Abstract
A milk membrane glycoprotein, MFG-E8 [milk fat globule-EGF (epidermal growth factor) factor 8], is expressed abundantly in lactating mammary glands in stage- and tissue-specific manners, and has been believed to be secreted in association with milk fat globules. In the present paper, we describe further up-regulation of MFG-E8 in involuting mammary glands, where the glands undergo a substantial increase in the rate of epithelial cell apoptosis, and a possible role of MFG-E8 in mediating recognition and engulfment of apoptotic cells through its specific binding to PS (phosphatidylserine). Immunoblotting and RNA blotting analyses revealed that both MFG-E8 protein and MFG-E8 mRNA were markedly increased in mammary tissue within 3 days of either natural or forced weaning (pup withdrawal) of lactating mice. Using immunohistochemical analysis of the mammary tissue cryosections, the MFG-E8 signal was detected around the epithelium of such involuting mammary glands, but was almost undetectable at early- and mid-lactation stages, although strong signals were obtained for milk fat globules stored in the alveolar lumen. Some signals double positive to a macrophage differentiation marker, CD68, and MFG-E8 were detected in the post-weaning mammary tissue, although such double-positive signals were much smaller in number than the MFG-E8 single-positive ones. Total MFG-E8 in milk was also increased in the post-weaning mammary glands and, furthermore, the free MFG-E8 content in the post-weaning milk, as measured by in vitro PS-binding and apoptotic HC11 cell-binding activities, was much higher than that of lactation. In addition, the post-weaning milk enhanced the binding of apoptotic HC11 cells to J774 macrophages. Sucrose density-gradient ultracentrifugation analyses revealed that such enhanced PS-binding activity of MFG-E8 was present in membrane vesicle fractions (density 1.05–1.13 g/ml), rather than milk fat globule fractions. The weaning-induced MFG-E8 might play an important role in the recognition and engulfment of apoptotic epithelial cells by the neighbouring phagocytic epithelial cells in involuting mammary glands.
Keywords: involution, lactation, mammary gland, milk fat globule-epidermal growth factor factor 8 (MFG-E8), weaning
Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; EGF, epidermal growth factor; ELMV, exosome-like membrane vesicle; MFG, milk fat globule; MFG-E8, MFG-EGF factor 8; MFGM, MFG membrane; PBST, PBS containing 0.05% (v/v) Tween 20; PI, propidium iodide; PS, phosphatidylserine; PC, phosphatidylcholine; TCA, trichloroacetic acid; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling
INTRODUCTION
After completion of lactation, the mammary glands undergo involution, regressing to a state like that of virgin animals. The post-lactational involution of the mammary glands can be divided into two distinct phases. The first phase is characterized by engorgement of the gland with milk and initial apoptosis of mammary epithelial cells, and the second one is characterized by biosynthesis of proteinases and intensive tissue remodelling [1–3]. The involuting mammary gland undergoes massive cell loss by apoptosis. Apoptotic mammary epithelial cells must be cleared immediately by phagocytes in involuting mammary glands to prevent inflammation and autoimmune response against intracellular antigens released from the dying cells [4]. Phagocytosis of apoptotic mammary epithelial cells by both macrophages and residual living epithelial cells has been demonstrated with convincing data, and three potential fates of apoptotic mammary epithelial cells were suggested: release into the lumen, phagocytosis by neighbouring alveolar epithelial cells and phagocytosis by macrophages [3,5]. As phagocytosis-related molecules associated with the apoptotic cell uptake, various receptors or ligands such as CD14, CD36, CD68, αvβ3 integrin and ABC1 (ATP-binding cassette 1) transporter, have been suggested [6], but detailed mechanisms for the recognition of apoptotic cells by phagocytes still remain to be investigated. Recently, another molecule, MFG-E8 [MFG (milk fat globule)-EGF (epidermal growth factor) factor 8] with an integrin-binding RGD motif and PS (phosphatidylserine)-binding domains has directly been identified by using MFG-E8-deficient mice as a tethering molecule between apoptotic cells and activated macrophages [7,8].
MFG-E8 was originally identified as a major component of MFGM (MFG membrane) surrounding the lipid droplets [9] and has therefore been assumed to be involved in milk fat secretion, although no experimental evidence has been reported. This MFG-E8 protein was cloned and characterized as 53- and 66-kDa glycoproteins associated peripherally with the MFGM [9]. This molecule consists of two repeated EGF-like domains (an RGD motif is located in the second EGF domain) on the N-terminal side and of two repeated C (discoidin-like) domains homologous with the C1 and C2 domains of blood coagulation Factors V and VIII. Orthologous proteins have been isolated in cow (MGP57/53 or PAS-6/7) [10,11], human (BA46 or lactadherin) [12,13] and rat (rAGS) [14]. Although MFG-E8 contains no apparent hydrophobic transmembrane regions, MFG-E8 has been shown to be a peripheral membrane protein and binds directly to the MFGM and cell membrane [11,15–17]. Both the native and recombinant MFG-E8 proteins bind in vitro to anionic phospholipids, especially PS [18–20]. This PS-binding of MFG-E8 has been reported to depend only on the C2 domain, but not the C1 domain, in the same manner as that of blood coagulation Factors V and VIII [21–23].
In our earlier studies, the expression of MFG-E8 in mouse mammary glands was shown to be up-regulated after parturition and was maintained during lactation even at a later stage of day 16 [24]. MFG-E8 expression at levels lower than that of lactating mammary glands has also been detected in various other tissues, including brain, lung, heart, kidney and spleen in some mammals, such as mouse, human and cow [24–26]. Very recently, a comprehensive analysis of gene expression in mouse mammary gland involution has been conducted by using the microarray technique, and revealed that MFG-E8 transcripts gradually increased to approx. 1.5 times the normalized intensity within 3 days after the forced weaning for l0-day lactating mice [6].
These previous experimental data on the structure, function and expression of MFG-E8 suggested to us that MFG-E8 might also be involved in recognition and clearance of the apoptotic cells during mammary gland involution. The main aims of the present study are to clarify the weaning-induced MFG-E8 expression in mammary glands and its PS-binding activity levels and to examine the possibility of apoptotic cell marking by mammary MFG-E8. Using milk recovered from involuting mammary glands, we demonstrated for the first time that MFG-E8 in the post-weaning milk exhibited high PS-binding activity and specifically bound to apoptosis-induced HC11 cells.
MATERIALS AND METHODS
Materials
The rabbit antibody against MFG-E8 was described previously [24], and anti-(mouse casein) antibody was prepared by the immunization of a rabbit using Freund's adjuvant with the casein purified by acid precipitation from mouse milk. Anti-(mouse CD68) and anti-(cytokeratin 18) (K18, clone Ks18.04) antibodies were purchased from Serotec and Progen respectively. All other reagents were from Sigma, unless noted otherwise.
