LUCsiRNA only; lane 8, FV and LUCsiRNA).
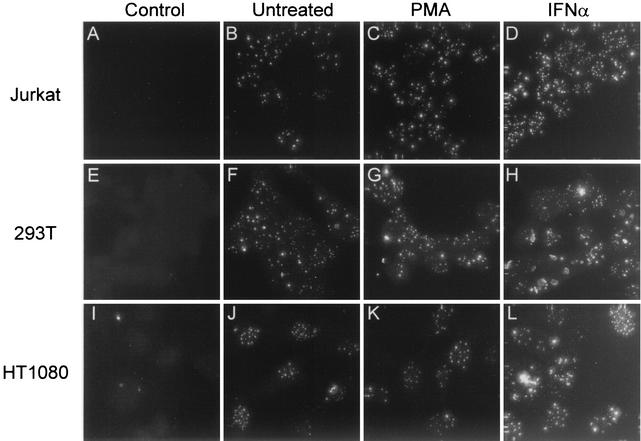
By using indirect immunofluorescence analysis, PML levels were assessed in these cell lines after treatment with PMA or IFN-α or in the absence of treatment (Fig. 2). HT1080 cells had the highest number of PML nuclear bodies per cell, but their intensity was slightly lower than that of Jurkat or 293T cells, which had fewer nuclear bodies per cell (Fig. 2, compare B, F, and J). Jurkat and 293T cells expressed similar levels of PML (Fig. 2B and F). Overall, there were no significant differences in endogenous PML levels in the cell types tested despite the differences in viral replication.
FIG. 2.
Analysis of endogenous PML expression in Jurkat, 293T, and HT1080 cells. Immunofluorescence analysis of endogenous PML levels measured in untreated cells (B, F, and J) and cells treated for 24 h with PMA (C, G, and K) or 1,000 U of IFN-α per ml (D, H, and L). Control (A, E, and I), no primary antibody added. PML expression in Jurkat cells (B, C, and D), 293T cells (F, G, and H), and HT1080 cells (J, K, and L) is shown.
One possible explanation for the increase in viral replication after PMA treatment is that PMA downregulates expression of PML, freeing Tas to promote FV replication. However, in all cases PMA treatment had no effect on PML levels (Fig. 2C, G, and K). As expected, in all cell types, IFN-α treatment resulted in significant increases in PML expression, which were similar for all three cell lines (Fig. 2D, H, and L). The absence of notable differences in PML levels and the observation that PMA has no effect on PML expression indicate that PML may not play a role in FV latency.
FV replication in the presence of endogenous PML.
To address the possibility that lytic FV replication in HT1080 cells was occurring only in a subset of cells with lower PML expression, HT1080 cells were infected with FV at a multiplicity of infection of 0.2; 48 h later, PML and FV antigen levels were assessed by indirect immunofluorescence analysis with antibodies against FV Gag (green) and PML (red) (Fig. 3A to D). In azidothymidine-treated cells, no FV expression was observed, as expected, but PML expression was evident (Fig. 3B). In untreated and PMA-treated cells, abundant FV replication was observed (Fig. 3A and C), and there were no discernible differences in PML levels between cells expressing FV Gag and those not expressing FV Gag. Treatment with 1,000 U of IFN-α per ml dramatically reduced the frequency of FV-positive cells (data not shown). However, even in the presence of nonphysiologic levels of interferon and a concomitant increase in PML levels, FV Gag was readily detectable in many cells (Fig. 3D).
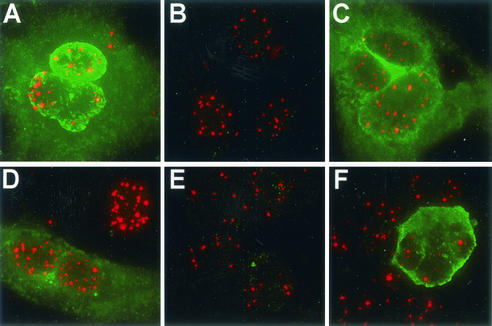
FIG. 3.
FV replication in the presence of endogenous PML. (A to D) expression of FV Gag (green) and PML (red) in HT1080 cells at 72 h after infection with FV. (A) Untreated control cells. (B) Cells treated with 50 μM azidothymidine at 12 h postinfection. (C) Cells treated with 50 ng of PMA per ml at 12 h postinfection. (D) Cells treated with 1,000 U of IFN-α per ml at 12 h postinfection. (E and F) Expression of FV Tas and Bet (green) and PML (red). (E) Untreated FV-infected Jurkat cells. (F) FV-infected Jurkat cells treated with 50 ng of PMA per ml for 72 h.
