Abstract
The chipmunk hibernation-specific HP-27 gene is expressed specifically in the liver and has a CpG-poor promoter. To reveal how the liver-specific transcription of the HP-27 gene is regulated, we performed yeast one-hybrid screening of a chipmunk liver cDNA library. A 5′-flanking sequence of the HP-27 gene, extending from −170 to −140 and containing an E-box (5′-CACGTG-3′), is essential for the liver-specific transcription of HP-27. We used this sequence as bait and found that a ubiquitously expressed transcription factor, USF (upstream stimulatory factor), bound to the E-box. In COS-7 cells, USF activated transcription from the HP-27 gene promoter. We then used bisulphite genomic sequencing to analyse the methylation status of the four CpG dinucleotides that lie in the 5′-flanking sequence of the HP-27 gene up to −450, to investigate how the ubiquitously expressed USF activates transcription of the HP-27 gene only in the liver, while its transcription is repressed elsewhere. The only difference in methylation in the tissues tested was in the CpG dinucleotide in the USF-binding site, which was hypomethylated in the liver, but highly methylated in the kidney and heart. The specific methylation of the CpG dinucleotide at the USF-binding site impeded both the binding of USF and its transcriptional activation of the HP-27 gene. Chromatin immunoprecipitation using anti-USF antibodies revealed that USF bound to the HP-27 gene promoter in the liver, but not in the kidney or heart. Thus CpG methylation at the USF-binding site functions in establishing and maintaining tissue-specific transcription from the CpG-poor HP-27 gene promoter.
Keywords: chipmunk, CpG methylation, hibernation, HP-27 gene, tissue-specific transcription, upstream stimulatory factor (USF)
Abbreviations: ChIP, chromatin immunoprecipitation; EMSA, electrophoretic mobility-shift assay; GFAP, glial fibrillary acidic protein; HP, hibernation-specific protein; IP, immunoprecipitation; rPL-I, rat placental lactogen-I; USF, upstream stimulatory factor
INTRODUCTION
Mammalian hibernation is a unique physiological adaptation that allows the sustainment of life at extremely low body temperatures. For most mammals that maintain a high steady body temperature throughout their adult lives, lowering the body temperature brings about the dysfunction of physiological systems, resulting in death. Only certain small mammals, primarily in the orders Rodentia, Insectivora and Chiroptera, can undergo hibernation, during which the body temperature falls to below 10 or even 5 °C [1]. Concomitantly, their heart and breathing rates also fall, and their metabolic rate is reduced to only a few percent of the euthermic level [2], resulting in a considerable conservation of energy [3]. These hibernation-associated physiological changes are assumed to be under genetic control. Although there is accumulating evidence for differential expression during hibernation [4–9], the molecular mechanisms underlying hibernation remain to be revealed.
In the squirrel family, some species, such as the chipmunk (Tamias asiaticus) and 13-lined ground squirrel (Citellus tridecemlineatus), hibernate; other species, such as the tree squirrel (Callosciurus caniceps), do not. The chipmunk hibernation-specific protein HP-27 was identified as a component of a 140-kDa complex that decreases drastically in the blood during hibernation [10]. The complex contains four proteins: HP-20, -25, -27 and -55. Of these, HP-20, -25, and -27 are homologous with each other and contain collagen-like Gly-Xaa-Yaa repeats near the N-terminus [5,10]. In the chipmunk and 13-lined ground squirrel, the HP-20, -25 and -27 genes are expressed specifically in the liver and are down-regulated during hibernation [5]. Although the tree squirrel genome also contains these genes, their expression is not detectable [5]. The chipmunk HP-20, -25 and -27 genes have well-conserved gene structures, and are thought to have arisen through gene duplication [11–13]. The liver-specific transcription of the chipmunk HP-20 and -25 genes is regulated by the liver-enriched transcription factors HNF-1 (hepatocyte nuclear factor 1) and -4 respectively [11,12]. In a previous study, we showed that the 170-bp 5′-flanking sequence of the chipmunk HP-27 gene contains the promoter for the liver-specific transcription, and that a transcription factor that binds to the region from −170 to −140 plays an important role in HP-27 gene transcription [13], although the molecular mechanisms leading to the liver-specific transcription have not been elucidated.
In the present study, we isolated cDNA clones for a transcription factor that bound to the HP-27 gene sequence from −170 to −140 by yeast one-hybrid screening, and found that the ubiquitously expressed transcription factor USF (upstream stimulatory factor) bound to the E-box (5′-CACGTG-3′) in this sequence and activated transcription of the HP-27 gene. These results prompted us to investigate the involvement of an epigenetic mechanism in the regulation of the tissue-specific transcription of the HP-27 gene. We found that CpG methylation at the USF-binding site in the HP-27 gene promoter was important for this liver-specific expression.
MATERIALS AND METHODS
Yeast one-hybrid screening
Six tandem copies of the HP-27 gene sequence from −170 to −140 were placed upstream of the minimal promoter in the pHISi vector (Clontech) to construct pHISi/CM27. pHISi/CM27 was linearized, and then yeast YM4271 (Clontech) was transformed with the construct. Next, the yeast strain harbouring pHISi/CM27 was transformed using the poly(ethylene glycol)/lithium acetate method with DNA from a chipmunk liver cDNA library [11] and plated on to medium lacking leucine and histidine. The library plasmids were rescued from the positive colonies and introduced into Escherichia coli HB101. The cDNA inserts were sequenced after they were subcloned into pBluescript.
EMSA (electrophoretic mobility-shift assay)
Mouse USF1, USF2a and USF2b were synthesized using an in vitro transcription/translation system (Promega). Nuclear extracts from chipmunk liver were prepared as described in [11]. Anti-USF1 and anti-USF2 antibodies were obtained from Santa Cruz Biotechnology. The following oligonucleotide was used as a probe: CM27G-170/-140, 5′-GGGTGCACACGTGACAGCCTGGTGGAAAGTC-3′. A mutant oligonucleotide CM27G-170/-140mut, 5′-GGGTGCACACaTGACAGCCTGGTGGAAAGTC-3′, and a methylated oligonucleotide Me-CM27G-170/-140, 5′-GGGTGCACAmeCGTGACAGCCTGGTGGAAAGTC-3′, were used as competitors. EMSAs and supershift assays were carried out as described in [11].
