Abstract
The cytosolic protein Bax plays a key role in apoptosis by migrating to mitochondria and releasing proapoptotic proteins from the mitochondrial intermembrane space. The present study investigates the movement of Bax in isolated rat neonatal cardiomyocytes subjected to simulated ischaemia (minus glucose, plus cyanide), using green fluorescent protein-tagged Bax as a means of imaging Bax movements. Simulated ischaemia induced Bax translocation from the cytosol to mitochondria, commencing within 20 min of simulated ischaemia and progressing for several hours. Under the same conditions, there was an increase in the active, phosphorylated forms of p38 MAPK (mitogen-activated protein kinase) and AMPK (AMP-activated protein kinase). The AMPK activators AICAR (5-aminoimidazole-4-carboxamide ribonucleoside) and metformin also stimulated Bax translocation. Inhibition of p38 MAPK with SB203580 attenuated the phosphorylation of the downstream substrates, MAPK-activated protein kinases 2 and 3, but not that of the upstream MAPK kinase 3, nor of AMPK. Under all conditions (ischaemia, AICAR and metformin), SB203580 blocked Bax translocation completely. It is concluded that Bax translocation to mitochondria is an early step in ischaemia and that it occurs in response to activation of p38 MAPK downstream of AMPK.
Keywords: AMP-activated protein kinase (AMPK), Bax, green fluorescent protein, imaging, ischaemia, phosphorylation
Abbreviations: AICAR, 5-aminoimidazole-4-carboxamide ribonucleoside; AMPK, AMP-activated protein kinase; AMPKK, AMPK kinase; GFP, green fluorescent protein; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MAPKAPK, MAPK-activated protein kinase; MKK3, MAPK kinase 3; MTR, MitoTracker Red; STKII, serine/threonine-protein kinase II; VDAC, voltage-dependent anion channel; ZMP, AICAR monophosphate
INTRODUCTION
Apoptosis contributes significantly to heart cell death during ischaemic injury. Apoptotic cells are evident in human myocardial infarcts [1,2], in animal models of cardiac ischaemia/reperfusion injury [3,4] and in cultured cardiomyocytes following hypoxia and reoxygenation [5–8]. As key regulators of apoptosis, Bcl-2 family proteins present potential sites of action of ischaemia. These proteins control the release of a spectrum of apoptogenic proteins from the mitochondrial intermembrane space to the cytosol. Some Bcl-2 family members inhibit the release and promote cell survival (e.g. Bcl-2 and Bcl-XL); others permeabilize the outer membrane to intermembrane space proteins and bring about apoptosis (e.g. Bax, Bak, Bad, Bid and Bik). Bax has a major role in outer membrane permeabilization. Simple addition of Bax to isolated mitochondria can permeabilize the outer membrane [9]. Conversely, Bax (plus Bak)-deficient cells are resistant to apoptosis [10]. Bax is normally resident in the cytosol but, under apoptotic stimuli, it migrates to mitochondria; here it inserts into the outer membrane, oligomerizes, and brings about outer membrane permeabilization (reviewed in [11]). Bax insertion is believed to occur via its C-terminal region following conformational changes. These changes also affect the N-terminus. In its inert, cytosolic state, the N-terminal region of Bax is unavailable to antibodies and to trypsin, but it becomes accessible after membrane insertion [12]. Thus membrane insertion exposes the N-terminus on the outside of the inner membrane.
Bax is abundant in neonatal cardiomyocytes [13,14]. It becomes down-regulated in adult cardiomyocytes [15], but is re-expressed under various conditions associated with increased apoptosis, e.g. hypertension [16], heart failure [17] and ischaemia [18–20]. In the present study, the movement of Bax during simulated ischaemia was investigated in isolated rat neonatal cardiomyocytes, using Bax tagged at its N-terminus with GFP (green fluorescent protein) as a means of imaging Bax distribution. The relationship between Bax translocation and activation of p38 MAPK (mitogen-activated protein kinase) was also explored, since p38 MAPK is generally considered to be involved in ischaemic injury [21–23]. We show that Bax translocates to mitochondria under simulated ischaemia and that the translocation requires active, phosphorylated p38 MAPK. In addition, we investigate the role of AMPK (AMP-activated protein kinase) in these events. AMPK is activated by phosphorylation during anaerobiosis in response to an increased AMP/ATP ratio (reviewed in [24,25]). It can also be activated by AICAR (5-aminoimidazole-4-carboxamide ribonucleoside), which is phosphorylated to form the AMP analogue, ZMP (AICAR monophosphate) [24], and by the antidiabetic drug metformin [26]. Use of these procedures implicates active, phosphorylated AMPK in the redistribution of Bax in ischaemia. It is concluded that p38 MAPK-mediated Bax migration to mitochondria is an early event in myocardial ischaemia and that it occurs downstream of AMPK activation.
MATERIALS AND METHODS
Cell culture and incubation
Primary cultures of ventricular cardiomyocytes were prepared from 14-day-old Sprague–Dawley rats and seeded on to either laminin-coated glass coverslips (for fluorescence imaging) or multiwell plates (for extraction; Techno Plastic, Trasadingen, Switzerland). The cells were maintained under CO2/air (1:19) at 37 °C in M199 medium (Sigma) containing 20 units/ml penicillin, 20 μg/ml streptomycin, 2 μg/ml vitamin B12 and 10% (w/v) foetal calf serum. For the expression of Bax tagged at its N-terminus with GFP, cells were transfected 2 days after seeding with a GFP–Bax construct in pEGFP (Clontech) as before [13]. Lipofectamine™ 2000 was used as transfection reagent according to the manufacturer's instructions (Life Technologies, Paisley, Scotland, U.K.). The Lipofectamine™-containing medium was replaced with fresh culture medium without Lipofectamine™ after 5 h. Cells were imaged 2–3 days after transfection. In all experiments, cells were preincubated in standard medium containing 100 mM NaCl, 4 mM KCl, 24 mM Hepes (pH 7.4), 1 mM MgSO4, 1 mM CaCl2, 1 mM KH2PO4 and 11 mM glucose for approx. 30 min. When added, SB205380 was included in the preincubation. Simulated ischaemia was imposed by switching to standard medium which lacked glucose but contained 2 mM NaCN (with or without SB205380).
Fluorescence imaging
Cells were imaged with an Olympus IX-70 fluorescence microscope and cooled CCD (charge-coupled-device) camera (Micromax 1401E; Princeton Instruments) using wavelengths 490 nm/530 nm (GFP) and 572 nm/625 nm [MTR (MitoTracker Red); Molecular Probes]. Cells were loaded with MTR by incubation with 10 ng of dye/ml for 10 min at room temperature (20–24 °C). Data were analysed by Metamorph software (Universal Imaging).
Cell extraction and Western blotting
For immunodetection of phosphorylated enzymes, cells were extracted in 100 mM NaCl/10 mM Tris/HCl (pH 7.2)/1 mM NaF/1 mM NaVO4/1 mM PMSF and protease inhibitors pepstatin, chymostatin, leupeptin and aprotinin (all at 1 μg/ml). Cell extracts were immediately denatured at 95 °C for 3 min in 4% (w/v) SDS, 1 mM 2-mercaptoethanol, 10 mM Tris/HCl (pH 6.8) and 10% (w/v) glycerol. For immunodetection of Bax in subcellular fractions, cells were scraped into 210 mM mannitol/70 mM sucrose/10 mM Tris/HCl (pH 7.2)/1 mM EGTA/10 mM KCl/1 mM MgCl2/protease inhibitors (as above) and disrupted by passage (five times) through a 25G needle. The homogenate was centrifuged at 1000 g for 5 min. The pellet was re-extracted, and the two low-speed supernatants were combined and centrifuged at 10000 g for 6 min to yield a pellet (mitochondria) and supernatant (containing cytosol). Fractions were analysed by standard SDS/PAGE and Western blotting [13]. The following primary antibodies were obtained from Cell Signaling Technology: polyclonals for p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), AMPK-α, phospho-AMPK-α (Thr172), phospho-MKK3 (where MKK3 is MAPK kinase 3; Ser189/Ser207), phospho-MAPKAPK 2/3 (where MAPKAPK 2/3 is MAPK-activated protein kinases 2/3; Thr334/Thr313) and JNK (c-Jun N-terminal kinase), and monoclonal for phospho-JNK (Thr183/Tyr185). The polyclonal for Bax (N-20) was from Santa Cruz Biotechnology, and the monoclonal for VDAC (voltage-dependent anion channel) was from Calbiochem. As secondary antibodies, we used peroxidase-conjugated goat anti-mouse IgG (Calbiochem) and anti-rabbit IgG (Bio-Rad). Bands were developed with the enhanced chemiluminescence reagent (Amersham Biosciences).
