Abstract
A series of fluorescent iron chelators has been synthesized such that a fluorescent function is covalently linked to a 3-hydroxypyridin-4-one. In the present study, the fluorescent iron chelators were loaded into isolated rat hepatocytes. The intracellular fluorescence was not only quenched by an addition of a highly lipophilic 8-hydroxyquinoline–iron(III) complex but also was dequenched by the addition of an excess of the membrane-permeable iron chelator CP94 (1,2-diethyl-3-hydroxypyridin-4-one). The time course of uptake of iron and iron chelation in single, intact cells was recorded on-line by using digital fluorescence microscopy. Intracellular concentrations of various fluorescent iron chelators were determined by using a spectrofluorophotometer subsequent to lysis of probe-loaded cells and were found to depend on their partition coefficients; the more hydrophobic the compound, the higher the intracellular concentration. An ex situ calibration method was used to determine the chelatable iron pool of cultured rat hepatocytes. CP655 (7-diethylamino-N-[(5-hydroxy-6-methyl-4-oxo-1,4-dihydropyridin-3-yl)methyl]-N-methyl-2-oxo-2H-chromen-3-carboxamide), which is a moderately lipophilic fluorescent chelator, was found to be the most sensitive probe for monitoring chelatable iron, as determined by the intracellular fluorescence increase induced by the addition of CP94. The concentration of the intracellular chelatable iron pool in hepatocytes was determined by this probe to be 5.4±1.3 μM.
Keywords: fluorescence microscopy, hepatocyte, 3-hydroxypyridin-4-one (HPO) fluorescent probe, intracellular labile iron, iron chelator, quenching
Abbreviations: ClogP, logarithm of calculated partition coefficient; CP94, 1,2-diethyl-3-hydroxypyridin-4-one; CP603, N-[2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethyl]-2-(7-(dimethylamino)-2-oxo-2H-chromen-4-yl)acetamide; CP623, N-[(3-hydroxy-1,6-dimethyl-4-oxo-1,4-dihydropyridin-2-yl)-methyl]-2-(7-(dimethylamino)-2-oxo-2H-chromen-4-yl)acetamide; CP645, 7-diethylamino-N-[(5-hydroxy-1-methyl-4-oxo-1,4-dihydropyridin-2-yl)methyl]-2-oxo-2H-chromene-3-carboxamide; CP655, 7-diethylamino-N-[(5-hydroxy-6-methyl-4-oxo-1,4-dihydropyridin-3-yl)methyl]-N-methyl-2-oxo-2H-chromen-3-carboxamide; CP800, 4- and 5-(2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethylcarbamoyl)-2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid; DFO, desferrioxamine-B; DTPA, diethylenetriaminepenta-acetic acid; HBSS, Hanks balanced salt solution; HPO, 3-hydroxypyridin-4-one
INTRODUCTION
Iron is an essential element in cellular metabolism and virtually all extracellular and intracellular iron is tightly bound to proteins [1]. However, a minor proportion of intracellular iron, termed either ‘labile iron pool’ [2] or ‘chelatable iron pool’ [3], is comparatively loosely bound to low-molecular-mass, low-affinity ligands such as phosphate and citrate, as well as to proteins and lipids [4,5]. The concentration of this iron pool is carefully controlled in normal tissue because labile iron is toxic when present in excess. In the presence of molecular oxygen, iron salts are able to redox cycle between iron(II) and iron(III) [6] and thereby generate highly reactive free radicals such as hydroxyl radicals, which lead to oxidative tissue damage [1,7]. Thus therapeutic iron chelators may have potential for the treatment of diseases such as stroke [8], heart attack [9] and neurodegenerative disorders [10], which have been reported to be associated with the presence of excess labile iron. DFO (desferrioxamine-B), the most widely used iron chelator in haematology, has a major disadvantage of being orally inactive [11] and thus has to be administered parentally. To overcome this problem, many laboratories have searched for alternatives to DFO, which are orally active and non-toxic. HPOs (3-hydroxypyridin-4-ones) are currently one of the main candidate groups [12,13]. To date, several HPO ligands have been widely investigated for iron chelation, both in iron-overload animal models and in thalassaemia patients [14–17]. In order to facilitate the comparison of the efficacy of such molecules, a reliable quantitative method that determines the effect of HPO ligands on the intracellular labile iron pool is required and also some knowledge of the intracellular distribution of these chelators is desirable. All iron-selective chelators used clinically have an overall efficiency of between 5 and 20% (percentage of dose excreted as iron complex) [18]. If this efficiency could be increased, lower doses would become possible, with an associated reduction of side effects. Thus knowledge of the factors that, for instance, enhance chelator access to the lysosome may have an important influence on chelator efficiency. In principle, the use of fluorescent chelators renders it possible to provide such information.
Over the past five decades, great effort has been devoted to developing suitable methods for the characterization of the labile iron pool [19–27]. However, many of these methods involve pretreatment of cells or tissues, often necessitating the destruction of the biological material, which can lead to mobilization of protein-bound iron. Moreover, these methods generally lack the requisite sensitivity and thus require large amounts of biological material. More recently, the fluorescence detection of iron has been investigated by several laboratories and found to be favourable in terms of sensitivity and versatility [28–31]. For these reasons, we considered it appropriate to investigate a series of iron-specific fluorescent probes [32,33] regarding their ability to chelate, monitor and determine the labile iron pool of isolated rat hepatocytes.
MATERIALS AND METHODS
Animals
Male Wistar rats (250–350 g) were obtained from the Zentrales Tierlaboratorium (Universitätsklinikum Essen). Animals were kept under standard conditions with free access to food and water. All animals received humane care in compliance with the institutional guidelines.
Chemicals
Leibovitz L-15 medium, 2,2′-dipyridyl, 8-hydroxyquinoline, 4,4′-dipyridyl (dihydrochloride), Chelex 100, ferrous ammonium sulphate, ferric chloride, DTPA (diethylenetriaminepenta-acetic acid), fetal calf serum and 1,10-phenanthroline were obtained from Sigma–Aldrich (Steinheim, Germany). Desferrioxamine mesylate (Desferal) was obtained from Novartis Pharma (Nuremberg, Germany). Collagen (Type R), dexamethasone and gentamicin were from Serva (Heidelberg, Germany), and DMSO, Triton X-100 and citric acid trisodium salt (dihydrate) were from Merck (Darmstadt, Germany). Falcon cell culture flasks and cell culture-grade Petri dishes were obtained from Becton Dickinson (Heidelberg, Germany), and glass coverslips were from Assistent (Sondheim/Röhn, Germany).