Mice care, weaning and sample preparation
Lactating Balb/c mice (8-week-old) purchased from Japan SLC were fed laboratory chow, and were cared for according to the Nagoya University guidelines for animal study. Litter size was standardized to six pups within 24 h postpartum. For ‘forced weaning’, the pups were separated from the mother mouse at day 2 or 10 of lactation. We designated day 20 after delivery the time of natural weaning, and hence the pups were separated from the mother at this time for ‘natural weaning’.
Mammary tissue and milk samples were always prepared from the number 4 glands (the numbering from head to tail) of each mouse at lactation or post-weaning stages as described. The tissue samples were excised from the glands and were homogenized immediately in each appropriate buffer for protein or RNA analyses, or frozen for histological analyses. For the milk sampling, the mouse was anaesthetized with Nembutal (Abbott Laboratories) and oxytocin (10 units per mouse; Teikoku Hormone MFG) was injected intraperitoneally to induce milk secretion. Then, the milk sample was collected from the mammary glands by milking manually using glass capillaries. The milk samples collected were either used immediately or kept frozen at −30 °C until use.
SDS/PAGE and immunoblotting
The mammary tissue samples were homogenized by sonication in 1× SDS/PAGE sample buffer [27] containing 4% 2-mercaptoethanol (12.5 μl per mg of tissue wet weight), boiled and centrifuged at 12000 g for 15 min to remove insoluble materials. As for the milk samples, whole milk was mixed directly with the sample buffer as above at the ratio of 1:30 (v/v). The tissue homogenates obtained (corresponding to 20 μg of tissue wet weight) or the milk samples (corresponding to 0.08 μl) were separated on SDS/10% polyacrylamide gels and transferred on to PVDF membranes. The membranes were blocked with 1× NETG solution [150 mM NaCl, 5 mM EDTA, pH 8.0, 0.05% (v/v) Triton X-100 and 0.25% (w/v) gelatin] for 1 h at room temperature (25 °C), incubated with primary antibody at 4 °C overnight and then with horseradish-peroxidase-conjugated secondary antibody at room temperature for 1 h. The protein bands were visualized with an ECL® (enhanced chemiluminescence) detection kit (Amersham Biosciences) and Light Capture System (AE-6962; ATTO). For the milk samples, proteins in the polyacrylamide gel were stained with Coomassie Brilliant Blue R-250.
RNA blotting
Total RNA was isolated from mammary glands using TRIzol® Reagent (Invitrogen), separated on denatured agarose gels, and blotted on to nylon membranes. The membrane was hybridized with the MFG-E8 cDNA probe, which had been labelled with [α-32P]dCTP using Rediprime II (Amersham Biosciences) and purified with a ProbeQuant G-50 Micro Column (Amersham Biosciences) in Ultra-Hyb buffer (Ambion) at 42 °C overnight. The membrane was washed and exposed to X-ray films.
Immunohistochemical analysis
Mammary glands were dissociated from lactating or involuting mice, and were immediately embedded and frozen with OCT compound (Tissue-Tec) at −80 °C. Cryosections (7–10 μm) were fixed with 100% methanol or 4% (w/v) paraformaldehyde at room temperature for 20 min, washed three times with PBS, blocked with PBS containing 2% BSA at room temperature for 30 min, and reacted with primary antibody at 4 °C overnight. Sections were then washed with PBS for 15 min and incubated with Alexa Fluor® 488 (green) or Alexa Fluor® 568 (red)-conjugated secondary antibody (Molecular Probes) and 100 ng/ml PI (propidium iodide) (Sigma) or 0.1 mM TOTO-3 iodine (Molecular Probes) at room temperature for 30 min. Finally, sections were washed three times with PBS and once with distilled water, and were observed by confocal laser-scanning fluorescence microscopy (Axioplan 2; Zeiss).
Fractionation of milk by sucrose density-gradient ultracentrifugation
Whole milk (corresponding to 5 μl) samples were layered on a linear sucrose gradient (10–70% sucrose in PBS) prepared with Gradient Mate device (BioComp) in a Beckman SW41 tube which was centrifuged at 200000 g for 18 h. Gradient fractions of 900 μl were collected from the top of the tube (12 fractions in total) and immediately used for the PS-binding analyses. The residual parts were subjected to TCA (trichloroacetic acid) precipitation, followed by SDS/10% PAGE and immunoblotting.
ELISA-based phospholipid-binding assay
The ELISA for MFG-E8 binding to solid-phase phospholipid was performed as described previously [25]. L-α-phosphatidyl-L-serine (Sigma) or L-α-phosphatidylcholine (Sigma) in methanol (10 μg/ml) was added to microwell plates (Nunc) (30 μl/well) followed by drying at 37 °C. The plates were washed three times between all subsequent steps with PBS containing 0.05% (v/v) Tween 20. The plates were blocked with 200 μl of PBS containing 0.25% (w/v) gelatin (blocking buffer). Whole milk diluted in 100 μl of PBS or each gradient fraction (50 μl) obtained by ultracentrifugation were added to wells, followed by incubation at 4 °C overnight. The plates were then incubated with anti-MFG-E8 serum and horseradish-peroxidase-labelled goat anti-(rabbit IgG) as the secondary antibody, and peroxidase activity was measured.
Detection of apoptotic mammary epithelial cells
For apoptosis induction, HC11 mammary epithelial cells were treated with 150 nM okadaic acid (Sigma) for 18 h. Apoptotic and non-apoptotic cells were fixed with 4% (w/v) paraformaldehyde for 20 min and permeabilized with 0.5% (v/v) Triton X-100 in PBS for 10 min at room temperature. Apoptotic cells were detected by using the Dead End Fluorometric TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) System (Promega) according to the manufacturer's instructions, or anti-(cleaved caspase 3) (Asp175) antibody (Cell Signaling Technology) followed by Alexa Fluor® 488-conjugated anti-rabbit IgG.
Binding assay of MFG-E8 to apoptotic mammary epithelial cells
Apoptotic and non-apoptotic cells were incubated at 37 °C for 1 h with whole-milk samples derived from lactating and involuting mice, which had been diluted 1:500 with serum-free Dulbecco's modified Eagle's medium. Cells were washed three times with PBS, fixed with 2% (w/v) paraformaldehyde for 20 min at room temperature, washed with PBST [PBS containing 0.05% (v/v) Tween 20], and blocked in PBS containing 2% (w/v) BSA for 30 min at room temperature. After brief washing with PBST, cells were reacted sequentially with anti-MFG-E8 antibody, Alexa Fluor® 488-conjugated goat anti-rabbit antibody and PI. Finally, cells were washed three times with PBST and twice with distilled water, and were observed by fluorescence microscopy.
For J774 macrophage binding, apoptotic HC11cells (5×105 cells/well of 24-well plate) were previously biotinylated with 1 mg/ml of biotinylation reagent (EL-Link™ sulpho-N-hydroxysuccinimido-biotin; Pierce) at 37 °C for 30 min, and then incubated with mouse milk samples as above. The biotinylated cells were added to J774 cells (5×105) in culture and were co-cultured for 1 h. Following extensive washing, both cell types were fixed with 4% (w/v) paraformaldehyde and were reacted with specific antibody against macrophage marker (CD68) for 18 h at 4 °C. Biotinylated HC11 and CD68-positive J774 cells were detected with Alexa Fluor® 568-conjugated streptavidin (Molecular Probes) and Alexa Fluor® 488-conjugated anti-rabbit IgG respectively. Nuclei were counter-stained with DAPI (4,6-diamidino-2-phenylindole).