Statistical analyses
Mean data values were compared with unpaired t test with Welch's correction. Where comparisons were made between more than two experimental groups, data were subjected to one-way ANOVA with Bonferroni's multiple comparison as a post hoc test.
RESULTS
Bax translocation to mitochondria during simulated ischaemia
The effects of energy deprivation on the intracellular distribution of Bax were investigated by incubating cardiomyocytes with cyanide (to inhibit the respiratory chain and mitochondrial ATP production) and in the absence of glucose (to limit glycolytic ATP production). Bax distribution was tracked by fluorescence imaging after transfecting the cells with a construct encoding Bax tagged at its N-terminus with GFP. In agreement with previous work in this laboratory [13], neonatal cardiomyocytes transfected with GFP–Bax had a negligible tendency to undergo apoptosis in the absence of stimulation (<4% of transfected cells became apoptotic within 60 h of transfection). As shown in Figure 1(A), Bax had a diffuse distribution throughout unstimulated cells. Simulated ischaemia (cyanide minus glucose), however, brought about a partial sequestration of GFP–Bax by intracellular organelles (Figure 1B).
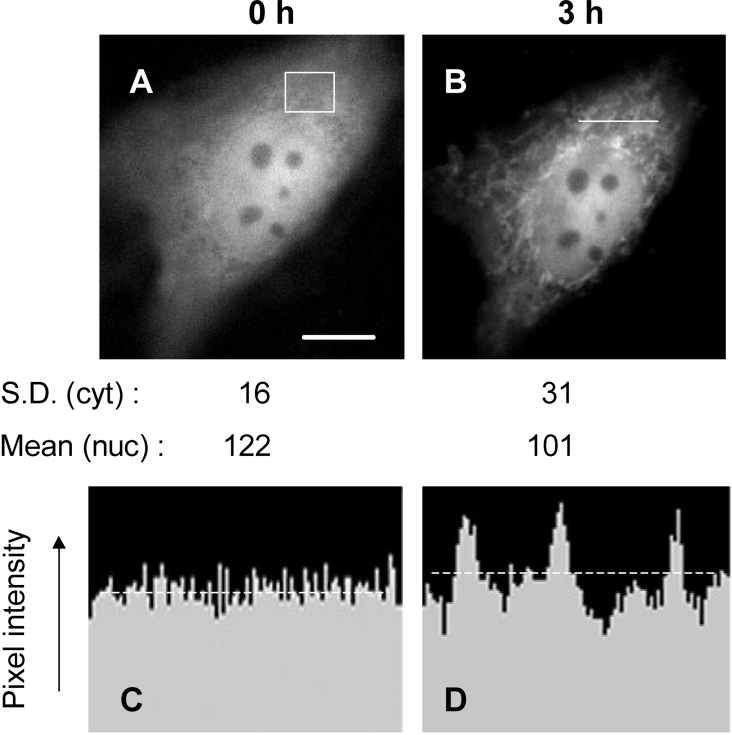
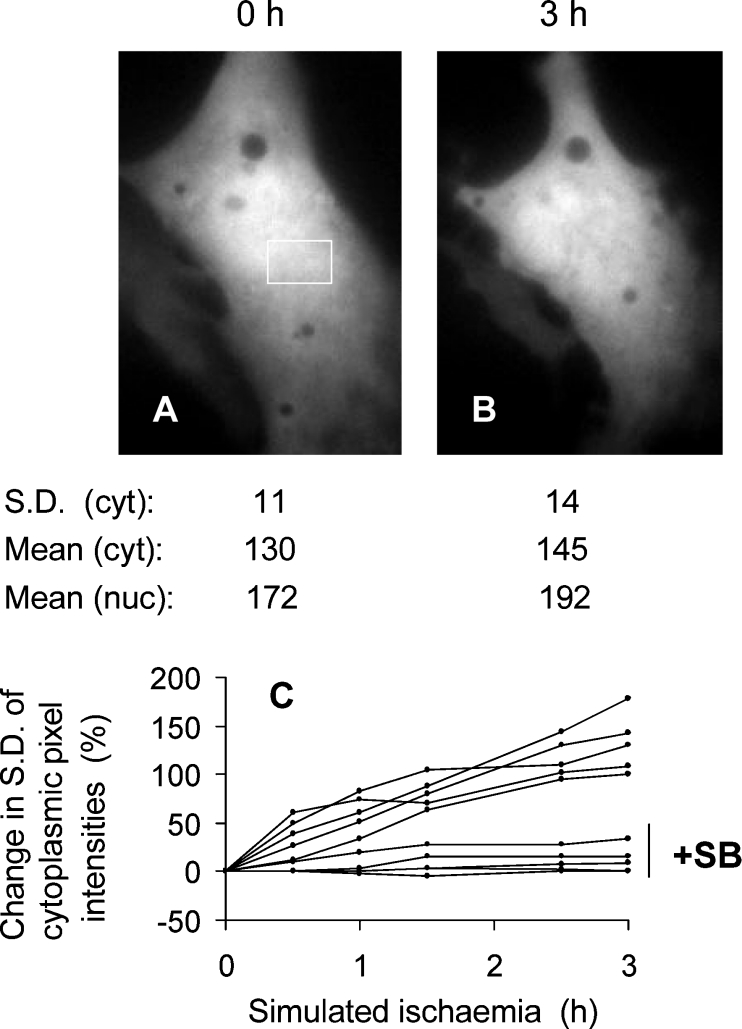
Figure 1. GFP–Bax sequestration during simulated ischaemia.
A cardiomyocyte transfected with GFP–Bax was preincubated in standard medium (the Materials and methods section) for 30 min, after which the medium was replaced with ‘ischaemic’ medium without glucose and containing cyanide. GFP–Bax was imaged immediately before the switch to ‘ischaemic’ medium (A) and 3 h after it (B). Scale bar (A), 10 μm. The standard deviations of pixel intensity values (S.D.) refer to the cytoplasmic (cyt) area boxed in (A). The mean nuclear (nuc) GFP–Bax fluorescence was obtained after defining the nuclear boundary from brightfield images. (C, D) Cross-sectional profiles of GFP–Bax fluorescence intensities before (C) and after (D) 3 h of simulated ischaemia. The position of the cross-section is given by the horizontal line in (B). The intensity profiles in (C, D) are corrected for background fluorescence and are drawn to the same scale. The broken lines indicate the mean pixel intensities.
It was important to ensure that the increase in organelle GFP–Bax relative to cytosolic GFP–Bax reflected a true translocation of GFP–Bax from cytosol to organelle (rather than any selective loss of cytosolic GFP–Bax and preservation of pre-existing organelle GFP–Bax). Figures 1(C) and 1(D) show cross-sectional profiles of GFP–Bax fluorescence along the same line within the cell before and after simulated ischaemia. The profile from the ischaemic state (Figure 1D) shows peaks and troughs that lie above and below the range of pixel intensities obtained before ischaemia (Figure 1C). Ischaemia also caused a small increase (18%) in mean cytoplasmic pixel intensity (Figures 1C and 1D, broken lines). Comparisons made along other lines in the cytoplasm confirmed these differences. At the same time, mean nuclear fluorescence was decreased by 17%. These analyses are consistent with net movement of GFP–Bax from the nucleus and cytosol to the organelle.
To ascertain that the GFP–Bax was sequestered by mitochondria, the transfected cells were also treated with the mitochondrial marker MTR, which is accumulated electrophoretically into mitochondria in response to the mitochondrial inner membrane potential. Figure 2 shows a typical GFP–Bax-transfected cell, loaded with MTR, and imaged after 3 h of simulated ischaemia for GFP–Bax (Figure 2A) and MTR (Figure 2B). Enlargements of part of the doubly labelled cell are given in Figures 2(C)–2(E). From the overlay (Figure 2E), it is clear that GFP–Bax co-localized with MTR, confirming that GFP–Bax translocates to the mitochondria of energy-deprived cardiomyocytes.