Construction of luciferase reporter plasmids
The construction of pCM27G-140/luc and pCM27G-170/luc was as described previously [13]. To create BglII and SalI sites upstream of −140 in pCM27G-140/luc, PCR was carried out using pCM27G-170/luc as the template with a primer complementary to the luciferase gene and the following primer: 5′-AGGAAGATGTGGATGGGTCGACCCCTGTGGTTATGCAAGGGT-3′. The PCR product was digested with BglII and HindIII, and was then subcloned between the BglII and HindIII sites of pGV-B. This plasmid was digested with BglII and SalI, and ligated with the following double-stranded oligonucleotides to construct pCM27G-170*/luc, Me-pCM27G-170*/luc, and pCM27G-140*/luc respectively: 5′-GATCGGGTGCACACGTGACAGCCTGGTGGAAAGTC-3′ and 5′-TCGAAGCTTTCCACCAGGCTGTCACGTGTGCACCC-3′; 5′-GATCGGGTGCACAmeCGTGACAGCCTGGTGGAAAGTC-3′ and 5′-TCGAAGCTTTCCACCAGGCTGTCAmeCGTGTGCACCC-3′; 5′-GATCTGATCAGCCTCGACTG-3′ and 5′-AGCTCAGTCGAGGCTGATCA-3′. pGV-B* was constructed by subcloning the following double-stranded oligonucleotide between the BglII and HindIII sites of pGV-B: 5′-GATCTGATCAGCCTCGACTG-3′ and 5′-TCGACAGTCGAGGCTGATCA-3′. The ligation reaction products were phenol-extracted, ethanol-precipitated and resuspended in TE (10 mM Tris/HCl and 1 mM EDTA, pH 8.0), and used for transfections without further manipulation.
Cell culture and transient transfection
HepG2 cells were obtained from RIKEN Bioresource Center, and grown in MEM (minimal essential medium) with 10% (v/v) foetal calf serum. COS-7 cells were cultured as described previously [12]. HepG2 and COS-7 cells were transfected using FuGENE6 (Roche) as described previously [12], and, after 24 h, luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega).
Immunoblotting
Tissue (25 mg) was suspended in 600 μl of cold NPB (10 mM Tris/HCl, pH 7.4, 2 mM MgCl2, 140 mM NaCl, 0.5 mM dithiothreitol and 0.5 mM PMSF) plus 0.1% (v/v) Triton X-100 using a Dounce homogenizer, then layered over a 600 μl cushion of 50% sucrose in NPB in a microfuge tube, and spun at 12000 g for 10 min at 4 °C. The pelleted nuclei were resuspended in gel sample buffer (62 mM Tris/HCl, pH 6.8, 2% SDS, 10% glycerol, 3% 2-mercaptoethanol and 0.04% Bromophenol Blue), boiled and subjected to SDS/10% PAGE and subsequent immunoblotting with antibodies against USF1 and USF2. Detection was performed using the SuperSignal detection system (Pierce).
Bisulphite sequencing
Genomic DNA was prepared using the GenElute Mammalian Genomic DNA Kit (Sigma). After being digested with XhoI, genomic DNA was subjected to sodium bisulphite treatment using the EZ DNA methylation Kit (Zymo Research). The DNA fragment containing the HP-27 gene promoter was amplified by PCR using the following primers: CM27-447(C/T)-F, 5′-AAGGGAGTGGTTTTTATTTAAAATTTAGTG-3′; CM27+100(C/T)-R, 5′-CTCACCCATTTCTAACCATAAACCCTTACA-3′. The PCR products were cloned into pBluescript-KS+, and five clones were sequenced for each tissue.
ChIP (chromatin immunoprecipitation)
ChIP was performed according to the Farnham Laboratory's protocol (http://www.genomecenter.ucdavis.edu/farnham/protocols/tissues.html) with minor modifications. Tissue (500 mg) was cut into small pieces, rinsed once with cold PBS and treated with 1% (w/v) formaldehyde in PBS at room temperature (20 °C) for 10 min. The cross-linking reaction was stopped by adding glycine to a final concentration of 0.125 M. The tissue was recovered by centrifugation at 1000 g for 3 min at 4 °C, rinsed once with cold PBS and then homogenized in 8 ml of cold PBS using a Dounce homogenizer. The cells were recovered by centrifugation at 1000 g for 5 min at 4 °C, resuspended in 4 ml of cell lysis buffer (5 mM Pipes, pH 8.0, 85 mM KCl, 0.5% Nonidet P40) plus protease inhibitors, homogenized using a Dounce homogenizer, and incubated on ice for 15 min. Nuclei were recovered by centrifugation at 3000 g for 5 min at 4 °C, and resuspended in 1 ml of nuclei lysis buffer (50 mM Tris/HCl, pH 8.1, 10 mM EDTA and 1% SDS) plus protease inhibitors. After the addition of 0.4 mg of glass beads (Sigma G-1277), the samples were sonicated until the DNA fragments were 600–1000 bp long. The samples were spun at 3000 g for 1 min at 4 °C, and the supernatant was diluted sixfold with IP (immunoprecipitation) dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris/HCl, pH 8.1, and 167 mM NaCl) plus protease inhibitors. For pre-clearing, 300 μl of the diluted sample per antibody was mixed with 6 mg of BSA, 0.2 μg of normal rabbit IgG and 20 μl of salmon sperm DNA/Protein A–agarose suspension (Upstate), rotated for 60 min at 4 °C and spun at 13000 g for 1 min at 4 °C. The supernatant was mixed with 0.5 μg of normal rabbit IgG or an antibody against USF1 or USF2, or no antibody was added, and rotated overnight at 4 °C. The samples were then mixed with 50 μl of salmon sperm DNA/Protein A–agarose suspension and rotated for 60 min at 4 °C. Immune complexes were collected by centrifugation at 2000 g for 1 min at 4 °C and the supernatant from the ‘no antibody’ sample was stored as the ‘total input sample’. The pelleted immune complexes were washed with 1.2 ml of dialysis buffer (50 mM Tris/HCl, pH 8.0, 2 mM EDTA and 0.2% Sarkosyl) twice, and then with 1.2 ml of IP wash buffer (100 mM Tris/HCl, pH 9.0, 500 mM LiCl, 1% Nonidet P40 and 1% deoxycholic acid) four times. Immune complexes were resuspended in 150 μl of IP elution buffer (50 mM NaHCO3 and 1% SDS), shaken on a vortex mixer for 15 min, and then centrifuged at 2000 g for 1 min at 20 °C. This elution step was repeated, and the eluted samples were combined. DNA–protein cross-links were reversed by incubation with 300 mM NaCl at 67 °C for 4 h for all samples, including the total input sample. The DNA was incubated further with proteinase K at 55 °C for 1 h, and was then purified using the GenElute Mammalian Genomic DNA Purification Kit (Sigma) according to the manufacturer's instructions. PCR was performed with the following set of primers: CM27-321F; 5′-AGTGTACAGTTGTTCTTTTGCTCAC-3′, CM27-19R; 5′-CTCTCCTAAGATCAATAGGTTGGCT-3′.
