Abstract
The six members of the insulin-like growth factor-binding protein family (IGFBP-1–6) are important components of the IGF (insulin-like growth factor) axis. In this capacity, they serve to regulate the activity of both IGF-I and -II polypeptide growth factors. The IGFBPs are able to enhance or inhibit the activity of IGFs in a cell- and tissue-specific manner. One of these proteins, IGFBP-5, also has an important role in controlling cell survival, differentiation and apoptosis. In this review, we report on the structural and functional features of the protein which are important for these effects. We also examine the regulation of IGFBP-5 expression and comment on its potential role in tumour biology, with special reference to work with breast cancer cells.
Keywords: extracellular matrix (ECM), glycosaminoglycan, insulin-like growth factor-I (IGF-I), insulin-like growth factor-binding protein 5 (IGFBP-5), mammary gland, proteolysis
Abbreviations: ADAM, adisintegrin and metalloprotease; AP-2, activator protein 2; CAT, chloramphenicol acetyltransferase; CBP-4, C-terminus of insulin-like growth factor-binding protein 4 (residues 151–232); C/EBP, CCAAT/enhancer-binding protein; ECM, extracellular matrix; ER, oestrogen receptor; ERK1/2, extracellular-signal-regulated protein kinase 1/2; FHL-2, four-and-a-half LIM domain 2; GAG, glycosaminoglycan; GH, growth hormone; IGF, insulin-like growth factor; IGFBP, IGF-binding protein; IGF-IR, IGF-I receptor; IGF-IIR, IGF-II receptor; IR, insulin receptor; IRS, IR substrate; MAPK, mitogen-activated protein kinase; NBP-4, N-terminus of IGFBP-4 (residues 3–82); OE2, oestradiol; OP-1, osteogenic protein-1; OPN, osteopontin; PAI-1, plasminogen activator inhibitor-1; PAPP, pregnancy-associated plasma protease; PGE2, prostaglandin E2; pSMC, porcine smooth-muscle cell; RA, retinoic acid; RASSF1C, isoform C of the Ras association family 1 protein group; RT, reverse transcription; SPR, surface plasmon resonance; tPA, tissue plasminogen activator; TSP-1, thrombospondin-1; VN, vitronectin
INTRODUCTION
The IGF (insulin-like growth factor) axis plays a crucial role in regulating cellular growth, differentiation and apoptosis [1,2]. As such, these molecules are important in the development and regulation of many tissues. For example, the IGF axis is involved in controlling the growth, differentiation and regression of the mammary gland during cycles of pregnancy, lactation and post-lactational involution [3]. The IGF axis is also known to be involved in the differentiation and growth of embryonic tissues [4] and it may also play a role in the process of tumorigenesis under some specific circumstances [5].
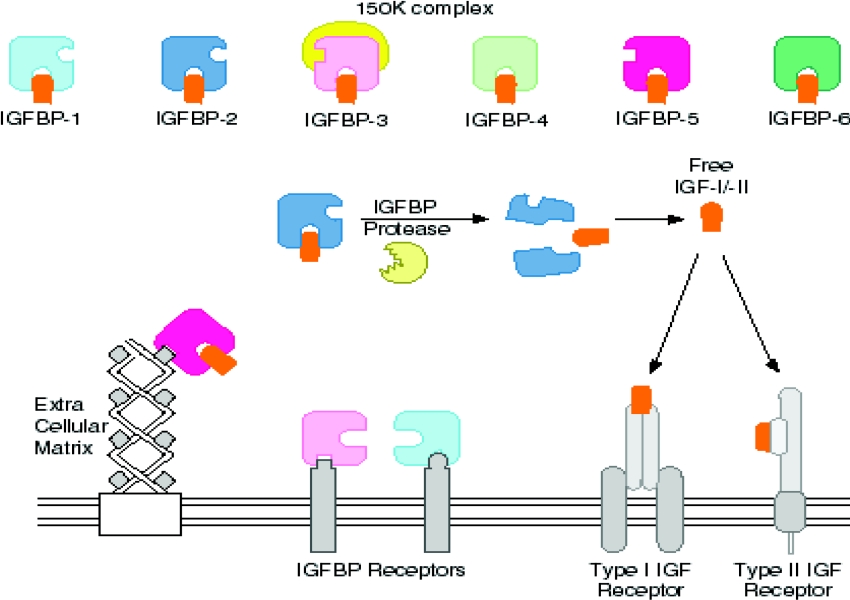
The IGF axis itself consists of two peptide growth factors, IGF-I and IGF-II [6,7], cell-surface IGF-I and -II receptors (IGF-IR and IGF-IIR) [8,9], six well-characterized soluble IGF-binding proteins (IGFBP-1–6) [10–15] and a family of partially characterized IGFBP proteases [16] (Figure 1). IGF-I and IGF-II comprise 70 and 67 amino acid residues respectively and exert their effects through the IGF-IR and IGF-IIR, although the growth-promoting effects of IGF-II occur specifically through the IGF-IR [17]. As suggested by their nomenclature, the IGFs show structural and functional homology with insulin. In addition, the IGF-IR is a heterotetrameric receptor comprising two α and two β subunits (α2β2) with homology with the IR (insulin receptor). On binding IGF-I, the IGF-IR displays intrinsic tyrosine kinase activity and activates intracellular signalling pathways that are similar to those described for the IR. The IGF-IIR is a large (approx. 280 kDa) single transmembrane protein with no homology with the IGF-IR or IR. The IGF-IIR is also known as the mannose 6-phosphate receptor. The signalling pathways activated by the IGF-IIR are not well defined.
Figure 1. The IGF axis consists of IGF-I and IGF-II polypeptides (orange), six IGFBPs (various colours), a family of IGFBP proteases (yellow) and cell-surface IGFBP receptor, IGF-IR and IGF-IIR (light grey).
Cell membrane receptors for IGFBP-1 and IGFBP-3 have been partially defined [211,212]. In the Figure, IGFBP-5 is shown interacting with components of the ECM at a site removed from the IGF-binding site. See text for further details.
The liver is the main source of circulating IGF-I, and expression of hepatic IGF-I is mainly under the control of pituitary GH (growth hormone) [18] and there is evidence for negative-feedback regulation by IGF-I at the level of the pituitary to inhibit GH secretion [19]. Although this pattern of regulation is similar to the properties displayed by classical endocrine hormones, IGF-I and IGF-II (like IGF-IR and IGF-IIR) are expressed in a wide variety of cells and their expression is regulated in a cell- and tissue-type-dependent manner. For this reason, the IGFs are not viewed as classical endocrine hormones, but rather as paracrine or autocrine growth factors.
IGFBPs are important members of the IGF axis. Six of these binding proteins have been cloned (IGFBP-1–6) and they vary in length from 201 to 289 residues. After various post-translational modifications, their molecular size determined by gel electrophoresis falls in the range 24–44 kDa. By analogy to IGF-I and IGF-II, IGFBPs are also secreted by many cell types, and the profile of expressed IGFBPs is also regulated in a cell- and tissue-type-dependent manner. The IGFBPs bind IGFs with high affinity (KD ∼0.1 nM) and over 95% of IGFs in serum and other biological fluids are bound to IGFBPs. As the affinity of IGFBPs for IGFs is higher than that of cell-surface IGF-IR (KD ∼1 nM), IGFs which are bound by soluble IGFBPs are restricted in accessing cell-surface IGF-IR. However, some IGFBPs can bind to ECM (extracellular matrix) GAGs (glycosaminoglycans) and other ECM proteins [20]. This reduces the affinity of the IGF–IGFBP interaction and has important consequences for the activity of both IGFs and IGFBPs. We discuss this phenomenon elsewhere in the review.
Other members of the IGF axis include a group of increasingly well-defined IGFBP proteases which act to hydrolyse IGFBPs. By analogy to the interaction with ECM components, proteolysis of IGFBPs also results in a large reduction in affinity between IGFBPs and IGFs, and potentially releases the growth factors to interact with cell-surface receptors. In addition to these features, IGFBPs are reported to have IGF-independent effects in some tissues [21] and, consequently, these proteins should be viewed as more than a reservoir of tightly bound IGFs.
We focus on the structure and function of one of these IGFBPs, IGFBP-5. This IGFBP has been shown to display all the properties discussed above and is established as a key member of the IGF axis. We review the initial discovery of this protein, its molecular cloning, primary/secondary structure and post-translational modifications, with special reference to the effects of these modifications on the biological activity of IGFBP-5. The most important of these post-translational modifications, proteolysis, is highlighted. We report briefly on the limited three-dimensional structural information that is available for the protein. We also review the literature relating to the architecture of the IGFBP-5 gene with special reference to regulation of mRNA expression through transcription factor-binding sites in the IGFBP-5 promoter. In an extended section, we report in detail the interactions of IGFBP-5 with other biomolecules and the structure–function aspects of these interactions.
We discuss the activity of IGFBP-5 in relation to IGF-dependent and -independent actions and how these are affected by complexation with other biomolecules. We also highlight the pleiotropic nature of the protein with respect to enhancement or inhibition of IGF activity and its cell-context-dependent association with either growth-promoting, differentiation or apoptotic actions. Finally, we review the literature relating to the presence and activity of IGFBP-5 in various tumours. In this section, we emphasize recent work with breast cancer cells and tissues.
It is important the reader is aware that, as indicated above, IGFBP-5 is only one of a family of six IGFBPs. These proteins all bind IGF-I and IGF-II with high affinity and share other specific properties. At appropriate locations within the review, we indicate where the activity of other IGFBPs may overlap with IGFBP-5 and deal briefly with such concepts as redundancy of function within the IGFBP family. For recent reviews on the structure and functional relationships within the whole IGFBP family, the reader is directed to two excellent reviews [22,23]. For a short review on some of the properties of IGFBP-5, see [24].
IGFBP-5: STRUCTURE
Primary structure
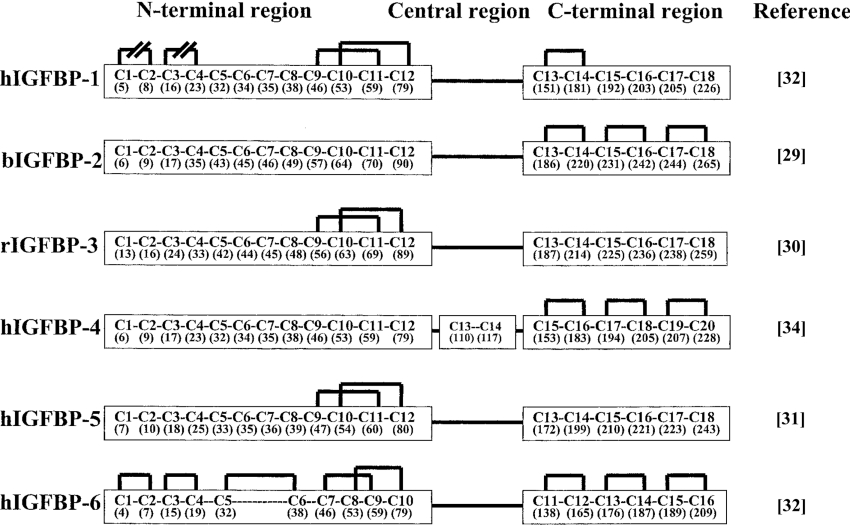
Cloning of mouse, rat, porcine and human IGFBP-5 cDNAs [25–28] indicated a high degree of sequence conservation. The protein comprises 252 residues (28.5 kDa) and is expressed with a hydrophobic signal sequence (19–20 residues) that is characteristic of secreted proteins. It is a slightly basic protein (pI ∼8.5). One of the most significant features of the primary sequence (along with the other IGFBPs) is the presence of a large number of highly conserved cysteine residues (Figure 2). There are 18 cysteine residues in IGFBP-5: 12 and six in the N- and C-terminal regions of the protein respectively. It has also been possible to partially assign disulphide bond linkages in the N- or C-terminal terminal domains of various IGFBPs [29–34] and for the entire IGFBP-6 protein [32] (Figure 3). These data indicated that the protein was completely disulphide-bonded within the N- and C-terminal regions of the protein, and this pattern of intra-domain disulphide bonding has led to a consensus model for IGFBP structure as comprising discrete N- and C-terminal domains joined by a ‘hinge region’ which has a less conserved and ordered structure. A report from Kalus et al. [31] indicates that Cys47 and Cys60 (C9 and C11) are disulphide-bonded, as are Cys54 and Cys80 (C10 and C12) (see Figure 3) to form the core of a ‘mini-IGFBP-5’ domain (see the next section). The assignment of these four N-terminal domain disulphide bonds was the same as that described for IGFBP-1, -3, -4 and -6 and has led to the suggestion that there are in fact two mini-domains within the N-terminus of IGFBP-5 (and by inference the other IGFBPs) with the core structure described by Kalus et al. [31] comprising the more C-terminal of these domains. Confirmation of this hypothesis, however, will require the full assignment of disulphide bonds within the N-terminal domain of IGFBP-5 and also a complete crystal structure for this region of the protein.
Figure 2. Sequence alignment of rat IGFBP-1–6.
Asterisks indicate the location of 18 conserved cysteine residues clustered in the N- and C-terminal domains for each IGFBP. The two extra cysteine residues in IGFBP-4 are underlined. When a residue is identical in three out of five of the IGFBPs, it is shown in bold. Dashes have been introduced in the N- and C-terminal domains to allow for better alignment of sequences. The central domain of the IGFBPs show less conserved sequence and these residues are bracketed. Figure reproduced from [14] with permission. © 1991 The American Society for Biochemistry and Molecular Biology.
Figure 3. Disulphide linkages in IGFBPs.
Cysteine residues are numbered sequentially from the N-terminus. Numbers in parentheses represent the actual residue number (the first residue in the mature peptide is designated residue 1). r, rat; b, bovine; h, human. Reproduced from D. Byun, S. Mohan, D. J. Baylink and X. Qin (2001), Localization of the IGF binding domain and evaluation of the role of cysteine residues in IGF binding in IGF binding protein-4, J. Endocrinol., 169, 135–143. © Society for Endocrinology (2001). Reproduced by permission.
There are two other important regions of primary sequence within the IGFBP-5 protein. The first lies in the C-terminal domain where there is a consensus long (L) heparin-binding sequence (BBBXXB; B=basic amino acid; X=any amino acid). This region of the protein (K206RKQCK211), together with neighbouring basic residues, is important in binding ECM components and other proteins (see the ‘Proteolysis’ section below) [35]. There is also a region in the central domain of IGFBP-5 which contains a short (S) heparin-binding consensus sequence between residues 131 and 140 [36]. Deletion mutagenesis studies have indicated that this central domain may be cryptic in nature and may only be fully effective in the absence of the C-terminal heparin-binding region [37]. Following proteolytic cleavage of IGFBP-5, this central heparin-binding domain may become exposed and display specific biological properties. The central hinge region within IGFBP-5 is also the site of major post-translational modifications of the protein. Proteolysis within this domain is discussed in the next section, especially with reference to its effects on the affinity of IGFBP-5 for IGF-I; however, the central domain of IGFBP-5 is also believed to contain glycosylation sites, and IGFBP-5 was first reported as an O-glycosylated protein following its secretion from the U2 human osteosarcoma cell line [38]. The secretion of glycosylated IGFBP-5 from other cell types has been confirmed [39,40] and, subsequently, an IGFBP-5 fragment purified from haemofiltrate was shown to be glycosylated on Thr152. The composition of the glycosyl moieties of IGFBP-5 has not been determined. It has been reported that glycosylation of some IGFBPs interferes with the ability of the proteins to bind to cell surfaces [41,42]. However, this has not been shown for IGFBP-5, and, given the extensive interactions of this binding protein with other structures, this is a topic worthy of further investigation. The central domain of IGFBPs also contains potential phosphorylation sites. The serine phosphorylation of IGFBP-1 and IGFBP-3 within this domain has been clearly demonstrated [43,44], and this post-translational modification has been shown to affect the affinities of these IGFBPs for IGF-I and ECM constituents. However, there is only limited evidence for the phosphorylation of IGFBP-5 [45], and the nature and occurrence of phosphorylation in this particular binding protein needs further confirmation.
Secondary and tertiary structure
Although the IGFBP-5 gene was cloned over 10 years ago [28], it is only recently that information relating to the three-dimensional structure of the protein has become available [31]. The crystallization of full-length IGFBPs has proven to be difficult. Such structural information that is available is derived from NMR or crystal structures of fragments or domains within the protein or has been inferred by homology modelling from partial structures of other IGFBPs. In 1998, Kalus et al. [31] reported the solution NMR structure of an N-terminal domain of IGFBP-5 comprising residues 40–92 termed ‘mini-IGFBP-5’ (see above). This domain was reported as a rigid globular structure having at its core three anti-parallel β-strands and containing two internal disulphide bonds (Cys47–Cys60 and Cys54–Cys80). Although this mini-domain bound both IGF-I and IGF-II, affinities were reduced 10- and 80-fold respectively compared with wild-type. These reduced affinities of truncated proteins were mainly due to much higher dissociation rates for the complexes formed with IGFs. The authors described a hydrophobic patch on the surface of mini-IGFBP-5 lying outwith the anti-parallel β-strand core containing residues Val49, Tyr50, Pro62, Lys68 and Leu75 which was important for binding IGF-II. Mutagenesis of five residues within this domain (Lys68, Pro69, Leu70, Leu73 and Leu74) to alter the hydrophobic properties of this region in the context of the full-length protein resulted in a 1000-fold decrease in affinity for IGF-I [46]. The mutant protein also had decreased ability to inhibit IGF-I biological action. One concern in such an extensive mutagenesis strategy is that the overall confirmation of the protein may be altered and that this, rather than specific residue substitution, may cause loss in recognition of ligand(s). The authors of this study indicated that the mutant protein was still susceptible to proteolysis following incubation with human fibroblast-conditioned medium, arguing that the overall conformation of the protein was not altered [46]. CD spectroscopic analysis of this mutant confirmed this finding [47].