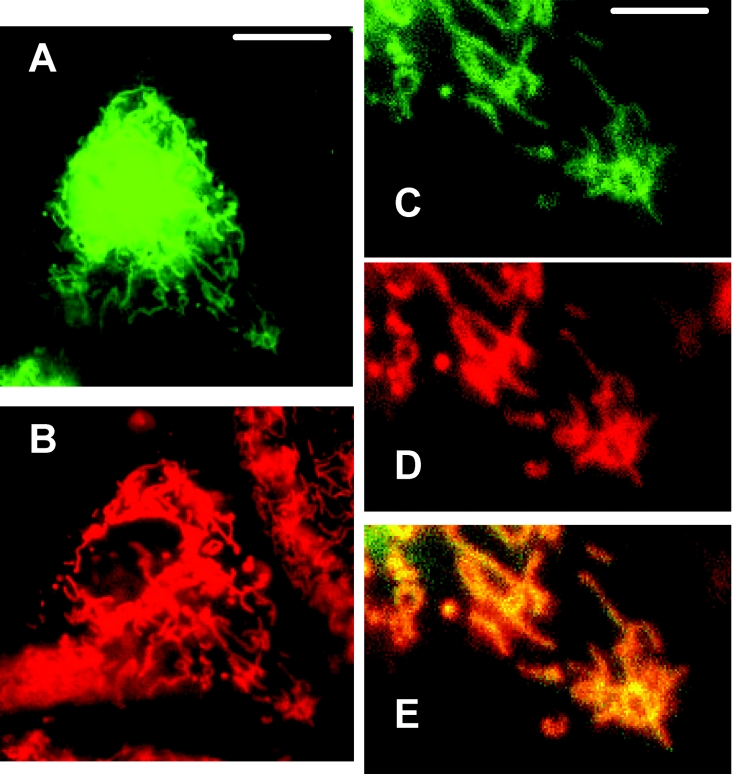
Figure 2. GFP–Bax sequestration by mitochondria during simulated ischaemia.
A cardiomyocyte culture expressing GFP–Bax was labelled with the mitochondrial marker, MTR, and subjected to simulated ischaemia for 3 h. (A, B) The Figures show the same cell imaged for GFP–Bax (A) and MTR (B); in (B), a neighbouring non-GFP–Bax-transfected cell is also seen. Scale bar, 10 μm. (C–E) The Figures show enlargements of the same cell imaged for GFP–Bax (C) and MTR (D), and an overlay of the two (E), where co-localization is given in yellow. Scale bar, 3 μm.
The involvement of p38 MAPK in ischaemia-induced Bax translocation
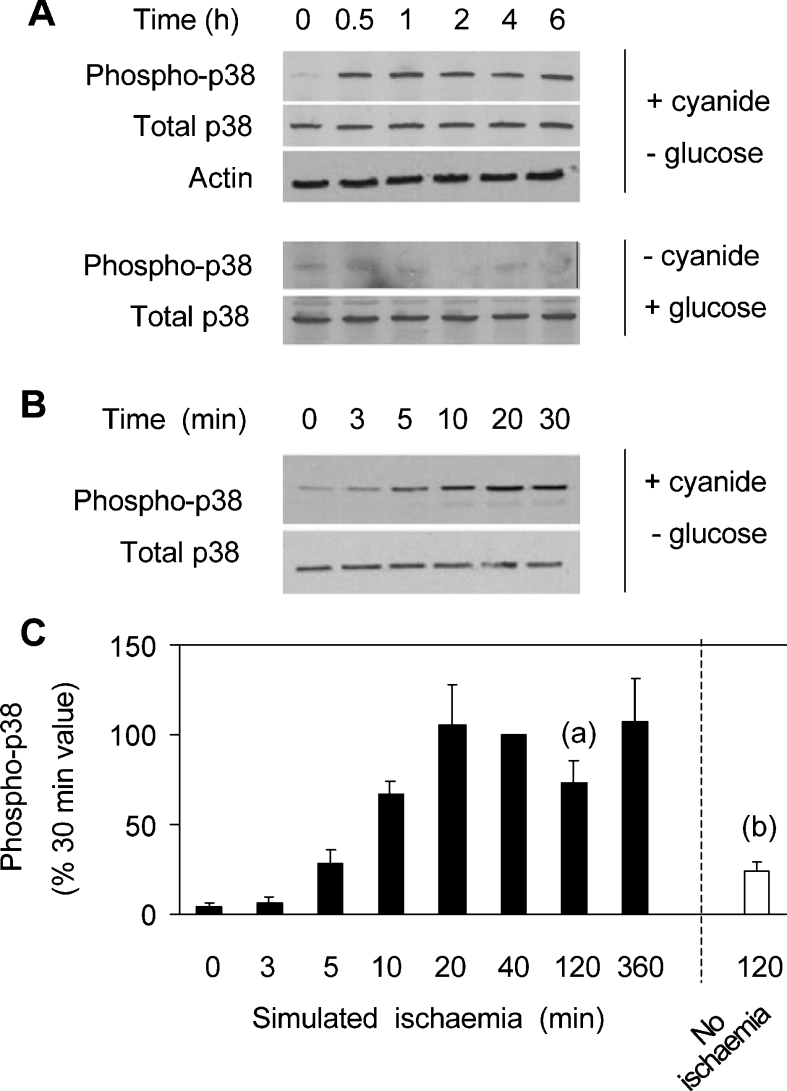
Simulated ischaemia also led to the activation of p38 MAPK. Activation was detected using an antibody specific for the active form of the enzyme phosphorylated on Thr180 and Tyr182. Total p38 MAPK was assayed with an antibody that recognizes the enzyme irrespective of its phosphorylation state. Actin was also measured to ensure constant cell protein. As shown in Figure 3(B), phosphorylation of p38 MAPK was detected within 5 min of simulated ischaemia. Phosphorylation was maximal at approx. 20 min, and was largely maintained for at least 6 h (Figures 3A and 3C). In some control experiments (i.e. minus CN, plus glucose), there was no detectable p38 MAPK phosphorylation (e.g. Figure 3A). Overall, however, a small degree of phosphorylation was detected after 2 h of control incubation (Figure 3C, open bar), which was significantly less than that observed under simulated ischaemia.
Figure 3. Ischaemia-induced changes in p38 MAPK phosphorylation.
(A, B) Cells were extracted after incubation for the times indicated with CN and no glucose (ischaemia), or with glucose and no CN (no ischaemia). Extracts were analysed in Western blots for phospho-p38 MAPK (phospho-p38), total p38 MAPK (total p38) and actin. (C) Densitometric data from blots are given as means±S.E.M. (n=6). P<0.01 for (a) with respect to (b).
Cell extracts were also analysed for active, phosphorylated JNK. No additional phosphorylation was detected at any time up to 5 h of simulated ischaemia. Increased JNK phosphorylation was seen after 6–8 h of ischaemia but, since this occurred long after Bax translocation had taken place, it was clearly not involved in Bax movement, and was not considered further (results not shown).
Since ischaemia activated both Bax translocation and p38 MAPK, we investigated the effect of p38 MAPK inhibition on Bax movement. Three p38 MAPK isoforms (α, χ and δ) are expressed in heart, of which the α isoform predominates [27]. As inhibitor we used 10 μM SB203580, which is sufficient for 98% inhibition of the α isoform, but negligible inhibition of either the χ or δ isoforms [28] (confirmation that p38 MAPK was substantially inhibited is given below; Figure 10). Figure 4 reports a typical cell subjected to ischaemia in the presence of SB203580. There was no discernible cytoplasmic sequestration of GFP–Bax.