RESULTS
USF binds the HP-27 gene promoter and activates transcription
Promoter analysis of the HP-27 gene in human hepatoma HepG2 cells has revealed that the 5′-flanking sequence from −170 to −140 is important for its liver-specific transcription [13]. To identify the transcription factor that binds to this 5′-flanking sequence, we performed yeast one-hybrid screening of a chipmunk liver cDNA library using six tandem copies of this sequence as bait. All of the positive clones encoded chipmunk USF2. USF1 and USF2 are known to bind to the E-box sequence (5′-CACGTG-3′), and the 5′-flanking sequence of the HP-27 gene has an E-box sequence from −163 to −158 (see Figure 1A).
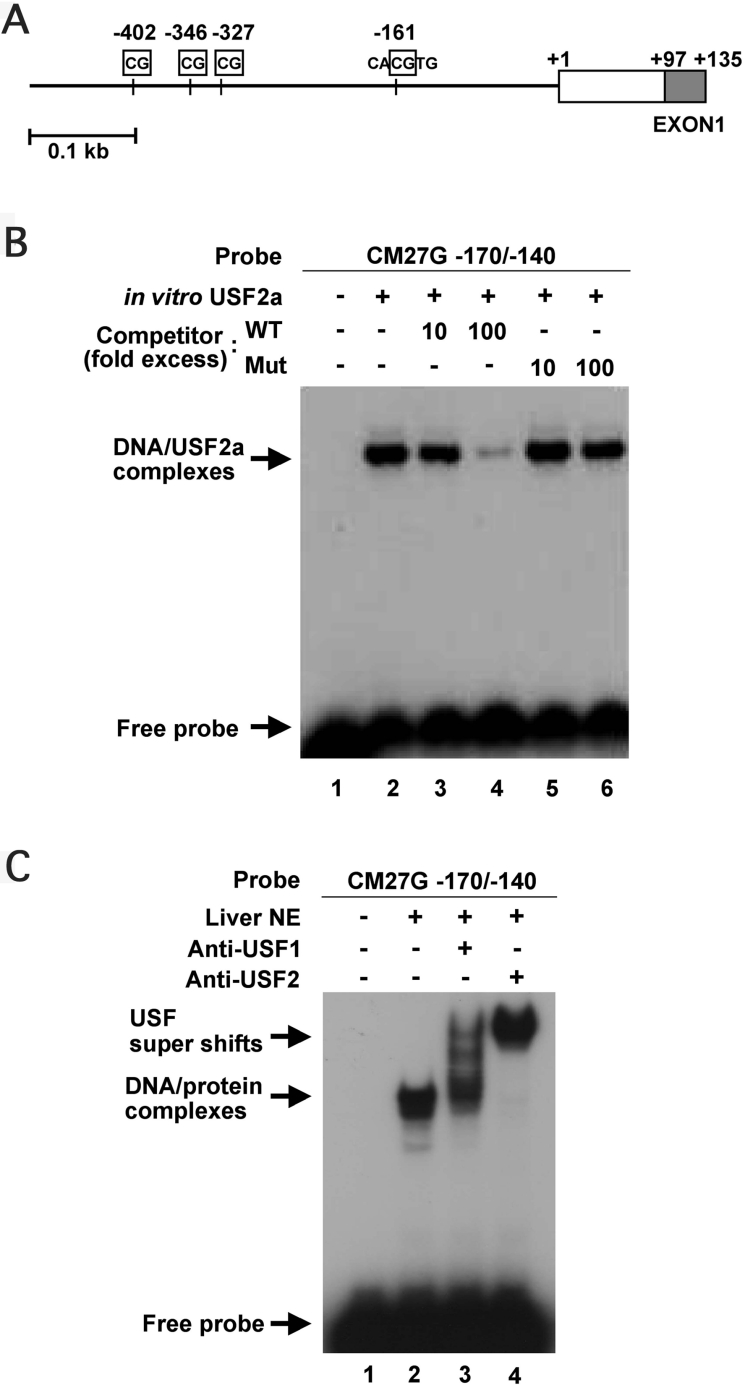
Figure 1. USF binds to the HP-27 gene promoter.
(A) Diagram of the HP-27 gene promoter region. The rectangle represents the first exon, and the shading shows the coding region. The vertical lines mark the locations of CpG dinucleotides. (B) EMSA of the HP-27 gene sequence from −170 to −140 binding to in vitro-translated USF2a. In vitro transcription/translation products of pcDNA3 (lane 1) or pcDNA3/mUSF2a (lanes 2–6) and 32P-labelled CM27G-170/-140 were used in the EMSA in the absence (lane 2) or presence of excess unlabelled oligonucleotide CM27G-170/-140 (WT) (lanes 3 and 4) or CM27G-170/-140mut (Mut) (lanes 5 and 6). (C) Supershift analysis using antibodies against USF1 and USF2. Antibody supershifts were observed after adding anti-USF1 antibodies (lane 3) or anti-USF2 antibodies (lane 4) during the incubation of 32P-labelled CM27G-170/-140 with nuclear extracts from chipmunk liver.