RESULTS
MFG-E8 protein and mRNA were increased by either natural or forced weaning in the mammary glands
In our previous study on the gene expression of MFGM proteins, including MFG-E8, in various gestation and lactation stages of mouse mammary glands, we have found that MFG-E8 was up-regulated similarly to the other milk protein in the lactation stage, and also that MFG-E8 was not down-regulated, but remained constant at the end of lactation before natural weaning [24]. This prompted us to examine the MFG-E8 expression in more detail in the different developmental stages of mammary gland, including involution.
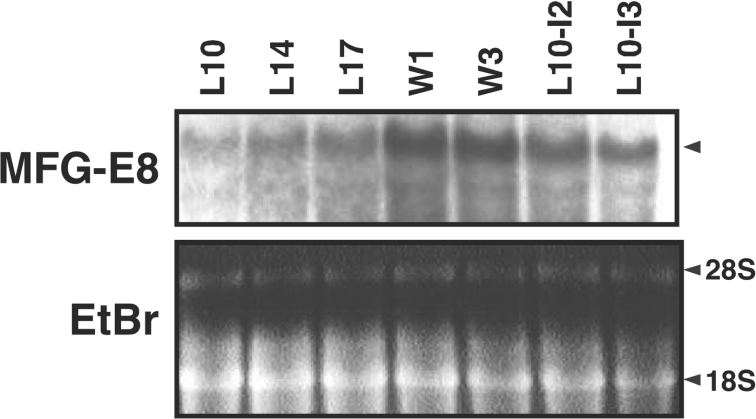
As shown in Figure 1, by forced weaning during the lactation stages as well as natural weaning at post-lactation, levels of MFG-E8 protein in the mammary tissue markedly increased, whereas the major milk proteins, α- and β-casein, remained constant or even decreased. Mammary epithelial marker protein K18 was also evenly detected. The MFG-E8 protein increase was more strongly induced by forced weaning at a mid-lactation stage (day 10) than at an early-lactation stage (day 2). Consistent with protein data, MFG-E8 transcripts were also up-regulated in the tissue during the post-weaning periods (Figure 2).
Figure 1. Increase in MFG-E8 protein in involuting mammary glands.
(A–C) Mammary glands were excised from two independent lactating and involuting mice. Tissue homogenates were solubilized with Laemmli buffer and separated by SDS/10% PAGE followed by blotting on to PVDF membranes. The membranes were sequentially probed with anti-MFG-E8, anti-caseins and anti-(cytokeratin 18) (K18) antibodies. (A) Day 2 of lactating mice, and the mice 1, 2, and 3 days after forced weaning from lactation day 2. (B) Day 10 of lactating mice, and the mice 1, 2, and 3 days after forced weaning from lactation day 10. (C) Days 14 and 17 of lactating mice, and mice 1 and 3 days after natural weaning (day 20 of lactation). Molecular-mass sizes are given in kDa.
Figure 2. Increase in MFG-E8 transcript in involuting mammary glands.
Mammary glands were excised from the mice during lactation [days 10, 14 and 17 (L10, L14 and L17 respectively)] and involution induced by natural [1 and 3 days after weaning (W1 and W3 respectively)] and forced [2 and 3 days after lactating day 10 (L10-I2 and L10-I3 respectively)] weaning. Total RNAs (20 μg) were separated and hybridized with 32P-labelled mouse MFG-E8 cDNA and 36B4 probes. The ribosomal 28 S and 18 S RNAs were detected by ethidium bromide (EtBr) staining.
MFG-E8 was detected immunohistologically around the epithelium of post-weaning mammary glands more strongly than lactating glands
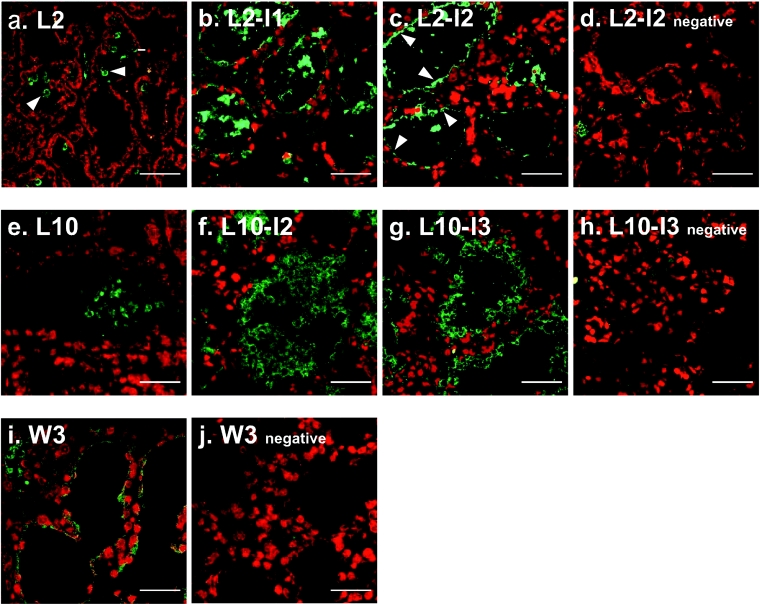
In the lactating mammary glands (L2 and L10), MFG-E8 was undetectable in the epithelium under the conditions used except for MFGs stored in the alveolar lumen (Figure 3). In contrast, in the involuting mammary glands 1 and 2 days after weaning at early-lactation day 2 (L2-I1 and L2-I2) and 3 days after the natural weaning (W3), MFG-E8 was clearly detected around or on the apical surface of the alveolar epithelium. In the involuting mammary glands 2 and 3 days after weaning at mid-lactation day 10 (L10-I2 and L10-I3 respectively), a thick MFG-E8-positive layer was observed along the epithelium in addition to the MFG-E8 signal around the epithelium. This strong signal was assumed to be MFGs translocated to the epithelium from the gland alveolar lumen. These histochemical results in addition to the biochemical data shown in Figures 1 and 2 suggest that the augmented expression of MFG-E8 protein was induced in mammary epithelial cells by weaning, and it could be induced not only after accomplishment of lactation, but also at any stage of lactation.
Figure 3. MFG-E8 in the epithelium of involuting mammary glands.
Mammary glands were excised from lactating day 2 (a) and day 10 (e) mice and involuting mice: 1 day (b) and 2 days (c and d) after pup withdrawal from lactating day 2 mice, 2 days (f) and 3 days (g and h) after pup withdrawal from lactating day 10 mice, and 3 days after natural weaning (i and j). Cryosections (10 μm in thickness) were prepared and probed with anti-MFG-E8 (a–c, e–g and i) or anti-V8 protease (d, h and j; negative control) antibody, and bound antibodies were detected with Alexa Fluor® 488-conjugated anti-rabbit antibody (green). Nuclei were counterstained with PI (red). Arrowheads in (a) show MFG-E8 seen in the lumen as large globules, and arrowheads in (c) indicate the MFG-E8 accumulated in the apical regions of epithelium. Scale bars, 50 μm.