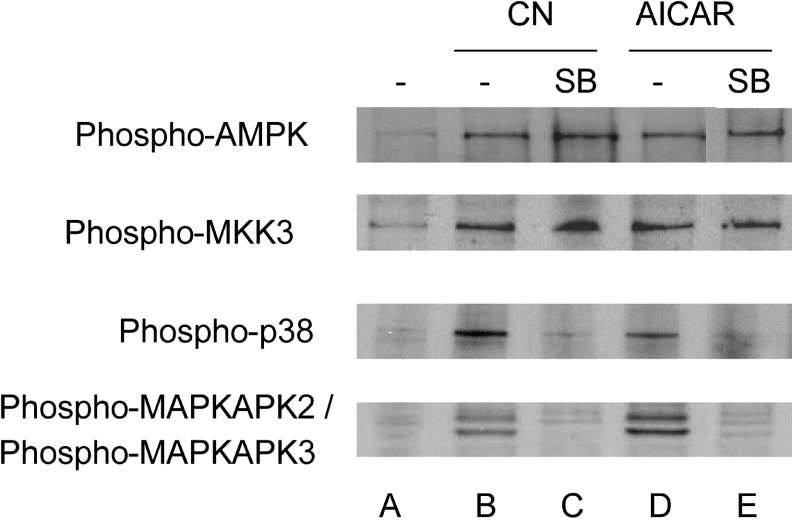
Figure 10. Effects of SB203580 on the phosphorylation of AMPK and enzymes of the p38 MAPK pathway.
Cells were extracted after a 2 h incubation in medium containing glucose (A), CN and no glucose (B, C), and glucose with AICAR (D, E). SB203580 (10 μM) was added in (C, E). The phosphoenzymes were detected in Western blots.
Figure 4. The lack of GFP–Bax sequestration during simulated ischaemia in the presence of SB203580.
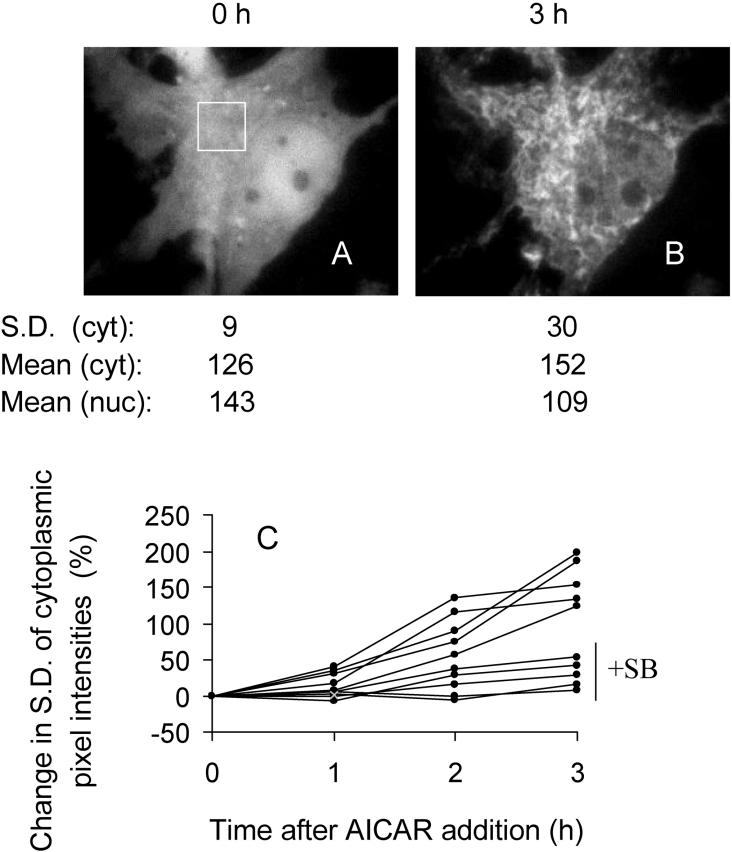
A GFP–Bax-transfected cell was preincubated in standard medium containing 10 μM SB203580 and imaged (A). The medium was then replaced with glucose-free medium containing CN and SB203580, and the cell was imaged 3 h later (B). The S.D. of cytoplasmic (cyt) pixel intensities refers to the area boxed in (A). The mean nuclear (nuc) and cytoplasmic fluorescence values were obtained after defining the boundaries from brightfield images. (C) Time courses of GFP–Bax sequestration according to the changes in S.D. of cytoplasmic pixel intensities. Each line was from a single cardiomyocyte in the presence or absence of 10 μM SB203580 (SB), as indicated.
In order to collate data of Bax translocation in the presence and absence of SB203580, GFP–Bax sequestration was quantified using the standard deviation (S.D.) of pixel intensity values. With a diffuse distribution, the S.D. value is relatively low, but the S.D. increases as GFP–Bax is sequestered. For example, in the cell of Figure 1, without SB203580, the S.D. of pixel intensities in the selected area of the cytoplasm increased approx. 2-fold following simulated ischaemia. In contrast, in the cell of Figure 4, with SB203580, the change in S.D. during ischaemia was much smaller. Transfected cells expressed GFP–Bax to different degrees and, to facilitate comparison of time series from different cells, the changes in S.D. values for each series were expressed as a percentage increase of the zero time values. This procedure shows that the mitochondria accumulated GFP–Bax progressively for at least 3 h during simulated ischaemia (Figure 4C, upper five curves), whereas there was little accumulation during ischaemia in the presence of SB203580 (Figure 4C, lower five curves). Analyses of 27 cells yielded a 130% increase in cytoplasmic S.D. after 3 h of simulated ischaemia, but negligible increase after ischaemia with SB203580 (Figure 5B).
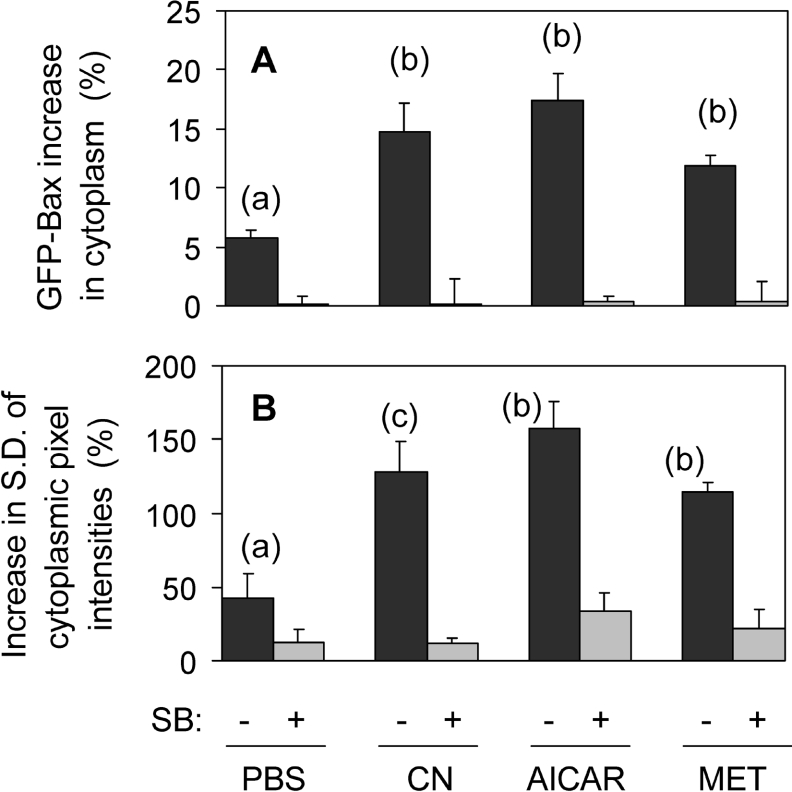
Figure 5. GFP–Bax sequestration induced by simulated ischaemia, AICAR and metformin, and its inhibition by SB203580.
GFP–Bax-transfected cardiomyocytes were incubated under simulated ischaemia in the absence of glucose (CN) or in glucose-replete medium with either 2 mM AICAR, 3 mM metformin (MET) or PBS (as control), and with or without 10 μM SB203580 (SB). GFP–Bax translocation was estimated after 3 h in two ways: (A) the cytoplasmic increase in GFP–Bax; this was calculated as the change (%) in cytoplasmic fluorescence minus the change (%) in total cell fluorescence. (B) Change (%) in S.D. of cytoplasmic pixel intensities. Results shown are the means±S.E.M. for the following number of cells without/with SB203580: 23/23 (PBS), 27/27 (CN), 14/14 (AICAR), 12/12 (MET). Statistical analyses: P<0.002 for (b) with respect to (a); P<0.01 for (c) with respect to (a).