To test whether USF could bind specifically to the 5′-flanking sequence of the HP-27 gene, EMSAs were carried out using in vitro translated mouse USF1, USF2a and USF2b, and a double-stranded oligonucleotide, CM27G-170/-140, containing the 5′-flanking sequence of the HP-27 gene from −170 to −140. Each USF formed a complex with CM27G-170/-140 (Figure 1B, and see Figure 5). In the tree squirrel, the HP-27 gene is not expressed [5], and has several base substitutions in the promoter region, including one at −160 in the E-box [13]. The tree squirrel-type base substitution at −160 decreased the promoter activity of a promoter-reporter plasmid pCM27G-170/luc, which carries the chipmunk HP-27 gene sequence from −170 to +89 upstream of the firefly luciferase gene by 50% [13]. We therefore performed EMSAs using CM27G-170/-140 and CM27G-170/-140mut, which had the tree squirrel-type base substitution at −160 within the E-box, as competitors (Figure 1B). The complex formation between USF and CM27G-170/-140 was competed by unlabelled CM27G-170/-140, but not by CM27G-170/-140mut. A similarly shifted band was observed when CM27G-170/-140 was incubated with chipmunk liver nuclear extracts, and this complex was super-shifted by the addition of anti-USF1 or anti-USF2 antibodies (Figure 1C).
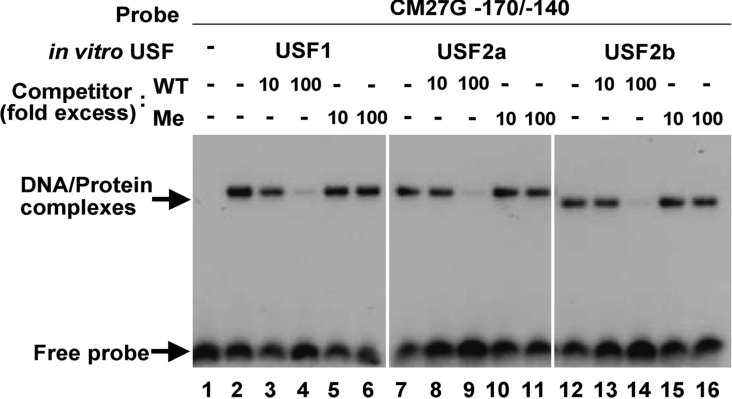
Figure 5. CpG methylation at the USF-binding site impedes USF binding.
In vitro transcription/translation products of pcDNA3 (lane 1), pcDNA3/mUSF1 (lanes 2–6), pcDNA3/mUSF2a (lanes 7–11) or pcDNA3/mUSF2b (lanes 12–16) were used in EMSAs with 32P-labelled CM27G-170/-140 in the absence (lanes 2, 7 and 12) or presence of excess unlabelled oligonucleotide CM27G-170/-140 (WT) (lanes 3, 4, 8, 9, 13 and 14) or Me-CM27G-170/-140 (Me) (lanes 5, 6, 10, 11, 15 and 16).
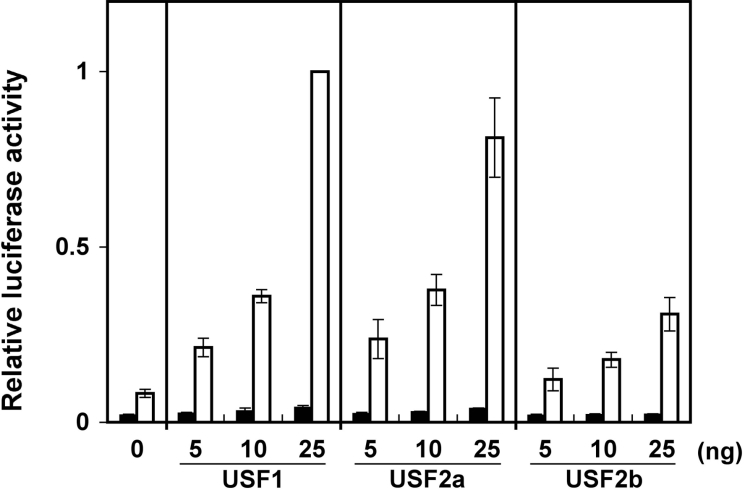
We then tested whether USF could activate transcription from the HP-27 gene promoter using the promoter-reporter plasmid pCM27G-170/luc. Upon the co-transfection of COS-7 cells with the mammalian expression constructs for mouse USF1, USF2a or USF2b, the luciferase activity of pCM27G-170/luc increased in a dose-dependent manner (Figure 2). These results indicate that USF binds to the 5′-flanking sequence of the HP-27 gene, and activates transcription from it.
Figure 2. USF activates transcription from the HP-27 gene promoter.
COS-7 cells were transfected with an HP-27 gene promoter-reporter plasmid, pCM27G-170/luc (white bars), or a promoterless reporter plasmid, pGV-B (black bars), together with a Renilla luciferase plasmid, pRL-SV40, which served as a control for transfection efficiency. The indicated amounts of the mammalian expression construct for mouse USF1, USF2a or USF2b were also added to the transfection mixture. The firefly luciferase activity was normalized to the Renilla luciferase activity, and the data are shown as the fold increase over the luciferase activity of pCM27G-170/luc plus pcDNA3/mUSF1 (25 ng). Results are means±S.E.M. for four separate experiments.
Tissue-specific CpG methylation at the USF-binding site
USF is a ubiquitously expressed transcription factor [14], and therefore it is not likely to account for the liver-specific expression of the HP-27 gene. In fact, pCM27G-170/luc showed significantly higher activity than a promoterless luciferase expression vector, pGV-B, in monkey kidney-derived COS-7 cells (see Figure 2), implying that the transcription of the HP-27 gene could be activated by USF even in non-hepatic cells. We therefore tested whether there was liver-specific expression of USF in the chipmunk. However, a Western blot analysis of nuclear extracts from the chipmunk liver, kidney, heart and lung with anti-USF1 or anti-USF2 antibodies revealed the presence of USF1 and USF2 in all the tissues examined (Figure 3A). In addition, the amounts of USF1 and USF2 in the liver were similar between non-hibernating and hibernating chipmunks (Figure 3B).
Figure 3. Western blot analysis of USF in chipmunk.