Further NMR and crystallographic studies confirmed the molecular details of the interaction between mini-IGFBP-5 and IGF-II [31,48], and this group also used the crystallographic co-ordinates of the mini-IGFBP-5–IGF-II complex together with NMR studies to design low-molecular-mass inhibitors of the interaction between the two proteins [48]. Although the affinity of interaction between such compounds and mini-IGFBP-5 was only in the micromolar range, such an approach may ultimately yield pharmaceutically useful reagents. In a subsequent study [33], a mini-domain within NBP-4 [N-terminus of IGFBP-4 (residues 3–82)] was shown to have a global fold almost identical with that described for the mini-IGFBP-5 protein. These authors then described crystallization of the ternary complex NBP-4–IGF-I–CBP-4 [C-terminus of IGFBP-4 (residues 151–232)], although the definition of CBP-4 remained poor in this structure. Nonetheless, CBP-4 was reported to increase the affinity of NBP-4 for IGF-I 3–6-fold. Although there is no information of the structure of the C-terminal domain of IGFBP-5, there are some studies which describe the structure of this domain in IGFBP-6 [49,50]. This binding protein (uniquely among the IGFBPs) demonstrates a 20–100-fold higher affinity for IGF-II compared with that for IGF-I [51], and it was suggested that the differential affinity displayed towards the growth factors may be attributed to residues within this C-terminal domain [50]. Caution must therefore be exercised when extrapolating from studies that examine structural elements within the C-terminal domain of IGFBP-6 and the same domain in other IGFBPs. Nevertheless, using a construct containing 27 residues of unstructured vector sequence followed by 80 residues from the C-terminal domain of IGFBP-6 (residues 161–240), Headey et al. [50] describe a structure analogous to a thyroglobulin type-1 fold with an initial α-helix connected by a loop to a three-stranded antiparallel β-sheet structure leading finally to a flexible disulphide-bonded second loop. NMR studies, together with site-directed mutagenesis, identified residues within this C-terminal domain that are important for interaction with IGF-II and indicate that this ligand-binding site is in close proximity to a positively charged sequence located within the first β-turn and β-bulge of the central β-sheet structure. This may provide a rationale for the reduction in affinity of IGFBPs for IGFs following the binding of the former proteins to ECM structures [50], a phenomenon widely reported for IGFBP-5. Further studies from this group using NMR techniques suggested some conformational changes involving these residues within the C-terminal domain of IGFBP-6 following IGF-II binding.
Recent papers have also reported the structures or properties of the C-terminal domains of IGFBP-1 and -4 [33,49,52,53]. The crystallization of a C-terminal fragment of IGFBP-1, isolated and purified from amniotic fluid, has also been reported [49]. This domain also demonstrated a thyroglobulin type-I fold, although the length of the α-helix in the IGFBP-1 structure is longer (19 residues) than that of the IGFBP-6 structure (15 residues). In addition, both the length of anti-parallel β-strands and the exact positioning of loops connecting elements of secondary structure vary between IGFBP-1 and IGFBP-6 C-terminal domain structures. A C-terminal fragment (residues 136–237) of IGFBP-4 has been purified from human haemofiltrate and has been produced recombinantly [52]. Although only limited information was provided from a crystal structure of this fragment, 1H-NMR spectroscopy suggested the predominance of α-helical components in this region.
In summary, there is no crystal or solution structure available for any full-length IGFBP. For IGFBP-5, limited structural information is available for an important IGF-binding region within the N-terminal domain of the protein. In recent years, some structural information has begun to appear in respect of the C-terminal domains of IGFBPs -1, -4 and -6. Given the high degree of homology between the IGFBP families, it could reasonably be expected that the structures described for the C-terminal domains for these IGFBPs may be represented to some extent in the IGFBP-5 C-terminal domain. However, confirmation of this observation requires further experimentation. Given the amount of interactions with other biomolecules which this region displays, such structural information is of paramount importance for the molecular dissection of these binding events.
IGFBP-5 GENE STRUCTURE AND PROMOTER REGULATION
Cloning of the rat and mouse IGFBP-5 genes [26,28] indicated that they both spanned some 17 kb and comprised four exons and three introns. The size of the gene was largely determined by the size of the first intron (10 kb) located between exons and 1 and 2. For the rat gene, a transcriptional start site was identified 772 nucleotides 5′ of an ATG translation initiation codon. The mouse IGFBP-5 gene is present on chromosome 1, where it is separated from the IGFBP-2 gene by only 5 kb and is arranged in a ‘tail-to-tail’ orientation with respect to that gene. Cloning of the human IGFBP-5 gene [25] revealed a similar organization to that described for the rodent genes. With a first intron of 25 kb and a total gene size of 33 kb, the human gene was somewhat larger than the rodent counterpart. However, as found for the mouse, the human IGFBP-5 gene was closely linked to the IGFBP-2 gene and was arranged in the same orientation with respect to that gene. A transcription initiation site was identified in a similar location to that of the rat gene, and limited promoter analysis indicated that 461 bp of sequence upstream from the mRNA cap site directed the expression of a CAT (chloramphenicol acetyltransferase) construct transfected into the MDA-MB-468 human breast cancer cell line. Subsequently, Rotwein and colleagues used deletion analysis of IGFBP-5 promoter–luciferase constructs transiently transfected into the differentiating mouse C2 myoblast cell line to show that 156 nt of 5′ flanking DNA sequence from the IGFBP-5 promoter retained 70% of the activity of a 1004 bp luciferase construct [54]. Further studies indicated that the same region of the mouse gene was active in transient transfection experiments in the human Hep G2 liver cell line and that a region at −70 to −34, which is footprinted with Hep G2 nuclear extracts, was responsible for the majority of this activity [54]. Further work involving co-transfection of human IGFBP-5 promoter–luciferase constructs and AP-2 (activator protein 2) constructs into a human dermal fibroblast cell line indicated up-regulated expression of the luciferase constructs by this transcription factor and identified regions at −152 to −127 and −57 to −30 as being important for binding AP-2 and conferring cAMP-responsiveness to the IGFBP-5 gene [55]. Following this work, a series of studies examined the regulation of IGFBP-5 expression in foetal rat osteoblasts [56–60]. McCarthy et al. [56] reported that AP-2 may be important in conferring PGE2 (prostaglandin E2) sensitivity on the IGFBP-5 promoter, although these PGE2-responsive elements were later reported to be further upstream (in regions at −2695 to −1470 and −989 to −332) than found previously [57]. These authors confirmed that the IGFBP-5 promoter in rat foetal osteoblasts was also sensitive to cAMP [57], confirming the findings reported for dermal fibroblasts [55]. Using deletion and point mutant analysis of the IGFBP-5 promoter in rat foetal osteoblasts, Gabbitas et al. [57] found that a region between −70 and +22 was required for basal promoter activity along with cortisol sensitivity and that this area also contained a putative E-box/c-Myb-binding motif. In an extensive study using similarly mutated IGFBP-5 promoter constructs, it was established that mutations in the E-box decreased basal promoter activity and also eliminated the stimulatory effect of PGE2 on luciferase expression [59]. The same study reported putative binding sites for the transcription factors C/EBP (CCAAT/enhancer-binding protein) and NF-1 (nuclear factor-1), and mutations in either of these elements reduced promoter activity. However, a very recent study with the human neuroblastoma cell line LAN-5 showed that mutation of CCAAT/enhancer element in a −83 to +53 IGFBP-5 promoter–luciferase construct increased the transcription of the transiently transfected plasmid [61]. These contradictory results may reflect cell-specific effects.
Studies using foetal rat osteoblast cultures have also indicated negative cis-acting regions in the IGFBP-5 promoter. For example, an inhibitory effect of OP-1 (osteogenic protein-1) was reported, and the location of this OP-1 inhibitory site was reported to be in the E-box/c-Myb-binding region described above [58,60]. In primary cultures of human osteoblasts and in a human osteoblast cell line, progesterone-responsive elements associated with a repeated CACCC box motif lying within the region −162 to −124 were identified. Thus, following co-transfection of the progesterone receptor-A isoform and IGFBP-5 proximal promoter–CAT constructs, progesterone treatment of cells results in an increase in CAT expression [62].
The other cell culture model used extensively to investigate the regulation of the IGFBP-5 promoter are mouse and human neuroblastoma cell lines [61,63]. Tanno et al. [63] reported two Myb-binding sites at −429 to −424 and −59 to −54. This latter site may correspond to the E-box/c-Myb site reported previously in foetal rat osteoblasts. This group also reported that the more distal site was important for Myb-directed stimulation of the IGFBP-5 promoter, although they found Myb stimulation of IGFBP-5 expression independently of binding to transactivation domains [63]. In addition, this study demonstrated an Akt-responsive element between −334 and −83 [63]. This may be of significance as IGF-I is known to activate the serine/threonine kinase Akt in a phosphoinositide 3-kinase-dependent manner to up-regulate IGFBP-5 expression [64]. Also using neuroblastoma cell lines, an RA (retinoic acid)-responsive element involving a CACCC tandem repeat lying between −147 and −137 was described, and this same element may contribute to RA-induced up-regulation of IGFBP-5 expression in rat osteoblasts [65], although it should be noted that RA is reported to decrease IGFBP-5 expression in other cell lines and primary culture systems [66,67]. Clearly, further studies are required to define the precise role of RA in the regulation of IGFBP-5 expression. It should be recalled that, in osteoblast-like cells, this motif was also described as constituting a progesterone-responsive element. Unlike those with osteoblast cells, studies with neuroblastoma cells indicated that the C/EBP-responsive element had a repressive effect on IGFBP-5 transcription [61]. Clearly there are cell-specific factors that are important in the regulation of IGFBP-5 expression, and further work is required to identify such factors that are involved in the control of IGFBP-5 gene expression, especially during growth, differentiation and apoptosis in different cell types. A summary of known transcription factor binding sites on the IGFBP-5 promoter is shown in Figure 4.
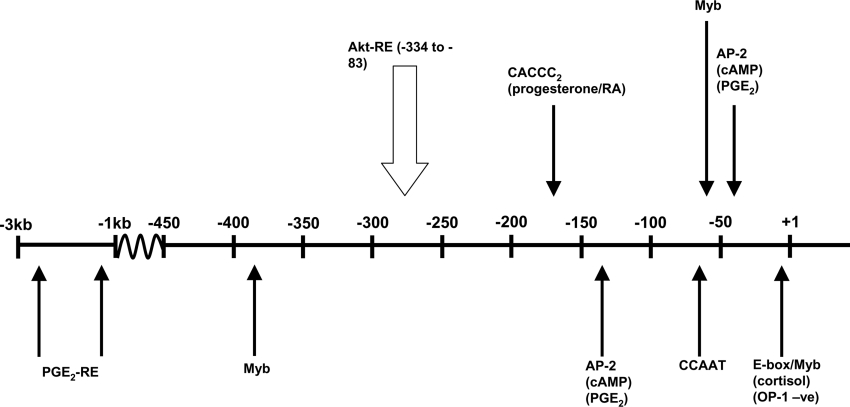
Figure 4. Location of putative transcription-factor-binding sites within the proximal promoter region of IGFBP-5, identified relative to the mRNA CAP site (+1).
Agents shown to regulate IGFBP-5 expression are indicated in parentheses below transcription factors. With the exception of OP-1, these act to increase IGFBP-5 expression. PGE2-RE, PGE2-responsive element.
IGFBP-5 PROTEOLYSIS
The earliest reports of IGFBP proteolysis describe an activity in late human pregnancy serum directed against IGFBP-3 [68,69]. Shortly thereafter this proteolytic activity was confirmed in pregnant mouse and rat serum [70,71], in human amniotic fluid and in seminal plasma [72,73]. The first report of proteolytic activity directed towards IGFBP-5 was described in conditioned medium from human fibroblast cultures [74], and this report also described the inhibition of IGFBP-5 proteolysis by pre-complexation of binding protein with IGF-I. This finding was confirmed as IGFBP-5 proteolytic activity and was subsequently identified and partially characterized in conditioned medium of cultures of the U-2 human osteosarcoma cell line [75,76], from cultures of follicle-stimulating hormone-stimulated rat granulosa cells [77] and in the T47D human breast carcinoma cell line [78]. IGFBP-5 derived from primary cultures of human dermal fibroblasts was also protected from proteolysis by pre-complexation with ECM components including heparin and heparan sulphate [79]. The presence of O-sulphate groups at positions 2 and 3 of the uronic acid monomer units of these GAGs was critical for this activity. Initial attempts at further characterization of the IGFBP-5 proteolytic activity secreted by human fibroblasts described a Ca2+-dependent serine protease which cleaved IGFBP-5 into non-IGF binding fragments of 22, 20 and 17 kDa. Subsequently, the same workers reported a molecular mass of 92 kDa for this serine protease, but also described a metalloprotease activity (or activities) in the molecular mass range 55–72 kDa secreted by human fibroblasts [19]. In these experiments, the serine protease was always the more active towards IGFBP-5. Confirmation of both serine protease and metalloprotease activity directed towards IGFBP-5 was then reported in conditioned medium from mouse osteoblast and neuroblastoma cell lines [80,81] and also in primary cultures of ovine articular chondrocytes [82].
Following these general descriptive studies of IGFBP-5 protease activity in different cell culture systems, researchers began to identify specific IGFBP-5 proteases. It had already been demonstrated that plasmin [83] and thrombin [84] acted to cleave IGFBP-5, and, subsequently, proteolysis of IGFBP-5 by the serine proteases cathepsin G and elastase was also described [85]. In 2000, an important paper [86] described the cloning and characterization of ADAM-12s (a disintegrin and metalloprotease 12 short-form) which is active on IGFBP-5, and Mohan and colleagues subsequently described the secretion of another member of this family (ADAM-9) by human osteoblast cultures [87]. At about the same time, the main serine protease secreted by human fibroblasts was identified as C1s, a member of the complement proteolytic cascade [88]. More recently, two groups have described the proteolysis of IGFBP-5 by PAPPs (pregnancy-associated plasma proteases) A and A2, which are present in several different biological fluids [89–91]. These proteases belong to the class of Zn2+-dependent metalloproteases and appear to cleave IGFBP-5 specifically between Ser143 and Lys144. As for the serine proteases, the activity of PAPP-A and PAPP-A2 is inhibited in the presence of IGF-I. A variant of PAPP-A, PAPP-Ai, which contains a highly basic 29-residue insert has been cloned and was shown to have proteolytic activity towards IGFBP-5 [92]. Most recently, a novel serine protease has been identified after being expressed in insect cells and purified to homogeneity from the growth medium [93]. Intriguingly, the protein has a region of sequence identity with the N-terminal 90-amino-acid sequence in the IGFBP family itself. Northern blotting analysis indicated a widespread expression pattern for this protease, with particularly high levels in uterus and placenta [93]. Further details as to the nature of this protease are awaited with interest.
The observation that IGFBP-5 is protected from proteolysis by engagement with IGF-I, heparin and other molecular components of the ECM, together with reports of biological activity of IGFBP-5 proteolytic fragments themselves, argues for the importance of the IGFBP-5 proteolytic axis in regulating the biological activity of the binding protein and also of IGF-I, IGF-II and ECM in controlling this process. The fact that the affinity of IGFBP-5 (and other IGFBPs) for IGF-I is greater than that of the IGF-IR led to the emergence of a paradigm that IGFBP proteolysis was required for the release of hormone from binding protein in order to allow subsequent binding to receptor and manifestation of IGF-I action. However, there is a literature which describes effects of unliganded intact IGFBP-5 and also activities associated with IGFBP-5 fragments. This is a controversial area, with several contradictory findings reported. For example, an early study found that a 22 kDa fragment of IGFBP-5 did not enhance the proliferative response of human foetal fibroblasts (plated on to ECM derived from the same cells or on to placental Type IV collagen) to added IGF-I, whereas the intact IGFBP-5 protein (100 ng/ml) appeared to enhance the proliferative response to IGF-I (20 ng/ml) by up to 100% [94]. The same inference of ablation of IGFBP-5-enhancing activity following proteolysis has been drawn in studies using protease-resistant forms of IGFBP-5 [95]. This study used pSMC (porcine smooth-muscle cell) cultures to demonstrate that a protease-resistant form of IGFBP-5 (but not the wild-type protein) inhibited IGF-I stimulation of protein and DNA synthesis and also inhibited IGF-I-induced cell migration in these cultures [95]. The conclusion drawn from these studies was that an endogenous proteolytic activity secreted by the cells inactivated wild-type IGFBP-5, but was unable to act on the protease-resistant molecule, resulting in differential inhibitory activities displayed by these proteins [95]. Indeed, these authors confirmed that protease-resistant IGFBP-5 was stable for up to 24 h in culture with these cells [95]. In a similar fashion, studies in cultures of rat growth-plate chondrocytes demonstrated that both N-terminal (residues 1–169) and C-terminal (residues 144–252) fragments of IGFBP-5 had no effect on IGF-I-stimulated cell proliferation, whereas the intact protein again increased IGF-I activity by up to 100% [96]. Intact IGFBP-5 was also reported to stimulate growth of primary cultures of human intestinal smooth-muscle cells via activation of the MAPK (mitogen-activated protein kinase) and ERK1/2 (extracellular-signal-regulated kinase 1/2) signalling pathways [97].
These early studies mainly focused on the effects that proteolysis of IGFBP-5 would have on IGF-I action and not on the possible IGF-independent effects of IGFBP-5 and its proteolytic fragments. However, working with neonatal mouse osteoblasts, Andress [98] and Andress and colleagues [99] demonstrated that IGFBP-5 fragments 1–169 and 201–218 (as well as intact IGFBP-5) caused serine phosphorylation of a 420 kDa putative IGFBP-5 receptor protein derived from cell membrane extracts. To date, this putative IGFBP-5 receptor protein has not been cloned or characterized further. A direct effect of IGFBP-5 fragment 201–218 in stimulating 35SO42− uptake into cultures of bovine vascular epithelial cells has been reported [100], although the EC50 for this effect (2 μM) is somewhat high. This same peptide also caused a direct change in phenotype when added to cultures of mesangial cells derived from rat glomerulus which was associated with filopodia formation and a rapid re-organization of the actin cytoskeleton [101]. The fragment 201–218 of IGFBP-5 has also been reported to displace intact binding protein from ECM structures [102] and therefore may have indirect effects through this mechanism. The area 201–218 in the C-terminal domain of the protein has been the subject of intense study (see below). Although proteolysis of IGFBPs has been commonly reported to generate non-IGF-binding fragments of the protein, there is some limited evidence that fragments of the binding protein may retain a reduced affinity for IGF-I [103], although the physiological significance of this observation has not been studied in any detail. IGFBP-5 fragments were reported to enhance mitogenesis in rat osteoblasts cells; however, recent studies using transgenic mice expressing IGFBP-5 fragments 1–162 and 1–193 under the control of an osteocalcin promoter showed no obvious changes in skeletal phenotype [104]. In contrast, transgenic mice expressing full-length IGFBP-5 from an osteocalcin promoter show decreases in bone trabecular volume, ECM/mineral ratios and osteoblast function [105,106]. Overexpression of IGFBP-5 in the osteoblastic cell line MC3T3-E1 also delayed or decreased the appearance of mRNA for bone-formation markers (e.g. osteocalcin and osteopontin) and inhibited the formation of bone nodules in confluent cell cultures [107]. Subsequent reports in IGFBP-5-overexpressing mice indicated that this bone phenotype was restricted to male mice and that IGFBP-5 transgenic animals also demonstrated increased neonatal mortality, growth inhibition and reduced muscle development, although, in these studies, IGFBP-5 expression was expressed ubiquitously from a CMV (cytomegalovirus) enhancer/β-actin promoter construct [108,109]. It would be interesting to test further the hypothesis that IGFBP-5 fragments have direct biological activity through transgenic routes such as these. Inhibition of IGFBP-5 proteolysis may have important biological and clinical implications. In an experimental model of osteoarthritis in dogs, it was shown that inhibition of the IGFBP-5 protease C1s in joint fluid increased the concentration of intact IGFBP-5 and IGF-I in the joint space [110]. This in turn was associated with an improvement in the structure of the joint during the development of osteoarthritis [110]. However, further studies have shown that IGFBP-5 (and other IGFBPs) are increased in chondrocytes and bone matrix during arthritic joint deterioration [39,111,112]. A positive correlation was reported between histological markers of joint pathology and IGFBP levels [111], and it was suggested that elevated IGFBPs sequestered IGFs, thus aiding the process of joint damage [39]. A review of the occurrence and physiological significance of IGFBP proteolysis has been published [113].