Synthesis of fluorescent probes
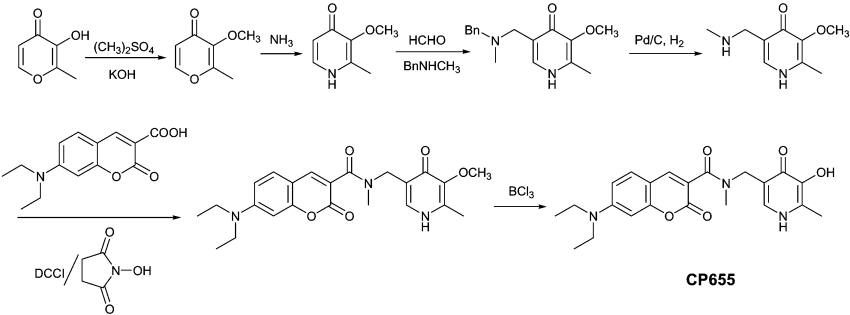
The fluorescent probes investigated for intracellular iron chelation and detection were synthesized as reported previously and the purity was assessed by elemental analysis [33]. As an example, the synthetic route employed for the fluorescent probe CP655 (7-diethylamino-N-[(5-hydroxy-6-methyl-4-oxo-1,4-dihydropyridin-3-yl)methyl]-N-methyl-2-oxo-2H-chromen-3-carboxamide) is summarized in Scheme 1. Briefly, the 3-hydroxy group was protected by using dimethyl sulphate under basic conditions, and the resulting protected maltol was reacted with ammonia to produce 2-methyl-3-methoxy-pyridin-4-one. 5-Methylaminomethylpyridinone was prepared by the reaction of the protected pyridinone with formaldehyde and benzylmethylamine under Mannich reaction conditions, followed by hydrogenation to remove the benzyl group. The fluorescent probe CP655 was synthesized by coupling the resulting 3-methoxy-2-methyl-5-methylaminomethyl-pyridin-4-one with an activated coumarin acid by 1,3-dicyclohexylcarbodi-imide/N-hydroxy succinimide, followed by BCl3 treatment to remove the protecting methyl group.
Scheme 1. Synthetic route of CP655.
Fluorescence measurements in cell-free systems
The spectra of the probes were scanned with both a PerkinElmer spectrophotometer (UV–visible Lambda 2S; PerkinElmer, Überlingen, Germany) at a speed of 120 nm/min and a PerkinElmer spectrofluorimeter (LS 50B; PerkinElmer, U.K.) at 240 nm/min. Spectra were not corrected for light intensity or detector sensitivity.
In cell-free systems, we determined the effect of various metal ions – Fe(II) (Fe(NH4)2(SO4)2·6H2O), Fe(III) (a mixture of 1 M FeCl3 and 4 M nitrilotriacetic acid), Zn(II) (ZnCl2), Ni(II) (NiSO4·6H2O), Cu(I) (CuCl), Cu(II) (CuCl2·2H2O), Co(II) (CoCl2) and Ca(II) (CaCl2·2H2O) – on the fluorescence of the following probes: CP603 (N-[2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethyl]-2-(7-(dimethylamino)-2-oxo-2H-chromen-4-yl)acetamide), CP623 (N-[(3-hydroxy-1,6-dimethyl-4-oxo-1,4-dihydropyridin-2-yl)methyl]-2-(7-(dimethylamino)-2-oxo-2H-chromen-4-yl)acetamide), CP645 (7-diethylamino-N-[(5-hydroxy-1-methyl-4-oxo-1,4-dihydropyridin-2-yl)methyl]-2-oxo-2H-chromene-3-carboxamide), CP655 and CP800 (4- and 5-(2-(3-hydroxy-2-methyl-4-oxopyridin-1(4H)-yl)ethylcarbamoyl)-2-(3-hydroxy-6-oxo-6H-xanthen-9-yl)benzoic acid) (Figure 1). The measurements were performed at room temperature (22 °C) by using freshly prepared Tris buffer (100 mM Tris) at pH 7.5. All buffer solutions were treated with Chelex 100 to minimize heavy-metal contamination.
Figure 1. Chemical structures of fluorescent iron chelators.
Cell culture
Hepatocytes were isolated from male Wistar rats as described previously [34]. For the fluorescence measurements, 1.7×105 cells/cm2 were seeded on to collagen-coated 6.15 cm2 glass coverslips in Petri dishes and cultured as described previously [30].
Intracellular fluorescence measurements
Experiments with hepatocytes were initiated 20–24 h after isolation of the cells. Cells were loaded with the probes (10 μM) at 37 °C for 10 min in HBSS (Hanks balanced salt solution; 137 mM NaCl, 5.4 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.4 mM KH2PO4, 0.4 mM MgSO4, 0.3 mM Na2HPO4 and 25 mM Hepes, pH 7.4).
The fluorescence intensity was measured by using an inverted microscope (Axiovert 135 TV; Zeiss, Oberkochen, Germany) equipped with an Attofluor imaging system (Atto Instruments, Rockville, MD, U.S.A.). Measurements were performed at 37 °C by using an excitation filter of 380±10 nm and monitoring an emission at 450–490 nm by using a band pass filter in experiments with CP603 and CP623, an excitation filter of 425±22.5 nm and an emission filter of 450–490 nm for CP645 and CP655 and an excitation filter of 488±10 nm and an emission filter of 520±20 nm for CP800.
The intracellular probe fluorescence was decreased or increased 5 min after the beginning of the measurements by either the addition of a lipophilic Fe(III) complex [Fe(III)-8-hydroxyquinoline; 15 μM final Fe(III) concentration] or several iron chelators (1 mM final concentration) to the supernatant. Fe(III) stock solutions were always freshly prepared from ferric chloride (10 mM) in the presence of the lipophilic iron ligand, 8-hydroxyquinoline (20 mM), in DMSO. Iron chelator stock solutions were freshly prepared in water or DMSO.