For the first 3 h of simulated ischaemia, the cardiomyocytes remained viable (as judged by the absence of nuclear staining by the live cell-impermeant ethidium homodimer; results not shown). During this time, the total cellular GFP–Bax fluorescence was essentially constant as the GFP–Bax was simply shifted between intracellular compartments. For example, the sequestration by mitochondria in the cell of Figure 1 resulted in a 17% loss of nuclear fluorescence and an 18% gain by the cytoplasm. In contrast, in the cell of Figure 4 (plus SB203580), the small change registered in cytoplasmic fluorescence was accompanied by a similar change in nuclear fluorescence. This provided a second means of quantifying GFP–Bax translocation. The shift to the cytoplasm was quantified as the percentage change in cytoplasmic fluorescence minus the percentage change in total cell fluorescence (legend, Figure 5). In this way, the increases in cytoplasmic GFP–Bax fluorescence were corrected for any small changes due to variations in focus and lamp intensity. Thus calculated, simulated ischaemia brought about a 15% increase in cytoplasmic GFP–Bax in 27 cells, but no change overall in the same number of cells subjected to ischaemia in the presence of SB203580 (Figure 5A).
There was a small increase in cytoplasmic GFP–Bax in control experiments with glucose-replete medium, without CN (PBS; Figure 5A). These controls also showed a small increase in S.D. of cytoplasmic pixel intensities (Figure 5B). Thus some translocation of GFP–Bax was apparent even under control conditions and this was also blocked by SB203580. This presumably resulted from the small activation of p38 MAPK during incubation under non-ischaemic conditions (Figure 3C). However, the SB203580-inhibited Bax translocation was increased 2.5–3-fold in the presence of CN (minus glucose) according to the two means of quantification (Figures 5A and 5B) Thus simulated ischaemia induced SB203580-sensitive translocation of GFP–Bax to mitochondria.
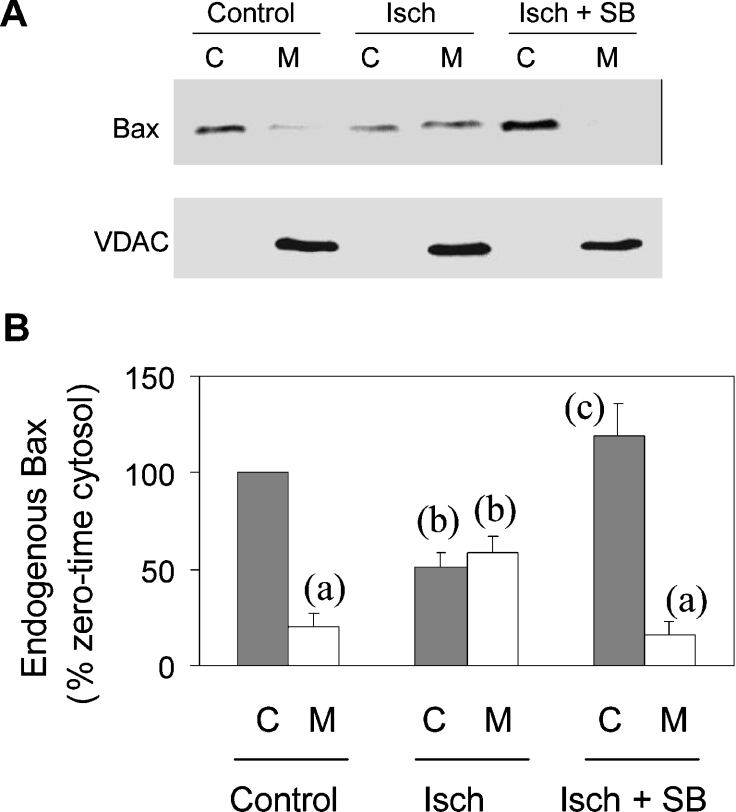
It was important to establish that the redistribution of GFP–Bax reflected the movement of endogenous Bax. Accordingly, cells subjected to simulated ischaemia were subfractionated into mitochondria- and cytosol-containing fractions, and the Bax distribution between the two was analysed in Western blots. Figure 6(A) shows that endogenous Bax was recovered predominantly in the cytosolic fraction of control cells and that it became approximately equally distributed between the two fractions after 3 h of simulated ischaemia. VDAC (mitochondrial outer membrane) was assayed to ensure consistent recovery of mitochondria in the mitochondrial fraction and its absence from the cytosolic fraction (Figure 6A). Data from a number of such experiments were analysed by densitometry (Figure 6B). These results show that the loss of endogenous Bax from the cytosolic fraction was accompanied by a corresponding gain of Bax in the mitochondrial fraction. The shift from cytosolic to mitochondrial fractions was blocked by SB203580. These data substantiate the conclusion from GFP–Bax imaging that Bax migrates to mitochondria under simulated ischaemia and that the migration is prevented by SB203580.
Figure 6. The translocation of endogenous Bax from cytosol to mitochondria during simulated ischaemia.
Cardiomyocytes were fractionated into cytosolic (C) and mitochondrial (M) fractions after incubation for 3 h with glucose (Control) or with CN and no glucose (Isch) in the presence (SB) and absence of 10 μM SB203580. (A) The fractions were analysed in Western blots for Bax and for VDAC as mitochondrial marker. (B) Densitometric analyses of Western blots; means±S.E.M. (six cell preparations). For each of the six series, the densitometric values were expressed as a percentage of the Control cytosolic value. Statistical analyses (a–c): P<0.05 for comparisons of columns bearing different letters; no significant difference between columns with the same letter.
Involvement of AMPK in Bax translocation
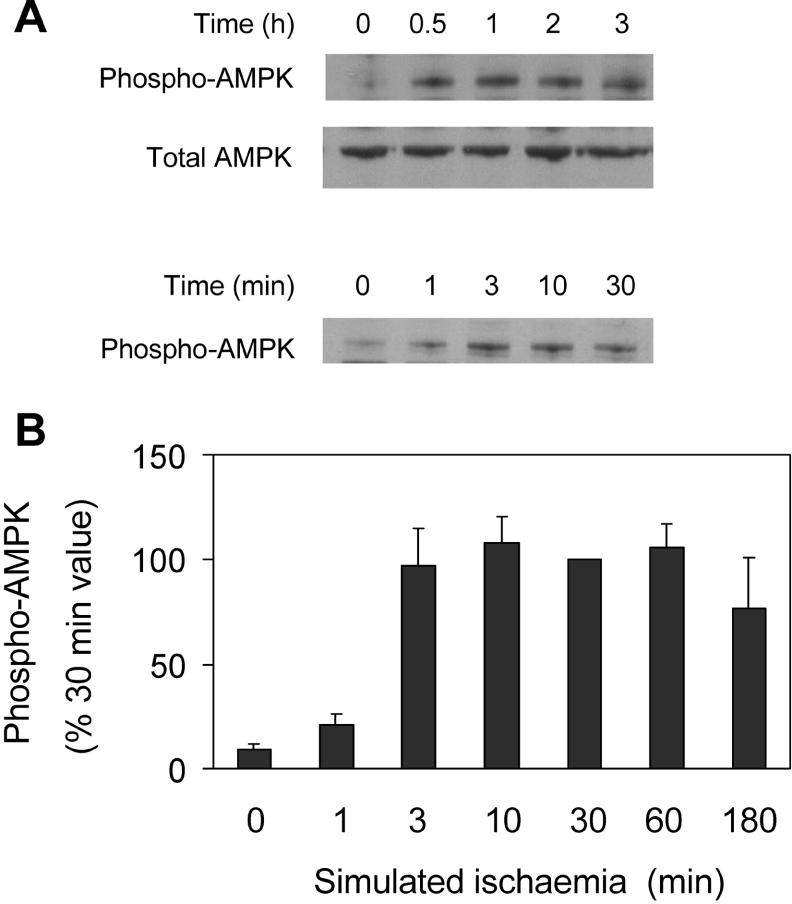
Ischaemia causes a marked increase in cytosolic [AMP], the principal sensor of which is AMPK [29]. AMP enables phosphorylation of the catalytic subunit, AMPK-α, at Thr172 by AMPKK (AMPK kinase), resulting in AMPK activation [30]. In the present study, activation was detected using an antibody specific for AMPK-α phosphorylated at Thr172. Simulated ischaemia brought about a rapid phosphorylation of AMPK-α (Figure 7A). In five such experiments, phosphorylation was near maximal after 3 min of ischaemia, and was largely maintained for 3 h (Figure 7B).