IGFBP-5: INTERACTIONS WITH ECM AND OTHER PROTEINS
Elsewhere in this review, we have indicated that IGFBP-5 binds to protein and GAG components of the ECM. In this section, we report in more detail on this phenomenon, on the structural elements within IGFBP-5 important for binding to ECM components and on the biological consequences of this interaction.
The first indication that IGFBPs interacted with biomolecules other than IGFs came with the observation that gel chromatography of heparinized plasma in a heparin-containing buffer caused a 70–80% shift in immunoreactive IGF from a high-molecular-mass complex (140 kDa) to an elution position characteristic of free IGF [114]. Following this initial study, later work by the same group using immunoblotting and immunohistochemical techniques identified IGFBP-5 associated with ECM preparations derived from human foetal fibroblasts [94]. This group demonstrated direct binding between IGFBP-5 and individual components of the ECM (Type III and IV collagen, laminin and fibronectin). A key finding in this study was that IGFBP-5 enhanced the growth response of human fibroblasts (plated on either ECM or Type IV collagen) to added IGF-I. IGFBP-5 has also been reported to bind to osteoblast and osteoclast-like cells [98], although, in this instance, no binding was observed to the ECM derived from these cells. However, a later study identified that IGFBP-5 associates with ECM derived from cultured mouse calvariae [115], and IGFBP-5 has also been described in ECM derived from human chondrocyte cultures and ECM derived from the clonal mouse osteoblastic cell line MC3T3-E1 [21,39], in ECM from primary cultures of rat osteoblast and periosteal cells [116], in membranes derived from ovine and bovine granulosa cell cultures [117,118] and in a canine mammary tumour cell line [119]. The overwhelming consensus is that IGFBP-5 is able to associate with ECM components and also with cell membrane structures derived from several different tissues, and a previous report has described a methodology for quantifying the amount of IGFBPs associated with ECM components [120].
Several studies have subsequently examined the biological significance of IGFBP-5–ECM association. Abrass et al. [121] have shown that both intact IGFBP-5 and also the heparin-binding domain defined between residues 201–218 are able to stimulate directly the migration of mesangial cells, and other studies have reported that ECM competes with cell membrane structures for binding to IGFBP-5 [122]. These studies also suggested that the enhancing activity of IGFBP-5 (with respect to IGF-I) was associated with a reduction in affinity between IGFBP-5 and IGF-I when the former was bound to the ECM. This may be physiologically relevant, as the affinity of soluble IGFBP-5 for IGF-I is an order of magnitude greater than that of the IGF-IR (see the Introduction). The reduction in affinity of IGFBP-5 for IGF-I when the former is bound to ECM suggested that this may provide a low-affinity ‘sink’ for IGFs, leading to release of growth factor to neighbouring IGF-IRs due to favourable kinetic and equilibrium binding constants that exist between IGFs, IGFBP-5 and IGF-IR under these circumstances. Although this is a view that has a large degree of support in the literature, recent work from our group [123] using biosensor technology has questioned this assumption (see below).
Arai et al. [79] reported that IGFBP-5 also bound to the GAGs heparan sulphate and dermatan sulphate and to other anionic polysaccharides, such as dextran sulphate, which share the structural motif of O-sulphation at positions at C-2 or C-3 of glucose monomers, similar to the O-sulphation present at these positions in the iduronic acid moiety of GAGs which bind IGFBP-5. Importantly, heparin was able to inhibit the interaction between IGF-I and IGFBP-5 and could separate preformed complexes of growth factor and binding protein [79]. This study indicated that a synthetic peptide spanning the region 201–218 inhibited the effect of heparin in disrupting IGF-I–IGFBP-5 interactions, providing further evidence that this region in the IGFBP-5 protein may be responsible for binding to GAGs [79]. As indicated by these authors, this peptide contains the motif (see the section on structure above) characteristic of a long (L) heparin-binding domain [79], and sequence analysis indicates that heparin-binding consensus sequences exist in all IGFBPs (with the exception of IGFBP-4) [14,35]. Although the majority of studies have focused on the effect of ECM/heparin binding on the biology of IGFBP-5 (and the closely related IGFBP-3), other IGFBPs interact with ECM components. For example, IGFBP-2 interacts with heparin if it is first pre-complexed with IGF-I [124]. For this binding protein, a different paradigm therefore exists for interaction with heparin and possibly other ECM components. We have confirmed these results using biosensor technology (J. Beattie and K. Phillips, unpublished work), and we believe that these findings for IGFBP-2 warrant further investigation.
IGFBP-5 has been reported to bind to other ECM proteins. Using co-immunoprecipitation techniques, Nam et al. [124a] demonstrated that IGFBP-5 bound to TSP-1 (thrombospondin-1) and OPN (osteopontin). The affinity of binding to these protein constituents of the ECM was in the low-nanomolar range (similar to the affinity of heparin binding). Other GAGs were able to disrupt the binding of IGFBP-5 and TSP-1/OPN, although the pattern of inhibition was somewhat complex. The 201–218 peptide fragment from IGFBP-5 was able to disrupt the interaction of IGFBP-5 with these ECM molecules, and a mutagenesis strategy within this region of IGFBP-5 suggested that residues Arg201 and Arg214 were the most important for binding TSP-1 and that Arg214, Lys217 and Arg218 were critical for interaction with OPN. Interestingly, and in contrast with the situation with heparin, binding of IGFBP-5 with either TSP-1 or OPN did not reduce the affinity of IGFBP-5 for IGF-I. In this study, the growth response of primary cultures of pSMCs to IGF-I was enhanced by the simultaneous addition of IGFBP-5. If the cells were grown on a matrix of TSP-1 or OPN, the enhancing effect of IGFBP-5 was augmented further. A recent study, however, indicated that if IGFBP-5 was added to pSMC cultures which had already been exposed to IGF-I and TSP-1, protein synthesis and cell migration were inhibited [125]. In this instance, it was shown that IGFBP-5 inhibited the interaction between TSP-1 and integrin-associated protein. At a signalling level, this prevented the enhancement of IGF-IR tyrosine phosphorylation and MAPK activity in response to IGF-I. Further investigations indicated that such an effect was due to the delay in the transfer of the tyrosine phosphatase SHP-2 (Src homology 2 domain-containing tyrosine phosphatase) to the IGF-IR, which in turn prolonged the IGF-IR tyrosine phosphorylation response. It would appear therefore that an inhibitory or enhancing response by IGFBP-5 can be dependent on whether cells are pre-incubated with the binding protein or potential binders of IGFBP-5 or whether experiments are performed using simultaneous or sequential addition of test substances. This means that data obtained in such experiments must be interpreted carefully and that careful attention to experimental design is required under these conditions.
It has been indicated that IGFBP-5 also bound to the ECM protein VN (vitronectin) [126]. As for TSP-1 and OPN, binding affinity of IGFBP-5 for VN was in the low-nanomolar range, and the interaction of the binding protein with VN did not affect the affinity for IGF-I. The region 201–218 of IGFBP-5 was also determined to be important in VN binding, and a mutational analysis suggested that the ability of IGFBP-5 to enhance IGF-I stimulation of smooth-muscle-cell migration in the presence of VN was dependent on its ability to bind both VN and IGF-I simultaneously. A non-IGF-binding mutant of IGFBP-5 had no effect in enhancing the cellular response to IGF-I, in contrast with other reports which indicated IGF-independent effects of ECM associated IGFBP-5 [99,127–129]. The interaction of IGFBP-5 with VN differs from that with other ECM components in that residues 131–141, lying within the central domain of IGFBP-5, are also involved in binding VN. In this respect, this binding interaction resembles that between an acid-labile subunit and IGFBP-5 [130]. It therefore appears that differences exist between the binding sites on IGFBP-5 for different ECM components and that this may effect the ability of IGFBP-5 to enhance or inhibit IGF-I action. Independent confirmation of the association of IGFBP-5 with VN has been reported [131], along with confirmation that tripartite complexes of VN–IGFBP-5–IGF-I are required to demonstrate IGFBP-5 enhancement of IGF-I-stimulated migration of the human breast cancer cell line MCF-7 when cells are grown on a substratum of VN. Recently, it has also been demonstrated that VN–IGFBP-5–IGF-I complexes can enhance protein synthesis and cell migration in human keratinocytes [132]. Many ECM proteins (including TSP-1, OPN and VN) bind to the integrin αVβ3, and there is evidence that this receptor must be occupied to allow cells to respond to IGF-I [133]. There are also studies in vitro and in vivo which use small non-peptide inhibitors of ligand binding to αVβ3 that inhibit IGF-I-stimulated DNA synthesis and migration of smooth-muscle cells [134]. This phenomenon is associated with hypophosphorylation of IGF-IR, and an attenuation of IGF-I-induced IGFBP-5 synthesis is also evident.
IGFBP-5 has also been reported to bind the ECM protein PAI-1 (plasminogen activator inhibitor-1) [135]. This protein plays an important role in regulating protease activity during ECM remodelling. The protein was identified following affinity chromatography of human fibroblast-conditioned medium on an Affigel column containing immobilized peptide 201–218 from IGFBP-5. Confirmation of binding to IGFBP-5 was obtained by co-immunoprecipitation studies using purified proteins. A mutagenic analysis of the interacting region of IGFBP-5 with PAI-1 indicated a 71% decrease in binding of the mutant R201A/K202N/K206N/K208N. This same mutant showed only minimally reduced GAG binding [136], indicating, once again, subtle differences in the nature of the binding sites on IGFBP-5 for ECM components. As for TSP-1, VN and OPN, PAI-1 complexation with IGFBP-5 did not affect the affinity of the latter molecule for IGF-I. The KD of PAI-1 for IGFBP-5 itself was reported at 90 nM, which is less than that of other ECM protein interactions with IGFBP-5. PAI-1 is a constituent of ECM preparations isolated from cultures of fibroblasts and is also produced by smooth-muscle cells, where it has an important role in regulating plasminogen activation and can control processes such as wound repair and ECM remodelling. IGFBP-5 has been shown to inhibit the activity of PAI-1 in an IGF-independent manner [135,137], causing an increase in plasmin activity, which in turn may have important consequences for the processes of wound repair and tissue remodelling.
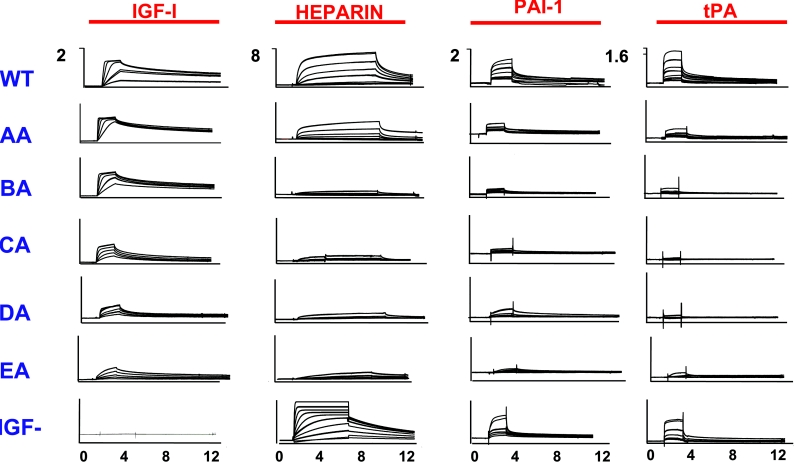
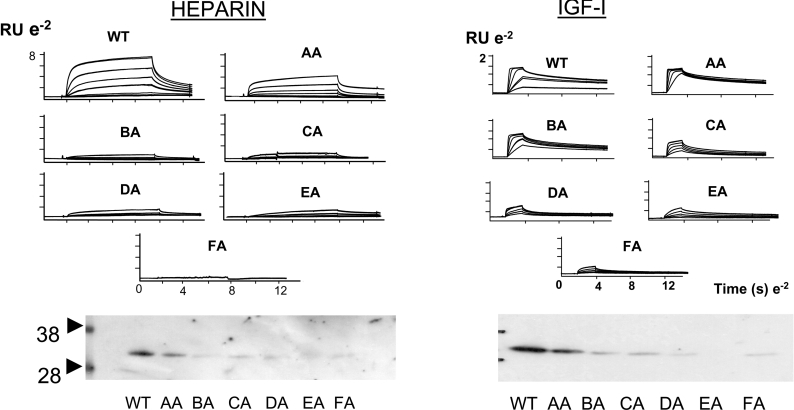
The kinetics and affinities of complex formation between biomolecules plays an important role in the biological activities of such complexes. In recent years, the technique of SPR (surface plasmon resonance) has been used to examine the kinetics and affinities of interaction between different biomolecules [52,138,139]. This technology, in which one partner of a binding pair is immobilized (usually covalently) on to the surface of a biosensor chip while a solution of interacting partner is passed across the chip surface has been used to probe the kinetics and affinities of interaction between intact and fragmented IGFBPs and IGF polypeptides [50,123,140,141] and has provided useful information on those regions within the IGFBPs which are important for binding IGF-I and IGF-II. We have discussed above the interaction of IGFBP-5 with various components of the ECM, and, in an attempt to gain more insight into the nature of these interactions, we have used the technique of SPR to probe these events in more detail. We have carried out an extensive analysis of the interaction of IGFBP-5 with immobilized IGF-I, heparin, PAI-1 and tPA (tissue plasminogen activator) surfaces. Such data are reported in the form of sensorgrams (Figure 5) which profile in real time the association of analyte (IGFBP-5) with immobilized ligand (IGF-I, heparin, PAI-1 and tPA respectively). Examination of the binding profiles allows both quantitative and qualitative information to be derived in relation to rate constants (Ka and Kd) and equilibrium constants (KD). For IGF-I, the KD for IGFBP-5 (derived as Kd/Ka) is approx. 0.2 nM, which agrees very closely with the values derived previously using solution-phase equilibrium-based binding methods. Inspection of the binding curves for the IGF-I–IGFBP-5 interaction suggest that the high binding affinity seen for this interaction is largely the result of the slow dissociation rate of the IGF-I–IGFBP-5 complex. For the interaction of IGFBP-5 with heparin, PAI-1 or tPA, it is clear that the binding complexes in each instance show a much more rapid dissociation than for the IGF-I–IGFBP-5 complex. Under these conditions, the binding complex reaches equilibrium more rapidly (plateau region on sensorgrams), and the equilibrium constant KD can be derived from a standard binding isotherm plot of analyte concentration against equilibrium response. This method indicates that the affinity of these ECM components for IGFBP-5 is ∼10–100-fold lower than for the IGF-I–IGFBP-5 complex and agrees with previous reports where these affinities have usually been determined by solution equilibrium-based methods [124a,126]. Together with the observation that the faster dissociation of these complexes leads to a more dynamic molecular interaction, these data can inform on the distribution of IGFBP-5 between IGF-I and ECM components. In relation to heparin, we have demonstrated that binding of this GAG and IGF-I to IGFBP-5 is mutually exclusive and that tripartite complexes of IGF-I–IGFBP-5-heparin do not form [141a]. We postulated therefore that free IGF-I may displace IGFBP-5 which is pre-bound to ECM structures, and indeed previous studies had demonstrated that addition of IGF-I to cultures of human fibroblasts was able to displace IGFBP-3 into the medium presumably from its location in ECM or cell membrane components [75,142]. We have shown subsequently the same effect for IGFBP-5 when IGF-I is added to monolayer cultures of the mouse mammary epithelial cell line HC11 [141a]. To investigate further the relationship between IGF-I and heparin-binding sites, an extensive series of mutagenesis studies have been undertaken [37,47,123,143–145]. Initially, it was shown that individual mutation of two highly conserved residues Gly203 and Gln209 within the 201–218 C-terminal heparin-binding domain of IGFBP-5 caused a 6–8-fold decrease in affinity for IGF-I and combined mutagenesis of these two residues resulted in a 10–30-fold reduction in affinity [47,144]. Although the Gly203 and Gln209 mutants did not show a decrease in heparin binding, a protein containing mutants in the same location, namely R201L/K202E/K206Q/R214A, had greatly reduced heparin binding [145]. This led the authors to suggest that the IGF-I- and heparin-binding sites on IGFBP-5 may overlap and provided a rationale for the failure to observe tripartite complexes of IGF-I–IGFBP-5–heparin in biosensor studies and also for the ability of IGF-I to displace heparin from preformed complexes of IGFBP-5–heparin and also to release IGFBP-5 from the ECM as a soluble species [145]. This hypothesis was supported following a cumulative mutagenesis analysis of the 201–218 region of IGFBP-5 which demonstrated a parallel loss in IGF-I and heparin binding in mutant proteins [123] (Figure 6). The loss of IGF-I affinity in the series of IGFBP-5 mutants was also reflected in the loss of their ability to inhibit the survival activity of IGF-I when added simultaneously to cultures of MCF-7 cells (M. Park, J. Shand, E. Tonner, M. Gamberoni, P. Accorsi, J. Beattie, G. J. Allan and D. J. Flint, unpublished work). The kinetics of the complexes formed between mutant IGFBPs and IGF-I were important in this respect with inhibitory activity associated with IGFBPs which formed more stable complexes as determined by lower dissociation constants. If IGFBP-5 is co-incubated with IGF-I and substratum, it enhances the activity of growth factor following addition of MCF-7 cells. Unlike the inhibitory effects of IGFBP-5, the affinity of mutants for IGF-I was not predictive of the ability of the proteins to enhance growth-factor activity. Instead, enhancing activity was related to the ability of complexes to dissociate from substratum and also the ability of IGF-I to dissociate from IGFBP-5 bound to substratum. Non-IGF-binding mutants did not enhance or inhibit IGF activity.
Figure 5. Biosensor analysis of WT, AA–EA and IGF− mutant IGFBP-5 binding to immobilized IGF-I, heparin, PAI-1 or tPA.
Details of mutant proteins are described in [123] and biosensor methodology is described in detail in [141a]. The IGF− mutant protein was originally described by Imai et al. [46] and shows no detectable binding to IGF-I, but has unimpaired binding to heparin, PAI-1 and tPA.
Figure 6. Analysis of the binding of wild-type (WT) and mutant (AA–FA) IGFBP-5 species to heparin and IGF-I was analysed by biosensor (upper panel) and blotting methodologies (lower panel).
See [123] for details of the IGFBP-5 mutants. Note the parallelism in the loss of affinity for both IGF-I and heparin with cumulative mutagenesis of positively charged residues within the region 201–218 of IGFBP-5. RU=resonance units; e−2=10−2; molecular-mass sizes are indicated in kDa. See the text for further details. Reproduced from Endocrinology, ‘Cumulative mutagenesis of the basic residues in the 201–218 region of insulin-like growth factor (IGF)-binding protein-5 results in progressive loss of both IGF-I binding and inhibition of IGF-I biological action' by G. J. Allan, E. Tonner, M. Szymanowska, J. H. Shand, S. M. Kelly, K. Phillips, R. A. Clegg, I. F. Gow, J. Beattie and D. J. Flint 147(1), pp. 338–349, with permission. © 2006, The Endocrine Society.