The CD68/MFG-E8 double-positive cells (activated macrophages) were also detected but in small numbers in the post-weaning mammary glands
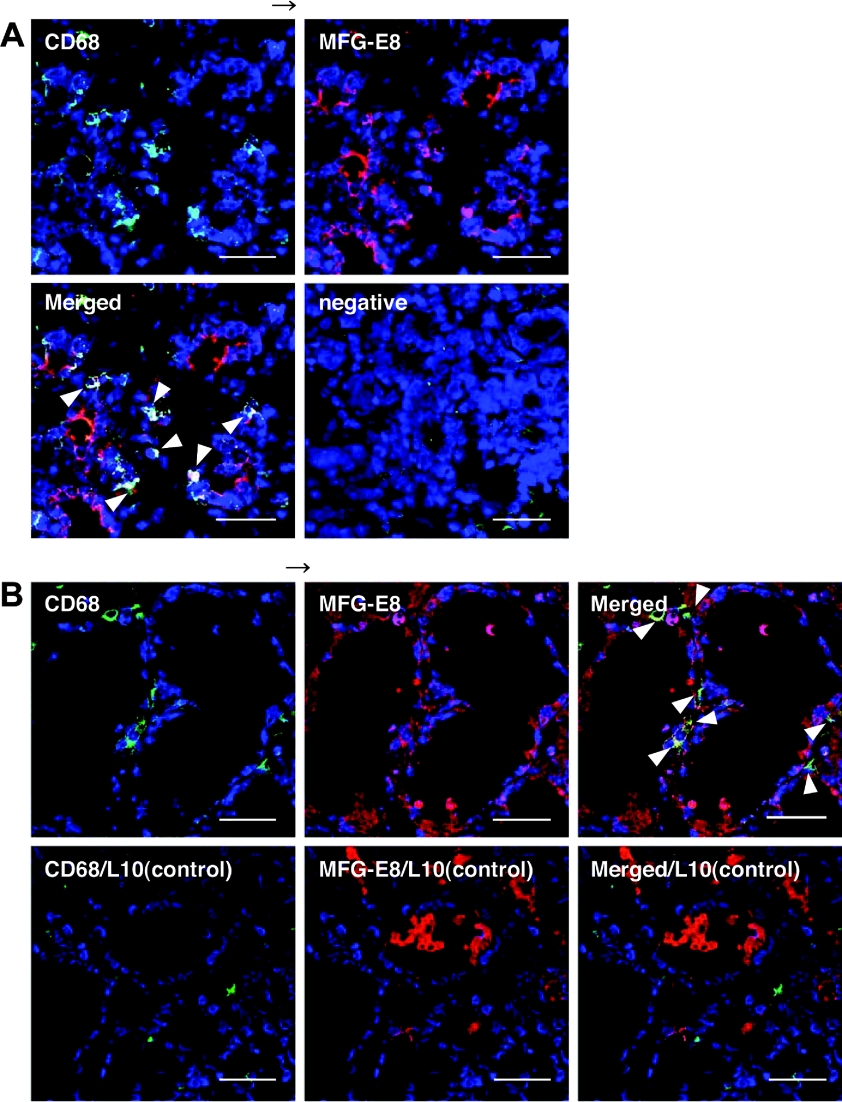
Several CD68-positive cells, activated macrophages, were also detected immunohistologically in the involuting mammary glands 2 or 3 days after the forced weaning at early- (day 2) or mid-lactation (day 10), and most of these CD68-positive cells were also positive to anti-MFG-E8 staining (Figure 4). Only a few signals were observed in the lactating mammary glands under the same conditions as for the immunostaining with anti-CD68 (Figure 4B). MFG-E8 expression and secretion by the mammary tissue macrophages might also contribute in part to the increase in MFG-E8 in the involuting mammary glands. However, the number of MFG-E8-positive- and CD68-negative signals were much more than that of the double-positive signals. Thus the MFG-E8 increase in the post-weaning mammary glands could be ascribed mainly to the mammary epithelial cells.
Figure 4. MFG-E8/CD68 (macrosialin)-double-positive cells in the involuting mammary glands.
Mammary glands were excised from involuting mice induced by 2-day pup withdrawal from lactating day 2 (A) and 3-day pup withdrawal from lactating day 10 (B, upper row). Mammary glands from lactating day 10 were used as a control (B, lower row). Cryosections (10 μm in thickness) were prepared, and probed with anti-CD68 (green), anti-MFG-E8 (red) or non-immune antibody as a negative control (in A). Nuclei were counterstained with TOTO-3 iodine (blue). Co-localization of CD68-positive macrophages and MFG-E8 in yellow are indicated by arrowheads. Scale bars, 50 μm.
MFG-E8 was secreted into milk by the post-weaning mammary glands and had an ability to bind to PS and apoptosis-induced HC11 cells in vitro
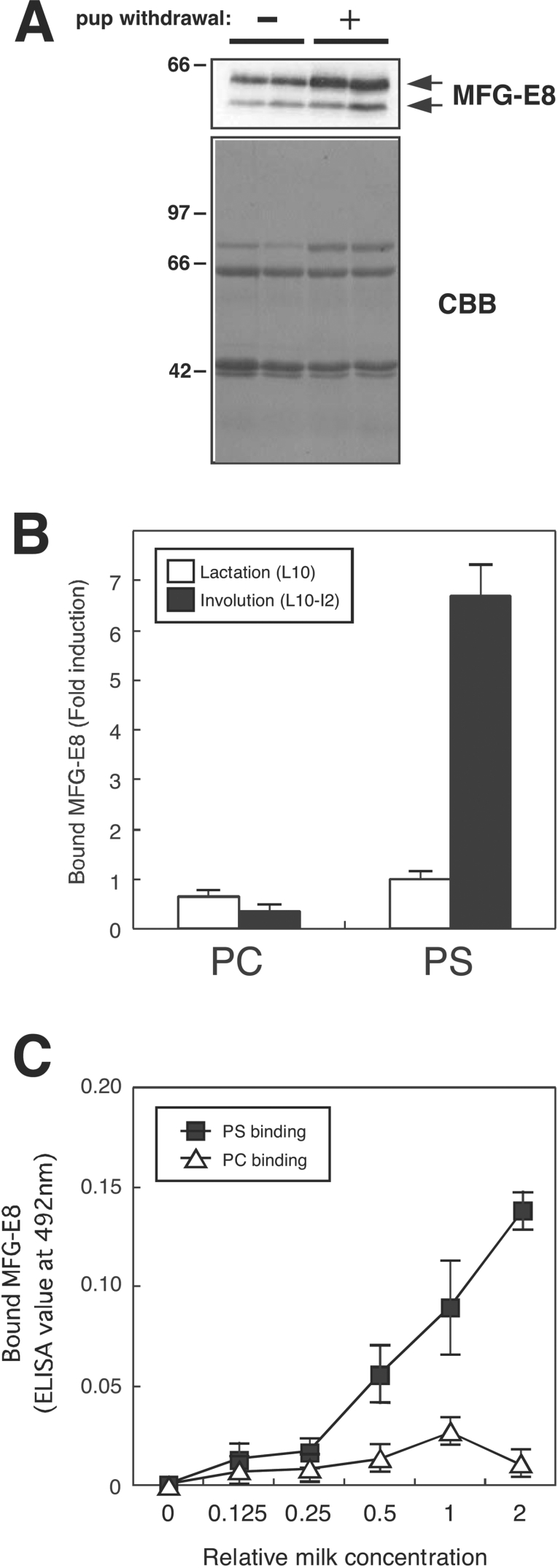
The immunoblotting analysis (Figure 5A) revealed that the total MFG-E8 content in milk recovered from the post-weaning mammary glands 2 days after the forced weaning at lactation day 2 (L2-I2) was higher than that of the corresponding lactating gland (lactation day 4, L4) on a protein weight basis. However, such a difference in MFG-E8 content between the two samples from lactating and involuting mammary glands was not so remarkable as compared with that of the mammary tissue samples (see Figure 1). In contrast with the total MFG-E8 content, the free MFG-E8 as measured by the PS-binding assay was markedly high in the milk from post-weaning mammary glands (Figure 5B). The PS-binding activity of MFG-E8 increased in a dose-dependent manner, while the PC (phosphatidylcholine)-binding activity was marginal (Figure 5C), consistent with our previous results using recombinant MFG-E8 expressed and secreted by COS7 cells [28].