Figure 7. Phosphorylation of AMPK during simulated ischaemia.
(A) Cardiomyocytes were subjected to simulated ischaemia for the indicated times, and phosphorylated and total AMPK were immunodetected in Western blots. (B) Phospho-AMPK was estimated densitometrically from immunoblots. Values are means±S.E.M. (five experiments).
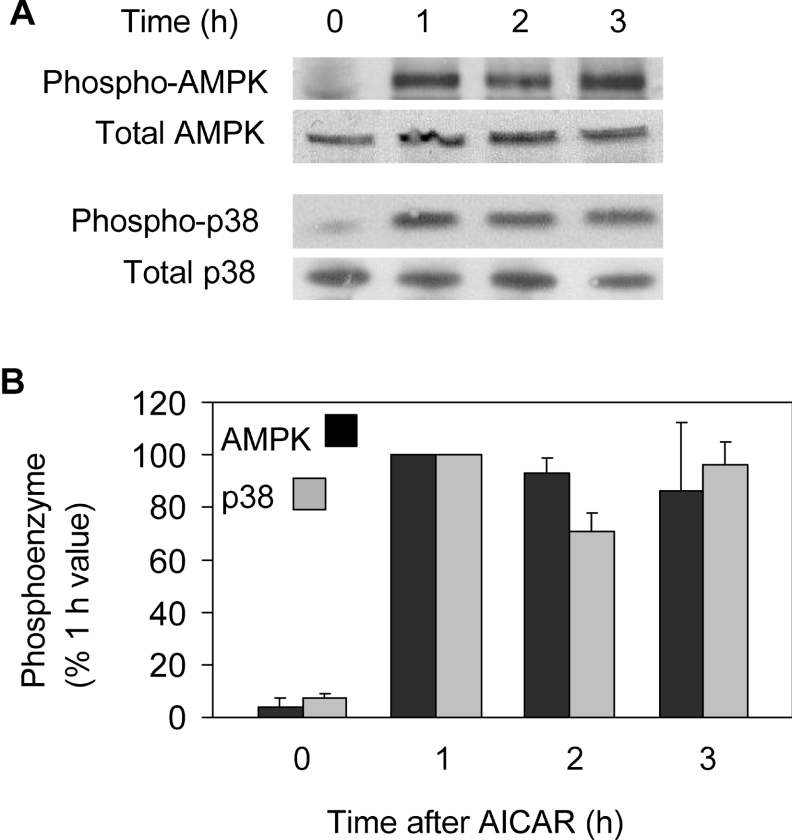
To investigate whether AMPK activation can lead to Bax translocation, we used the adenosine analogue AICAR. AICAR is cell-permeable and, after entering cells, is phosphorylated to produce the AMP analogue ZMP. ZMP mimics the action of AMP on AMPK. In agreement, AICAR markedly increased phosphorylation of AMPK, without affecting total AMPK (Figure 8A). AICAR treatment also led to phosphorylation of p38 MAPK (Figure 8A). The increased phosphorylation of both enzymes was maintained for at least 3 h (Figure 8B).
Figure 8. Phosphorylation of AMPK and p38 MAPK during incubation with AICAR.
(A) Cells were extracted after 0–3 h of treatment with AICAR and analysed for total and phosphorylated AMPK and p38 MAPK (p38) in Western blots. (B) Densitometric results from blots are given as means±S.E.M. (four experiments).
Figures 9(A) and 9(B) show typical images of GFP–Bax-transfected cardiomyocytes before and after treatment with AICAR under non-ischaemic conditions (i.e. plus glucose, minus cyanide). GFP–Bax was clearly sequestered during this time, leading to a 3-fold increase in S.D. of cytoplasmic pixel intensities. Overlay images (GFP–Bax and MTR; not shown) confirmed that GFP–Bax was sequestered by mitochondria. As with simulated ischaemia, the translocation of GFP–Bax to mitochondria resulted in an increase in mean cytoplasmic fluorescence and a loss from the nucleus (21 and 22% respectively). In five such experiments, sequestration of GFP–Bax continued for at least 3 h and was largely prevented by SB203580 (Figure 9C). Analyses of 14 cells indicated that the SB203580-sensitive GFP–Bax translocation in the presence of AICAR was at least as great as that under simulated ischaemia (Figures 5A and 5B). Thus the AICAR-induced changes in GFP–Bax location had the same characteristics as those brought about by simulated ischaemia and both appear to be mediated via p38 MAPK. As a further check, we used metformin (dimethyl biguanide), which activates AMPK without changing the AMP/ATP ratio [26]. In the present study, metformin also induced phosphorylation of p38 MAPK (∼ 75% of that produced by AICAR after 2 h; results not shown) and stimulated Bax translocation in an SB203580-dependent manner (Figure 5).
Figure 9. GFP–Bax sequestration induced by AICAR.
A GFP–Bax-transfected cardiomyocyte was preincubated for 30 min in standard medium (see the Materials and methods section), imaged (A), and then 2 mM AICAR was added; 3 h later, the cell was imaged again (B). The S.D. of cytoplasmic (cyt) pixel intensities relate to the area delineated in (A). The mean cytoplasmic and nuclear (nuc) fluorescence values (mean) were obtained after defining the relevant boundaries from brightfield images. (C) The time course of S.D. change in 10 cells incubated with or without 10 μM SB203580 (SB).
To confirm activation of the p38 MAPK pathway, we also examined the phosphorylation state of enzymes immediately upstream and downstream of p38 MAPK. Both simulated ischaemia and AICAR caused phosphorylation of MKK3 (Figure 10), which phosphorylates p38 MAPK but not JNK or ERK (extracellular-signal-regulated kinase) [31]. As substrate of p38 MAPK, we examined MAPKAPK2 (46 kDa) and MAPKAPK3 (42 kDa) using an antibody reactive with the phosphorylated forms of both, and both bands appeared after ischaemia and after AICAR treatment (Figure 10). Moreover, SB203580 blocked phosphorylation of p38 MAPK and its downstream substrate MAPKAPK2/3 during simulated ischaemia and in the presence of AICAR, but had no effect on the phosphorylation of the upstream kinase MKK3 or of AMPK. Thus SB203580 blockade of Bax translocation induced by simulated ischaemia and by AICAR under non-ischaemic conditions may be attributed to p38 MAPK inhibition.
DISCUSSION
Ischaemia and reperfusion lead to both necrotic and apoptotic cell death. In part, the relative proportions reflect the degree of energy deprivation (see [32] and references therein). Thus, in acute myocardial infarction, the severely ischaemic core of the infarct becomes necrotic, but the less energetically compromised cells in the hypoxic border regions undergo apoptosis [2–4]. In addition, whereas cells become necrotic after prolonged ischaemia or anoxia, they become increasingly apoptotic when the ischaemic/anoxic period is cut short by reperfusion [6,7,33,34] or reoxygenation [5]. There is evidence that apoptosis is initiated in the ischaemic period, when the energy state is low, but completed after reperfusion when an adequate energy state is restored [35]. It is important therefore to understand which apoptotic reactions occur during energy deprivation and how they are initiated. Here, we address the question of Bax movement to mitochondria, an early step in the apoptotic pathway.