Clearly many factors will be involved in determining the distribution of IGFBP-5 between IGFs and various components of the ECM and other IGFBP-5-binding proteins. These include issues such as affinity and stability of binding complexes, local concentrations of different binding partners, conformationally allowable complex formation, proteolysis of IGFBP-5 and also the presence and concentration in the local pericellular environment of other GAG-binding IGFBPs. In addition, the activity of IGFBP-5 may depend on the nature of the ECM in the local environment [146]. Obtaining a full molecular profile at the level of the cell for all of these potential molecular complexes (especially in real time) is clearly a challenging task. Nonetheless, without this detailed information, interpretation of the observed biological effects of IGF at a cellular level may be problematic.
Although the majority of studies have examined the interaction of IGFBP-5 with GAGs or protein components of the ECM, there are reports of interaction of IGFBP-5 with other structures and proteins which are not associated with the ECM. For example, IGFBP-5 has been shown to bind the inorganic bone matrix hydroxyapatite [83]. This interaction differs from those described previously in two respects. First, the binding of IGFBP-5 to hydroxyapatite beads is a lower-affinity interaction (KD 0.21 μM). Secondly, binding of IGFBP-5 and hydroxyapatite was actually enhanced by the peptide fragment 201–218. As indicated above, this peptide competed with the binding of IGFBP-5 to GAG and protein components of the ECM. Also somewhat unusually, the paper by Campbell and Andress [83] reported that peptide 201–218 itself bound to intact IGFBP-5 and increased its affinity for IGF-I 3-fold. This observation has not been reported in other laboratories, although the authors of this paper postulate that binding of this peptide to IGFBP-5 may be related to various anecdotal reports of the ability of IGFBP-5 to dimerize [83]. IGFBP-5 has also been reported to bind αs2 casein micelles [137], and these structures, like hydroxyapatite, contain a high concentration of calcium phosphate. Using a yeast two-hybrid screen, IGFBP-5 has been shown to interact with the transcriptional co-activator protein FHL-2 (four-and-a-half LIM domain 2) [147], although this study did not propose a functional role for the interaction. Confirmation of this binding interaction was demonstrated when both proteins were overexpressed in the U-2 human osteosarcoma cell line and they were shown to co-localize in the cell nucleus. It remains to be seen whether the proteins interact under normal physiological conditions. Interestingly, IGFBP-5 has also been shown to localize in the nucleus of vascular smooth-muscle cells, and there is some evidence that it may possess transcriptional activity when fused to the DNA-binding domain of Gal4 [148]. Whether IGFBP-5 acts as a transcriptional co-activator along with FHL-2 remains to be determined. The yeast two-hybrid screening methodology has also demonstrated the association of IGFBP-5 and RASSF1C (isoform C of the Ras association family 1 protein group) [149]. Another study demonstrated that IGFBP-5 increased the phosphorylation status of ERK1/2 in mouse osteoblasts and also increased cell proliferation [150]. The observation that siRNA (small interfering RNA) directed towards RASSF1C mRNA inhibited this effect of IGFBP-5 led the authors to conclude that the effects of IGFBP-5 on ERK1/2 phosphorylation were mediated by RASSF1C [149]. This interesting area is worthy of further investigation.
IGFBP-5 AND CANCER
Mammary gland
As both IGF-I and IGF-II have well described proliferative actions in several cell types, many research groups have investigated the role of both growth factors in the aetiology and progression of various tumours. The largest body of work in this area has examined different models of mammary cancer including work in breast cancer cell lines, primary cultures and animal models of mammary cancer.
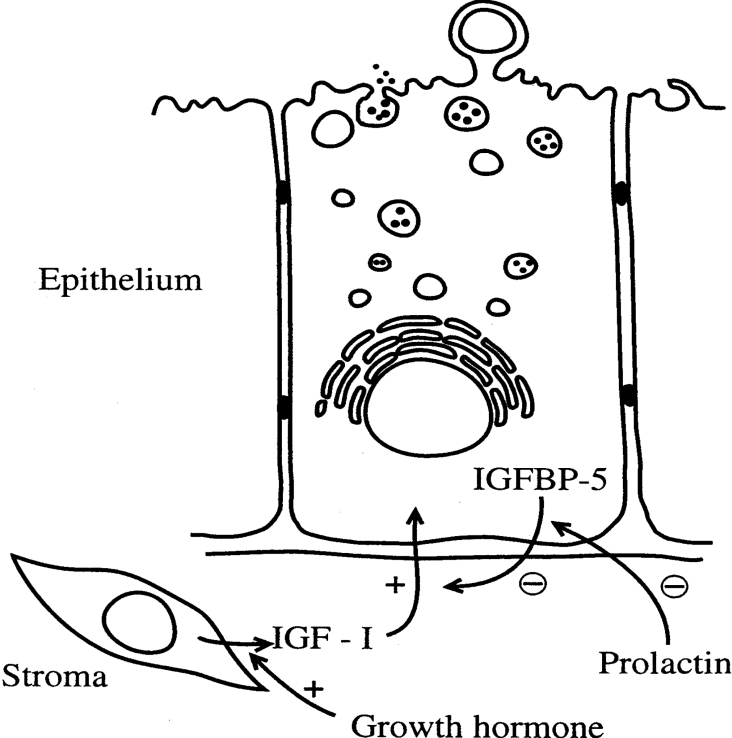
The mammary gland undergoes cycles of growth, differentiation and regression that are associated with cycles of pregnancy, lactation and involution. In this process, mammary epithelial cells undergo cell division and differentiation to secrete milk components and are then reduced in number by the process of apoptosis at the end of lactation. As such, an examination of the regulation of this process of cell division and death in the gland under normal physiological conditions may aid in an understanding of the factors that are involved in the uncontrolled process of epithelial cell division that is characteristic of tumorigenesis. It is well established that the IGF axis plays an important role in the regulation of mammary epithelial cell function [3,151,152], and, depending on the cellular context, IGFBP-5 can have both inhibitory and enhancing effects on IGF-I activity in the gland. As has been discussed above, IGFBP-5 interacts with various ECM constituents, undergoes proteolysis and is regulated by hormones which are also important in controlling mammary gland function. These features have significance when the role of IGFBP-5 in normal and abnormal mammary epithelial cell function has been investigated. In normal mammary gland, one of the most interesting recent observations has been increased IGFBP-5 expression during the early stages of apoptosis [137,153–156]. Involution of the mammary gland can be achieved by withdrawal of pituitary hormone (GH and prolactin) support [156], by litter removal or by teat sealing the gland [155]. In each of these conditions, IGFBP-5 expression is increased within 24 h. This is before major structural changes in the gland are seen, suggesting that the protein may play an early role in cellular apoptosis and mammary gland regression. Using transgenic mice that express IGFBP-5 under the control of a β-lactoglobulin promoter, Tonner et al. [154] demonstrated that IGFBP-5 was up-regulated specifically in the mammary gland during pregnancy and lactation. Phenotypically, these animals showed abnormal mammary gland development and demonstrated reduced cellular proliferation rates and increased cell death. These differences were most apparent at parturition and early lactation, when IGFBP-5 levels increased sharply. The mammary epithelial cell has been confirmed as the location of IGFBP-5 synthesis and secretion [153,155,157], and it is postulated that the binding protein is secreted from the epithelial cell around parturition and gains access to the basal aspect of the epithelial cell surface through the leaky tight junctions which exist between the cells at this time (Figure 7). In this location, excess IGFBP-5 can neutralize IGF-I secreted by stromal cells in the tissue and thus induce apoptotic activity in the gland. In support of this theory, long R3-IGF-I (an IGF-I analogue which has low affinity for IGFBPs) partially restored lactation in transgenic animals. At approx. 48 h after parturition, tight junctions between mammary epithelial cells are less permeable, and this may be the reason for partial restoration of function in the gland of transgenic animals at this time. Furthermore, addition of IGFBP-5 to primary cultures of mouse mammary epithelial cells and to the FSK-7 mouse mammary epithelial cell line inhibits IGF-I activation of IRS-1 (IR substrate-1), protein kinase B and FKHRL-1 (forkhead in rhabdomyosarcoma-like 1), providing direct evidence for the ability of IGFBP-5 to act as an apoptotic factor by inhibiting the action of local IGF-I [153]. Although increased IGFBP-5 levels around parturition were associated with epithelial cell apoptosis, it should be noted that IGFBP-5 mRNA is also present in epithelial cells in mid- to late pregnancy, when the cells are undergoing division and differentiation and IGFBP-5 is also secreted by these cells in culture [158]. Lactogenic hormones stimulate IGFBP-5 secretion in both the HC11 mouse mammary epithelial cell line [158a] and in primary cultures of mammary epithelial cells grown on EHS (Engelbreth–Holm–Swarm) matrix [158b]. In addition, microarray analysis of selected gene expression in virgin, pregnant, lactating and involuting mouse mammary gland indicates that IGFBP-5 expression is elevated both in mid-pregnancy and in early involution [159,160]. As indicated above, IGFBP-5 contains a progesterone-responsive element within the proximal promoter, and it has been suggested that progesterone may maintain elevated IGFBP-5 levels during pregnancy [62]. It is therefore possible that IGFBP-5 may also have a differentiating activity in the mammary gland and that the activity of the protein may depend on local IGF-I/IGFBP-5 ratios. In support of this, IGFBP-5 has been reported to have differentiation activity in other cell culture systems [24,161,162].
Figure 7. Stromal cell derived IGF-I and epithelial cell-derived IGFBP-5 interact to regulate mammary epithelial cell function.
During lactation, IGF-I maintains secretory function in the mammary epithelial cell. At this stage, the activity of the pituitary hormones GH (stimulating IGF-I secretion) and prolactin (inhibiting IGFBP-5 secretion) act to maintain a high IGF-I/IGFBP-5 ratio. During mammary gland regression and apoptosis of epithelial cells, IGFBP-5 concentrations increase and neutralize the survival factor activity of IGF-I. See the text for further details.
With this information on the potential role of IGFBP-5 in normal mammary gland apoptosis and differentiation, it is instructive to examine the work which has focused on the expression and function of the protein in mammary cancer models. These studies have used both primary cultures and breast cancer cell lines from human and rodent species. In addition, a few in vivo studies have been performed. Among the first tasks of early researchers was to profile the IGFBP species which were expressed in mammary tumorigenic cell lines. In contrast with the case with normal mammary epithelial cell function, this is a large body of literature, and here we discuss only the most salient observations from these data with respect to IGFBP-5. The first demonstration of the expression and secretion of IGFBP-5 by human breast cancer cell lines came in 1992 when Sheikh et al. [163] reported the presence of IGFBP-5 mRNA and protein expression in four ER (oestrogen receptor)-positive cell lines (MCF-7, T47D, ZR75 and BT474) and also in two ER-negative cell lines (Hs578T and MDA-MB-468). IGFBP-5 expression was not found in a third ER-negative cell line (MDA-MB-231). It was also reported that IGF-I increased IGFBP-5 levels (both mRNA and protein) in cultures of MCF-7 cells [164]. Enhanced IGFBP-5 expression was also evident following treatment of cells with OE2 (oestradiol), and combined treatment with IGF-I and OE2 resulted in a synergistic effect on IGFBP-5 expression. As IGF-I and OE2 also had synergistic effects on cell growth, it was deemed unlikely that the induced IGFBP-5 inhibited the stimulatory activity of IGF-I. This differs somewhat from the conclusions drawn regarding the pro-apoptotic functions of IGFBP-5 in involuting mammary gland, although no indication of the concentrations of IGFBP-5 achieved in cultures of MCF-7 cells was given. In a subsequent study, Dubois et al. [165] reported IGFBP-5 secretion by an invasive subclone of MCF-7 (MCF-7/6), whereas IGFBP-5 protein was undetectable in an non-invasive MCF-7 subclone (MCF-7/AZ). Both cell lines were ER-positive, and the differential pattern of IGFBP-5 secretion suggested that the binding protein may play a role in determining the phenotype of these subclones, although experiments designed to examine this hypothesis were not reported. IGF-I was also shown to stimulate IGFBP-5 secretion in the ER-negative cell line MDA-MB-468 [166], although there was no effect on IGFBP-5 mRNA expression, suggesting regulation at a post-transcriptional level in this particular cell line. In support of this finding, it was shown that IGF-I regulation of IGFBP-5 levels in the T47D cell line was partly due to the ability of the growth factor to bind IGFBP-5 and protect the protein from proteolysis [78]. This phenomenon has been reported elsewhere in this review and was confirmed by the report that IGF-I analogues which had reduced affinity for IGFBP-5 had decreased ability to effect IGFBP-5 levels [78]. Treatment of T47D cells with an anti-IGF-IR blocking monoclonal antibody attenuated the effect of the growth factor, demonstrating that IGF-I also had effects which were dependent on interaction with the IGF-IR. As IGF-I did not influence the levels of IGFBP-5 mRNA in these cells, the authors proposed that this receptor-dependent effect of IGF-I may be to regulate the expression of an IGFBP-5 protease [78]. IGF-I has also been reported to displace IGFBP-5 from ECM secreted by a mammary cell line [119] and this may be another non-receptor-mediated route by which IGF-I increases levels of soluble IGFBP-5 in cell-conditioned medium.
Of special interest was the observation that medroxyprogesterone acetate and two anti-oestrogens (RU486 and Org2058) inhibited the growth of T47D cells [167]. As these compounds decreased IGFBP-5 levels, the somewhat counterintuitive deduction was made that IGFBP-5 may act as a potential growth promoter in these cells in much the same way that IGFBP-5 has been reported to directly promote osteoblast cell growth [150]. However, subsequent reports [168–172] in which IGFBP-5 mRNA and protein levels in MCF-7 cell cultures were increased by treatment with either anti-oestrogens or vitamin D3-related compounds demonstrated that this alteration in the IGFBP-5 profile was associated with inhibition of cellular growth. In addition, treatment of MCF-7 cells with an IGFBP-5 antisense oligonucleotide decreased IGFBP-5 accumulation and resulted in an enhancement of basal DNA synthesis. Confirmation of the effects of vitamin D3 were provided by the demonstration that it could reduce the level of IGF-I-stimulated tyrosine phosphorylation of the signalling intermediates IRS-1 and -2 in MCF-7 cells [170]. In addition, IGFBP-5 may sensitize breast cancer cells to the growth-inhibitory properties of other agents [173]. It appears therefore that the regulation of IGFBP-5 expression and subsequent effects on cell growth in breast cancer cells is cell-line-dependent.
IGFBP-5 mRNA and protein was also reported in breast cancer tissues and in primary cultures of breast cancer cells [174–177]. For example, Pekonen et al. [177] using RT (reverse transcription)–PCR detected IGFBP-5 mRNA in each of 47 human breast tumour samples and reported that tumour tissue had higher levels of binding protein than the surrounding normal tissue. IGFBP-5 protein was also detected by ligand blotting in 80 breast cancer specimens [176], and the binding protein was found in the stromal compartment in the N-nitrosourea- and DMBA (7,12-dimethylbenz[a]anthracene)-induced rat mammary tumour models [174,175]. In the study by Manni et al. [175], IGFBP-5 was reported in the stromal compartment of the gland. The overwhelming body of evidence indicates that IGFBP-5 is expressed in mammary epithelial cells, and therefore the presence of IGFBP-5 in the stromal compartment may be indicative of epithelial–stromal cell communication.
It is only recently that functional studies on IGFBP-5 activity in breast cancer have been addressed. Working with the ER-negative human breast cancer cell line Hs578T, Perks et al. [178] reported that exogenously added IGFBP-5 could inhibit apoptosis induced by the ceramide analogue C2 or by the integrin-binding tripeptide RGD (Arg-Gly-Asp). As Hs578T cells do not express the IGF-IR, and as it was also demonstrated that a non-IGF-binding mutant of IGFBP-5 was able to attenuate C2-induced apoptosis in these cells, this suggested that the effects of IGFBP-5 were IGF-independent. Therefore, in this cell culture model, IGFBP-5 was viewed perhaps somewhat unusually as a survival factor. Further studies from this group [179] using inhibitors of cell signalling pathways suggested that IGFBP-5 exerted its cell-survival activity through the intracellular kinases PKC (protein kinase C) and sphingosine kinase which regulate the balance between ceramide and the survival factor sphingosine 1-phosphate [180]. McCaig et al. [179] also demonstrate that IGFBP-5 attenuates apoptosis in the IGF-responsive cell line MCF-7. However, the findings are somewhat complicated by the observation that the non-IGF-binding IGFBP-5 mutant inhibited the ability of IGF-I to confer protection against C2-induced apoptosis in this cell line, suggesting that the protective effect of IGFBP-5 was, at least in part, due to its ability to bind IGF-I. The ability of the non-IGF-binding mutant IGFBP-5 to block IGF-I survival action in MCF-7 cells was postulated to be the result of IGFBP-5-mediated disruption of the interaction between ECM components and cell-surface integrin receptors and, elsewhere in this review, we have indicated that appropriate occupancy of these receptors is required for IGF-I to exert its biological actions.
Given an excess of soluble IGFBP-5, all endogenous IGF-I would be sequestered and thus the net effect on cell survival would depend on the IGFBP-5/IGF-I ratio. In an experimental sense, it is difficult to conceive how one might differentiate between the contribution of IGFBP-5 and IGF-I to cell survival in such an IGF-I-responsive cell line. Nonetheless, these observations bear a striking similarity to the proposal of dual action of IGFBP-5 in normal mammary gland, where apoptotic function and enhancing/differentiating activity may be regulated by local IGFBP-5/IGF-I ratios.
Inhibition of growth and DNA synthesis has been observed in MDA-MB-231 and Hs578T cells transfected with IGFBP-5 and cell-cycle arrest at the G2/M transition was also demonstrated [173]. Caspase-dependent apoptosis was described in transfected cells and IGFBP-5 has been shown to activate both caspase 8 and caspase 9 [173]. Tumours developed by injection of transfected MDA-MB-231 cells into nude mice were smaller and of lower incidence than tumours derived by injection of control vector-transfected cells. As Hs578T cells do not respond to IGF-I and as growth of MDA-MB-231 cells was not affected by a specific monoclonal antibody which blocks the activity of the IGF-IR, the effects of IGFBP-5 in the two cell lines appeared to be IGF-independent [173]. The authors demonstrated that the addition of intact or proteolytic fragments of IGFBP-5 did not affect apoptosis in these cells, indicating that the mode of action of IGFBP-5 was probably via intracrine mechanisms [173,181]. IGFBP-5 contains a classical bipartite nuclear localization signal in the 201–218 region of the protein and, in transfected cells, it was found in both nuclear and cytoplasmic compartments, although nuclear transport of IGFBP-5 was required for activity of the protein [173].