This study was extended to reactivation of latent FV in FV-infected Jurkat cells. In untreated FV-infected Jurkat cells, abundant PML was observed in the absence of detectable FV Tas and/or Bet (Fig. 3E). As expected, following PMA treatment a dramatic increase in Tas/Bet-positive cells was observed (data not shown), but abundant PML was also expressed in the same cells (Fig. 3F). These results indicate that FV replication can proceed in the presence of PML and that expression of FV protein is independent of endogenous PML levels in untreated HT1080 cells and PMA-treated FV-infected Jurkat cells.
Endogenous PML does not colocalize with Tas or Bet.
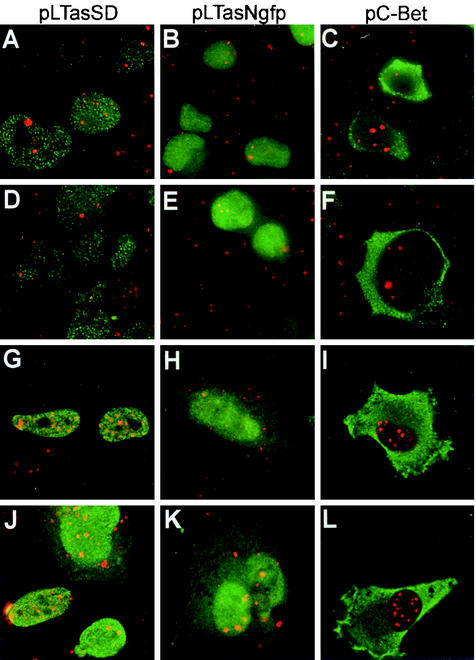
Colocalization of overexpressed PML and FV Tas has been observed in cells that support lytic replication (27). Our attempts to increase FV expression in transfected 293T cells by sequestering endogenous PML with the PML-binding region of Tas proved unsuccessful (Fig. 1). We next asked if Tas or the putative PML-binding region of Tas colocalized with endogenous PML in HT1080 cells that are fully permissive and those that support latent FV infection. HT1080 or 293T cells were transfected with the plasmid pLTasSD (expressing Tas), pLTasNgfp (expressing the N terminus of Tas), or pC-Bet (expressing Bet), all of which contain the putative PML binding region (Fig. 1A, horizontal). PML was detected with an anti-PML monoclonal antibody, and FV proteins were detected with a polyclonal antiserum that reacts with the amino terminus of Tas. Colocalization of PML and the FV proteins was determined by analyzing deconvolved z-sections collected with Deltavision microscopy.
No significant levels of colocalization, which would be denoted by yellow, between PML and any of the FV proteins was observed in 293T cells (Fig. 4A to C) or 293T cells treated with 1,000 U of IFN-α per ml (Fig. 4D to F). Similar results were obtained for HT1080 cells (Fig. 4G to L). Some yellow, indicating possible colocalization, was observed in pLTasSD-transfected cells (Fig. 4G and J) and pLTasNgfp-transfected cells (Fig. 4E and K). However, upon closer examination by removing the red channel and looking for concentrated areas of green where the PML nuclear bodies are located, we determined that the yellow areas were most likely due to the coincident juxtaposition of the two proteins rather than physical association. These data indicate that even under overexpression conditions, the amino-terminal PML-binding region of Tas does not colocalize with endogenous PML. The lack of colocalization between Tas and endogenous PML further supports the idea that endogenous PML does not play an important role in FV replication.
FIG. 4.
Proteins containing the amino terminus of Tas do not efficiently colocalize with endogenous PML. Deltavision microscopy was performed on 293T and HT1080 cells transfected with the indicated vectors to detect colocalization of FV Tas and Bet (green) and endogenous PML (red). (A to C) Untreated 293T cells. (D to F) 293T cells treated with 1,000 U of IFN-α per ml. (G to I) Untreated HT1080 cells. (J to L) HT1080 cells treated with 1,000 U of IFN-α per ml. (A, D, G, and J) pLTasSD transfected. (B, E, H, and K) pLTasNgfp transfected. (C, F, I, and L) pC-Bet transfected. Colocalization (yellow) was assessed after 0.25-μm sections were collected and deconvolved and levels were normalized. See Materials and Methods for details.