Determination of intracellular chelatable iron
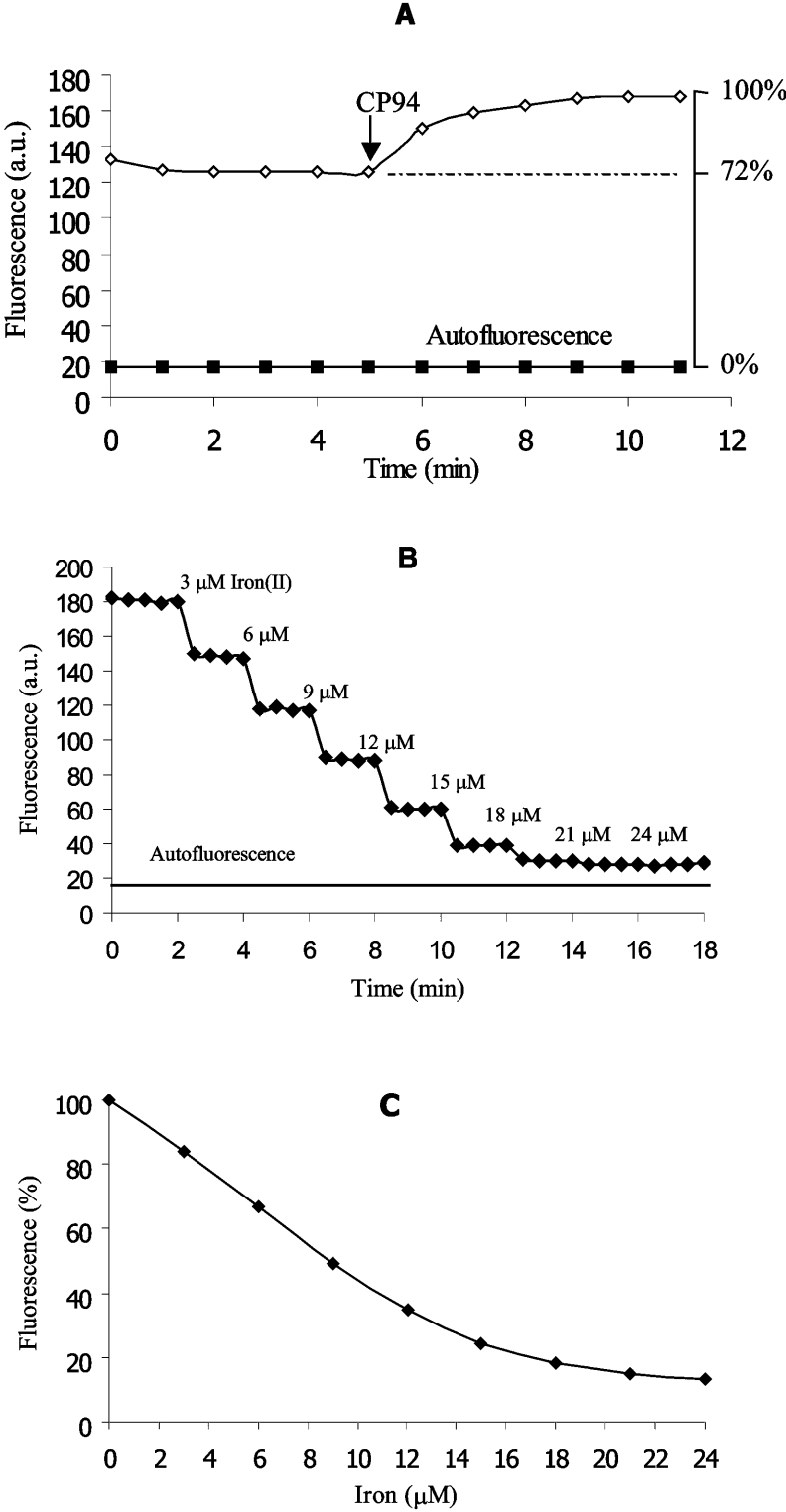
As an example, CP655 (10 μM) was loaded into hepatocytes at 37 °C for 10 min. After loading, the intracellular probe fluorescence was recorded for 5–10 min. Then, the cell-permeable non-fluorescent iron chelator CP94 (1,2-diethyl-3-hydroxypyridin-4-one) was added in excess (1 mM), leading to an increase in probe fluorescence that stabilized after a period of 5–10 min (Figure 2A). The autofluorescence from unloaded cells was set at 0% probe fluorescence intensity. The fluorescence intensity of the unquenched probe after the addition of excess CP94 was set at 100% (Figure 2A). Thus the relative fluorescence intensity of the initial state at which the fluorescence of the probe had been quenched by intracellular labile iron, but not yet dequenched by the addition of CP94, could be calculated.
Figure 2. Intracellular fluorescence dequenching and ex situ calibration of CP655 fluorescence quenching.
(A) The intracellular fluorescence (λex=425±22.5 nm, λem=450–490 nm) of hepatocytes loaded with CP655 (10 μM, for 10 min, in HBSS, 37 °C) was recorded by using digital fluorescence microscopy. The initial hepatocellular CP655 fluorescence was increased by the addition of the non-fluorescent chelator CP94 (1 mM). The autofluorescence of unloaded hepatocytes was recorded in parallel experiments by using the same instrument settings. (B) The fluorescence intensity of CP655 (60 μM), as recorded in cytosolic medium, was quenched by successive additions of iron(II). The fluorescence of the cytosolic medium without the probe was recorded by using the same instrument settings. (C) Using the data obtained in (B), the fluorescence intensity of iron-free CP655 (60 μM) was set at 100% relative probe fluorescence intensity; 0% fluorescence is equal to the fluorescence of cytosolic medium without CP655.
The intracellular concentration of CP655 (and other fluorescent probes) was determined as reported previously for the indicator Phen Green SK [30] by using a spectrofluorophotometer (RF-1501; Shimadzu, Tokyo, Japan). CP655 was used at a concentration of 60 μM, which was calculated to be the intracellular probe concentration, for an ex situ calibration in the ‘cytosolic’ medium to quantify the cellular chelatable iron. The cytosolic medium contained 100 mM KCl, 5 mM Na2HPO4, 4 mM ATP, 2 mM MgCl2, 10 mM imidazole, 6.85 mM glucose, 0.138 mM pyruvate, 1.5 mM L-lactate, 0.23 mM sodium citrate, 2.99 mM potassium phosphate and a 1:50 ‘complete’ amino acid mixture in Eagle minimum essential medium (pH 7.2) (Sigma–Aldrich). The final medium was treated with Chelex 100. Ascorbic acid (2 mM) and glutathione (4.5 mM) were added to the medium when measuring the quenching effect of Fe(II) on probe fluorescence (both freshly prepared). The background fluorescence of the cytosolic medium in the absence of CP655 was set at 0% probe fluorescence intensity; the fluorescence intensities of CP655 (60 μM) in the absence of Fe(II) were termed as ‘unquenched’ values and set at 100% (Figure 2C). Small quantities of iron(II) were added to the supernatant at short time intervals and the fluorescence was recorded until the quenching levelled to stable values (Figure 2B). Based on these plots, a standard curve was obtained (Figure 2C).