It is apparent that, for breast epithelial tumour cells, there are contradictory findings in relation to enhancing or inhibitory effects of IGFBP-5. Although some of these contradictions can be explained by features such as cell- and context-specific effects, it is clearly important that further experiments are conducted into IGFBP-5 action in breast cancer. In addition, it is clear that IGFBP-5 may exert its effects through multiple mechanisms. These mechanisms can include IGF-dependent and -independent effects. They may also be contingent on the regulation of IGFBP-5 secretion by breast cancer cells and tissue by steroid hormones and other circulating hormones and growth factors (e.g. prolactin, GH, insulin and IGF-I itself). The role of IGFBP-5 in breast cancer may be modified by proteolysis and/or the association of IGFBP-5 with the ECM and other proteins. These areas pose the most challenging tasks for future research.
Prostate
The importance of the IGF axis in prostate biology has been clearly established [182], and IGF-I has been shown to regulate the growth and division of both normal and neoplastic prostate epithelial cells [183]. These results, together with the report of a strong positive correlation between serum IGF-I levels and subsequent risk of prostate cancer [183a], has led to an examination of the role of several members of the IGF axis in benign and malignant hyperplasia of the prostate. The role of the IGF axis (principally IGFBPs) in either pharmacological- or castration-induced involution of the prostate has also been investigated. Initial studies described an increase in IGFBP-5 mRNA expression in stromal cells derived from benign hyperplastic prostate tissue from the low levels that exist in normal prostate tissue [73]. In subsequent studies, IGFBP-5 expression was reported to be highest in stromal cell cultures derived from the trans-urethral region of the prostate gland [184]. In addition, IGFBP-5 is also expressed in the prostate cancer cell line PC-3 [186,187]. An in situ hybridization and immunohistochemical study of IGFBP-5 expression in benign and malignant human prostate tissue indicated an increase in IGFBP-5 protein in epithelial cells derived from malignant prostate [185]. However, in situ hybridization studies indicated that IGFBP-5 mRNA expression was restricted to stromal cells, and these authors suggested that IGFBP-5 released by stromal cells may associate with epithelial cells and provide a route for intercellular communication [185]. This was analogous to the situation described in the mammary gland, although, in this tissue, IGFBP-5 expression is believed to be restricted to epithelial cells. Several studies have reported on the effects of prostate regression (achieved by castration or inhibition of testosterone 5α-reductase) on IGFBP-5 expression. Usually, IGFBP-5 expression was increased in involuting prostate, irrespective of the method by which involution was achieved [188–191] and such findings are similar to those described for the involuting mammary gland, where IGFBP-5 expression was also increased [154]. Unlike the mammary gland, however, substantial evidence for a causative role for IGFBP-5 in inducing apoptosis in prostate cells, and thus involution of the gland is largely lacking. Indeed, in some studies, it has been shown that there is a large temporal dissociation between the appearance of apoptotic cells in the involuting prostate and peak levels of IGFBP-5 expression in the tissue [191]. In addition, some immunohistochemical data indicated that IGFBP-5 did not co-stain with apoptotic cell markers in histological sections of involuting prostate [191]. Also, in contrast with studies in involuting mammary gland, expression of other IGFBPs was increased in involuting prostate [189,190]. These observations therefore cast some doubt as to the role of IGFBP-5 in apoptosis of involuting prostate.
In contrast with the involuting prostate, recent studies with prostate tumour cells have suggested a growth-enhancing effect of IGFBP-5 [192–194]. For example, stable transfection of IGFBP-5 in the prostate tumour cell line LNCaP resulted in faster growth of cells compared with vector-transfected controls [194]. Apoptosis was induced by androgen withdrawal in both transfected and untransfected cells, but IGF-I rescued only transfected cells from apoptosis [194]. This implied that IGFBP-5 in some way enhanced the anti-apoptotic action of IGF-I. In confirmation of this hypothesis, the same group demonstrated that tumours derived from LNCaP cells stably transfected with IGFBP-5 proceed more rapidly to androgen-independent growth following castration of host rats [193]. Treatment of Shionogi prostate tumour cells with antisense oligonucleotides to IGFBP-5 inhibited the growth of these cells compared with control vector-transfected cells, again implying a stimulatory effect for IGFBP-5 [193]. Such dual behaviour of IGFBP-5, i.e. up-regulated during apoptosis, but also implicated in stimulation of cellular growth and differentiation, is again reminiscent of the profile and behaviour of this protein in the mammary gland and in other tissues. Although IGFBP-5 expression is increased during apoptosis in prostate and mammary gland, an isolated report shows down-regulation of IGFBP-5 during apoptosis in rat cerebellar granule cells [195]. The overwhelming evidence, however, suggests that IGFBP-5 is up-regulated during apoptosis in cells of epithelial origin. It is somewhat disappointing that there has been little research published in the last 5 years with respect to IGFBP-5 and prostate gland function. Given the high incidence of benign and malignant hyperplasia of the prostate, it is an area that is worthy of further research.
Ovary
Ovarian granulosa cells express IGFBP-5 [196], and the expression of IGFBP-5 has also been reported in ovarian cancer cell lines [197,198]. Although the expression of IGFBP-5 is known to be associated with follicular atresia in the rat ovary [40], there are little data regarding the role of IGFBP-5 in either a growth-stimulatory or -inhibitory role in ovarian cells. One study indicated that IGFBP-5 inhibited gonadotropin-releasing hormone-stimulated growth of a human ovarian adenocarcinoma cell line, and that this effect may have been due to the sequestration of endogenously produced IGF-II [198a]. In general, however, this is a much neglected area, and further research into the role of the IGF axis in ovarian carcinomas is required.
Other cancers
Using techniques such as RT–PCR, microarray analysis, in situ hybridization and immunohistochemistry, the presence of IGFBP-5 in numerous tumours and cancer cell lines has been reported [67,68,199–209]. Although the pattern of IGFBP-5 expression in these cell lines varies greatly, in most tumour cells, levels of IGFBP-5 mRNA and protein were increased [207,208]. This led to the suggestion that increased levels of IGFBP-5 may either enhance the action of locally available IGF-I, leading to a stimulation of the process of tumorigenesis or, conversely, that IGFBP-5 may inhibit the action of local IGFs and attenuate tumour progression. Unfortunately, supporting experimental evidence for either of these hypotheses is largely absent in these studies. Schneider et al. [202] did report that transfection and overexpression of IGFBP-5 in mouse osteosarcoma cells resulted in a reduced basal proliferation of cells and also a reduced cellular growth response in the presence of IGF-I, suggesting both an IGF-dependent and an -independent route of action for IGFBP-5. It was also reported that addition of exogenous IGFBP-5 to cultures of a human papilloma virus-negative cervical cancer cell line decreased the viability of the cells [210]. Conversely, the silencing of IGFBP-5 expression in two neuroblastoma cell lines using micro-RNA interference inhibited both the growth and differentiation of these cells [61]. In essence, however, a larger body of experimental work is required to establish a functional role for IGFBP-5 in the regulation of tumour cell biology.
CONCLUSIONS
There has clearly been a great deal of progress in research into the structure and function of IGFBP-5 since the protein was first cloned 14 years ago. However, there are still some large gaps in our knowledge in relation to the properties of this protein. A description of the three-dimensional structure of IGFBP-5 (and other IGFBPs) is still awaited. Achievement of this goal would help in the interpretation of IGFBP-5 activity and may go some way to explaining the complicated biological actions of the protein. Similarly, a concentrated effort needs to be made to dissect further the IGFBP-5 promoter. Relatively few transcription factors have been identified as interacting with the promoter, and it would be particularly interesting to investigate factors which might be active during cellular apoptosis. Footprinting IGFBP-5 promoter regions with nuclear extracts from cells undergoing apoptosis may prove to be a useful initial strategy. In addition, it is highly likely that other IGFBP-5 proteases will be reported in the near future. More information about the sites of proteolysis and the potential activity of proteolytic fragments would be especially useful. It is also likely that new proteins or other biomolecules which interact with IGFBP-5 will be reported. This may lead to a re-evaluation of the biological properties of the protein. In this regard, the recent demonstration of the nuclear location of IGFBP-5 and its potential to interact with other nuclear proteins is particularly interesting. In general, the mechanisms of action of IGFBP-5 also require clarification. To non-experts in the field, these can appear confusing. A distillation of some of the mechanisms proposed in this review show that IGFBP-5 may act in any one (or combination) of the following mechanisms: (i) through an IGF-independent action in IGF-null cells via regulation of the ceramide (and potentially other signalling pathways); (ii) through IGF-independent interactions with ECM–integrin-binding events acting to promote cell detachment and apoptosis; (iii) by simultaneous association with ECM and IGFs to provide a pool of growth factor in the vicinity of IGF receptors and thus enhance their activities; (iv) through an excess of soluble binding protein acting to sequester IGF-I and thus inhibit the activity of the growth factor.
Clearly such list of potential mechanisms is not exhaustive (see below), and more than one mechanism may operate at the individual cellular level at any one time. In addition, such effects are going to be influenced by the family of IGFBP-5 proteases, by the regulation of IGFBP-5 expression (e.g. by IGF-I itself) and also by the nature of the ECM in a particular cell and tissue type, by the local concentrations of each of the components in this molecular axis and also by the presence of other IGFBPs. A complete dissection of all these features in the activity of IGFBP-5 is clearly a daunting task; however, it is to be hoped that future research in each of these areas will shed further light on the properties and actions of this important protein.
References
- 1.Gluckman P., Klempt N., Guan J., Mallard C., Sirimanne E., Dragunow M., Klempt M., Singh K., Williams C., Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem. Biophys. Res. Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 2.Valentinis B., Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 2001;54:133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadsell D. L., Bonnette S. G., Lee A. V. Genetic manipulation of the IGF-I axis to regulate mammary gland development and function. J. Dairy Sci. 2002;85:365–377. doi: 10.3168/jds.S0022-0302(02)74083-6. [DOI] [PubMed] [Google Scholar]
- 4.Accili D., Nakae J., Kim J. J., Park B. C., Rother K. I. Targeted gene mutations define the roles of insulin and IGF-I receptors in mouse embryonic development. J. Pediatr. Endocrinol. Metab. 1999;12:475–485. doi: 10.1515/jpem.1999.12.4.475. [DOI] [PubMed] [Google Scholar]
- 5.Khandwala H. M., McCutcheon I. E., Flyvbjerg A., Friend K. E. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr. Rev. 2000;21:215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 6.Salmon W. D., Jr, Daughaday W. H., Jr A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med. 1957;49:825–836. [PubMed] [Google Scholar]
- 7.Daughaday W. H., Parker K. A., Borowsky S., Trivedi B., Kapadia M. Measurement of somatomedin-related peptides in fetal, neonatal, and maternal rat serum by insulin-like growth factor (IGF) I radioimmunoassay, IGF-II radioreceptor assay (RRA), and multiplication-stimulating activity RRA after acid–ethanol extraction. Endocrinology. 1982;110:575–581. doi: 10.1210/endo-110-2-575. [DOI] [PubMed] [Google Scholar]
- 8.Rechler M. M., Nissley S. P., King G. L., Moses A. C., Van Obberghen-Schilling E. E., Romanus J. A., Knight A. B., Short P. A., White R. M. Multiplication stimulating activity (MSA) from the BRL 3A rat liver cell line: relation to human somatomedins and insulin. J. Supramol. Struct. Cell. Biochem. 1981;15:253–286. doi: 10.1002/jsscb.1981.380150305. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld R. G., Hintz R. L. Characterization of a specific receptor for somatomedin C (SM-C) on cultured human lymphocytes: evidence that SM-C modulates homologous receptor concentration. Endocrinology. 1980;107:1841–1848. doi: 10.1210/endo-107-6-1841. [DOI] [PubMed] [Google Scholar]
- 10.Baxter R. C., Martin J. L. Binding proteins for insulin-like growth factors in adult rat serum: comparison with other human and rat binding proteins. Biochem. Biophys. Res. Commun. 1987;147:408–415. doi: 10.1016/s0006-291x(87)80136-5. [DOI] [PubMed] [Google Scholar]
- 11.Binkert C., Landwehr J., Mary J. L., Schwander J., Heinrich G. Cloning, sequence analysis and expression of a cDNA encoding a novel insulin-like growth factor binding protein (IGFBP-2) EMBO J. 1989;8:2497–2502. doi: 10.1002/j.1460-2075.1989.tb08386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkman A., Groffen C., Kortleve D. J., Geurts van Kessel A., Drop S. L. Isolation and characterization of a cDNA encoding the low molecular weight insulin-like growth factor binding protein (IBP-1) EMBO J. 1988;7:2417–2423. doi: 10.1002/j.1460-2075.1988.tb03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin J. L., Willetts K. E., Baxter R. C. Purification and properties of a novel insulin-like growth factor-II binding protein from transformed human fibroblasts. J. Biol. Chem. 1990;265:4124–4130. [PubMed] [Google Scholar]
- 14.Shimasaki S., Shimonaka M., Zhang H. P., Ling N. Identification of five different insulin-like growth factor binding proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human. J. Biol. Chem. 1991;266:10646–10653. [PubMed] [Google Scholar]
- 15.Shimonaka M., Schroeder R., Shimasaki S., Ling N. Identification of a novel binding protein for insulin-like growth factors in adult rat serum. Biochem. Biophys. Res. Commun. 1989;165:189–195. doi: 10.1016/0006-291x(89)91053-x. [DOI] [PubMed] [Google Scholar]
- 16.Rajah R., Katz L., Nunn S., Solberg P., Beers T., Cohen P. Insulin-like growth factor binding protein (IGFBP) proteases: functional regulators of cell growth. Prog. Growth Factor Res. 1995;6:273–284. doi: 10.1016/0955-2235(95)00012-7. [DOI] [PubMed] [Google Scholar]
- 17.Osborne C. K., Coronado E. B., Kitten L. J., Arteaga C. I., Fuqua S. A., Ramasharma K., Marshall M., Li C. H. Insulin-like growth factor-II (IGF-II): a potential autocrine/paracrine growth factor for human breast cancer acting via the IGF-I receptor. Mol. Endocrinol. 1989;3:1701–1709. doi: 10.1210/mend-3-11-1701. [DOI] [PubMed] [Google Scholar]
- 18.Scott C. D., Martin J. L., Baxter R. C. Rat hepatocyte insulin-like growth factor I and binding protein: effect of growth hormone in vitro and in vivo. Endocrinology. 1985;116:1102–1107. doi: 10.1210/endo-116-3-1102. [DOI] [PubMed] [Google Scholar]
- 19.Melmed S., Yamashita S., Yamasaki H., Fagin J., Namba H., Yamamoto H., Weber M., Morita S., Webster J., Prager D. IGF-I receptor signalling: lessons from the somatotroph. Recent Prog. Horm. Res. 1996;51:189–215. [PubMed] [Google Scholar]
- 20.Arai T., Parker A., Busby W., Jr, Clemmons D. R. Heparin, heparan sulfate, and dermatan sulfate regulate formation of the insulin-like growth factor-I and insulin-like growth factor-binding protein complexes. J. Biol. Chem. 1994;269:20388–20393. [PubMed] [Google Scholar]
- 21.Mohan S., Nakao Y., Honda Y., Landale E., Leser U., Dony C., Lang K., Baylink D. J. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J. Biol. Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 22.Clemmons D. R. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr. Rev. 2001;22:800–817. doi: 10.1210/edrv.22.6.0449. [DOI] [PubMed] [Google Scholar]
- 23.Firth S. M., Baxter R. C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M. R., Wolf E., Hoeflich A., Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J. Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- 25.Allander S. V., Larsson C., Ehrenborg E., Suwanichkul A., Weber G., Morris S. L., Bajalica S., Kiefer M. C., Luthman H., Powell D. R. Characterization of the chromosomal gene and promoter for human insulin-like growth factor binding protein-5. J. Biol. Chem. 1994;269:10891–10898. [PubMed] [Google Scholar]
- 26.Kou K., Jenkins N. A., Gilbert D. J., Copeland N. G., Rotwein P. Organization, expression, and chromosomal location of the mouse insulin-like growth factor binding protein 5 gene. Genomics. 1994;20:412–418. doi: 10.1006/geno.1994.1195. [DOI] [PubMed] [Google Scholar]