Figure 5. Increased amounts and PS-binding of MFG-E8 in the milk from involuting mammary glands.
(A) Milk was collected from lactating day 2 mice and involuting mice, 2 days after pup withdrawal from lactating day 2 mice. Aliquots (0.08 μl) of whole-milk samples (corresponding to 0.08 μl) were separated by SDS/10% PAGE followed by immunoblotting with anti-MFG-E8 (upper panel) or Coomassie Brilliant Blue (CBB) staining (lower panel). Molecular-mass sizes are indicated in kDa. (B) Whole-milk samples as in (A) were diluted 1:1000 in PBS and added to the microwell plates, which had been coated with L-α-phosphatidyl-L-serine (PS) or L-α-phosphatidylcholine (PC). Bound MFG-E8 was detected as described in the Materials and methods section. Results are means±S.D. for four independent experiments. (C) Milk samples as in (B) were serially diluted in PBS and processed as above. Concentration of milk diluted 1:1000 in PBS was expressed as 1. Results are means±S.D. for four independent experiments.
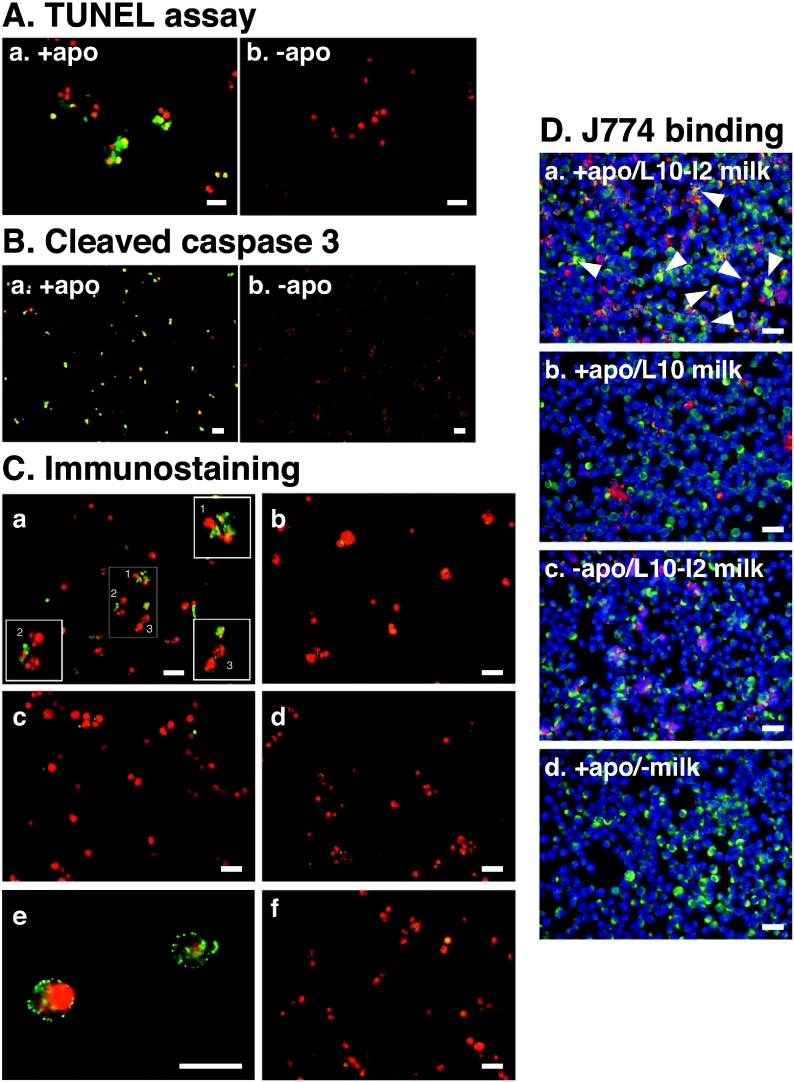
To extend this finding further, milk samples recovered from the post-weaning mammary glands 2 days after the forced weaning at lactation day 2 (L2-I2) were subjected to an intact-cell-based binding assay. A mouse mammary epithelial cell line, HC11, was treated with okadaic acid for the apoptosis induction, and was subsequently incubated with the culture medium supplemented with the MFG-E8-containing milk from the post-weaning mammary glands. Most mammary epithelial cells treated with okadaic acid were positive by TUNEL assay (Figure 6A) and immunostaining with anti-(cleaved caspase 3) antibody (Figure 6B), suggesting the successful induction of apoptosis by treatment with okadaic acid. Several apoptosis-induced HC11 cells exhibited chromatin condensation, but none of the control healthy cells was clearly labelled with MFG-E8 of milk recovered from the post-weaning mammary glands (Figure 6C, panels a and b). Further magnification of the apoptotic cells revealed speckled localization of MFG-E8 on the cell surface (Figure 6C, panel e). When cultured in the medium without the MFG-E8-containing milk, even the apoptosis-induced HC11 cells were completely negative to the anti-MFG-E8 staining (Figure 6C, panel d). The cultured HC11 cells itself expressed and secreted MFG-E8 into the culture medium (results not shown). The level of such endogenous MFG-E8 was assumed to be much lower than that of milk from the post-weaning mammary gland and to be below the detection limit of our experimental conditions.
Figure 6. Specific binding of MFG-E8 derived from involuting mammary glands to apoptotic mammary epithelial cells.