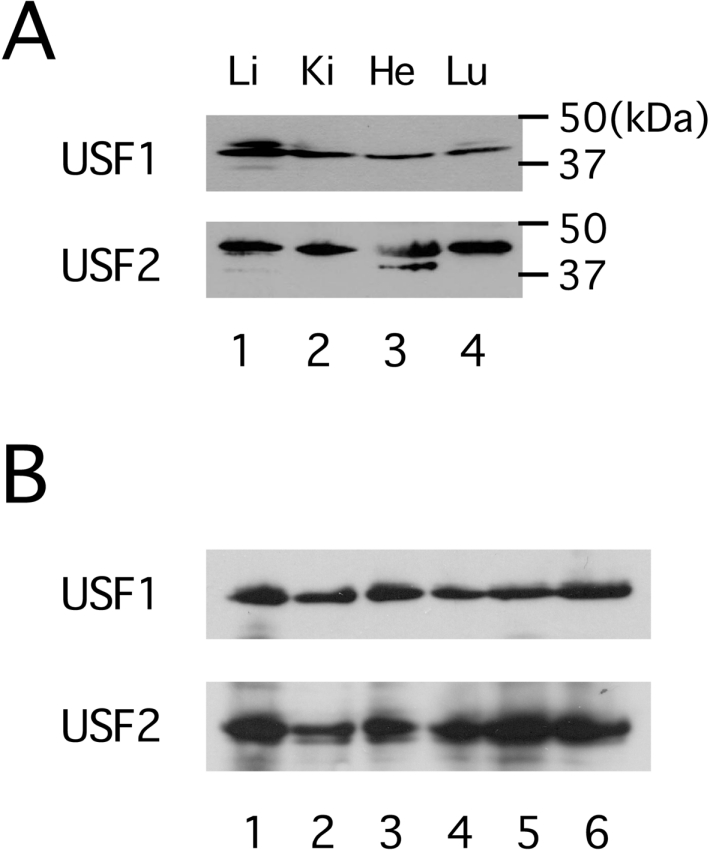
(A) Ubiquitous expression of USF. Nuclear extracts from chipmunk liver (Li, lane 1), kidney (Ki, lane 2), heart (He, lane 3) and lung (Lu, lane 4) were subjected to immunoblotting. The filter was first probed with antibodies against USF1, and then reprobed with antibodies against USF2. Molecular-mass sizes are given in kDa. (B) Comparison of the amounts of USF in the liver between non-hibernating and hibernating chipmunks. Liver nuclear extracts from three individual non-hibernating active chipmunks (lanes 1–3) and three individual hibernating chipmunks (lanes 4–6) were subjected to immunoblotting as in (A).
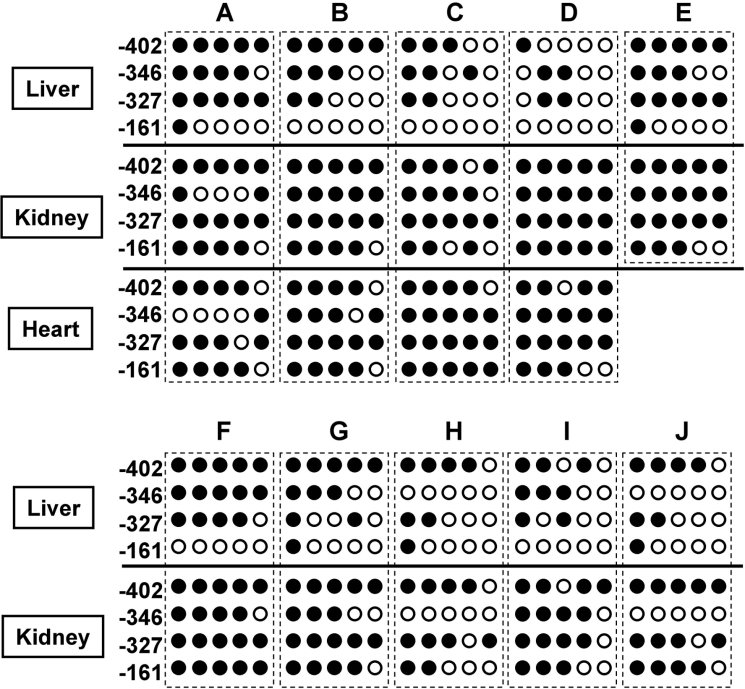
The USF-binding site in the HP-27 gene promoter has a CpG dinucleotide, a potential target for DNA methylation in mammals. Some mammalian genes exhibit an inverse correlation between the extent of DNA methylation and gene activity [15–18]. To determine whether CpG methylation in the USF-binding site correlated with the liver-specific expression of the HP-27 gene, we prepared chromosomal DNA from the liver, kidney and heart of five non-hibernating active chipmunks, and analysed the methylation status of the CpG dinucleotides using the bisulphite sequencing method (Figure 4). There are only four CpG dinucleotides in the 5′-flanking sequence up to −450 (Figure 1A), and the HP-27 gene promoter is categorized as a CpG-poor promoter. Although there were no major differences among the tissues in the methylation status of the three CpG dinucleotides at −402, −346 and −327, the CpG dinucleotide within the USF-binding site at −161 was hypomethylated in the liver and highly methylated in the kidney and heart, revealing that the methylation status of the CpG dinucleotide at −161 reflected the tissue-specificity of the HP-27 gene expression. Since the HP-27 gene expression is down-regulated during hibernation [5], we also prepared chromosomal DNA from the liver and kidney of hibernating chipmunks and analysed the methylation status. Similar to the tissues from non-hibernating animals, the CpG dinucleotide at −161 was hypomethylated in the liver and highly methylated in the kidney, indicating that the CpG methylation at −161 is not involved in the hibernation-associated regulation of the HP-27 gene.
Figure 4. The cytosine methylation profile of the four CpG sites in the HP-27 gene promoter.
Genomic DNA was prepared from the liver, kidney and heart of five non-hibernating active chipmunks (A–E), and the liver and kidney of five hibernating chipmunks (F–J), and the methylation status was analysed by bisulphite sequencing. Each column of circles represents the cytosine methylation pattern obtained from individual clones. Open circles indicate unmethylated CpG sites; filled circles indicate methylated CpG sites.