Assessment of the distribution of fluorescent HPOs in hepatocytes by using laser scanning microscopy
A laser scanning microscope (LSM 510; Zeiss) equipped with an argon laser was used. The objective lens was a ×63 NA 1.40 Plan-Apochromat. The pinhole was set at 118 μm, producing confocal optical slices of approx. 1.0 μm in thickness. Fluorescence of CP645 and CP800 excited at 458 and 488 nm respectively was collected through a 475 and 505 nm long-pass filter respectively.
Cell viability
The uptake of the vital dye propidium iodide (5 μg/ml) was determined at the end of some experiments in order to detect loss of cell viability. The red fluorescence of propidium iodide excited at 543 nm was collected through a 560 nm long-pass filter when laser scanning microscopy was used; by using digital fluorescence microscopy, propidium iodide was detected at λex=535±17.5 nm and λem.≥590 nm.
Statistics
All experiments with hepatocytes were performed in duplicate and repeated at least three times with cells from different animals. Cellular fluorescence intensities given are averages of 15–20 hepatocytes at the respective time points. Experiments in the cell-free system were repeated at least twice. Traces presented in the Figures are representative for all the corresponding experiments performed. The results are expressed as means±S.D.
RESULTS
Physicochemical properties of HPO fluorescent iron chelators
All of the fluorescent iron chelators studied (Figure 1) are bidentate ligands and bind with iron(III) as 3:1 ligand–metal complexes [13]. The chelating portion of these bidentate molecules is composed of two charged oxygen atoms, which are classified as ‘hard’ donor atoms and hence are selective for tribasic metal cations over dibasic cations. These chelators possess comparatively high iron(III) affinities and high pFe3+ values [13]. The pFe3+ value is a useful measure of the ability of a ligand to bind iron(III) at pH 7.4 and is defined as the negative logarithm of the free iron(III) concentration, calculated for a total ligand concentration of 10−5 M and a total iron concentration of 10−6 M at pH 7.4.
The influence of various biological factors on probe fluorescence in a cell-free system
Neither decreasing the fluorescence intensity by addition of iron(III) or iron(II) nor the fluorescence increase following addition of CP94 led to a shift of the value of the maximum excitation or emission wavelength of all probes investigated (results not shown). The fluorescence of the five probes, as well as their quenching by iron(II) or iron(III) in imidazole buffer (10 mM, pH 7.4), was not affected by changes of ionic strength (KCl; 0–200 mM) and the presence of different electrolytes (KCl, KBr, KI, KNO3, NaCl and Na2SO4; 100 mM each), but was influenced by changes in pH (5.2–8.8) and the presence of cellular iron ligands such as phosphate (0–30 mM; results not shown). At lower pH values, the affinity of the pyridinone chelating moiety of the probes for iron(III) successively decreases, leading to a lower probe sensitivity for iron. Because phosphate competes for iron with these probes, a higher concentration of iron was required to quench the probe fluorescence to the same level.
Although the HPO-based probes are selective for iron(III), they are also highly responsive to the addition of iron(II). The reason for this phenomenon is that iron(II) is immediately oxidized after combining with the probes to form iron(III) complexes, even in the presence of physiological levels of ascorbic acid (results not shown). The response to both iron(III) and iron(II) was found to be strongly dose-dependent when the ligand-to-metal molar ratio was higher than 3:1. At a ligand-to-metal ratio of 3:1, fluorescence was maximally quenched. In contrast with iron(III) and iron(II), the fluorescence intensities of the five probes were found to be only slightly quenched by the presence of Zn(II), Ni(II), Cu(I), Co(II) and Ca(II) (10 μM; see Table 2).
Table 2. Quenching of HPO fluorescent iron chelators by various metal ions in a cell-free system.
The fluorescence of CP603, CP623, CP645, CP655 (5 μM each) and CP800 (0.5 μM) in Tris buffer (50 mM, pH 7.5, 20 °C) was recorded at their respective excitation and emission maxima by using a PerkinElmer spectrofluorimeter. The metal ions were added as their corresponding salts (see the Materials and methods section) at a final concentration of 10 μM. The decrease in fluorescence is expressed as a percentage of the initial fluorescence intensity (set at 0% quenching) with the buffer as blank without probe (set at 100%). Spectra were not corrected for light intensity, detector sensitivity and photobleaching of the probes.
| Fluorescence (%) | |||||
|---|---|---|---|---|---|
| CP655 | CP645 | CP623 | CP603 | CP800 | |
| Fe3+ | 92 | 93 | 97 | 96 | 66 |
| Fe2+* | 92 | 94 | 97 | 97 | 63 |
| Zn2+ | 15 | 11 | 17 | 14 | 9 |
| Ni2+ | 7 | 11 | 7 | 10 | 6 |
| Cu+* | 10 | 11 | 8 | 6 | 9 |
| Cu2+ | 42 | 50 | 49 | 40 | 31 |
| Co2+ | 11 | 12 | 15 | 11 | 8 |
| Ca2+ | 3 | 8 | 5 | 8 | 4 |
* Measurements were performed in the presence of the reducing equivalent ascorbic acid (200 μM).