Bax is located largely in the cytosol of healthy cells [36], but some Bax can be detected in mitochondria [12], where it is believed to be heterodimerized (and inactivated) with antiapoptotic Bcl-XL and Bcl-2. In agreement, GFP-tagged Bax assumed a largely diffuse distribution throughout the cytoplasm and nucleus of unstimulated cardiomyocytes, although in some images, including Figure 1(A), a small degree of GFP–Bax sequestration was just discernible in the perinuclear region. Secondary labelling of such cells with MTR (results not shown) confirmed that, when discernible, this represented a small amount of mitochondrial GFP–Bax in pre-ischaemic cells. GFP–Bax redistribution from this basal state was analysed in terms of movement to (Figure 5A), and sequestration within (Figure 5B), the cytoplasm. Since sequestration was restricted to mitochondria (Figure 2), these forms of analysis provided means of comparing GFP–Bax migration to mitochondria under the various experimental conditions. They revealed that simulated ischaemia markedly stimulates the translocation of GFP–Bax from the cytosol and nucleus to mitochondria over several hours. The translocation was substantiated by immunodetection of increased endogenous Bax in the mitochondrial fraction of cell lysates following simulated ischaemia (Figure 6). Thus Bax migrates to mitochondria under simulated ischaemia and this may be used to investigate how ischaemia triggers the apoptotic pathway.
We show that Bax migration was linked to p38 MAPK. Simulated ischaemia rapidly activated p38 MAPK, but had no detectable effect on JNK phosphorylation for at least 6 h. This broadly agrees with the report that, whereas ischaemia and reperfusion activate both the p38 MAPK and JNK pathways in heart, only the p38 MAPK pathway is activated by ischaemia alone [37]. Increased phosphorylation of p38 MAPK was detected as early as 5 min into simulated ischaemia, clearly preceding the earliest detection of Bax movement (20 min). Moreover, p38 MAPK inhibition by SB203580 completely blocked Bax translocation. A link between p38 MAPK activation and Bax migration to mitochondria has also been observed in other systems, i.e. NO-induced apoptosis in neuronal cells [38,39] and UVB light-induced apoptosis in keratinocytes [40]. It appears, therefore, that a range of cellular insults induce Bax translocation via p38 MAPK.
A key aspect of Bax translocation in ischaemia is how p38 MAPK is activated by the energy stress. The main sensor of the cellular energy state is AMPK, which becomes activated under various forms of energy stress, including hypoxia and ischaemia. Activation involves phosphorylation of the α subunit [30], brought about by an AMPKK, e.g. STKII (serine/threonine-protein kinase II) [41], and reversed by protein phosphatases 2A and 2B [42]. AMPK is activated by increase in the AMP/ATP ratio [25], but the mechanism is not fully clear. AMP binding to AMPK may make it a better substrate for AMPKK [43]. However, in heart, very mild ischaemia, insufficient to produce measurable change in the AMP/ATP ratio, was reported to activate AMPK, and this was attributed to a second AMPKK, in addition to STKII [44]. The binding of AMPK to glycogen granules may also influence its activity with protein targets [45]. AMPK plays a major role in the cellular adaptation to hypoxia by promoting anaerobic energy metabolism (glucose uptake and glycolysis) and by suppressing energy-requiring biosynthetic processes (e.g. fatty acid, sterol and glycogen syntheses) [24,43]. Recent studies of the role of AMPK in the stimulation of glucose uptake during energy stress have revealed a possible link between AMPK and p38 MAPK under these conditions. Thus AMPK and p38 MAPK were both activated along with glucose uptake in adult cardiomyocytes subjected to energy stress with the mitochondrial uncoupling agent 2,4-dinitrophenol, but transfection with a dominant-negative AMPK mutant suppressed the enhancement of both p38 MAPK and glucose transport [46]. Conversely, in a liver-derived cell line, overexpression of a constitutively active form of AMPK resulted in the activation of both p38 MAPK and glucose transport [47]. The current data agree with these indications. First, ischaemia stimulated the phosphorylation of both AMPK and p38 MAPK. Secondly, the stimulation of AMPK by AICAR and metformin also produced p38 MAPK phosphorylation. Thirdly, AICAR and metformin both stimulated Bax translocation in an SB203580-sensitive manner. Fourthly, the locus of SB203580 inhibition was p38 MAPK, since the phosphorylations of p38 MAPK and downstream substrates (MAPKAPK 2/3) were largely abolished by the inhibitor, whereas the phosphorylations of the upstream MKK3 and of AMPK were unaffected. Taken as a whole, our results indicate that activation of the p38 MAPK pathway leading to Bax translocation occurs downstream of AMPK in energy-stressed heart cells.
There are conflicting reports of the effects of AMPK activation on apoptosis, and the role of AMPK may be cell- and system-specific. An inhibitory action of AICAR and AMPK was observed on glucocorticoid-induced apoptosis [48]. In contrast, sustained activation of AMPK with AICAR promoted apoptosis in neuroblastoma [49,50] and liver [51] cells, and in a pancreatic β-cell line [52] although, in three of these reports, these effects were attributable to JNK [49,51,52], rather than p38 MAPK. It should be stressed, however, that the current data relate strictly to Bax translocation, an initial step in apoptosis, rather than execution of subsequent reactions of the apoptotic pathway and the formation of apoptotic cells. Indeed, ischaemia alone, as in the present study, leads only to necrosis; apoptotic cell death requires reperfusion [6,7,33,34], when JNK is known to be activated [37]. Thus, whereas p38 MAPK seems to be instrumental in the early event of Bax translocation in ischaemia, completion of the apoptotic pathway after reperfusion may require additional signalling pathways. This is in line with the finding that ischaemia alone produces caspase 3 activation, but it does not lead to DNA fragmentation until the tissue is reperfused [35].
Acknowledgments
We are grateful to the British Heart Foundation (PG 2000009) for financial support and to Dr Tony Michael (Department of Clinical Developmental Sciences, St George's, University of London, London, U.K.) for advice.
References
- 1.Veinot J. P., Gattinger D. A., Fliss H. Early apoptosis in human myocardial infarcts. Hum. Pathol. 1997;28:85–92. doi: 10.1016/s0046-8177(97)90039-3. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G., Quaini F., Sala R., Lagrasta C., Corradi D., Bonacina E., Gambert S. R., Cigola E., Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J. Mol. Cell. Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 3.Bialik S., Geenen D. L., Sasson I. E., Cheng R., Horner J. W., Evans S. M., Lord E. M., Koch C. J., Kitsis R. N. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng W., Kajstura J., Nitahara J. A., Li B., Reiss K., Liu Y., Clark W. A., Krajewski S., Reed J. C., Olivetti G., et al. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp. Cell Res. 1996;226:316–327. doi: 10.1006/excr.1996.0232. [DOI] [PubMed] [Google Scholar]
- 5.Kang P. M., Haunstetter A., Aoki H., Usheva A., Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ. Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umansky S. R., Cuenco G. M., Khutzian S. S., Barr P. J., Tomei L. D. Post-ischaemic apoptotic death of rat neonatal cardiomyocytes. Cell Death Differ. 1995;2:236–241. [PubMed] [Google Scholar]
- 8.Tanaka M., Ito H., Adachi S., Akimoto H., Nishikawa T., Kasajima T., Marumo F., Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured rat neonatal cardiomyocytes. Circ. Res. 1994;75:426–433. doi: 10.1161/01.res.75.3.426. [DOI] [PubMed] [Google Scholar]
- 9.von Ahsen O., Renken C., Perkins G., Kluck R. M., Bossy Wetzel E., Newmeyer D. D. Preservation of mitochondrial structure and function after Bid and Bax mediated cytochrome c release. J. Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei M. C., Zong W. X., Cheng E. H. Y., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacCregor G. R., Thompson C. B., Korsmeyer S. J. Proapoptotic Bax and Bak; a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonsson B. Bax and other pro-apoptotic Bcl-2 family ‘killer proteins’ and their victim, the mitochondrion. Cell Tissue Res. 2001;306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- 12.Goping I. S., Gross A., Lavoie N., Nguyen N., Jemmerson R., Roth K., Korsmeyer S. J., Shore G. C. Regulated targeting of Bax to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capano M., Crompton M. Biphasic translocation of Bax to mitochondria. Biochem. J. 2002;367:169–178. doi: 10.1042/BJ20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Q., Hsu Y.-T. Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis; development of a GFP-Bax-stable H9cl cell line for apoptosis analysis. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H477–H487. doi: 10.1152/ajpheart.00879.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cook S. A., Sugden P. H., Clerk A. Regulation of Bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes. Circ. Res. 1999;85:940–949. doi: 10.1161/01.res.85.10.940. [DOI] [PubMed] [Google Scholar]