CpG methylation at the USF-binding site inhibits the binding of USF
To investigate whether CpG methylation at the USF-binding site affected the binding of USF, we performed EMSAs using the double-stranded oligonucleotide probe CM27G-170/-140 and its methylated form Me-CM27G-170/-140, in which the cytosine residues in the CpG dinucleotides on both strands were methylated (Figure 5). Incubation of in vitro translated USF1, USF2a or USF2b with CM27G-170/-140 resulted in the formation of a protein–DNA complex. This complex was competed by the addition of a 100-fold excess of unlabelled CM27G-170/-140. However, the addition of a 100-fold excess of unlabelled Me-CM27G-170/-140 did not affect the complex formation. These results suggested that CpG methylation at the USF-binding site impedes the binding of USF.
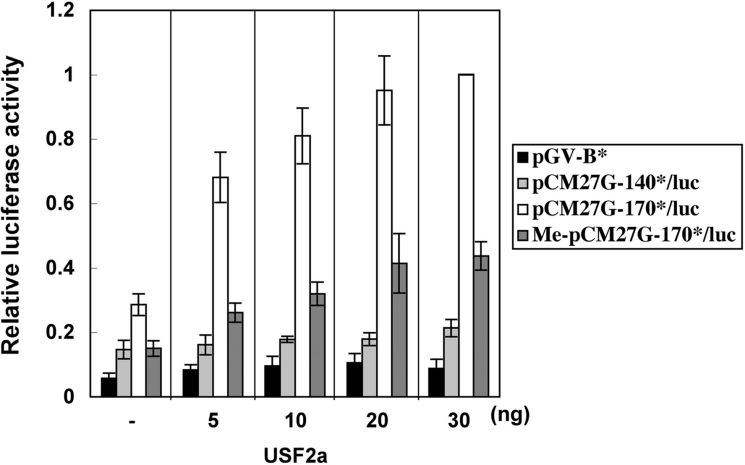
We also evaluated the effect of CpG methylation at the USF-binding site on USF-mediated transcriptional activation using promoter assays. Since the full CpG methylation of promoter-reporter plasmids by SssI methylase abolished the luciferase expression (results not shown), to address this issue, three promoter-reporter plasmids, pCM27G-170*/luc, Me-pCM27G-170*/luc and pCM27G-140*/luc, were constructed by ligating a double-stranded oligonucleotide containing the HP-27 gene sequence from −170 to −140, its CpG-methylated form, or an irrelevant double-stranded oligonucleotide into pCM27G-140/luc upstream of −140, and the ligation mixtures were used for transfection after being purified by phenol extraction and ethanol precipitation [19,20]. As shown in Figure 6, the activity of Me-pCM27G-170*/luc was 50% lower than that of pCM27G-170*/luc, and was comparable with that of pCM27G-140*/luc in HepG2 cells. Furthermore, USF2a did not activate the transcription of Me-pCM27G-170*/luc as efficiently as it did that of pCM27G-170*/luc, and similar results were obtained with USF1 and USF2b (results not shown). These results indicated that the CpG methylation impedes the USF-mediated transcriptional activation.
Figure 6. CpG methylation at the USF-binding site impedes HP-27 gene promoter activity.
HepG2 cells were transfected with the indicated amounts of pcDNA3/mUSF2a together with the indicated HP-27 gene promoter-reporter constructs and pRL-SV40. The firefly luciferase activity was normalized to the Renilla luciferase activity, and the data are shown as the fold increase in luciferase activity over that of pCM27G-170*/luc plus pcDNA3/mUSF2a (30 ng). Results are means±S.E.M. for four separate experiments.
To explore whether CpG methylation within the USF-binding site interfered with the binding of USF in vivo, we carried out ChIP assays using chipmunk liver, kidney and heart, and anti-USF1 and anti-USF2 antibodies. The presence of the HP-27 gene promoter in the chromatin immunoprecipitates was analysed by PCR using a specific pair of primers spanning the USF-binding site in the HP-27 gene promoter. The data in Figure 7 provide evidence for the occupancy by USF-1 and USF-2 of the HP-27 gene promoter in the liver, but not in the kidney and heart.
Figure 7. ChIP assay of USF binding to the HP-27 gene promoter in vivo.
ChIP was performed with chromatin from the chipmunk liver, kidney and heart using anti-USF1 and anti-USF2 antibodies, normal rabbit IgG or no antibody (no Ab). Following DNA purification, the samples were analysed by PCR using a primer set specific for the HP-27 gene promoter. A portion of the total input sample was also examined by PCR.
DISCUSSION
The chipmunk hibernation-specific HP-20, -25 and -27 genes are expressed specifically in the liver [5]. These genes have well-conserved gene structures, and are considered to have arisen through gene duplication [11–13]. In the present study, we found that, in contrast with the HP-20 and -25 genes, whose liver-specific transcription is regulated by the liver-enriched transcription factors HNF-1 and -4 respectively [11,12], the transcription of the HP-27 gene in the liver is activated by the ubiquitous transcription factor USF, which binds to the E-box sequence (5′-CACGTG-3′) in the HP-27 gene promoter. This finding led us to investigate whether DNA methylation was involved in the mechanisms underlying the silencing of the HP-27 gene in non-expressing tissues. In the present study, we showed that: (i) the CpG dinucleotide at the USF-binding site is hypomethylated in the liver, and the methylation status is inversely correlated with expression of HP-27 among tissues; (ii) specific methylation of the CpG dinucleotide at the USF-binding site impedes both the binding of USF and its transcriptional activation; and (iii) USF binds to the HP-27 gene promoter in the liver, but not in non-expressing tissues. These results indicate that HP-27 gene transcription is activated by the ubiquitously expressed transcription factor USF in the liver, and that, in other non-expressing tissues, CpG methylation at the USF-binding site in the HP-27 gene promoter inhibits the binding of USF, resulting in the repression of HP-27 gene expression.