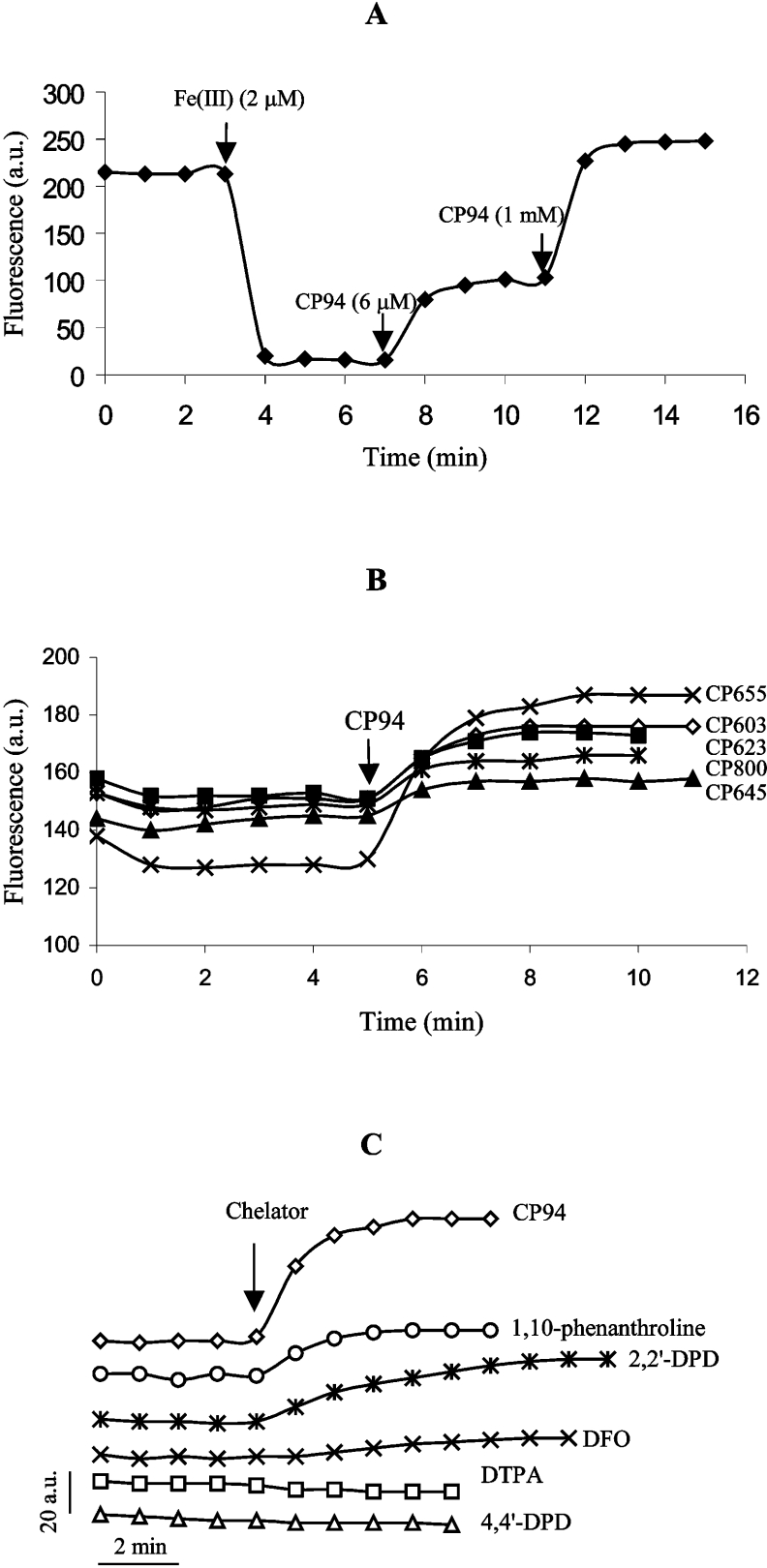
The addition of a strong iron chelator to a preformed iron–probe complex led to removal of iron, thereby releasing the iron-free probe accompanied by an increase in fluorescence. Thus, after the fluorescence of CP655 (6 μM) had been quenched by iron (2 μM), the addition of an equivalent concentration (6 μM) of CP94 led to recovery of approximately half of the initial fluorescence (Figure 3A). This indicates that the two ligands have similar affinity coefficients. Addition of a large excess of CP94 to the probe–iron complex led to a CP655 fluorescence recovery slightly above the initial value (Figure 3A). This phenomenon is attributable to the presence of trace iron contaminants in the buffer, which cause a partial decrease in the initial fluorescence. It was anticipated that this phenomenon would not cause problems in experiments utilizing intact cells, i.e. cellular iron loading by the probe, as the 3:1 ligand-to-iron complex (molecular mass >1000 Da) is predicted to be poorly membrane-permeable [35].
Figure 3. Evaluation of fluorescence dequenching of pyridinone fluorescent probes.
(A) Fluorescence (λex=416 nm, λem=474 nm) of CP655 (6 μM) was quenched by the addition of 2 μM iron(III) and then recovered after addition of 6 μM and 1 mM CP94. (B) Effect of CP94 (1 mM) on the intracellular fluorescence of hepatocytes that had been loaded with various probes (10 μM each). (C) Effect of various chelators [CP94, 1,10-phenanthroline, 2,2′-dipyridyl (2,2′-DPD), DFO and DTPA; 1 mM each] and the non-chelating analogue of 2,2′-dipyridyl, 4,4′-dipyridyl (4,4′-DPD) (1 mM), on hepatocellular CP655 fluorescence.
Evaluation of the fluorescent probes for determining intracellular chelatable iron in hepatocytes
The intracellular fluorescence of all the probes was quenched by the addition of the iron(III)–8-hydroxyquinoline complex (1:2 molar ratio) and the resulting fluorescence remained stable for at least 30 min (results not shown). The intracellular probe fluorescence was not only decreased by the addition of exogenous iron, but was also increased by the addition of membrane-permeable iron chelators, such as CP94 (1 mM). Amongst the five fluorescent probes, CP655 was found to be the most sensitive towards the addition of CP94 at 10 μM probe-loading conditions (Figure 3B). Thus a range of CP655 concentrations (1–20 μM) was used to load hepatocytes. In the range 1–10 μM, it was found that the dequenching effect of CP94 increased with the concentration of CP655 (results not shown). Further increasing the CP655 concentration did not lead to a corresponding increase in the CP94-induced dequenching effect. Thus 10 μM CP655 was found to be an optimal concentration for loading hepatocytes. None of the pyridinone fluorescent probes had any cytotoxic effect on hepatocytes under the experimental conditions studied.