IFN partially abrogates reactivation of latent FV by PMA.
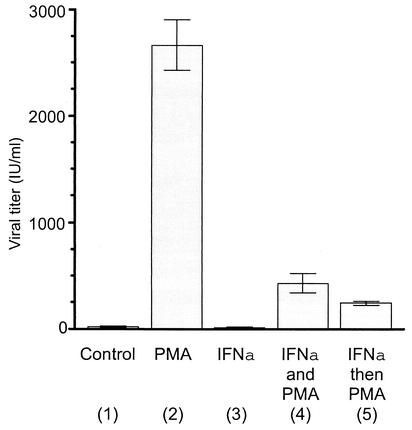
The antiviral effects of interferons on FV replication in fully permissive cell types is well documented (27-30). To address whether IFN-α could limit the reactivation of FV by PMA, FV-infected Jurkat cells were treated with IFN-α and then treated with PMA or concurrently treated with IFN-α and PMA (Fig. 5). PMA treatment alone resulted in a dramatic increase in FV titers, as expected (Fig. 5, column 2). IFN-α treatment alone had no effect on titer (Fig. 5, column 3). Concurrent treatment with IFN-α and PMA resulted in an approximately sixfold decrease in titer compared to PMA alone (Fig. 5, compare columns 2 and 4), but under these conditions, titers were still about 20-fold above those in untreated cells (Fig. 5, compare columns 1 and 4). Pretreatment with IFN-α for 12 h prior to the addition of PMA further attenuated the PMA effect, but the titers observed remained about 12-fold above those in untreated cells (Fig. 5, compare columns 1 and 5).
FIG. 5.
Interferon attenuates PMA-mediated reactivation of latent FV. At 72 h posttransfection of FV-infected Jurkat cells, titers were determined in triplicate on FAB cells. Column 1, untreated FV-infected Jurkat cells; column 2, cells treated with 50 ng of PMA per ml; column 3, cells treated with 1,000 U of IFN-α per ml; column 4, cells treated simultaneously with 50 ng of PMA per ml and 1,000 U of IFN-α per ml; column 5, cells treated with 1,000 U of IFN-α per ml for 12 h, after which 50 ng of PMA per ml was added.
These results indicate that the antiviral effects of IFN-α can attenuate the reactivation of latent FV by PMA but not completely suppress it. Whether PML is the only antiviral mediator under these circumstances is not known. Experiments in PML-deficient cells, however, indicate that PML is the key IFN-mediated factor in limiting FV replication (27).
Depletion of endogenous PML by RNA interference.
RNA interference (siRNA) with PML-specific oligonucleotides was performed in an effort to determine if endogenous PML expression plays a role in restricting FV expression in cells that are not normally permissive for FV replication. Double-stranded 21-nucleotide RNA oligonucleotides corresponding to a conserved region of PML intron 2 (PMLsiRNA) or a control RNA duplex corresponding to the firefly luciferase gene (LUCsiRNA) were transfected into 293T cells, and PML expression was examined 72 h later by indirect immunofluorescence analysis.
Random fields visualized by Deltavision microscopy are shown in Fig. 6A. Transfection with PMLsiRNA significantly reduced the levels of PML expression (right panels) compared to the control LUCsiRNA (left panels). Next, the pFV vector was transfected into 293T cells after siRNA treatment. Duplicate cultures were either left untreated or treated with PMA, and protein expression was analyzed by Western blotting. Transfection of either control LUCsiRNA or PMLsiRNA had negligible effects on FV protein expression in the absence of PMA treatment (Fig. 6B, lanes 1 to 4). Treatment of transfected cells with PMA resulted in an increase in FV protein expression (Fig. 6B, lanes 6 and 8).
Interestingly, LUCsiRNA-treated cells showed a higher level of FV protein expression than PMLsiRNA-treated cells following PMA stimulation (Fig. 6B, compare lanes 6 and 8). In contrast to our initial prediction, these data indicate that depletion of PML does not enhance FV replication in the absence or presence of PMA. In fact, multiple experiments suggest that depletion of PML by siRNA may partially abrogate FV replication following PMA treatment. These data demonstrate that endogenous PML is not an important factor in restricting FV replication in 293T cells.