(A and B) HC11 cells were cultured as described in the Materials and methods section and apoptosis was induced. Apoptotic cells (+apo) and non-apoptotic healthy cells (−apo) were assessed using TUNEL assay (A) or immunostaining with anti-(cleaved caspase 3) antibody (B). (C) Whole-milk samples from lactating (panel b) and involuting (panels a, c, e and f) mammary glands as described in Figure 5 were diluted and added to apoptotic (panels a, b and d–f) or non-apoptotic (panel c) HC11 mammary epithelial cells. No milk was added as a control (panel d). Following incubation and washing, each well was incubated with anti-MFG-E8 (panels a–e) or anti-V8 (panel f) antibody, and bound antibodies were detected with Alexa Fluor® 488-conjugated anti-rabbit antibody (green). Nuclei were counterstained with PI (red). In panel a, numbered cells in the centre section are shown with further magnification in the corresponding numbered squares in the same panel. Much further magnification of apoptotic cells with bound MFG-E8 are shown in panel e. (D) Apoptotic (panels a, b and d) or non-apoptotic (panel c) HC11 cells were labelled by biotinylation, pre-treated with lactating (panel b) or involuting (panels a and c) milk, and incubated with J774 macrophages. In panel c, cells were pre-treated with no milk. Biotinylated HC11 cells and CD68-positive J774 macrophages were detected with Alexa Fluor® 568-conjugated streptavidin (red) and Alexa Fluor® 488-conjugated anti-rabbit IgG (green) respectively. Nuclei were counterstained with DAPI (blue). Note that co-localization of biotinylated apoptotic HC11 cells and CD68-positive J774 macrophages is obvious in panel a, as indicated by arrowheads. Scale bars, 50 μm.
To confirm that MFG-E8 acts as a link between apoptotic cells and phagocytes such as macrophages, apoptotic cells were labelled by biotinylation, incubated with the MFG-E8-containing milk, and then added to J774 macrophages. The apoptotic macrophages obviously bound to apoptotic HC11 cells more frequently than they bound to J774 cells, when pre-treated with the MFG-E8-containing milk from the post-weaning mammary glands (Figure 6D, panel a). Much less co-localization with macrophages of the apoptotic cells pre-treated with lactating milk (Figure 6D, panel b) or the non-apoptotic cells pre-treated with post-weaning milk (Figure 6D, panel c) was observed. These results support the idea that MFG-E8 in the post-weaning milk plays a role in tethering of apoptotic cells to phagocytes during involution.
Predominant distribution of MFG-E8 PS-binding activity in the milk membrane vesicle fractions
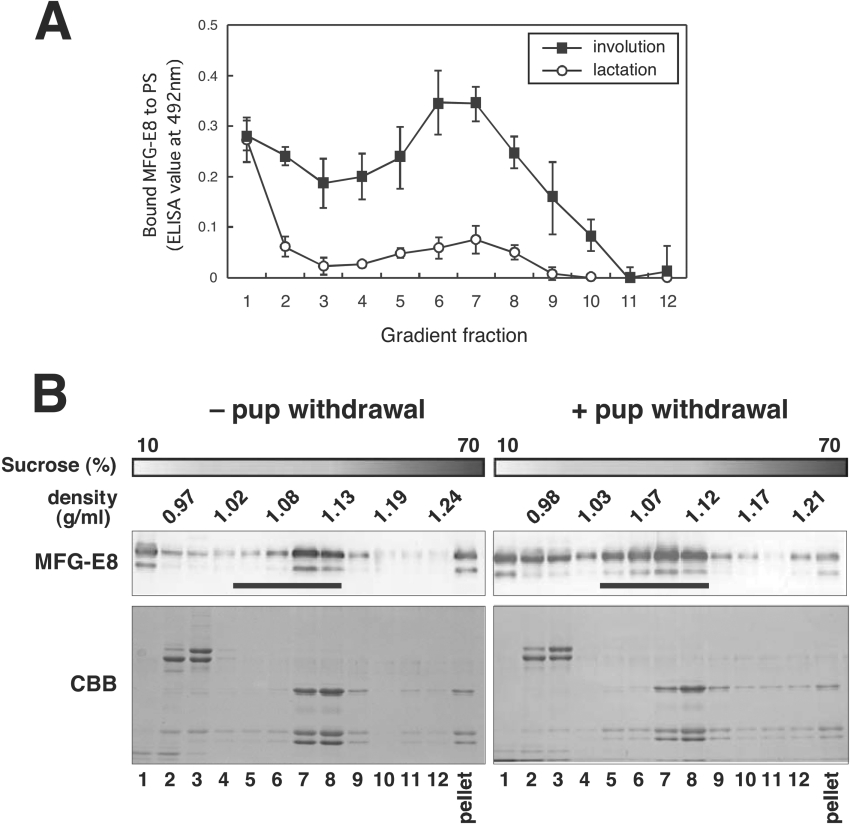
It has been reported that substantial amounts of MFG-E8 are also present in the whey fraction [29–31], which is free from fat, although it was originally identified as a major component of MFGM [9]. To better understand the increase in PS-binding of MFG-E8 of milk retained in involuting mammary glands, whole-milk samples were subjected to fractionation on sucrose density gradient ranging from 10 to 70% concentration. As shown in Figure 7(A), fractions 2–10 derived from involuting glands exhibited significantly higher MFG-E8 PS-binding activity over those from lactating ones, and except for the low-density top fraction containing MFGs, the PS-binding activity was peaked in fractions 6 and 7 with densities ranging from 1.07 to 1.12, which met the criteria for ELMV (exosome-like membrane vesicle) fractions [32]. On the other hand, PC-binding activity of these fractions from both lactating and involuting mice was negligible (results not shown).
Figure 7. Predominant distribution of MFG-E8 with PS-binding activity in the milk ELMV fractions.
(A) Whole-milk samples (5 μl) as described in Figure 5 were fractionated by sucrose density-gradient (10–70%) ultracentrifugation. Each density-gradient fraction (900 μl per fraction) was collected from the top, added to PS- or PC-coated plates, and processed as described in Figure 5. Results are means±S.D. for four independent experiments. (B) Each gradient fraction (900 μl per fraction) was collected from the top (corresponding to lane 1), precipitated with TCA and separated by SDS/10% PAGE followed by immunoblotting with anti-MFG-E8 antibody (upper panels) or Coomassie Brilliant Blue (CBB) staining (lower panels). Pellets were directly dissolved in Laemmli buffer and processed as above. ELMV fractions (lanes 5–8, and 17–21) are indicated by bars.
Using immunoblotting, MFG-E8 protein of milk from lactating glands was detected mainly in the top, middle and pellet fractions (Figure 7B, left-hand panels). Among the three fractions, only the top fraction containing MFGs exhibited reasonable PS-binding activity. In contrast, the milk samples from post-weaning glands showed the increased MFG-E8 protein as a whole, especially in the intermediate fractions, with densities ranging from 1.04 to 1.12 (Figure 7B, right-hand panels), which nearly met the profile of MFG-E8 PS-binding activity (Figure 7A).