Addition of a large excess of CP94 strongly enhanced the intracellular CP655 fluorescence of cultured hepatocytes (Figure 3C). As the affinity constants of both CP94 and CP655 are similar (see Figure 3A) and the intracellular CP94 concentration was estimated to be at least 20-fold higher than that of CP655, it was assumed that the CP655 fluorescence was almost fully recovered. 2,2′-Dipyridyl and 1,10-phenanthroline, which are both highly membrane-permeable metal chelators with a selectivity favouring iron(II) over iron(III), induced some dequenching of CP655 but were less effective than CP94. Since Fe(II) undergoes oxidation upon chelation by CP655 (see above), the lower effectiveness of 2,2′-dipyridyl and 1,10-phenanthroline can be explained by their lower iron(III) affinity constants (pFe3+ values of 2,2′-dipyridyl, 1,10-phenanthroline and CP94 are 16.3, 14.1 and 19.7 respectively [13,36]). The inability of the two former chelators to completely remove the iron from the CP655–iron(III) complex is thus to be expected. The non-chelating 2,2′-dipyridyl analogue, 4,4′-dipyridyl, did not affect CP655 fluorescence intensity. DTPA, a highly hydrophilic iron(III) chelator, which does not cross membranes [37], was also found not to increase the intracellular probe fluorescence (Figure 3C). Addition of DFO, a powerful hydrophilic chelator for iron(III) that only slowly enters cells, resulted in a low rate of dequenching.
Intracellular concentrations of fluorescent HPOs
Hepatocytes were loaded with the probes under the same conditions as described above and the intracellular probe concentrations were quantified from their released fluorescence intensities after cell lysis. Assuming a mean volume of 7.8 pl per hepatocyte [31], the mean intracellular probe concentrations for CP603, CP623, CP645, CP655 and CP800 were calculated to be 23.6±2.2, 18.9±1.7, 163±26, 60.5±4.4 and 9.9±1.6 μM respectively. CP623, which possesses the lowest ClogP (logarithm of calculated partition coefficient) value amongst the five probes (Table 1), was found to achieve the lowest intracellular concentration. The intracellular probe concentrations were found to increase with increasing ClogP value, the highest being achieved by CP645. The intracellular concentration of CP800 was found to be the lowest of the group and this could be explained by Lipinski's rules [35].
Table 1. Physicochemical properties of fluorescent iron chelators.
The maximum absorption and emission wavelengths of all fluorescent iron chelators (10 μM) were measured in Mops buffer (25 mM) at pH 7.4. The ClogP values were calculated by using Chemoffice 6.0 from Cambridgesoft (Cambridge, U.K.).
| Ab.* | Em.† | Molecular mass (Da) | Number of H donors | Number of H acceptors | ClogP | |
|---|---|---|---|---|---|---|
| CP603 | 387 | 473 | 397 | 2 | 8 | −0.14 |
| CP623 | 387 | 474 | 397 | 2 | 8 | −0.39 |
| CP645 | 432 | 470 | 397 | 2 | 8 | 0.69 |
| CP655 | 416 | 477 | 411 | 2 | 8 | 0.43 |
| CP800 | 494 | 520 | 526 | 4 | 10 | 0.90 |
* Absorption wavelength (nm).
† Emission wavelength (nm).
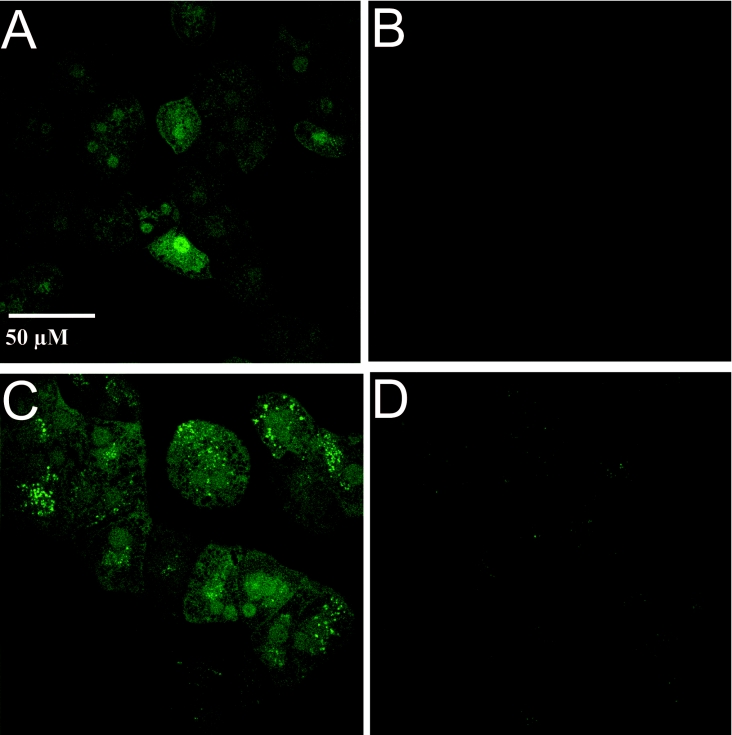
Using quantitative laser scanning microscopy, the intracellular fluorescence intensities of CP800 and CP645 were found to strongly exceed cellular autofluorescence (Figure 4). Analysis of the subcellular distribution of the HPO fluorescent sensors led to the observation that they were mainly distributed in the nucleus and the cytosol, i.e. the main intracellular sites of iron toxicity [38]. In contrast with CP800, CP645 was also readily detectable within the endosomal/lysosomal apparatus of the cells.
Figure 4. Intracellular distribution of CP800 and CP645 in cultured rat hepatocytes.
Cells were loaded with CP800 or CP645 by incubation with 10 μM probe for 10 min in HBSS (37 °C). The intracellular fluorescence was scanned by using a laser scanning microscope (LSM 510). (A) The fluorescence of CP800-loaded cells was excited at 488 nm and a 505 nm long-pass filter was used for emission. (B) The autofluorescence of unloaded cells at the same instrument settings is shown. (C) The fluorescence of CP645-loaded cells was excited at 458 nm by using a 475 nm long-pass filter for emission. (D) The autofluorescence of unloaded cells at the same instrument settings as used for (C) is shown.
The intracellular chelatable iron pool of hepatocytes as determined with HPO fluorescent sensors
The intracellular chelatable iron pool of hepatocytes was assessed from the initial relative fluorescence intensity within hepatocytes and the respective ex situ calibration curve for each probe. Thus, given an initial relative fluorescence intensity for CP655 of 72.1±7.0% within cells (Figure 2A), 5.4±1.3 μM iron(II) was required to reach this value when calibrating ex situ (Figure 2C). As the total iron content of these cells has been determined to amount to 4.7±1.5 nmol/106 hepatocytes [30], and the volume of a single hepatocyte to be 7.8 pl [31], the chelatable iron as a percentage of the total hepatocellular iron was estimated to be 0.90±0.21%. The intracellular chelatable iron concentrations as determined by using the other pyridinone fluorescent probes (CP603, CP623, CP645 and CP800) were slightly lower but in the same range (results not shown).