DISCUSSION
A key aspect of FV latency is the necessity to limit production of the FV transactivator Tas. Because Tas transactivates its own promoter, resulting in a positive-feedback loop, FV replication can be viewed as an on-off switch. Once sufficient Tas is produced, the switch is turned on and replication commences. We have recently shown that a small increase in the amount of Tas can induce a switch from latent to lytic replication (24), indicating that given sufficient levels of functional Tas, latently infected cells are fully permissive for FV replication. Thus, the presence of a potent Tas inhibitor, whose function can be overcome following PMA or mitogen treatment, could explain differences in cell permissivity. The only known FV inhibitor is the interferon-induced PML protein, which suppresses FV replication by binding and inactivating Tas (27). Although PML expression is induced following IFN treatment, many cell types express PML in the absence of IFN stimulation. These observations led to the hypothesis that endogenous PML may play a role in FV latency.
In this study, however, we have shown that endogenous PML does not play a major role in FV latency. We have shown that there is no correlation between the level of endogenous PML expression and permissivity to infection. Cell lines that support lytic replication express levels of PML similar to those in cell lines that support latent infection. Expression of PML is not altered by treatment with PMA, a known activator of latent FV. In addition, FV replication can proceed in cells expressing high levels of PML protein. In contrast to PML overexpression, endogenous PML does not colocalize with Tas even after upregulation of PML by IFN-α treatment. Finally, depletion of endogenous PML by RNA interference did not result in activation of transfected pFV. These data suggest that there are negative regulators other than endogenous PML which mediate FV latency.
It has been shown previously that overexpression of PML in cell types that are normally fully permissive for FV replication results in a dramatic decrease in FV gene expression (27). In contrast to the situation in lytically infected cells, in this report we have shown that endogenous PML expression cannot account for the lack of FV transcription during latency, suggesting that other factors limit FV replication in latently infected cells. In addition to PML, overexpression of FV Bet prior to infection is known to greatly attenuate FV replication in permissive cell types (1, 31, 32). The precise mechanism of Bet function under these conditions is not fully understood. The use of reporter assays to show that Bet can directly inhibit Tas transactivation of either the long terminal repeat or internal promoter has been unsuccessful (16).
In cells that support latent infection, we have recently shown that Bet can inhibit upregulation of internal promoter basal activity by PMA, thereby limiting the amount of Tas produced (24). However, because Bet is produced in latently infected cells, it is likely that Tas is also produced. Given that a positive-feedback loop is operant at the internal promoter and that Bet does not directly interfere with Tas function, even small amounts of Tas would be expected to result in virus replication. Because this is not the case, we propose that there are additional Tas inhibitors in latently infected cells, and reactivation of latent FV would only occur if expression of Tas was sufficient to overcome any such inhibitors.
Subcellular localization of PML protein may explain why endogenous PML does not limit FV replication but increased expression of PML does limit FV replication. Endogenous PML is primarily found localized to nuclear bodies, and an increase in the number and size of nuclear bodies is observed after IFN treatment or overexpression (33). It is possible that PML localized to nuclear bodies is unavailable to bind and inactivate Tas, while free PML can do so effectively. The lack of Tas colocalization to nuclear bodies (Fig. 4) supports the idea that PML localized to nuclear bodies is not capable of binding Tas. Increasing PML expression may provide a pool of free PML capable of interfering with Tas function. In contrast, because endogenous PML is primarily localized to nuclear bodies, there may not be sufficient amounts of free PML to act upon Tas.
There is evidence that free PML retains its ability to inhibit Tas function. PML lacking its coiled-coil domain and forms of PML that are not modified by sumoylation both fail to localize to nuclear bodies but retain their Tas-inhibitory properties (27). Interestingly, activation of transfected pFV by PMA was completely blocked when nuclear body structure was disrupted by cotransfection of the human cytomegalovirus immediate-early 1 protein (IE1) (data not shown), which is known to redistribute PML from nuclear bodies to a diffuse nuclear pattern (17). Because IE1 has complex effects on the host cell in addition to disruption of nuclear bodies, this finding is not conclusive for a role of free PML in Tas inhibition. It will be worthwhile to determine if disruption of nuclear body structure by more specific means results in a concomitant decrease in FV replication.