DISCUSSION
Apoptosis of mammary epithelial cells upon weaning is a critical developmental process in mammary glands, and the rapid removal of apoptotic cells ensures appropriate tissue remodelling for the subsequent pregnancy. During the involution process, a set of genes has been reported to be transcrptionally regulated [6] and assumed to be associated with clearance of apoptotic cells. However, it has not been well documented how apoptotic mammary epithelial cells are recognized, engulfed and eliminated by phagocytic cells.
In the present study, the MFG-E8 protein in mammary tissue was demonstrated by immunoblotting to increase on a tissue wet weight basis after weaning (Figure 1). Rigorous determination of expression at protein level would not be easy because milk proteins, including MFG-E8, had accumulated in the gland alveolar lumen before weaning and the mammary gland tissue samples could not completely be free from milk in the lumen. Based on the histochemical observation (Figure 3) and the biochemical data of MFG-E8 (Figure 5A), we can conclude that the MFG-E8 protein increase observed in the tissue samples of involuting mammary gland (Figure 1) is the sum of increased MFG-E8 protein in both the cellular and the secreted proteins. Moreover, at the resolution of fluorescence microscopic observation, it is unclear whether the MFG-E8 protein localized in the intracellular space or on the apical surface of the alveolar epithelium. Thus, strictly speaking, MFG-E8 protein around the epithelium would include the MFG-E8 which had been secreted in the lumen and subsequently bound to the surface of apoptotic epithelial cells, because MFG-E8 in the milk from involuting mammary glands in fact bound to apoptotic HC11 cells (Figure 6). In either event, it is presumed that up-regulation of MFG-E8 expression at the protein level is induced in involuting mammary glands. Such an increase in MFG-E8 protein is consistent with the up-regulation at a transcriptional level as shown by RNA blotting (Figure 2) as well as the previously reported microarray data [6].
Fewer numbers of CD68/MFG-E8-double-positive cells were detected in the involuting mammary glands after weaning at either early- (day 2) or mid- (day 10) lactation stage (Figure 4). These findings are consistent with the results of the previous work showing that macrophages were present in the mammary glands only in low numbers during lactation and in the initial stage of involution and that their numbers began to increase on day 3 post-weaning and continue to increase up until day 10 post-weaning [33]. These immunohistological observations suggest that macrophages were indeed recruited in the involuting mammary glands and expressed MFG-E8. However, the CD68/MFG-E8-double-positive cells were detected in limited parts of the mammary gland, whereas MFG-E8-positive cells were found in the major part of alveolar epithelium. Our present data, together with the results of previous work [33], suggest that activated macrophages is not the major cause of the MFG-E8 increase in involuting mammary glands, at least during a few days after weaning. The mammary epithelial cells themselves would act as non-professional phagocytes and express and secrete MFG-E8 for the effective phagocytosis of apoptotic bodies shed in the lumen and/or neighbouring apoptotic epithelial cells.
The MFG-E8 concentration in the post-weaning milk was suggested by immunoblotting to be markedly higher than that of milk from lactating glands (Figure 5A). During mammary gland involution, the milk constituents remaining in the alveolar lumen ought to be cleared through pinocytosis and/or phagocytosis by professional and/or non-professional cells. However, within 2 days of weaning, no large changes in the major milk protein, casein, have yet been caused in the alveolar lumen, as demonstrated by SDS/10% PAGE analysis of whole milk (Figure 5A). Therefore it could be presumed that MFG-E8 increased in the milk of involuting mammary glands, which is exceptional for milk proteins. It is of interest to note that the binding activities of MFG-E8 in the milk from lactating glands to the solid-phase PS and the apoptosis-induced HC11 cells were much lower than that of the post-weaning milk (Figures 5 and 6), even though the milk from lactating mammary glands indeed contained a substantial amount of MFG-E8 (Figure 5A). Moreover, MFG-E8 in the post-weaning milk could tether apoptotic HC11 cells to J774 macrophages efficiently (Figure 6D). Such differences in PS-binding activity per MFG-E8 protein between the two milk samples from involuting and lactating mammary glands was more remarkable when the milk samples were fractionated by density gradient ultracentrifugation (Figure 7). The MFG-E8 protein contents in the top and middle fractions (numbers 1 and 7 respectively) were comparable between the two milk samples based on their immunoblotting band intensities. In accordance with the MFG-E8 protein contents, the PS-binding activities of the top fractions of the two samples were almost equal. These top fractions with a density below 1.0 are rich in MFGs. Interestingly, however, the middle fraction (number 7) of the milk from lactating glands exhibited markedly low PS-binding activity in spite of the presence of MFG-E8 protein. In other words, the PS-binding activity of MFG-E8 was suppressed in the lactating mammary glands as compared with that of the involuting glands. These results indicate that the weaning-induced increase in the PS-binding activity of MFG-E8 is due not only to the MFG-E8 protein increase, but also to some unknown quality changes such as the association state of MFG-E8 with fat globules in milk. It would be of special interest to determine whether MFG-E8 in the post-weaning milk associates with PS of MFGs or some other phospholipid membranes in milk described below.
In milk, in addition to association with MFGs, MFG-E8 is known to be present in the whey fraction, free from fat [29–31]. In our previous study, MFG-E8 was also found to be present in the membrane vesicle fraction of culture supernatant of mammary epithelial COMMA-1D cells [28]. In the present study, we demonstrated for the first time that MFG-E8 also exists in the exosome-like membrane fractions (densities ranging from 1.04 to 1.12) in milk (Figure 5). Thus it would be likely that MFG-E8 associating with the ELMVs contribute to the weaning-induced increase or lactation-dependent suppression in the PS-binding activity of MFG-E8 in milk.
Since the present paper was first submitted, two independent groups have reported that MFG-E8 is a critical protein for mammary gland remodelling during the involution process with MFG-E8-knockout mice [34,35]. A deficiency of MFG-E8 caused delayed clearance of apoptotic mammary epithelial cells as well as MFGs and impaired involution and inflammation of mammary glands, strongly supporting our results that MFG-E8 mediated phagocytosis of apoptotic mammary epithelial cells.
Acknowledgments
We thank Dr David Flint for critical reading of this manuscript. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture in Japan (to N. A. and T. M.).