These results also indicate that a base substitution at −160 in the USF-binding site is likely to be involved in the lack of HP-27 gene expression in the tree squirrel. This tree squirrel-type base substitution impeded the binding of USF (Figure 1B) and reduced the promoter activity [13]. Although both the hibernating and non-hibernating species of the squirrel family have the HP-20, -25 and -27 genes, their expression is observed only in the hibernating species [5]. For the full promoter activity of the chipmunk HP-27 gene, the 170-bp 5′-flanking sequence is necessary, and the 69-bp 5′-flanking sequence still retains approx. 10% of the promoter activity, which is activated by HNF-1 binding to the sequence from −54 to −40 [21]. The tree squirrel HP-27 gene has base substitutions in the HNF-1-binding site as well, which abolish the binding of and transactivation by HNF-1 [21]. Similarly, the tree squirrel HP-25 gene has a base substitution in the HNF-4-binding site, which is essential for the promoter activity of the chipmunk HP-25 gene [11], and this mutation is the possible cause of the lack of HP-25 gene expression in the tree squirrel [22]. It is notable that the tree squirrel HP-25 and -27 genes have accumulated mutations in the transcription factor-binding sites that are essential for the transcription of the chipmunk HP-25 and -27 genes.
The methylation of genomic DNA at CpG dinucleotides is a major epigenetic modification of the mammalian genome [23]. It functions in several aspects of gene expression, such as genomic imprinting, X chromosome inactivation, immobilization of transposons and suppression of transcriptional noise [24–26]. CpG methylation is also believed to ensure the silencing of tissue-specific genes in non-expressing cells [15]. To date, most investigations of the role of DNA methylation have focused on CpG islands, which are stretches of DNA with a higher frequency of CpG dinucleotides than is found in the rest of the genome [27,28]. In these situations, CpG methylation contributes generally to transcriptional suppression, either by blocking the binding of transcription factors to their CpG-containing binding sites [15] or by recruiting methyl-CpG-binding proteins, which in turn recruit chromatin-remodelling machinery to induce the formation of a repressive chromatin structure [29]. In contrast, with respect to CpG-poor promoters, the potential role for DNA methylation in tissue-specific gene expression is less well established [25,30]. Although an inverse correlation between gene expression and promoter methylation has been observed for genes that have a CpG-poor promoter, such as the rat placental lactogen-I (rPL-I), prolactin and growth hormone family genes [31,32], it is still not clear whether the mechanisms whereby DNA methylation operates on genes with CpG-rich promoters are similar to those preventing the transcription of genes with CpG-poor promoters. The results in the present study provide an example in which CpG methylation in the CpG-poor HP-27 gene promoter is important for its tissue-specific transcription.
Cell- or tissue-specific gene expression is generally thought to be regulated by the interaction of basal and cell- or tissue-specific transcription factors with cis-acting promoter elements [33]. Alternatively, tissue-specific gene expression may be accomplished by the activation of transcription by ubiquitous transcription factors only in the expressing tissues. The existence of transcription factors such as c-Myb [34], c-Myc/Myn [35], E2F [19], CREB (cAMP-response-element-binding protein) [36], AP2 (activator protein 2) [37] and NF-κB (nuclear factor κB) [38], which are incapable of binding to methylated forms of their recognition sequences, gives evidence of this mechanism. For some CpG island promoters, CpG methylation contributes to tissue-specific gene expression by blocking the binding of transcription factors [30,39]. For example, methylation of a CpG dinucleotide within a STAT3 (signal transducer and activator of transcription 3)-binding site in the CpG-rich GFAP (glial fibrillary acidic protein) gene promoter is critical for astrocyte-specific GFAP expression [40]. Such a mechanism also works to regulate the tissue-specificity of HP-27 gene expression, which is established by CpG methylation that prevents the ubiquitous transcriptional factor USF from binding to the HP-27 gene promoter in non-expressing tissues.
To our knowledge, this is the first evidence that the tissue-specific expression of genes that have a CpG-poor promoter can be regulated by CpG methylation that blocks the binding of the critical transcription factor in non-expressing tissues. Since the overexpression of MeCP2 suppresses the promoter activity of the CpG-poor rPL-I gene [32], it is of interest to investigate whether the regulation of higher-order chromatin structures by DNA methylation is involved in HP-27 gene silencing in non-expressing tissues. Such information would give further insight into the role of CpG methylation in the tissue-specific expression of genes with a CpG-poor promoter.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture in Japan.
References
- 1.Swan H. New York: Elsevier; 1972. Thermoregulation and Bioenergetics. [Google Scholar]
- 2.Johansson B. W. Heart and circulation in hibernators. In: Fisher K. C., Dawe A. R., Lyman C. P., Schonbaum E., South E. F., editors. Mammalian Hibernation III. London: Oliver and Boyd; 1967. pp. 346–355. [Google Scholar]
- 3.Kayser C. New York: Pergamon Press; 1961. The Physiology of Natural Hibernation. [Google Scholar]
- 4.Srere H. K., Wang L. C. W., Martin S. L. Central role for differential gene expression in mammalian hibernation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7119–7123. doi: 10.1073/pnas.89.15.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takamatsu N., Ohba K., Kondo J., Kondo N., Shiba T. Hibernation-associated gene regulation of plasma proteins with collagen-like domain in mammalian hibernators. Mol. Cell. Biol. 1993;13:1516–1521. doi: 10.1128/mcb.13.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamatsu N., Kojima M., Taniyama M., Ohba K., Uematsu T., Segawa C., Tsutou S., Watanabe M., Kondo J., Kondo N., Shiba T. Expression of multiple α1-antitrypsin-like genes in hibernating species of the squirrel family. Gene. 1997;204:127–132. doi: 10.1016/s0378-1119(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 7.Soukri A., Valverde F., Hafid N., Elkebbaj M. S., Serrano A. Occurrence of a differential expression of the glyceraldehyde-3-phosphate dehydrogenase gene in muscle and liver from euthermic and induced hibernating jerboa (Jaculus orientalis) Gene. 1996;181:139–145. doi: 10.1016/s0378-1119(96)00494-5. [DOI] [PubMed] [Google Scholar]