DISCUSSION
The determination of the intracellular chelatable iron pool of rat hepatocytes in the present study is based on the properties of HPO fluorescent probes, which are quenched by the intracellular chelatable iron pool and dequenched by the presence of excess high-affinity iron chelator. This method can detect both increases and decreases in the iron pool at a single cell level with high sensitivity.
The intracellular probe concentrations were found to depend on their partition coefficients. Within the group under investigation, enhanced lipophilicity led to greater membrane penetration [15,33]. Although the partition coefficient is an important factor which influences the intracellular probe concentration, the mechanism for the accumulation of these probes in hepatocytes remains uncertain. Microfluorographs obtained by using laser scanning microscopy indicated that within CP645-loaded hepatocytes, the fluorescence of some organelles was far stronger than that of other cellular structures (Figure 4C). This phenomenon did not occur in the CP800-loaded cells (Figure 4A). However, similar phenomena occurred when autofluorescence of unloaded cells was monitored at the appropriate instrument settings (compare Figure 4D with Figure 4B). Nevertheless, the distribution of fluorescent probes indicates that the pyridinone class of chelators gains access not only to the cytoplasm but also to the nucleus and vesicular organelles located at the cell periphery (most likely to be lysosomes/endosomes). These preliminary distribution studies are currently being extended by more detailed investigations utilizing specific lysosomal markers. The design of lysosomotrophic chelators could mobilize this local iron pool more efficiently and therefore, in principle, increase the overall efficiency of iron chelation.
Porter et al. [15] found that compounds with partition coefficients above 0.3 in the iron-free form are more effective at mobilizing iron from hepatocytes than those with lower values (<0.2). The observation that the ClogP value for CP655 is 0.43, combined with our finding that CP655 was the most sensitive probe for the hepatocyte chelatable iron pool, indicated that CP655 would be the most effective chelator in cultured hepatocytes. Thus this probe was adopted in order to monitor the concentration of intracellular chelatable iron. The value obtained with CP655 was 5.4±1.3 μM, which is close to the concentration of hepatocyte chelatable iron (6.2±1.8 μM) that has been previously reported by using the 1,10-phenanthroline-based indicator Phen Green SK in the same cells (assuming a mean volume of 7.8 pl – rather than 5 pl – per hepatocyte; F. Petrat, unpublished work; see [30,31]).
The major advantage of the new fluorescent iron chelators reported in the present study, in comparison with the previously introduced iron indicators Phen Green SK and calcein, resides in their high selectivity for iron. In a cell-free system, the HPO-based indicators respond much less to other biologically important bivalent metal ions such as Cu2+, Zn2+, Ni2+ and Co2+ (Table 2), in contrast with the high sensitivity of calcein for Ni2+ (82%), Cu2+ (96%), Co2+ (74%) and Zn2+ (38%) [29] and of Phen Green SK for Cu2+ (70%) [30] respectively. The corresponding values for CP655 are Ni2+ (7%), Cu2+ (42%), Co2+ (11%) and Zn2+ (15%). The other advantage of the HPO fluorescent probes is that they remain membrane-permeable and are not hydrolysed after permeating the cell membrane, therefore enabling the probes to be distributed not only within the cytosol but also into intracellular organelles, for instance, lysosomes.
Acknowledgments
We thank Ms B. Lammers (Institut für Physiologische Chemie, Universitatsklinikum Essen, Germany) for the isolation and cultivation of the cells and Ms M. Holzhauser (Institut für Physiologische Chemie) for her excellent technical assistance. This work was supported by Natural Medicine Research Institute, King's College London.
References
- 1.Crichton R. R. 2nd edn. Chichester: John Wiley and Sons; 2001. Inorganic Biochemistry of Iron Metabolism. [Google Scholar]
- 2.Esposito B. P., Epsztejn S., Breuer W., Cabantchik Z. I. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal. Biochem. 2002;304:1–18. doi: 10.1006/abio.2002.5611. [DOI] [PubMed] [Google Scholar]
- 3.Petrat F., de Groot H., Sustmann R., Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B., Gutteridge J. M. C. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 5.Hamed M. Y., Hider R. C., Silver J. The competition between enterobactin and glutathione for iron. Inorg. Chim. Acta. 1982;66:13–18. [Google Scholar]
- 6.Halliwell B., Gutteridge J. M. C. 3rd edn. Oxford, U.K.: Oxford University Press; 1999. Free Radicals in Biology and Medicine; pp. 48–55. [Google Scholar]
- 7.Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J. Biol. Chem. 1989;264:20509–20512. [PubMed] [Google Scholar]
- 8.Komara J. S., Nayini N. R., Bialick H. A., Indrieri R. J., Evans A. T., Garritano A. M., Hoehner T. J., Jacobs W. A., Huang R. R., Krause G. S., et al. Brain iron delocalization and lipid peroxidation following cardiac arrest. Ann. Emerg. Med. 1986;15:384–389. doi: 10.1016/s0196-0644(86)80171-8. [DOI] [PubMed] [Google Scholar]
- 9.Reddy B. R., Kloner R. A., Przyklenk K. Early treatment with deferoxamine limits myocardial ischaemic/reperfusion injury. Free Radical Biol. Med. 1989;7:45–52. doi: 10.1016/0891-5849(89)90099-3. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach M., Ben-Shachar D., Riederer P., Youdim M. B. H. Altered brain metabolism of iron as a cause of neurodegenerative disease? J. Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 11.Hershko C., Konijn A. M., Link G. Iron chelators for thalassaemia. Br. J. Haematol. 1998;101:399–406. doi: 10.1046/j.1365-2141.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 12.Tilbrook G. S., Hider R. C. Iron chelators for clinical use. In: Sigel A., Sigel H., editors. Metal Ions in Biological Systems. New York: Marcel Dekker; 1998. pp. 691–730. [PubMed] [Google Scholar]