The current study indicates that factors other than PML are important in mediating FV latency. The effects of overexpressed or IFN-induced PML on FV replication are evident (27). However, FVs have apparently evolved a replication strategy that does not induce IFN (14, 29). Therefore, the role of PML in FV biology remains unclear. Perhaps PML is important in controlling viral spread following reactivation of latent FV, while an as yet unidentified factor(s) limits FV replication in latently infected cells.
Acknowledgments
We are grateful to Michael Emerman for critical reading of the manuscript. We thank Martin Lochelt (Heidelberg) for the Bel1 antiserum, Ali Saib (Hôpital Saint-Louis) for the infected rabbit serum, and Dusty Miller (FHCRC) for pLNCZ and pLN.
This work was supported by NCI grant CA81297 to M.L.L. C.D.M. was funded by NCI training grant T32 CA80416 and by a Helen Riaboff Whiteley fellowship.
REFERENCES
- 1.Bock, M., M. Heinkelein, D. Lindemann, and A. Rethwilm. 1998. Cells expressing the human foamy virus (HFV) accessory Bet protein are resistant to productive HFV superinfection. Virology 250:194-204. [DOI] [PubMed] [Google Scholar]
- 2.Chelbi-Alix, M. K., F. Quignon, L. Pelicano, M. H. Koken, and H. de The. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 72:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel, M. T., M. Koken, O. Romagne, S. Barbey, A. Bazarbachi, M. Stadler, M. C. Guillemin, L. Degos, C. Chomienne, and H. de The. 1993. PML protein expression in hematopoietic and acute promyelocytic leukemia cells. Blood 82:1858-1867. [PubMed] [Google Scholar]
- 4.de The, H., C. Lavau, A. Marchio, C. Chomienne, L. Degos, and A. Dejean. 1991. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66:675-684. [DOI] [PubMed] [Google Scholar]
- 5.Dyck, J. A., G. G. Maul, W. H. Miller, Jr., J. D. Chen, A. Kakizuka, and R. M. Evans. 1994. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 76:333-343. [DOI] [PubMed] [Google Scholar]
- 6.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 7.Erlwein, O., and A. Rethwilm. 1993. BEL-1 transactivator responsive sequences in the long terminal repeat of human foamy virus. Virology 196:256-268. [DOI] [PubMed] [Google Scholar]
- 8.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 20:7266-7273. [DOI] [PubMed] [Google Scholar]
- 9.Falcone, V., M. Schweizer, A. Toniolo, D. Neumann-Haefelin, and A. Meyerhans. 1999. Gamma interferon is a major suppressive factor produced by activated human peripheral blood lymphocytes that is able to inhibit foamy virus-induced cytopathic effects. J. Virol. 73:1724-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 11.Goddard, A. D., J. Borrow, P. S. Freemont, and E. Solomon. 1991. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science 254:1371-1374. [DOI] [PubMed] [Google Scholar]
- 12.He, F., W. S. Blair, J. Fukushima, and B. R. Cullen. 1996. The human foamy virus Bel-1 transcription factor is a sequence-specific DNA binding protein. J. Virol. 70:3902-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, F., J. D. Sun, E. D. Garrett, and B. R. Cullen. 1993. Functional organization of the Bel-1 trans activator of human foamy virus. J. Virol. 67:1896-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooks, J. J., and B. Detrick-Hooks. 1979. Simian foamy virus-induced immunosuppression in rabbits. J. Gen. Virol. 44:383-390. [DOI] [PubMed] [Google Scholar]
- 15.Kakizuka, A., W. H. Miller, Jr., K. Umesono, R. P. Warrell, Jr., S. R. Frankel, V. V. Murty, E. Dmitrovsky, and R. M. Evans. 1991. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66:663-674. [DOI] [PubMed] [Google Scholar]
- 16.Keller, A., K. M. Partin, M. Lochelt, H. Bannert, R. M. Flugel, and B. R. Cullen. 1991. Characterization of the transcriptional trans activator of human foamy retrovirus. J. Virol. 65:2589-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korioth, F., G. G. Maul, B. Plachter, T. Stamminger, and J. Frey. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229:155-158. [DOI] [PubMed] [Google Scholar]