References
- 1.Green K. A., Streuli C. H. Apoptosis regulation in the mammary gland. Cell. Mol. Life Sci. 2004;61:1867–1883. doi: 10.1007/s00018-004-3366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lund J. M., Alexopoulou L., Sato A., Karow M., Adams N. C., Gale N. W., Iwasaki A., Flavell R. A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monks J., Geske F. J., Lehman L., Fadok V. A. Do inflammatory cells participate in mammary gland involution? J. Mammary Gland Biol. Neoplasia. 2002;7:163–176. doi: 10.1023/a:1020351919634. [DOI] [PubMed] [Google Scholar]
- 4.Walker N. I., Bennett R. E., Kerr J. F. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am. J. Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- 5.Abrahams V. M., Kim Y. M., Straszewski S. L., Romero R., Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am. J. Reprod. Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson R. W., Wayland M. T., Lee J., Freeman T., Watson C. J. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature (London) 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 8.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 9.Stubbs J. D., Lekutis C., Singer K. L., Bui A., Yuzuki D., Srinivasan U., Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. U.S.A. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki N., Kishi M., Taniguchi Y., Adachi T., Nakamura R., Matsuda T. Molecular cloning of glycoprotein antigens MGP57/53 recognized by monoclonal antibodies raised against bovine milk fat globule membrane. Biochim. Biophys. Acta. 1995;1245:385–391. doi: 10.1016/0304-4165(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 11.Hvarregaard J., Andersen M. H., Berglund L., Rasmussen J. T., Petersen T. E. Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. Eur. J. Biochem. 1996;240:628–636. doi: 10.1111/j.1432-1033.1996.0628h.x. [DOI] [PubMed] [Google Scholar]
- 12.Larocca D., Peterson J. A., Urrea R., Kuniyoshi J., Bistrain A. M., Ceriani R. L. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991;51:4994–4998. [PubMed] [Google Scholar]
- 13.Couto J. R., Taylor M. R., Godwin S. G., Ceriani R. L., Peterson J. A. Cloning and sequence analysis of human breast epithelial antigen BA46 reveals an RGD cell adhesion sequence presented on an epidermal growth factor-like domain. DNA Cell Biol. 1996;15:281–286. doi: 10.1089/dna.1996.15.281. [DOI] [PubMed] [Google Scholar]
- 14.Ogura K., Nara K., Watanabe Y., Kohno K., Tai T., Sanai Y. Cloning and expression of cDNA for O-acetylation of GD3 ganglioside. Biochem. Biophys. Res. Commun. 1996;225:932–938. doi: 10.1006/bbrc.1996.1274. [DOI] [PubMed] [Google Scholar]
- 15.Basch J. J., Farrell H. M., Greenberg R. Identification of the milk fat globule membrane proteins. I. Isolation and partial characterization of glycoprotein B. Biochim. Biophys. Acta. 1976;448:589–598. doi: 10.1016/0005-2736(76)90112-7. [DOI] [PubMed] [Google Scholar]
- 16.Peterson J. A., Couto J. R., Taylor M. R., Ceriani R. L. Selection of tumor-specific epitopes on target antigens for radioimmunotherapy of breast cancer. Cancer Res. 1995;55:5847–5851. [PubMed] [Google Scholar]
- 17.Mather I. H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000;83:203–247. doi: 10.3168/jds.S0022-0302(00)74870-3. [DOI] [PubMed] [Google Scholar]
- 18.Andersen M. H., Berglund L., Rasmussen J. T., Petersen T. E. Bovine PAS-6/7 binds αvβ5 integrins and anionic phospholipids through two domains. Biochemistry. 1997;36:5441–5446. doi: 10.1021/bi963119m. [DOI] [PubMed] [Google Scholar]
- 19.Andersen M. H., Graversen H., Fedosov S. N., Petersen T. E., Rasmussen J. T. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 20.Peterson J. A., Patton S., Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate. 1998;74:143–162. doi: 10.1159/000014020. [DOI] [PubMed] [Google Scholar]
- 21.Pellequer J. L., Gale A. J., Griffin J. H., Getzoff E. D. Homology models of the C domains of blood coagulation factors V and VIII: a proposed membrane binding mode for FV and FVIII C2 domains. Blood Cells Mol. Dis. 1998;24:448–461. doi: 10.1006/bcmd.1998.0214. [DOI] [PubMed] [Google Scholar]
- 22.Macedo-Ribeiro S., Bode W., Huber R., Quinn-Allen M. A., Kim S. W., Ortel T. L., Bourenkov G. P., Bartunik H. D., Stubbs M. T., Kane W. H., Fuentes-Prior P. Crystal structures of the membrane-binding C2 domain of human coagulation factor V. Nature (London) 1999;402:434–439. doi: 10.1038/46594. [DOI] [PubMed] [Google Scholar]
- 23.Pratt K. P., Shen B. W., Takeshima K., Davie E. W., Fujikawa K., Stoddard B. L. Structure of the C2 domain of human factor VIII at 1.5 Å resolution. Nature (London) 1999;402:439–442. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]
- 24.Aoki N., Ishii T., Ohira S., Yamaguchi Y., Negi M., Adachi T., Nakamura R., Matsuda T. Stage specific expression of milk fat globule membrane glycoproteins in mouse mammary gland: comparison of MFG-E8, butyrophilin, and CD36 with a major milk protein, β-casein. Biochim. Biophys. Acta. 1997;1334:182–190. doi: 10.1016/s0304-4165(96)00091-8. [DOI] [PubMed] [Google Scholar]
- 25.Andersen M. H., Berglund L., Rasmussen J. T., Petersen T. E. Bovine PAS-6/7 binds αvβ5 integrins and anionic phospholipids through two domains. Biochemistry. 1997;36:5441–5446. doi: 10.1021/bi963119m. [DOI] [PubMed] [Google Scholar]
- 26.Oshima K., Aoki N., Negi M., Kishi M., Kitajima K., Matsuda T. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem. Biophys. Res. Commun. 1999;254:522–528. doi: 10.1006/bbrc.1998.0107. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Oshima K., Aoki N., Kato T., Kitajima K., Matsuda T. Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur. J. Biochem. 2002;269:1209–1218. doi: 10.1046/j.1432-1033.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- 29.Feuerhake F., Sigg W., Hofter E. A., Dimpfl T., Welsch U. Immunohistochemical analysis of Bcl-2 and Bax expression in relation to cell turnover and epithelial differentiation markers in the non-lactating human mammary gland epithelium. Cell Tissue Res. 2004;299:47–58. doi: 10.1007/s004419900127. [DOI] [PubMed] [Google Scholar]
- 30.Butler J. E., Pringnitz D. J., Martens C. L., Crouch N. Bovine-associated mucoprotein: I. Distribution among adult and fetal bovine tissues and body fluids. Differentiation. 1980;17:31–40. doi: 10.1111/j.1432-0436.1980.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 31.Mather I. H., Bruder G., Jarasch E. D., Heid H. W., Johnson V. G. Protein synthesis in lactating guinea-pig mammary tissue perfused in vitro. II. Biogenesis of milk-fat-globule membrane proteins. Exp. Cell Res. 1984;151:277–282. doi: 10.1016/0014-4827(84)90378-1. [DOI] [PubMed] [Google Scholar]
- 32.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 33.Lund L. R., Romer J., Thomasset N., Solberg H., Pyke C., Bissell M. J., Dano K., Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanayama R., Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16886–16891. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atabai K., Fernandez R., Huang X., Ueki I., Kline A., Li Y., Sadatmansoori S., Smith-Steinhart C., Zhu W., Pytela R., Werb Z., Sheppard D. Mfge8 is critical for mammary gland remodeling during involution. Mol. Biol. Cell. 2005;16:5528–5537. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]