- 27.White M. E., Diao R., Hathaway M. R., Mickelson J., Dayton W. R. Molecular cloning and sequence analysis of the porcine insulin-like growth factor binding protein-5 complementary deoxyribonucleic acid. Biochem. Biophys. Res. Commun. 1996;218:248–253. doi: 10.1006/bbrc.1996.0044. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Ling N., Shimasaki S. Cloning of the rat insulin-like growth factor binding protein-5 gene and DNA sequence analysis of its promoter region. Biochem. Biophys. Res. Commun. 1993;190:1045–1052. doi: 10.1006/bbrc.1993.1154. [DOI] [PubMed] [Google Scholar]
- 29.Forbes B. E., Turner D., Hodge S. J., McNeil K. A., Forsberg G., Wallace J. C. Localization of an insulin-like growth factor (IGF) binding site of bovine IGF binding protein-2 using disulfide mapping and deletion mutation analysis of the C-terminal domain. J. Biol. Chem. 1998;273:4647–4652. doi: 10.1074/jbc.273.8.4647. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto R., Ono M., Fujiwara H., Higashihashi N., Yoshida M., Enjoh-Kimura T., Sakano K. Binding sites and binding properties of binary and ternary complexes of insulin-like growth factor-II (IGF-II), IGF-binding protein-3, and acid-labile subunit. J. Biol. Chem. 1997;272:27936–27942. doi: 10.1074/jbc.272.44.27936. [DOI] [PubMed] [Google Scholar]
- 31.Kalus W., Zweckstetter M., Renner C., Sanchez Y., Georgescu J., Grol M., Demuth D., Schumacher R., Dony C., Lang K., Holak T. A. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. EMBO J. 1998;17:6558–6572. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann G. M., Bach L. A. The N-terminal disulfide linkages of human insulin-like growth factor-binding protein-6 (hIGFBP-6) and hIGFBP-1 are different as determined by mass spectrometry. J. Biol. Chem. 1999;274:14587–14594. doi: 10.1074/jbc.274.21.14587. [DOI] [PubMed] [Google Scholar]
- 33.Siwanowicz I., Popowicz G. M., Wisniewska M., Huber R., Kuenkele K. P., Lang K., Engh R. A., Holak T. A. Structural basis for the regulation of insulin-like growth factors by IGF binding proteins. Structure. 2005;13:155–167. doi: 10.1016/j.str.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Standker L., Braulke T., Mark S., Mostafavi H., Meyer M., Honing S., Gimenez-Gallego G., Forssmann W. G. Partial IGF affinity of circulating N- and C-terminal fragments of human insulin-like growth factor binding protein-4 (IGFBP-4) and the disulfide bonding pattern of the C-terminal IGFBP-4 domain. Biochemistry. 2000;39:5082–5088. doi: 10.1021/bi992513s. [DOI] [PubMed] [Google Scholar]
- 35.Hodgkinson S. C., Napier J. R., Spencer G. S., Bass J. J. Glycosaminoglycan binding characteristics of the insulin-like growth factor-binding proteins. J. Mol. Endocrinol. 1994;13:105–112. doi: 10.1677/jme.0.0130105. [DOI] [PubMed] [Google Scholar]
- 36.Twigg S. M., Kiefer M. C., Zapf J., Baxter R. C. A central domain binding site in insulin-like growth factor binding protein-5 for the acid-labile subunit. Endocrinology. 2000;141:454–457. doi: 10.1210/endo.141.1.7375. [DOI] [PubMed] [Google Scholar]
- 37.Song H., Shand J. H., Beattie J., Flint D. J., Allan G. J. The carboxy-terminal domain of IGF-binding protein-5 inhibits heparin binding to a site in the central domain. J. Mol. Endocrinol. 2001;26:229–239. doi: 10.1677/jme.0.0260229. [DOI] [PubMed] [Google Scholar]
- 38.Conover C. A., Kiefer M. C. Regulation and biological effect of endogenous insulin-like growth factor binding protein-5 in human osteoblastic cells. J. Clin. Endocrinol. Metab. 1993;76:1153–1159. doi: 10.1210/jcem.76.5.7684391. [DOI] [PubMed] [Google Scholar]
- 39.Olney R. C., Tsuchiya K., Wilson D. M., Mohtai M., Maloney W. J., Schurman D. J., Smith R. L. Chondrocytes from osteoarthritic cartilage have increased expression of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) and -5, but not IGF-II or IGFBP-4. J. Clin. Endocrinol. Metab. 1996;81:1096–1103. doi: 10.1210/jcem.81.3.8772582. [DOI] [PubMed] [Google Scholar]
- 40.Onoda N., Li D., Mickey G., Erickson G., Shimasaki S. Gonadotropin-releasing hormone overcomes follicle-stimulating hormone's inhibition of insulin-like growth factor-5 synthesis and promotion of its degradation in rat granulosa cells. Mol. Cell. Endocrinol. 1995;110:17–25. doi: 10.1016/0303-7207(95)03511-5. [DOI] [PubMed] [Google Scholar]
- 41.Firth S. M., Baxter R. C. Characterisation of recombinant glycosylation variants of insulin-like growth factor binding protein-3. J. Endocrinol. 1999;160:379–387. doi: 10.1677/joe.0.1600379. [DOI] [PubMed] [Google Scholar]
- 42.Marinaro J. A., Neumann G. M., Russo V. C., Leeding K. S., Bach L. A. O-glycosylation of insulin-like growth factor (IGF) binding protein-6 maintains high IGF-II binding affinity by decreasing binding to glycosaminoglycans and susceptibility to proteolysis. Eur. J. Biochem. 2000;267:5378–5386. doi: 10.1046/j.1432-1327.2000.01575.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoeck W. G., Mukku V. R. Identification of the major sites of phosphorylation in IGF binding protein-3. J. Cell Biochem. 1994;56:262–273. doi: 10.1002/jcb.240560220. [DOI] [PubMed] [Google Scholar]
- 44.Jones J. I., D'Ercole A. J., Camacho-Hubner C., Clemmons D. R. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones J. I., Gockerman A., Clemmons D. R. San Antonio, TX, U.S.A.: 74th Annual Endocrine Society Meeting; 1992. Insulin-like growth factor binding protein-5 (IGFBP-5) binds to extracellular matrix and is phosphorylated. 24–27 June, 1992; p. 372. (abstract) [Google Scholar]
- 46.Imai Y., Moralez A., Andag U., Clarke J. B., Busby W. H., Jr, Clemmons D. R. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J. Biol. Chem. 2000;275:18188–18194. doi: 10.1074/jbc.M000070200. [DOI] [PubMed] [Google Scholar]
- 47.Shand J. H., Beattie J., Song H., Phillips K., Kelly S. M., Flint D. J., Allan G. J. Specific amino acid substitutions determine the differential contribution of the N- and C-terminal domains of insulin-like growth factor (IGF)-binding protein-5 in binding IGF-I. J. Biol. Chem. 2003;278:17859–17866. doi: 10.1074/jbc.M300526200. [DOI] [PubMed] [Google Scholar]
- 48.Zeslawski W., Beisel H. G., Kamionka M., Kalus W., Engh R. A., Huber R., Lang K., Holak T. A. The interaction of insulin-like growth factor-I with the N-terminal domain of IGFBP-5. EMBO J. 2001;20:3638–3644. doi: 10.1093/emboj/20.14.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sala A., Capaldi S., Campagnoli M., Faggion B., Labo S., Perduca M., Romano A., Carrizo M. E., Valli M., Visai L., et al. Structure and properties of the C-terminal domain of insulin-like growth factor binding protein-1 isolated from human amniotic fluid. J. Biol. Chem. 2005;280:29812–29819. doi: 10.1074/jbc.M504304200. [DOI] [PubMed] [Google Scholar]
- 50.Headey S. J., Leeding K. S., Norton R. S., Bach L. A. Contributions of the N- and C-terminal domains of IGF binding protein-6 to IGF binding. J. Mol. Endocrinol. 2004;33:377–386. doi: 10.1677/jme.1.01547. [DOI] [PubMed] [Google Scholar]
- 51.Kiefer M. C., Schmid C., Waldvogel M., Schlapfer I., Futo E., Masiarz F. R., Green K., Barr P. J., Zapf J. Characterization of recombinant human insulin-like growth factor binding proteins 4, 5, and 6 produced in yeast. J. Biol. Chem. 1992;267:12692–12699. [PubMed] [Google Scholar]
- 52.Fernandez-Tornero C., Lozano R. M., Rivas G., Jimenez M. A., Standker L., Diaz-Gonzalez D., Forssmann W. G., Cuevas P., Romero A., Gimenez-Gallego G. Synthesis of the blood circulating C-terminal fragment of insulin-like growth factor (IGF)-binding protein-4 in its native conformation: crystallization, heparin and IGF binding, and osteogenic activity. J. Biol. Chem. 2005;280:18899–18907. doi: 10.1074/jbc.M500587200. [DOI] [PubMed] [Google Scholar]
- 53.Carrick F. E., Hinds M. G., McNeil K. A., Wallace J. C., Forbes B. E., Norton R. S. Interaction of insulin-like growth factor (IGF)-I and -II with IGF binding protein-2: mapping the binding surfaces by nuclear magnetic resonance. J. Mol. Endocrinol. 2005;34:685–698. doi: 10.1677/jme.1.01756. [DOI] [PubMed] [Google Scholar]
- 54.Kou K., Mittanck D. W., Fu C., Rotwein P. Structure and function of the mouse insulin-like growth factor binding protein 5 gene promoter. DNA Cell Biol. 1995;14:241–249. doi: 10.1089/dna.1995.14.241. [DOI] [PubMed] [Google Scholar]
- 55.Duan C., Clemmons D. R. Transcription factor AP-2 regulates human insulin-like growth factor binding protein-5 gene expression. J. Biol. Chem. 1995;270:24844–24851. doi: 10.1074/jbc.270.42.24844. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy T. L., Casinghino S., Mittanck D. W., Ji C. H., Centrella M., Rotwein P. Promoter-dependent and -independent activation of insulin-like growth factor binding protein-5 gene expression by prostaglandin E2 in primary rat osteoblasts. J. Biol. Chem. 1996;271:6666–6671. doi: 10.1074/jbc.271.12.6666. [DOI] [PubMed] [Google Scholar]
- 57.Gabbitas B., Pash J. M., Delany A. M., Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-binding protein-5 in bone cell cultures by transcriptional mechanisms. J. Biol. Chem. 1996;271:9033–9038. doi: 10.1074/jbc.271.15.9033. [DOI] [PubMed] [Google Scholar]
- 58.Yeh L. C., Adamo M. L., Duan C., Lee J. C. Osteogenic protein-1 regulates insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding protein-5 (IGFBP-5) gene expression in fetal rat calvaria cells by different mechanisms. J. Cell Physiol. 1998;175:78–88. doi: 10.1002/(SICI)1097-4652(199804)175:1<78::AID-JCP9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Ji C., Chen Y., Centrella M., McCarthy T. L. Activation of the insulin-like growth factor-binding protein-5 promoter in osteoblasts by cooperative E box, CCAAT enhancer-binding protein, and nuclear factor-1 deoxyribonucleic acid-binding sequences. Endocrinology. 1999;140:4564–4572. doi: 10.1210/endo.140.10.7061. [DOI] [PubMed] [Google Scholar]
- 60.Yeh L. C., Lee J. C. Identification of an osteogenic protein-1 (bone morphogenetic protein-7)-responsive element in the promoter of the rat insulin-like growth factor-binding protein-5 gene. Endocrinology. 2000;141:3278–3286. doi: 10.1210/endo.141.9.7643. [DOI] [PubMed] [Google Scholar]
- 61.Cesi V., Giuffrida M. L., Vitali R., Tanno B., Mancini C., Calabretta B., Raschella G. C/EBPα and β mimic retinoic acid activation of IGFBP-5 in neuroblastoma cells by a mechanism independent from binding to their site. Exp. Cell Res. 2005;305:179–189. doi: 10.1016/j.yexcr.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Boonyaratanakornkit V., Strong D. D., Mohan S., Baylink D. J., Beck C. A., Linkhart T. A. Progesterone stimulation of human insulin-like growth factor-binding protein-5 gene transcription in human osteoblasts is mediated by a CACCC sequence in the proximal promoter. J. Biol. Chem. 1999;274:26431–26438. doi: 10.1074/jbc.274.37.26431. [DOI] [PubMed] [Google Scholar]
- 63.Tanno B., Negroni A., Vitali R., Pirozzoli M. C., Cesi V., Mancini C., Calabretta B., Raschella G. Expression of insulin-like growth factor-binding protein 5 in neuroblastoma cells is regulated at the transcriptional level by c-Myb and B-Myb via direct and indirect mechanisms. J. Biol. Chem. 2002;277:23172–23180. doi: 10.1074/jbc.M200141200. [DOI] [PubMed] [Google Scholar]
- 64.Kulik G., Klippel A., Weber M. J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong Y., Canalis E. Insulin-like growth factor (IGF) I and retinoic acid induce the synthesis of IGF-binding protein 5 in rat osteoblastic cells. Endocrinology. 1995;136:2000–2006. doi: 10.1210/endo.136.5.7536661. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y., Mohan S., Linkhart T. A., Baylink D. J., Strong D. D. Retinoic acid regulates insulin-like growth factor-binding protein expression in human osteoblast cells. Endocrinology. 1996;137:975–983. doi: 10.1210/endo.137.3.8603611. [DOI] [PubMed] [Google Scholar]
- 67.Schmid C., Schlapfer I., Gosteli-Peter M. A., Froesch E. R., Zapf J. Expression, effects, and fate of IGFBP-5 are different in normal and malignant osteoblastic cells. Prog. Growth Factor Res. 1995;6:167–173. doi: 10.1016/0955-2235(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 68.Giudice L. C., Irwin J. C., Dsupin B. A., Pannier E. M., Jin I. H., Vu T. H., Hoffman A. R. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum. Reprod. 1993;8:1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- 69.Hossenlopp P., Segovia B., Lassarre C., Roghani M., Bredon M., Binoux M. Evidence of enzymatic degradation of insulin-like growth factor-binding proteins in the 150K complex during pregnancy. J. Clin. Endocrinol. Metab. 1990;71:797–805. doi: 10.1210/jcem-71-4-797. [DOI] [PubMed] [Google Scholar]
- 70.Davenport M. L., Clemmons D. R., Miles M. V., Camacho-Hubner C., D'Ercole A. J., Underwood L. E. Regulation of serum insulin-like growth factor-I (IGF-I) and IGF binding proteins during rat pregnancy. Endocrinology. 1990;127:1278–1286. doi: 10.1210/endo-127-3-1278. [DOI] [PubMed] [Google Scholar]
- 71.Fielder P. J., Thordarson G., English A., Rosenfeld R. G., Talamantes F. Expression of a lactogen-dependent insulin-like growth factor-binding protein in cultured mouse mammary epithelial cells. Endocrinology. 1992;131:261–267. doi: 10.1210/endo.131.1.1377124. [DOI] [PubMed] [Google Scholar]
- 72.Claussen M., Zapf J., Braulke T. Proteolysis of insulin-like growth factor binding protein-5 by pregnancy serum and amniotic fluid. Endocrinology. 1994;134:1964–1966. doi: 10.1210/endo.134.4.7511097. [DOI] [PubMed] [Google Scholar]
- 73.Lee K. O., Oh Y., Giudice L. C., Cohen P., Peehl D. M., Rosenfeld R. G. Identification of insulin-like growth factor-binding protein-3 (IGFBP-3) fragments and IGFBP-5 proteolytic activity in human seminal plasma: a comparison of normal and vasectomized patients. J. Clin. Endocrinol. Metab. 1994;79:1367–1372. doi: 10.1210/jcem.79.5.7525634. [DOI] [PubMed] [Google Scholar]
- 74.Camacho-Hubner C., Busby W. H., Jr, McCusker R. H., Wright G., Clemmons D. R. Identification of the forms of insulin-like growth factor-binding proteins produced by human fibroblasts and the mechanisms that regulate their secretion. J. Biol. Chem. 1992;267:11949–11956. [PubMed] [Google Scholar]
- 75.Conover C. A. A unique receptor-independent mechanism by which insulin like growth factor I regulates the availability of insulin-like growth factor binding proteins in normal and transformed human fibroblasts. J. Clin. Invest. 1991;88:1354–1361. doi: 10.1172/JCI115441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanzaki S., Hilliker S., Baylink D. J., Mohan S. Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and -5 proteases. Endocrinology. 1994;134:383–392. doi: 10.1210/endo.134.1.7506211. [DOI] [PubMed] [Google Scholar]
- 77.Conover C. A., Baker B. K., Bale L. K., Clarkson J. T., Liu F., Hintz R. L. Human hepatoma cells synthesize and secrete insulin-like growth factor Ia prohormone under growth hormone control. Regul. Pept. 1993;48:1–8. doi: 10.1016/0167-0115(93)90330-b. [DOI] [PubMed] [Google Scholar]
- 78.Shemer J., Yaron A., Werner H., Shao Z. M., Sheikh M. S., Fontana J. A., LeRoith D., Roberts C. T., Jr Regulation of insulin-like growth factor (IGF) binding protein-5 in the T47D human breast carcinoma cell line by IGF-I and retinoic acid. J. Clin. Endocrinol. Metab. 1993;77:1246–1250. doi: 10.1210/jcem.77.5.7521344. [DOI] [PubMed] [Google Scholar]
- 79.Arai T., Arai A., Busby W. H., Jr, Clemmons D. R. Glycosaminoglycans inhibit degradation of insulin-like growth factor-binding protein-5. Endocrinology. 1994;135:2358–2363. doi: 10.1210/endo.135.6.7527332. [DOI] [PubMed] [Google Scholar]
- 80.Fowlkes J. L., Thrailkill K. M., George-Nascimento C., Rosenberg C. K., Serra D. M. Heparin-binding, highly basic regions within the thyroglobulin type-1 repeat of insulin-like growth factor (IGF)-binding proteins (IGFBPs) -3, -5, and -6 inhibit IGFBP-4 degradation. Endocrinology. 1997;138:2280–2285. doi: 10.1210/endo.138.6.5182. [DOI] [PubMed] [Google Scholar]
- 81.Matsumoto T., Tsurumoto T., Goldring M. B., Shindo H. Differential effects of IGF-binding proteins, IGFBP-3 and IGFBP-5, on IGF-I action and binding to cell membranes of immortalized human chondrocytes. J. Endocrinol. 2000;166:29–37. doi: 10.1677/joe.0.1660029. [DOI] [PubMed] [Google Scholar]
- 82.Sunic D., McNeil J. D., Andress D. L., Belford D. A. Insulin-like growth factor binding protein-5 proteolytic activity in ovine articular chondrocyte culture. Biochim. Biophys. Acta. 1998;1425:567–576. doi: 10.1016/s0304-4165(98)00110-x. [DOI] [PubMed] [Google Scholar]
- 83.Campbell P. G., Andress D. L. Insulin-like growth factor (IGF)-binding protein-5-(201–218) region regulates hydroxyapatite and IGF-I binding. Am. J. Physiol. 1997;273:E1005–E1013. doi: 10.1152/ajpendo.1997.273.5.E1005. [DOI] [PubMed] [Google Scholar]
- 84.Zheng B., Clarke J. B., Busby W. H., Duan C., Clemmons D. R. Insulin-like growth factor-binding protein-5 is cleaved by physiological concentrations of thrombin. Endocrinology. 1998;139:1708–1714. doi: 10.1210/endo.139.4.5945. [DOI] [PubMed] [Google Scholar]
- 85.Gibson T. L., Cohen P. Inflammation-related neutrophil proteases, cathepsin G and elastase, function as insulin-like growth factor binding protein proteases. Growth Horm. IGF Res. 1999;9:241–253. doi: 10.1054/ghir.1999.0115. [DOI] [PubMed] [Google Scholar]
- 86.Loechel F., Fox J. W., Murphy G., Albrechtsen R., Wewer U. M. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem. Biophys. Res. Commun. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- 87.Mohan S., Thompson G. R., Amaar Y. G., Hathaway G., Tschesche H., Baylink D. J. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 88.Busby W. H., Jr, Nam T. J., Moralez A., Smith C., Jennings M., Clemmons D. R. The complement component C1s is the protease that accounts for cleavage of insulin-like growth factor-binding protein-5 in fibroblast medium. J. Biol. Chem. 2000;275:37638–37644. doi: 10.1074/jbc.M006107200. [DOI] [PubMed] [Google Scholar]