- 8.Andrews M. T., Squire T. L., Bowen C. M., Rollins M. B. Low-temperature carbon utilization is regulated by novel gene activity in the heart of a hibernating mammal. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8392–8397. doi: 10.1073/pnas.95.14.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer B. B., Barnes B. M., Lowell B. B., Grujic D. Differential regulation of uncoupling protein gene homologues in multiple tissues of hibernating ground squirrels. Am. J. Physiol. 1998;275:R1232–R1238. doi: 10.1152/ajpregu.1998.275.4.R1232. [DOI] [PubMed] [Google Scholar]
- 10.Kondo N., Kondo J. Identification of novel blood proteins specific for mammalian hibernation. J. Biol. Chem. 1992;267:473–478. [PubMed] [Google Scholar]
- 11.Kojima M., Takamatsu N., Ishii T., Kondo N., Shiba T. HNF-4 plays a pivotal role in the liver-specific transcription of the chipmunk HP-25 gene. Eur. J. Biochem. 2000;267:4635–4641. doi: 10.1046/j.1432-1327.2000.01499.x. [DOI] [PubMed] [Google Scholar]
- 12.Ono M., Hosoe Y., Azuma S., Shoji M., Nara K., Kondo N., Shiba T., Takamatsu N. HNF-1 regulates the liver-specific transcription of the chipmunk HP-20 gene. Gene. 2001;277:121–127. doi: 10.1016/s0378-1119(01)00699-0. [DOI] [PubMed] [Google Scholar]
- 13.Ono M., Kojima-Kawagoe M., Kondo N., Shiba T., Takamatsu N. Comparative study of HP-27 gene promoter activities between the chipmunk and tree squirrel. Gene. 2003;302:193–199. doi: 10.1016/s0378-1119(02)01152-6. [DOI] [PubMed] [Google Scholar]
- 14.Sirito M., Lin Q., Maity T., Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res. 1994;22:427–433. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden S., Cedar H. Role of DNA methylation in the regulation of transcription. Curr. Opin. Genet. Dev. 1994;4:255–259. doi: 10.1016/s0959-437x(05)80052-8. [DOI] [PubMed] [Google Scholar]
- 16.Beard C., Li E., Jaenisch R. Loss of methylation activates Xist in somatic but not in embryonic cells. Cell. 1995;65:2325–2334. doi: 10.1101/gad.9.19.2325. [DOI] [PubMed] [Google Scholar]
- 17.Bird A. P. Gene number, noise reduction and biological complexity. Trends Genet. 1995;11:94–100. doi: 10.1016/S0168-9525(00)89009-5. [DOI] [PubMed] [Google Scholar]
- 18.Siegfried Z., Cedar H. DNA methylation: molecular lock. Curr. Biol. 1997;7:305–307. doi: 10.1016/s0960-9822(06)00144-8. [DOI] [PubMed] [Google Scholar]
- 19.Campenero M. R., Armstrong M. I., Flemington E. K. CpG methylation as a mechanism for the regulation of E2F activity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6481–6486. doi: 10.1073/pnas.100340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y.-X., Jean J-C., Williams M. C. Cytosine methylation of an Sp1 site contributes to organ-specific and cell-specific regulation of expression of the lung epithelial gene T1α. Biochem. J. 2000;350:883–890. [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M., Kojima-Kawagoe M., Ito M., Kondo N., Shiba T., Takamatsu N. HNF-1 regulates the promoter activity of the HP-27 gene. Zool. Sci. 2004;21:393–396. doi: 10.2108/zsj.21.393. [DOI] [PubMed] [Google Scholar]
- 22.Kojima M., Shiba T., Kondo N., Takamatsu N. The tree squirrel HP-25 gene is a pseudogene. Eur. J. Biochem. 2001;268:5997–6002. doi: 10.1046/j.0014-2956.2001.02572.x. [DOI] [PubMed] [Google Scholar]
- 23.Gruenbaum Y., Stein R., Cedar H., Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981;124:67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
- 24.Bird A. P., Wolffe A. P. Methylation-induced repression: belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 25.Jones P. A., Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 26.Reik W., Dean W., Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 27.Bird A. P. CpG island as gene markers in the vertebrate nucleus. Trends Genet. 1987;3:342–347. [Google Scholar]
- 28.Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J. Mol. Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 29.Ordway J. M., Curran T. Methylation matters: modeling a manageable genome. Cell Growth Differ. 2002;13:149–162. [PubMed] [Google Scholar]
- 30.Shiota K. DNA methylation profiles of CpG islands for cellular differentiation and development in mammals. Cytogenet. Genome Res. 2004;105:325–334. doi: 10.1159/000078205. [DOI] [PubMed] [Google Scholar]
- 31.Ngo V., Gourdji D., Laverriere J. N. Site-specific methylation of the rat prolactin and growth hormone promoters correlates with gene expression. Mol. Cell. Biol. 1996;16:3245–3254. doi: 10.1128/mcb.16.7.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho J. H., Kimura H., Minami T., Ohgane J., Hattori N., Tanaka S., Shiota K. DNA methylation regulates placental lactogen I gene expression. Endocrinology. 2001;142:3389–3396. doi: 10.1210/endo.142.8.8347. [DOI] [PubMed] [Google Scholar]
- 33.Roeder R. G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 1996;21:327–334. [PubMed] [Google Scholar]
- 34.Klempnauer K. H. Methylation-sensitive DNA binding by v-myb and c-myb proteins. Oncogene. 1993;8:111–115. [PubMed] [Google Scholar]
- 35.Prendergast G. C., Lawe D., Ziff E. B. Association of Myn, the murine homologue of Max, with c-myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991;65:395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- 36.Weih F., Nitsch D., Reik A., Schutz G., Becker P. B. Analysis of CpG methylation and genomic footprinting at the tyrosine aminotransferase gene: DNA methylation alone is not sufficient to prevent protein binding in vivo. EMBO J. 1991;10:2559–2567. doi: 10.1002/j.1460-2075.1991.tb07796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comb M., Goodman H. M. CpG methylation inhibits proenkephalin gene expression and binding of the transcription factor AP-2. Nucleic Acids Res. 1990;18:3975–3982. doi: 10.1093/nar/18.13.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirillov A., Kistler B., Mostoslavsky R., Cedar H., Wirth T., Bergman Y. A role for nuclear NF-κB in B-cell-specific demethylation of the Igκ locus. Nat. Genet. 1996;13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 39.Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J. Cell. Biochem. 2003;88:899–910. doi: 10.1002/jcb.10464. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa T., Nakashima K., Namihira M., Ochiai W., Uemura A., Yanagisawa M., Fujita N., Nakao M., Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]