- 16.Fortuna M., Ravassa S., Etayo J. C., Diez J. Overexpression of Bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats. Hypertension. 1998;32:280–286. doi: 10.1161/01.hyp.32.2.280. [DOI] [PubMed] [Google Scholar]
- 17.Kawai K., Qin F. Z., Shite J., Mao W. K., Fukuoka S., Liang C. S. Importance of antioxidant and antiapoptotic effects of β receptor blockers in heart future therapy. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1003–H1012. doi: 10.1152/ajpheart.00797.2003. [DOI] [PubMed] [Google Scholar]
- 18.Misao J., Hayakawa Y., Ohno M., Kato S., Fujiwara T., Fujiwara H. Expression of bcl-2, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 19.Liu L. X., Azhar G., Gao W., Zhang X., Wei J. Y. Bcl-2 and Bax expression in adult rat hearts after coronary occlusion. Am. J. Physiol. 1998;275:H315–H322. doi: 10.1152/ajpregu.1998.275.1.R315. [DOI] [PubMed] [Google Scholar]
- 20.Anversa P., Leri A., Kajstura J., Nadal-Ginard B. Myocyte growth and cardiac repair. J. Mol. Cell. Cardiol. 2002;34:91–99. doi: 10.1006/jmcc.2001.1506. [DOI] [PubMed] [Google Scholar]
- 21.Mackay K., Mochly-Rosen D. An inhibitor of p38 mitogen activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischaemia and reperfusion. Circulation. 1999;274:6272–6279. [Google Scholar]
- 22.Ma X. L., Kumar S., Gao F., Louden C. S., Lopez B. L., Christopher T. A., Wang C. L., Lee J. C., Feuerstein G. Z., Yue T. L. Inhibition of p38 mitogen activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischaemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 23.Schneider S., Chen W., Hou J., Steenbergen C., Murphy E. Inhibition of p38 MAPK α/β reduces ischaemic injury and does not block protective effects of preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 24.Carling D. AMP-activated protein kinase: balancing the scales. Biochimie. 2005;87:87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Hardie D. G., Carling D. The AMP-activated protein kinase. Fuel gauge of the mammalian cell. Eur. J. Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 26.Fryer L. G. D., Parbu-Patel A., Carling D. The antidiabetic drugs rosiglitazone and metformin stimulate the AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 27.Lemke L. E., Bloem L. J., Esterman M., Sandusky G., Vlahos C. J. Decreased p38 MAPK activity in end-stage failing human myocardium: p38 MAPKα is the predominant isoform expressed in human heart. J. Mol. Cell. Cardiol. 2000;33:1527–1540. doi: 10.1006/jmcc.2001.1415. [DOI] [PubMed] [Google Scholar]
- 28.Davies S. P., Riddy H., Caivan M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsin A. S., Bertrand L., Rider M. H., Deprez I., Beualoye C., Vincent M. F., Van den Bergh G., Carling D., Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 30.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R., Carling D., Hardie D. G. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine-172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 31.Brancho D., Tanaka N., Jaeschke A., Ventura J. J., Kelkar J., Tanaka Y., Kyuuma M., Takeshita T., Flavell R. A., Davis R. J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1699–1706. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiraishi J., Tatsumi T., Keira N., Akashi K., Mano A., Yamanaka S., Matoba S., Asayama J., Yaoi T., Fushiki S., et al. Important role of energy dependent mitochondrial pathways in cultured rat cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1637–H1647. doi: 10.1152/ajpheart.2001.281.4.H1637. [DOI] [PubMed] [Google Scholar]
- 33.Fliss H., Gattinger D. Apoptosis in ischaemic and reperfused rat myocardium. Circ. Res. 1996;79:959–965. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z. Q., Nakamura M., Wang N. P., Wilcox J. N., Shearer S., Ronson R. S., Guyton R. A., Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc. Res. 2000;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 35.Freude B., Masters T. N., Robiczek F., Fokin A., Kostin S., Zimmermann R., Ullmann C., Losenz-Meyer S., Schaper J. Apoptosis is initiated by myocardial ischaemia and executed during reperfusion. J. Mol. Cell. Cardiol. 2000;32:197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 36.Wolter G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X., Youle R. J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogoyevitch M. A., Gillespie-Brown J., Ketterman A. J., Fuller S. J., Ben-Levy R., Ashworth A., Marshall C. J., Sugden P. H. Stimulation of the stress-activated mitogen activated protein kinase subfamilies in perfused heart. Circ. Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa Y., Kusaka E., Enokida Y., Ikenchi T., Hatanaka H. Regulation of Bax translocation through phosphorylation at Ser-70 of Bcl-2 by MAP kinase in NO-induced neuronal apoptosis. Mol. Cell. Neurosci. 2003;24:451–459. doi: 10.1016/s1044-7431(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 39.Ghatan S., Larner S., Kinoshito Y., Hetman M., Patel L., Xia Z. G., Youle R. J., Morrison R. S. p38 MAP kinase mediates Bax translocation in nitric oxide induced apoptosis in neurons. J. Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Laethem A., Van Keldst S., Lippens S., Declerq W., Vandenabeele P., Janssens S., Vandenheede J. R., Garmyn M., Agostinis P. Activation of p38 MAP kinase is required for Bax translocation to mitochondria, cytochrome c release and apoptosis induced by UVB irradiation in human keratinocytes. FASEB J. 2004;18:U281–U313. doi: 10.1096/fj.04-2285fje. [DOI] [PubMed] [Google Scholar]
- 41.Shaw R. J., Kosmatka M., Bardesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein S. C., Woods A., Jones N. A., Davison M. D., Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Marthy S., Waller M., Gupta A., Adams J. J., Katsis F., van Denderen B., et al. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 44.Altarejos J. Y., Taniguchi M., Clanachan A. S., Lopaschuk G. D. Myocardial ischaemia differentially regulates LKB1 and an alternate 5′-AMP-activated protein kinase kinase. J. Biol. Chem. 2005;280:183–190. doi: 10.1074/jbc.M411810200. [DOI] [PubMed] [Google Scholar]
- 45.Polekhina G., Gupta A., Michell B. J., van Denderen B., Feil S. C., Jennings I. G., Campbell D. J., Witters L. A., Parker M. W., Kemp B. E., et al. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr. Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 46.Pelletier A., Joly E., Prentki M., Coderre L. Adenosine-5′-monophosphate-activated protein kinase and p38 mitogen activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology. 2005;146:2285–2294. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 47.Xi X., Han J., Zhang J.-Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen activated protein kinase. J. Biol. Chem. 2001;276:41029–41034. doi: 10.1074/jbc.M102824200. [DOI] [PubMed] [Google Scholar]
- 48.Stefanelli C., Stanic F., Bonovita F., Flamigni F., Pignatti C., Guarnieri C., Caldarera C. M. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleotide, a cell permeable activator of AMP activated protein kinase. Biochem. Biophys. Res. Commun. 1998;243:821–826. doi: 10.1006/bbrc.1998.8154. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Gil M., Pesi R., Perna S., Allegrini S., Giannecchini M., Camici M., Tozzi M. 5′-Aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. J. Neurosci. 2003;117:811–826. doi: 10.1016/s0306-4522(02)00836-9. [DOI] [PubMed] [Google Scholar]
- 50.Jung J. E., Lee J., Ha J., Kim S. S., Cho Y. H., Baik H. H., Kang I. 5′-Aminoimidazole-4-carboxamide-ribonucleotide enhances oxidative stress-induced apoptosis through activation of nuclear factor-κβ in mouse Neuro2a neuroblastoma cells. Neurosci. Lett. 2004;354:197–200. doi: 10.1016/j.neulet.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Meisse D., Van de Casteele M., Beauloye C., Hainault I., Kefas B. A., Rider M. H., Foufelle F., Hue L. Sustained activation of AMP-activated protein kinase induces a c-Jun N-terminal kinase activation in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- 52.Kefas B. A., Cai Y., Ling Z., Heimberg H., Hue L., Pipeleers D., Van de Casteele M. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun N-terminal kinase. J. Mol. Endocrinol. 2003;30:151–161. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]