- 89.Byun D., Mohan S., Yoo M., Sexton C., Baylink D. J., Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J. Clin. Endocrinol. Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- 90.Laursen L. S., Overgaard M. T., Soe R., Boldt H. B., Sottrup-Jensen L., Giudice L. C., Conover C. A., Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 91.Overgaard M. T., Boldt H. B., Laursen L. S., Sottrup-Jensen L., Conover C. A., Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J. Biol. Chem. 2001;276:21849–21853. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- 92.Soe R., Overgaard M. T., Thomsen A. R., Laursen L. S., Olsen I. M., Sottrup-Jensen L., Haaning J., Giudice L. C., Conover C. A., Oxvig C. Expression of recombinant murine pregnancy-associated plasma protein-A (PAPP-A) and a novel variant (PAPP-Ai) with differential proteolytic activity. Eur. J. Biochem. 2002;269:2247–2256. doi: 10.1046/j.1432-1033.2002.02883.x. [DOI] [PubMed] [Google Scholar]
- 93.Hou J., Clemmons D. R., Smeekens S. Expression and characterization of a serine protease that preferentially cleaves insulin-like growth factor binding protein-5. J. Cell. Biochem. 2005;94:470–484. doi: 10.1002/jcb.20328. [DOI] [PubMed] [Google Scholar]
- 94.Jones J. I., Gockerman A., Busby W. H., Jr, Camacho-Hubner C., Clemmons D. R. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J. Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imai Y., Busby W. H., Jr, Smith C. E., Clarke J. B., Garmong A. J., Horwitz G. D., Rees C., Clemmons D. R. Protease-resistant form of insulin-like growth factor-binding protein 5 is an inhibitor of insulin-like growth factor-I actions on porcine smooth muscle cells in culture. J. Clin. Invest. 1997;100:2596–2605. doi: 10.1172/JCI119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiepe D., Andress D. L., Mohan S., Standker L., Ulinski T., Himmele R., Mehls O., Tonshoff B. Intact IGF-binding protein-4 and -5 and their respective fragments isolated from chronic renal failure serum differentially modulate IGF-I actions in cultured growth plate chondrocytes. J. Am. Soc. Nephrol. 2001;12:2400–2410. doi: 10.1681/ASN.V12112400. [DOI] [PubMed] [Google Scholar]
- 97.Kuemmerle J. F., Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J. Biol. Chem. 2002;277:20563–20571. doi: 10.1074/jbc.M200885200. [DOI] [PubMed] [Google Scholar]
- 98.Andress D. L. Heparin modulates the binding of insulin-like growth factor (IGF) binding protein-5 to a membrane protein in osteoblastic cells. J. Biol. Chem. 1995;270:28289–28296. [PubMed] [Google Scholar]
- 99.Sunic D., McNeil J. D., Rayner T. E., Andress D. L., Belford D. A. Regulation of insulin-like growth factor-binding protein-5 by insulin-like growth factor I and interleukin-1α in ovine articular chondrocytes. Endocrinology. 1998;139:2356–2362. doi: 10.1210/endo.139.5.5983. [DOI] [PubMed] [Google Scholar]
- 100.Booth B. A., Boes M., Dake B. L., Caldwell E. E., Weiler J. M., Bar R. S. Effect of IGFBP-derived peptides on incorporation of 35SO4 into proteoglycans. Growth Horm. IGF Res. 2000;10:224–229. doi: 10.1054/ghir.2000.0158. [DOI] [PubMed] [Google Scholar]
- 101.Berfield A. K., Andress D. L., Abrass C. K. IGFBP-5(201–218) stimulates Cdc42GAP aggregation and filopodia formation in migrating mesangial cells. Kidney Int. 2000;57:1991–2003. doi: 10.1046/j.1523-1755.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- 102.Parker A., Rees C., Clarke J., Busby W. H., Jr, Clemmons D. R. Binding of insulin-like growth factor (IGF)-binding protein-5 to smooth-muscle cell extracellular matrix is a major determinant of the cellular response to IGF-I. Mol. Biol. Cell. 1998;9:2383–2392. doi: 10.1091/mbc.9.9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Standker L., Wobst P., Mark S., Forssmann W. G. Isolation and characterization of circulating 13-kDa C-terminal fragments of human insulin-like growth factor binding protein-5. FEBS Lett. 1998;441:281–286. doi: 10.1016/s0014-5793(98)01497-5. [DOI] [PubMed] [Google Scholar]
- 104.Durant D., Pereira R., Stadmeyer L., Canalis E. Transgenic mice expressing selected insulin-like growth factor-binding protein-5 fragments do not exhibit enhanced bone formation. Growth Horm. IGF Res. 2004;14:319–327. doi: 10.1016/j.ghir.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Atti E., Boskey A. L., Canalis E. Overexpression of IGF-binding protein 5 alters mineral and matrix properties in mouse femora: an infrared imaging study. Calcif. Tissue Int. 2005;76:187–193. doi: 10.1007/s00223-004-0076-2. [DOI] [PubMed] [Google Scholar]
- 106.Devlin R. D., Du Z., Buccilli V., Jorgetti V., Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- 107.Durant D., Pereira R. M., Canalis E. Overexpression of insulin-like growth factor binding protein-5 decreases osteoblastic function in vitro. Bone. 2004;35:1256–1262. doi: 10.1016/j.bone.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 108.Salih D. A., Mohan S., Kasukawa Y., Tripathi G., Lovett F. A., Anderson N. F., Carter E. J., Wergedal J. E., Baylink D. J., Pell J. M. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salih D. A., Tripathi G., Holding C., Szestak T. A., Gonzalez M. I., Carter E. J., Cobb L. J., Eisemann J. E., Pell J. M. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clemmons D. R., Busby W. H., Jr, Garmong A., Schultz D. R., Howell D. S., Altman R. D., Karr R. Inhibition of insulin-like growth factor binding protein 5 proteolysis in articular cartilage and joint fluid results in enhanced concentrations of insulin-like growth factor 1 and is associated with improved osteoarthritis. Arthritis Rheum. 2002;46:694–703. doi: 10.1002/art.10222. [DOI] [PubMed] [Google Scholar]
- 111.Iwanaga H., Matsumoto T., Enomoto H., Okano K., Hishikawa Y., Shindo H., Koji T. Enhanced expression of insulin-like growth factor-binding proteins in human osteoarthritic cartilage detected by immunohistochemistry and in situ hybridization. Osteoarthritis Cartilage. 2005;13:439–448. doi: 10.1016/j.joca.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Sharp C. A., Brown S. J., Davie M. W., Magnusson P., Mohan S. Increased matrix concentrations of IGFBP-5 in cancellous bone in osteoarthritis. Ann. Rheum. Dis. 2004;63:1162–1165. doi: 10.1136/ard.2003.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bunn R. C., Fowlkes J. L. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol. Metab. 2003;14:176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 114.Clemmons D. R., Underwood L. E., Chatelain P. G., Van Wyk J. J. Liberation of immunoreactive somatomedin-C from its binding proteins by proteolytic enzymes and heparin. J. Clin. Endocrinol. Metab. 1983;56:384–389. doi: 10.1210/jcem-56-2-384. [DOI] [PubMed] [Google Scholar]
- 115.Hakeda Y., Kawaguchi H., Hurley M., Pilbeam C. C., Abreu C., Linkhart T. A., Mohan S., Kumegawa M., Raisz L. G. Intact insulin-like growth factor binding protein-5 (IGFBP-5) associates with bone matrix and the soluble fragments of IGFBP-5 accumulated in culture medium of neonatal mouse calvariae by parathyroid hormone and prostaglandin E2-treatment. J. Cell Physiol. 1996;166:370–379. doi: 10.1002/(SICI)1097-4652(199602)166:2<370::AID-JCP15>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y., Shu H., Ji C., Casinghino S., Kim K., Gundberg C. M., Centrella M., McCarthy T. L. Insulin-like growth factor binding proteins localize to discrete cell culture compartments in periosteal and osteoblast cultures from fetal rat bone. J. Cell. Biochem. 1998;71:351–362. [PubMed] [Google Scholar]
- 117.Monget P., Pisselet C., Monniaux D. Expression of insulin-like growth factor binding protein-5 by ovine granulosa cells is regulated by cell density and programmed cell death in vitro. J. Cell. Physiol. 1998;177:13–25. doi: 10.1002/(SICI)1097-4652(199810)177:1<13::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 118.Ingman W. V., Owens P. C., Armstrong D. T. Differential regulation by FSH and IGF-I of extracellular matrix IGFBP-5 in bovine granulosa cells: effect of association with the oocyte. Mol. Cell. Endocrinol. 2000;164:53–58. doi: 10.1016/s0303-7207(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 119.Oosterlaken-Dijksterhuis M. A., Kwant M. M., Slob A., Hellmen E., Mol J. A. IGF-I and retinoic acid regulate the distribution pattern of IGFBPs synthesized by the canine mammary tumor cell line CMT-U335. Breast Cancer Res. Treat. 1999;54:11–23. doi: 10.1023/a:1006107703745. [DOI] [PubMed] [Google Scholar]
- 120.Zheng B., Clemmons D. R. Methods for preparing extracellular matrix and quantifying insulin-like growth factor-binding protein binding to the ECM. Methods Mol. Biol. 2000;139:221–230. doi: 10.1385/1-59259-063-2:221. [DOI] [PubMed] [Google Scholar]
- 121.Abrass C. K., Berfield A. K., Andress D. L. Heparin binding domain of insulin-like growth factor binding protein-5 stimulates mesangial cell migration. Am. J. Physiol. 1997;273:F899–F906. doi: 10.1152/ajprenal.1997.273.6.F899. [DOI] [PubMed] [Google Scholar]
- 122.Booth B. A., Boes M., Andress D. L., Dake B. L., Kiefer M. C., Maack C., Linhardt R. J., Bar K., Caldwell E. E., Weiler J., et al. IGFBP-3 and IGFBP-5 association with endothelial cells: role of C-terminal heparin binding domain. Growth Regul. 1995;5:1–17. [PubMed] [Google Scholar]
- 123.Allan G. J., Tonner E., Szymanowska M., Shand J. H., Kelly S. M., Phillips K., Clegg R. A., Gow I. F., Beattie J., Flint D. J. Cumulative mutagenesis of the basic residues in the 201–218 region of insulin-like growth factor binding protein (IGFBP)-5 results in progressive loss of both IGF-I binding and inhibition of IGF-I biological action. Endocrinology. 2006;147:338–349. doi: 10.1210/en.2005-0582. [DOI] [PubMed] [Google Scholar]
- 124.Arai T., Busby W., Jr, Clemmons D. R. Binding of insulin-like growth factor (IGF) I or II to IGF-binding protein-2 enables it to bind to heparin and extracellular matrix. Endocrinology. 1996;137:4571–4575. doi: 10.1210/endo.137.11.8895319. [DOI] [PubMed] [Google Scholar]
- 124a.Nam T. J., Busby W. H., Jr, Rees C., Clemmons D. R. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–1106. doi: 10.1210/endo.141.3.7386. [DOI] [PubMed] [Google Scholar]
- 125.Moralez A. M., Maile L. A., Clarke J., Busby W. H., Jr, Clemmons D. R. Insulin-like growth factor binding protein-5 (IGFBP-5) interacts with thrombospondin-1 to induce negative regulatory effects on IGF-I actions. J. Cell. Physiol. 2005;203:328–334. doi: 10.1002/jcp.20343. [DOI] [PubMed] [Google Scholar]
- 126.Nam T., Moralez A., Clemmons D. Vitronectin binding to IGF binding protein-5 (IGFBP-5) alters IGFBP-5 modulation of IGF-I actions. Endocrinology. 2002;143:30–36. doi: 10.1210/endo.143.1.8596. [DOI] [PubMed] [Google Scholar]
- 127.Kanatani M., Sugimoto T., Nishiyama K., Chihara K. Stimulatory effect of insulin-like growth factor binding protein-5 on mouse osteoclast formation and osteoclastic bone-resorbing activity. J. Bone Miner. Res. 2000;15:902–910. doi: 10.1359/jbmr.2000.15.5.902. [DOI] [PubMed] [Google Scholar]
- 128.Miyakoshi N., Richman C., Kasukawa Y., Linkhart T. A., Baylink D. J., Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J. Clin. Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richman C., Baylink D. J., Lang K., Dony C., Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 130.Twigg S. M., Baxter R. C. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J. Biol. Chem. 1998;273:6074–6079. doi: 10.1074/jbc.273.11.6074. [DOI] [PubMed] [Google Scholar]
- 131.Kricker J. A., Towne C. L., Firth S. M., Herington A. C., Upton Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology. 2003;144:2807–2015. doi: 10.1210/en.2002-221086. [DOI] [PubMed] [Google Scholar]
- 132.Hyde C., Hollier B., Anderson A., Harkin D., Upton Z. Insulin-like growth factors (IGF) and IGF-binding proteins bound to vitronectin enhance keratinocyte protein synthesis and migration. J. Invest. Dermatol. 2004;122:1198–1206. doi: 10.1111/j.0022-202X.2004.22527.x. [DOI] [PubMed] [Google Scholar]
- 133.Maile L. A., Imai Y., Clarke J. B., Clemmons D. R. Insulin-like growth factor I increases αvβ3 affinity by increasing the amount of integrin-associated protein that is associated with non-raft domains of the cellular membrane. J. Biol. Chem. 2002;277:1800–1805. doi: 10.1074/jbc.M108380200. [DOI] [PubMed] [Google Scholar]
- 134.Nichols T. C., du Laney T., Zheng B., Bellinger D. A., Nickols G. A., Engleman W., Clemmons D. R. Reduction in atherosclerotic lesion size in pigs by αvβ3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circ. Res. 1999;85:1040–1045. doi: 10.1161/01.res.85.11.1040. [DOI] [PubMed] [Google Scholar]
- 135.Nam T. J., Busby W., Jr, Clemmons D. R. Insulin-like growth factor binding protein-5 binds to plasminogen activator inhibitor-I. Endocrinology. 1997;138:2972–2978. doi: 10.1210/endo.138.7.5230. [DOI] [PubMed] [Google Scholar]
- 136.Arai T., Clarke J., Parker A., Busby W., Jr, Nam T., Clemmons D. R. Substitution of specific amino acids in insulin-like growth factor (IGF) binding protein 5 alters heparin binding and its change in affinity for IGF-I response to heparin. J. Biol. Chem. 1996;271:6099–6106. doi: 10.1074/jbc.271.11.6099. [DOI] [PubMed] [Google Scholar]
- 137.Flint D. J., Tonner E., Allan G. J. Insulin-like growth factor binding proteins: IGF-dependent and -independent effects in the mammary gland. J. Mammary Gland Biol. Neoplasia. 2000;5:65–73. doi: 10.1023/a:1009567316520. [DOI] [PubMed] [Google Scholar]
- 138.Hahnefeld C., Drewianka S., Herberg F. W. Determination of kinetic data using surface plasmon resonance biosensors. Methods Mol. Med. 2004;94:299–320. doi: 10.1385/1-59259-679-7:299. [DOI] [PubMed] [Google Scholar]
- 139.Wu S. J., Chaiken I. Biosensor analysis of receptor-ligand interactions. Methods Mol. Biol. 2004;249:93–110. doi: 10.1385/1-59259-667-3:93. [DOI] [PubMed] [Google Scholar]
- 140.Robinson S. A., Rosenzweig S. A. Synthesis and characterization of biotinylated forms of insulin-like growth factor-1: topographical evaluation of the IGF-1/IGFBP-2 and IGFBP-3 interface. Biochemistry. 2004;43:11533–11545. doi: 10.1021/bi049082k. [DOI] [PubMed] [Google Scholar]
- 141.Yan X., Forbes B. E., McNeil K. A., Baxter R. C., Firth S. M. Role of N- and C-terminal residues of insulin-like growth factor (IGF) binding protein-3 in regulating IGF complex formation and receptor activation. J. Biol. Chem. 2004;279:53232–53240. doi: 10.1074/jbc.M409345200. [DOI] [PubMed] [Google Scholar]
- 141a.Beattie J., Phillips K., Shand J. H., Szymanowska M., Flint D. J., Allan G. J. Molecular recognition characteristics in the insulin-like growth factor (IGF)-insulin-like growth factor binding protein-3/5 (IGFBP-3/5) heparin axis. J. Mol. Endocrinol. 2005;34:163–175. doi: 10.1677/jme.1.01656. [DOI] [PubMed] [Google Scholar]
- 142.Martin J. L., Ballesteros M., Baxter R. C. Insulin-like growth factor-I (IGF-I) and transforming growth factor-β1 release IGF-binding protein-3 from human fibroblasts by different mechanisms. Endocrinology. 1992;131:1703–1710. doi: 10.1210/endo.131.4.1382959. [DOI] [PubMed] [Google Scholar]
- 143.Allan G. J., Zannoni A., McKinnell I., Otto W. R., Holzenberger M., Flint D. J., Patel K. Major components of the insulin-like growth factor axis are expressed early in chicken embryogenesis, with IGF binding protein (IGFBP)-5 expression subject to regulation by Sonic Hedgehog. Anat. Embryol. 2003;207:73–84. doi: 10.1007/s00429-003-0321-x. [DOI] [PubMed] [Google Scholar]
- 144.Bramani S., Song H., Beattie J., Tonner E., Flint D. J., Allan G. J. Amino acids within the extracellular matrix (ECM) binding region (201–218) of rat insulin-like growth factor binding protein (IGFBP)-5 are important determinants in binding IGF-I. J. Mol. Endocrinol. 1999;23:117–123. doi: 10.1677/jme.0.0230117. [DOI] [PubMed] [Google Scholar]
- 145.Song H., Beattie J., Campbell I. W., Allan G. J. Overlap of IGF- and heparin-binding sites in rat IGF-binding protein-5. J. Mol. Endocrinol. 2000;24:43–51. doi: 10.1677/jme.0.0240043. [DOI] [PubMed] [Google Scholar]
- 146.Zheng B., Duan C., Clemmons D. R. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J. Biol. Chem. 1998;273:8994–9000. doi: 10.1074/jbc.273.15.8994. [DOI] [PubMed] [Google Scholar]
- 147.Amaar Y. G., Thompson G. R., Linkhart T. A., Chen S. T., Baylink D. J., Mohan S. Insulin-like growth factor-binding protein 5 (IGFBP-5) interacts with a four and a half LIM protein 2 (FHL2) J. Biol. Chem. 2002;277:12053–12060. doi: 10.1074/jbc.M110872200. [DOI] [PubMed] [Google Scholar]
- 148.Xu Q., Li S., Zhao Y., Maures T. J., Yin P., Duan C. Evidence that IGF binding protein-5 functions as a ligand-independent transcriptional regulator in vascular smooth muscle cells. Circ. Res. 2004;94:E46–E54. doi: 10.1161/01.RES.0000124761.62846.DF. [DOI] [PubMed] [Google Scholar]
- 149.Amaar Y. G., Baylink D. J., Mohan S. Ras-association domain family 1 protein, RASSF1C, is an IGFBP-5 binding partner and a potential regulator of osteoblast cell proliferation. J. Bone Miner. Res. 2005;20:1430–1439. doi: 10.1359/JBMR.050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Andress D. L., Birnbaum R. S. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J. Biol. Chem. 1992;267:22467–22472. [PubMed] [Google Scholar]
- 151.Neuenschwander S., Schwartz A., Wood T. L., Roberts C. T., Jr, Henninghausen L., LeRoith D. Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J. Clin. Invest. 1996;97:2225–2232. doi: 10.1172/JCI118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruan W., Kleinberg D. L. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 1999;140:5075–5081. doi: 10.1210/endo.140.11.7095. [DOI] [PubMed] [Google Scholar]