- 18.Linial, M., L. 1999. Foamy viruses are unconventional retroviruses. J. Virol. 73:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lochelt, M., M. Aboud, and R. M. Flugel. 1993. Increase in the basal transcriptional activity of the human foamy virus internal promoter by the homologous long terminal repeat promoter in cis. Nucleic Acids Res. 21:4226-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lochelt, M., S. F. Yu, M. L. Linial, and R. M. Flugel. 1995. The human foamy virus internal promoter is required for efficient gene expression and infectivity. Virology 206:601-610. [DOI] [PubMed] [Google Scholar]
- 21.Lochelt, M., H. Zentgraf, and R. M. Flugel. 1991. Construction of an infectious DNA clone of the full-length human spumaretrovirus genome and mutagenesis of the bel 1 gene. Virology 184:43-54. [DOI] [PubMed] [Google Scholar]
- 22.Maul, G. G., D. Negorev, P. Bell, and A. M. Ishov. 2000. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129:278-287. [DOI] [PubMed] [Google Scholar]
- 23.Meiering, C. D., K. E. Comstock, and M. L. Linial. 2000. Multiple integrations of human foamy virus in persistently infected human erythroleukemia cells. J. Virol. 74:1718-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meiering, C. D., and M. L. Linial. 2002. Reactivation of a complex retrovirus is controlled by a molecular switch and is inhibited by a viral protein. Proc. Natl. Acad. Sci., 99:15130-15135. [DOI] [PMC free article] [PubMed]
- 25.Meiering, C. D., C. Rubio, C. May, and M. L. Linial. 2001. Cell-type-specific regulation of the two foamy virus promoters. J. Virol. 75:6574-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, M., and P. G. Pelicci. 2001. PML interaction with p53 and its role in apoptosis and replicative senescence. Oncogene 20:7250-7256. [DOI] [PubMed] [Google Scholar]
- 27.Regad, T., A. Saib, V. Lallemand-Breitenbach, P. P. Pandolfi, H. de The, and M. K. Chelbi-Alix. 2001. PML mediates the interferon-induced antiviral state against a complex retrovirus via its association with the viral transactivator. EMBO J. 20:3495-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes-Feuillette, A., J. Lasneret, S. Paulien, W. Ogunkolade, J. Peries, and M. Canivet. 1990. Effects of human recombinant alpha and gamma and of highly purified natural beta interferons on simian Spumavirinae prototype (simian foamy virus 1) multiplication in human cells. Res. Virol. 141:31-43. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes-Feuillette, A., F. Saal, J. Lasneret, M. Santillana-Hayat, and J. Peries. 1987. Studies on in vitro interferon induction capacity and interferon sensitivity of simian foamy viruses. Arch. Virol. 97:77-84. [DOI] [PubMed] [Google Scholar]
- 30.Sabile, A., A. Rhodes-Feuillette, F. Z. Jaoui, J. Tobaly-Tapiero, M. L. Giron, J. Lasneret, J. Peries, and M. Canivet. 1996. In vitro studies on interferon-inducing capacity and sensitivity to IFN of human foamy virus. Res. Virol. 147:29-37. [DOI] [PubMed] [Google Scholar]
- 31.Saib, A., M. H. Koken, P. van der Spek, J. Peries, and H. de The. 1995. Involvement of a spliced and defective human foamy virus in the establishment of chronic infection. J. Virol. 69:5261-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saib, A., J. Peries, and H. de The. 1993. A defective human foamy provirus generated by pregenome splicing. EMBO J. 12:4439-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stadler, M., M. K. Chelbi-Alix, M. H. Koken, L. Venturini, C. Lee, A. Saib, F. Quignon, L. Pelicano, M. C. Guillemin, C. Schindler, et al. 1995. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene 11:2565-2573. [PubMed] [Google Scholar]
- 34.Wang, Z. G., L. Delva, M. Gaboli, R. Rivi, M. Giorgio, C. Cordon-Cardo, F. Grosveld, and P. P. Pandolfi. 1998. Role of PML in cell growth and the retinoic acid pathway. Science 279:1547-1551. [DOI] [PubMed] [Google Scholar]
- 35.Weis, K., S. Rambaud, C. Lavau, J. Jansen, T. Carvalho, M. Carmo-Fonseca, A. Lamond, and A. Dejean. 1994. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell 76:345-356. [DOI] [PubMed] [Google Scholar]
- 36.Yu, S. F., and M. L. Linial. 1993. Analysis of the role of the bel and bet open reading frames of human foamy virus by with a new quantitative assay. J. Virol. 67:6618-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu, S. F., J. Stone, and M. L. Linial. 1996. Productive persistent infection of hematopoietic cells by human foamy virus. J. Virol. 70:1250-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]