- 13.Liu Z. D., Hider R. C. Design of clinically useful iron(III)-selective chelators. Med. Res. Rev. 2002;22:26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- 14.Porter J. B., Huehns E. R., Hider R. C. The development of iron chelating drugs. Baillieres Clin. Haematol. 1989;2:257–292. doi: 10.1016/s0950-3536(89)80018-6. [DOI] [PubMed] [Google Scholar]
- 15.Porter J. B., Morgan J., Hoyes K. P., Burke L. C., Huehns E. R., Hider R. C. Relative oral efficacy and acute toxicity of hydroxypyridin-4-one iron chelators in mice. Blood. 1990;76:2389–2396. [PubMed] [Google Scholar]
- 16.Porter J. B., Singh S., Katherine P. H., Epemolu O., Abeysinghe R. D., Hider R. C. Lessons from preclinical and clinical studies with 1,2-diethyl-3-hydroxypyridin-4-one, CP94 and related compounds. Adv. Exp. Med. Biol. 1994;356:361–370. doi: 10.1007/978-1-4615-2554-7_38. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z. D., Lu S. L., Hider R. C. In vivo iron mobilization evaluation of hydroxypyridinones in 59Fe-ferritin-loaded rat model. Biochem. Pharmacol. 1999;57:559–566. doi: 10.1016/s0006-2952(98)00319-0. [DOI] [PubMed] [Google Scholar]
- 18.Porter J. B. Evaluation of new iron chelators for clinical use. Acta Haematol. 1996;95:13–25. doi: 10.1159/000203852. [DOI] [PubMed] [Google Scholar]
- 19.Bauminger E. R., Cohen S. G., Ofer S., Rachmilewitz E. A. Quantitative studies of ferritinlike iron in erythrocytes of thalassemia, sickle-cell anemia, and hemoglobin Hammersmith with Mossbauer spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 1979;76:939–943. doi: 10.1073/pnas.76.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauminger E. R., Nowik I. Iron in Parkinson disease, blood diseases, malaria and ferritin. Hyperfine Interact. 1998;111:159–170. [Google Scholar]
- 21.Gower J. D., Healing G., Green C. J. Determination of desferrioxamine-available iron in biological tissues by high-pressure liquid chromatography. Anal. Biochem. 1989;180:126–130. doi: 10.1016/0003-2697(89)90099-7. [DOI] [PubMed] [Google Scholar]
- 22.Öllinger K., Roberg K. Nutrient deprivation of cultured rat hepatocytes increases the desferrioxamine-available iron pool and augments the sensitivity to hydrogen peroxide. J. Biol. Chem. 1997;272:23707–23711. doi: 10.1074/jbc.272.38.23707. [DOI] [PubMed] [Google Scholar]
- 23.Cammack R., Cooper C. E. Electron paramagnetic resonance spectroscopy of iron complexes and iron-containing proteins. Methods Enzymol. 1993;227:353–384. doi: 10.1016/0076-6879(93)27014-8. [DOI] [PubMed] [Google Scholar]
- 24.Cairo G., Tacchini L., Pogliaghi G., Anzon E., Tomasi A., Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the ‘free’ iron pool. J. Biol. Chem. 1995;270:700–703. doi: 10.1074/jbc.270.2.700. [DOI] [PubMed] [Google Scholar]
- 25.Flatmark T., Tangeras A. Mitochondrial ‘non-heme non-FeS iron’ and its significance in the cellular metabolism of iron. In: Brown E. B., Aisen P., Fielding J., Crichton R. R., editors. Proteins of Iron Metabolism. New York: Grune and Stratton; 1976. pp. 349–358. [Google Scholar]
- 26.Yegorov D. Y., Kozlov A. V., Azizova O. A., Vladimirov Y. A. Simultaneous determination of Fe(III) and Fe(II) in water solutions and tissue homogenates using desferal and 1,10-phenanthroline. Free Radical Biol. Med. 1993;15:565–574. doi: 10.1016/0891-5849(93)90158-q. [DOI] [PubMed] [Google Scholar]
- 27.Savory J., Herman M. M. Advances in instrumental methods for the measurement and speciation of trace metals. Ann. Clin. Lab. Sci. 1999;29:118–126. [PubMed] [Google Scholar]
- 28.Breuer W., Epsztejn S., Millgram P., Cabantchik Z. I. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am. J. Physiol. 1995;268:C1354–C1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- 29.Epsztejn S., Kakhlon O., Glickstein H., Breuer W., Cabantchik Z. I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 1997;248:31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 30.Petrat F., Rauen U., de Groot H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999;29:1171–1179. doi: 10.1002/hep.510290435. [DOI] [PubMed] [Google Scholar]
- 31.Petrat F., de Groot H., Rauen U. Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch. Biochem. Biophys. 2000;376:74–81. doi: 10.1006/abbi.2000.1711. [DOI] [PubMed] [Google Scholar]
- 32.Luo W., Ma Y. M., Quinn P. J., Hider R. C., Liu Z. D. Design, synthesis and properties of novel iron(III)-specific fluorescent probes. J. Pharm. Pharmacol. 2004;56:529–536. doi: 10.1211/0022357023196. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y. M., Luo W., Quinn P. J., Liu Z. D., Hider R. C. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J. Med. Chem. 2004;47:6349–6362. doi: 10.1021/jm049751s. [DOI] [PubMed] [Google Scholar]
- 34.de Groot H., Brecht M. Reoxygenation injury in rat hepatocytes: mediation by O2−/H2O2 liberated by sources other than xanthine oxidase. Biol. Chem. Hoppe-Seyler. 1991;372:35–41. doi: 10.1515/bchm3.1991.372.1.35. [DOI] [PubMed] [Google Scholar]
- 35.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z. D., Piyamongkol S., Liu D. Y., Khodr H. H., Lu S. L., Hider R. C. Synthesis of 2-amido-3-hydroxypyridin-4-(1H)-ones: novel iron chelators with enhanced pFe3+ values. Bioorg. Med. Chem. 2001;9:563–573. doi: 10.1016/s0968-0896(00)00273-x. [DOI] [PubMed] [Google Scholar]
- 37.Scheiber B., Goldenberg H. Uptake of iron by isolated rat hepatocytes from a hydrophilic impermeable ferric chelate, Fe(III)-DTPA. Arch. Biochem. Biophys. 1996;326:185–192. doi: 10.1006/abbi.1996.0064. [DOI] [PubMed] [Google Scholar]
- 38.Sturm B., Bistrich U., Schranzhofer M., Sarsero J. P., Rauen U., Scheiber-Mojdehkar B., de Groot H., Ioannou P., Petrat F. Friedreich's ataxia, no changes in mitochondrial labile iron in human lymphoblasts and fibroblasts: a decrease in antioxidative capacity? J. Biol. Chem. 2005;280:6701–6708. doi: 10.1074/jbc.M408717200. [DOI] [PubMed] [Google Scholar]