- 153.Marshman E., Green K. A., Flint D. J., White A., Streuli C. H., Westwood M. Insulin-like growth factor binding protein 5 and apoptosis in mammary epithelial cells. J. Cell Sci. 2003;116:675–682. doi: 10.1242/jcs.00263. [DOI] [PubMed] [Google Scholar]
- 154.Tonner E., Barber M. C., Allan G. J., Beattie J., Webster J., Whitelaw C. B., Flint D. J. Insulin-like growth factor binding protein-5 (IGFBP-5) induces premature cell death in the mammary glands of transgenic mice. Development. 2002;129:4547–4557. doi: 10.1242/dev.129.19.4547. [DOI] [PubMed] [Google Scholar]
- 155.Tonner E., Barber M. C., Travers M. T., Logan A., Flint D. J. Hormonal control of insulin-like growth factor-binding protein-5 production in the involuting mammary gland of the rat. Endocrinology. 1997;138:5101–5107. doi: 10.1210/endo.138.12.5619. [DOI] [PubMed] [Google Scholar]
- 156.Tonner E., Quarrie L., Travers M., Barber M., Logan A., Wilde C., Flint D. Does an IGF-binding protein (IGFBP) present in involuting rat mammary gland regulate apoptosis? Prog. Growth Factor Res. 1995;6:409–414. doi: 10.1016/0955-2235(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 157.Allar M. A., Wood T. L. Expression of the insulin-like growth factor binding proteins during postnatal development of the murine mammary gland. Endocrinology. 2004;145:2467–2477. doi: 10.1210/en.2003-1641. [DOI] [PubMed] [Google Scholar]
- 158.Wood T. L., Richert M. M., Stull M. A., Allar M. A. The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands. J. Mammary Gland Biol. Neoplasia. 2000;5:31–42. doi: 10.1023/a:1009511131541. [DOI] [PubMed] [Google Scholar]
- 158a.Phillips K., Park A. M., Quamie L. H., Boutinaud M., Flint D. J., Beattie J. Regulation of IGFBP-5 and -2 during differentiation of the HC11 mouse mammory epithelial cell line. J. Mol. Endocrinol. 2003;31:197–208. doi: 10.1677/jme.0.0310197. [DOI] [PubMed] [Google Scholar]
- 158b.Lochrie J. D., Phillips K., Tonner E., Flint D. J., Allan G. J., Price N. C., Beattie J. Insulin-like growth factor binding protein (IGFBP)-5 is upregulated during both differentiation and apoptosis in primary cultures of mouse mammary epithelial cells. J. Cell. Physiol. 2006 doi: 10.1002/jcp.20587. 10.1002/jcp.20587. [DOI] [PubMed]
- 159.Clarkson R. W., Watson C. J. Microarray analysis of the involution switch. J. Mammary Gland Biol. Neoplasia. 2003;8:309–319. doi: 10.1023/b:jomg.0000010031.53310.92. [DOI] [PubMed] [Google Scholar]
- 160.Rudolph M. C., McManaman J. L., Hunter L., Phang T., Neville M. C. Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J. Mammary Gland Biol. Neoplasia. 2003;8:287–307. doi: 10.1023/b:jomg.0000010030.73983.57. [DOI] [PubMed] [Google Scholar]
- 161.Cheng H. L., Shy M., Feldman E. L. Regulation of insulin-like growth factor-binding protein-5 expression during Schwann cell differentiation. Endocrinology. 1999;140:4478–4485. doi: 10.1210/endo.140.10.7051. [DOI] [PubMed] [Google Scholar]
- 162.Rotwein P., James P. L., Kou K. Rapid activation of insulin-like growth factor binding protein-5 gene transcription during myoblast differentiation. Mol. Endocrinol. 1995;9:913–923. doi: 10.1210/mend.9.7.7476973. [DOI] [PubMed] [Google Scholar]
- 163.Sheikh M. S., Shao Z. M., Clemmons D. R., LeRoith D., Roberts C. T., Jr, Fontana J. A. Identification of the insulin-like growth factor binding proteins 5 and 6 (IGFBP-5 and 6) in human breast cancer cells. Biochem. Biophys. Res. Commun. 1992;183:1003–1010. doi: 10.1016/s0006-291x(05)80290-6. [DOI] [PubMed] [Google Scholar]
- 164.McGuire W. L., Jr, Jackson J. G., Figueroa J. A., Shimasaki S., Powell D. R., Yee D. Regulation of insulin-like growth factor-binding protein (IGFBP) expression by breast cancer cells: use of IGFBP-1 as an inhibitor of insulin-like growth factor action. J. Natl. Cancer Inst. 1992;84:1336–1341. doi: 10.1093/jnci/84.17.1336. [DOI] [PubMed] [Google Scholar]
- 165.Dubois V., Couissi D., Schonne E., Remacle C., Trouet A. Intracellular levels and secretion of insulin-like-growth-factor-binding proteins in MCF-7/6, MCF-7/AZ and MDA-MB-231 breast cancer cells: differential modulation by estrogens in serum-free medium. Eur. J. Biochem. 1995;232:47–53. doi: 10.1111/j.1432-1033.1995.tb20779.x. [DOI] [PubMed] [Google Scholar]
- 166.Sheikh M. S., Shao Z. M., Hussain A., Clemmons D. R., Chen J. C., Roberts C. T., Jr, LeRoith D., Fontana J. A. Regulation of insulin-like growth factor-binding-protein-1, 2, 3, 4, 5, and 6: synthesis, secretion, and gene expression in estrogen receptor-negative human breast carcinoma cells. J. Cell. Physiol. 1993;155:556–567. doi: 10.1002/jcp.1041550314. [DOI] [PubMed] [Google Scholar]
- 167.Coutts A., Murphy L. J., Murphy L. C. Expression of insulin-like growth factor binding proteins by T-47D human breast cancer cells: regulation by progestins and antiestrogens. Breast Cancer Res. Treat. 1994;32:153–164. doi: 10.1007/BF00665766. [DOI] [PubMed] [Google Scholar]
- 168.Huynh H., Yang X. F., Pollak M. A role for insulin-like growth factor binding protein 5 in the antiproliferative action of the antiestrogen ICI 182780. Cell Growth Differ. 1996;7:1501–1506. [PubMed] [Google Scholar]
- 169.Parisot J. P., Leeding K. S., Hu X. F., DeLuise M., Zalcberg J. R., Bach L. A. Induction of insulin-like growth factor binding protein expression by ICI 182,780 in a tamoxifen-resistant human breast cancer cell line. Breast Cancer Res. Treat. 1999;55:231–242. doi: 10.1023/a:1006274712664. [DOI] [PubMed] [Google Scholar]
- 170.Rozen F., Pollak M. Inhibition of insulin-like growth factor I receptor signaling by the vitamin D analogue EB1089 in MCF-7 breast cancer cells: a role for insulin-like growth factor binding proteins. Int. J. Oncol. 1999;15:589–594. doi: 10.3892/ijo.15.3.589. [DOI] [PubMed] [Google Scholar]
- 171.Rozen F., Yang X. F., Huynh H., Pollak M. Antiproliferative action of vitamin D-related compounds and insulin-like growth factor-binding protein 5 accumulation. J. Natl. Cancer Inst. 1997;89:652–656. doi: 10.1093/jnci/89.9.652. [DOI] [PubMed] [Google Scholar]
- 172.Swami S., Raghavachari N., Muller U. R., Bao Y. P., Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res. Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 173.Butt A. J., Dickson K. A., Jambazov S., Baxter R. C. Enhancement of tumor necrosis factor-α-induced growth inhibition by insulin-like growth factor-binding protein-5 (IGFBP-5), but not IGFBP-3 in human breast cancer cells. Endocrinology. 2005;146:3113–3122. doi: 10.1210/en.2004-1408. [DOI] [PubMed] [Google Scholar]
- 174.Huynh H. In vivo regulation of the insulin-like growth factor system of mitogens by human chorionic gonadotropin. Int. J. Oncol. 1998;13:571–557. doi: 10.3892/ijo.13.3.571. [DOI] [PubMed] [Google Scholar]
- 175.Manni A., Badger B., Wei L., Zaenglein A., Grove R., Khin S., Heitjan D., Shimasaki S., Ling N. Hormonal regulation of insulin-like growth factor II and insulin-like growth factor binding protein expression by breast cancer cells in vivo: evidence for stromal epithelial interactions. Cancer Res. 1994;54:2934–2942. [PubMed] [Google Scholar]
- 176.McGuire S. E., Hilsenbeck S. G., Figueroa J. A., Jackson J. G., Yee D. Detection of insulin-like growth factor binding proteins (IGFBPs) by ligand blotting in breast cancer tissues. Cancer Lett. 1994;77:25–32. doi: 10.1016/0304-3835(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 177.Pekonen F., Nyman T., Ilvesmaki V., Partanen S. Insulin-like growth factor binding proteins in human breast cancer tissue. Cancer Res. 1992;52:5204–5207. [PubMed] [Google Scholar]
- 178.Perks C. M., Bowen S., Gill Z. P., Newcomb P. V., Holly J. M. Differential IGF-independent effects of insulin-like growth factor binding proteins (1–6) on apoptosis of breast epithelial cells. J. Cell. Biochem. 1999;75:652–664. doi: 10.1002/(sici)1097-4644(19991215)75:4<652::aid-jcb11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 179.McCaig C., Perks C. M., Holly J. M. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J. Cell. Sci. 2002;115:4293–4303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- 180.Mathias S., Pena L. A., Kolesnick R. N. Signal transduction of stress via ceramide. Biochem. J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schedlich L. J., Young T. F., Firth S. M., Baxter R. C. Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J. Biol. Chem. 1998;273:18347–18352. doi: 10.1074/jbc.273.29.18347. [DOI] [PubMed] [Google Scholar]
- 182.Peehl D. M., Cohen P., Rosenfeld R. G. The role of insulin-like growth factors in prostate biology. J. Androl. 1996;17:2–4. [PubMed] [Google Scholar]
- 183.Cohen P., Peehl D. M., Lamson G., Rosenfeld R. G. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J. Clin. Endocrinol. Metab. 1991;73:401–407. doi: 10.1210/jcem-73-2-401. [DOI] [PubMed] [Google Scholar]
- 183a.Li L., Yu H., Schumacher F., Casey G., Witte J. S. Relation of serum insulin-like growth factor I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States) Cancer Causes Control. 2003;14:721–726. doi: 10.1023/a:1026383824791. [DOI] [PubMed] [Google Scholar]
- 184.Boudon C., Rodier G., Lechevallier E., Mottet N., Barenton B., Sultan C. Secretion of insulin-like growth factors and their binding proteins by human normal and hyperplastic prostatic cells in primary culture. J. Clin. Endocrinol. Metab. 1996;81:612–617. doi: 10.1210/jcem.81.2.8636277. [DOI] [PubMed] [Google Scholar]
- 185.Tennant M. K., Thrasher J. B., Twomey P. A., Birnbaum R. S., Plymate S. R. Insulin-like growth factor-binding proteins (IGFBP)-4, -5, and -6 in the benign and malignant human prostate: IGFBP-5 messenger ribonucleic acid localization differs from IGFBP-5 protein localization. J. Clin. Endocrinol. Metab. 1996;81:3783–3792. doi: 10.1210/jcem.81.10.8855838. [DOI] [PubMed] [Google Scholar]
- 186.Conover C. A., Perry J. E., Tindall D. J. Endogenous cathepsin D-mediated hydrolysis of insulin-like growth factor-binding proteins in cultured human prostatic carcinoma cells. J. Clin. Endocrinol. Metab. 1995;80:987–993. doi: 10.1210/jcem.80.3.7533776. [DOI] [PubMed] [Google Scholar]
- 187.Kimura G., Kasuya J., Giannini S., Honda Y., Mohan S., Kawachi M., Akimoto M., Fujita-Yamaguchi Y. Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Int. J. Urol. 1996;3:39–46. doi: 10.1111/j.1442-2042.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 188.Guenette R. S., Tenniswood M. The role of growth factors in the suppression of active cell death in the prostate: an hypothesis. Biochem. Cell Biol. 1994;72:553–559. doi: 10.1139/o94-074. [DOI] [PubMed] [Google Scholar]
- 189.Nickerson T., Huynh H. Vitamin D analogue EB1089-induced prostate regression is associated with increased gene expression of insulin-like growth factor binding proteins. J. Endocrinol. 1999;160:223–229. doi: 10.1677/joe.0.1600223. [DOI] [PubMed] [Google Scholar]
- 190.Nickerson T., Pollak M., Huynh H. Castration-induced apoptosis in the rat ventral prostate is associated with increased expression of genes encoding insulin-like growth factor binding proteins 2, 3, 4 and 5. Endocrinology. 1998;139:807–810. doi: 10.1210/endo.139.2.5912. [DOI] [PubMed] [Google Scholar]
- 191.Thomas L. N., Cohen P., Douglas R. C., Lazier C., Rittmaster R. S. Insulin-like growth factor binding protein 5 is associated with involution of the ventral prostate in castrated and finasteride-treated rats. Prostate. 1998;35:273–278. doi: 10.1002/(sici)1097-0045(19980601)35:4<273::aid-pros6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 192.Gleave M. E., Miyake H. Castration-induced upregulation of insulin-like growth factor binding protein-5 potentiates IGF-1 and accelerates androgen-independent progression in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:S16. doi: 10.1038/sj.pcan.4500440. [DOI] [PubMed] [Google Scholar]
- 193.Miyake H., Nelson C., Rennie P. S., Gleave M. E. Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3′-kinase pathway. Endocrinology. 2000;141:2257–2265. doi: 10.1210/endo.141.6.7520. [DOI] [PubMed] [Google Scholar]
- 194.Miyake H., Pollak M., Gleave M. E. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res. 2000;60:3058–3064. [PubMed] [Google Scholar]
- 195.Roschier M., Kuusisto E., Suuronen T., Korhonen P., Kyrylenko S., Salminen A. Insulin-like growth factor binding protein 5 and type-1 insulin-like growth factor receptor are differentially regulated during apoptosis in cerebellar granule cells. J. Neurochem. 2001;76:11–20. doi: 10.1046/j.1471-4159.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 196.Adashi E. Y., Resnick C. E., Hernandez E. R., Hurwitz A., Rosenfeld R. G. Ovarian granulosa cell-derived insulin-like growth factor (IGF) binding proteins: release of low molecular weight, high-affinity IGF-selective species. Mol. Cell. Endocrinol. 1990;74:175–184. doi: 10.1016/0303-7207(90)90222-t. [DOI] [PubMed] [Google Scholar]
- 197.Hofmann J., Wegmann B., Hackenberg R., Kunzmann R., Schulz K. D., Havemann K. Production of insulin-like growth factor binding proteins by human ovarian carcinoma cells. J. Cancer Res. Clin. Oncol. 1994;120:137–142. doi: 10.1007/BF01202191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Krywicki R. F., Figueroa J. A., Jackson J. G., Kozelsky T. W., Shimasaki S., Von Hoff D. D., Yee D. Regulation of insulin-like growth factor binding proteins in ovarian cancer cells by oestrogen. Eur. J. Cancer. 1993;29A:2015–2019. doi: 10.1016/0959-8049(93)90464-q. [DOI] [PubMed] [Google Scholar]
- 198a.Ho M. N., Delgado C. H., Owens G. A., Steller M. A. Insulin-like growth factor-II participates in the biphasic effect of a gonadotropin releasing hormone agonist on ovarian cancer cell growth. Fertil. Steril. 1997;67:870–876. doi: 10.1016/s0015-0282(97)81399-4. [DOI] [PubMed] [Google Scholar]
- 199.Drescher B., Lauke H., Hartmann M., Davidoff M. S., Zumkeller W. Immunohistochemical pattern of insulin-like growth factor (IGF) I, IGF II and IGF binding proteins 1 to 6 in carcinoma in situ of the testis. Mol. Pathol. 1997;50:298–303. doi: 10.1136/mp.50.6.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cheung C., Vesey D., Cotterill A., Douglas M., Gobe G., Nicol D., Johnson D. Altered messenger RNA and protein expressions for insulin-like growth factor family members in clear cell and papillary renal cell carcinomas. Int. J. Urol. 2005;12:17–28. doi: 10.1111/j.1442-2042.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 201.Gong Y., Ballejo G., Alkhalaf B., Molnar P., Murphy L. C., Murphy L. J. Phorbol esters differentially regulate the expression of insulin-like growth factor-binding proteins in endometrial carcinoma cells. Endocrinology. 1992;131:2747–2754. doi: 10.1210/endo.131.6.1280205. [DOI] [PubMed] [Google Scholar]
- 202.Schneider M. R., Zhou R., Hoeflich A., Krebs O., Schmidt J., Mohan S., Wolf E., Lahm H. Insulin-like growth factor-binding protein-5 inhibits growth and induces differentiation of mouse osteosarcoma cells. Biochem. Biophys. Res. Commun. 2001;288:435–442. doi: 10.1006/bbrc.2001.5785. [DOI] [PubMed] [Google Scholar]
- 203.Stolf B. S., Carvalho A. F., Martins W. K., Runza F. B., Brun M., Hirata R., Jr, Jordao Neves E., Soares F. A., Postigo-Dias J., Kowalski L. P., Reis L. F. Differential expression of IGFBP-5 and two human ESTs in thyroid glands with goiter, adenoma and papillary or follicular carcinomas. Cancer Lett. 2003;191:193–202. doi: 10.1016/s0304-3835(02)00679-1. [DOI] [PubMed] [Google Scholar]
- 204.Takahashi M., Papavero V., Yuhas J., Kort E., Kanayama H. O., Kagawa S., Baxter R. C., Yang X. J., Gray S. G., Teh B. T. Altered expression of members of the IGF-axis in clear cell renal cell carcinoma. Int. J. Oncol. 2005;26:923–931. [PubMed] [Google Scholar]
- 205.Vorwerk P., Wex H., Hohmann B., Mohnike K., Schmidt U., Mittler U. Expression of components of the IGF signalling system in childhood acute lymphoblastic leukaemia. Mol. Pathol. 2002;55:40–45. doi: 10.1136/mp.55.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wegmann B. R., Schoneberger H. J., Kiefer P. E., Jaques G., Brandscheid D., Havemann K. Molecular cloning of IGFBP-5 from SCLC cell lines and expression of IGFBP-4, IGFBP-5 and IGFBP-6 in lung cancer cell lines and primary tumours. Eur. J. Cancer. 1993;29A:1578–1584. doi: 10.1016/0959-8049(93)90298-t. [DOI] [PubMed] [Google Scholar]
- 207.Wulbrand U., Remmert G., Zofel P., Wied M., Arnold R., Fehmann H. C. mRNA expression patterns of insulin-like growth factor system components in human neuroendocrine tumours. Eur. J. Clin. Invest. 2000;30:729–739. doi: 10.1046/j.1365-2362.2000.00700.x. [DOI] [PubMed] [Google Scholar]
- 208.Yi H. K., Hwang P. H., Yang D. H., Kang C. W., Lee D. Y. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur. J. Cancer. 2001;37:2257–2263. doi: 10.1016/s0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 209.Zumkeller W., Muller D., Muller S., Gunther C., Westphal M. Expression and synthesis of insulin-like growth factor-binding proteins in human glioma cell lines. Int. J. Oncol. 1998;12:129–135. doi: 10.3892/ijo.12.1.129. [DOI] [PubMed] [Google Scholar]
- 210.Higo H., Duan C., Clemmons D. R., Herman B. Retinoic acid inhibits cell growth in HPV negative cervical carcinoma cells by induction of insulin-like growth factor binding protein-5 (IGFBP-5) secretion. Biochem. Biophys. Res. Commun. 1997;239:706–709. doi: 10.1006/bbrc.1997.7499. [DOI] [PubMed] [Google Scholar]
- 211.Jones J. I., Gockerman A., Busby W. H., Jr, Wright G., Clemmons D. R. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg-Gly-Asp sequence. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oh Y., Muller H. L., Pham H., Rosenfeld R. G. Demonstration of receptors for insulin-like growth factor binding protein-3 on Hs578T human breast cancer cells. J. Biol. Chem. 1993;268:26045–26048. [PubMed] [Google Scholar]