Abstract
A gene, phl, encoding a phenylacetyl-CoA ligase was cloned from a phage library of Penicillium chrysogenum AS-P-78. The presence of five introns in the phl gene was confirmed by reverse transcriptase-PCR. The phl gene encoded an aryl-CoA ligase closely related to Arabidopsis thaliana 4-coumaroyl-CoA ligase. The Phl protein contained most of the amino acids defining the aryl-CoA (4-coumaroyl-CoA) ligase substrate-specificity code and differed from acetyl-CoA ligase and other acyl-CoA ligases. The phl gene was not linked to the penicillin gene cluster. Amplification of phl in an autonomous replicating plasmid led to an 8-fold increase in phenylacetyl-CoA ligase activity and a 35% increase in penicillin production. Transformants containing the amplified phl gene were resistant to high concentrations of phenylacetic acid (more than 2.5 g/l). Disruption of the phl gene resulted in a 40% decrease in penicillin production and a similar reduction of phenylacetyl-CoA ligase activity. The disrupted mutants were highly susceptible to phenylacetic acid. Complementation of the disrupted mutants with the phl gene restored normal levels of penicillin production and resistance to phenylacetic acid. The phenylacetyl-CoA ligase encoded by the phl gene is therefore involved in penicillin production, although a second aryl-CoA ligase appears to contribute partially to phenylacetic acid activation. The Phl protein lacks a peptide-carrier-protein domain and behaves as an aryl-capping enzyme that activates phenylacetic acid and transfers it to the isopenicillin N acyltransferase. The Phl protein contains the peroxisome-targeting sequence that is also present in the isopenicillin N acyltransferase. The peroxisomal co-localization of these two proteins indicates that the last two enzymes of the penicillin pathway form a peroxisomal functional complex.
Keywords: aryl-capping enzyme, isopenicillin N acyltransferase (IAT), Penicillium chrysogenum, phenylacetic acid, phenylacetyl-CoA ligase
Abbreviations: 6-APA, 6-aminopenicillanic acid; ArCP, aromatic-acid-carrier protein; CP, complex production; DP, defined production; DPhl, disrupted in phl; DTT, dithiothreitol; IAT, isopenicillin N acyltransferase; NRPS, non-ribosomal peptide synthetase; PAA, phenylacetic acid; PAA-CoA, phenylacetyl-CoA; PCP, peptide-carrier protein; RT, reverse transcriptase
INTRODUCTION
The pathway for penicillin biosynthesis has been largely elucidated [1,2], although the molecular mechanisms underlying some of the reactions, e.g. the activation of PAA (phenylacetic acid) and the amino acid epimerization reactions that are catalysed by ACV (δ-L-α-aminoadipyl-L-cysteinyl-D-valine) synthetase, remain obscure [3]. The Penicillium chrysogenum genes pcbAB, pcbC and penDE, encoding the enzymes that catalyse the three central penicillin biosynthetic reactions, have been characterized [4–8].
In the last reaction of the penicillin biosynthetic pathway, the α-aminoadipyl side chain of isopenicillin N is removed and replaced by a phenylacetate (or phenoxyacetate) residue [5]. This reaction is catalysed by the IAT (isopenicillin N acyltransferase), a complex enzyme with several catalytic activities [9–11]. The IAT is able to attach PAA efficiently to 6-APA (6-aminopenicillanic acid), but requires that the PAA is activated previously in the form of a thioester with CoA (PAA-CoA, phenylacetyl-CoA) [10,11].
The mechanism of activation of PAA has remained obscure for decades despite its industrial interest. There was early evidence for the existence of a PAA-activating activity [12,13]. Although partial purification of the enzyme was achieved [13], the enzyme was very labile and no significant characterization of its biochemical properties could be made.
An acetyl-CoA synthase gene (acuA or facA) able to activate several fatty acids in vitro was reported [14]. However, when the gene acuA ( facA) encoding the acetyl-CoA synthase [15,16] was mutated, it did not affect penicillin biosynthesis [16], suggesting that the authentic PAA-CoA ligase was a different enzyme.
Gledhill et al. [17] described in a patent application the purification of the PAA-CoA ligase and sequenced the N-terminal end of the protein. A DNA fragment containing the putative PAA-CoA ligase gene was cloned from P. chrysogenum BW1901. However, the patent work did not provide genetic evidence to prove that the cloned gene corresponded to the authentic PAA-CoA ligase. Gene disruption in P. chrysogenum is difficult to perform because of frequent ectopic integration of the exogenous DNA during disruption experiments, but gene inactivation is required to prove unequivocally the involvement of a specific gene in a biosynthetic reaction. We have previously optimized gene disruption in P. chrysogenum [18] using the two-marker strategy [19].
Taking into account the precursors involved in the biosynthesis of benzylpenicillin, its pathway adjusts to the model of hybrid polyketide–non-ribosomal-peptide secondary metabolites. Activation of short-chain fatty acids and aromatic acids and their sequential condensation with amino acids during the biosynthesis of hybrid polyketide–polypeptide secondary metabolites is a subject of great interest. The modules of NRPSs (non-ribosomal peptide synthetases) are involved in amino acid activation [20]. Fatty acids and organic acids are activated as acyladenylates and later transferred to a PCP (peptide-carrier protein) domain equivalent to the ACP (acyl-carrier protein) domain of polyketide synthases. In this regard, the exact role of the protein encoded by the PAA-CoA ligase gene is unknown.
It was, therefore, necessary to perform a detailed genetic analysis of the possible involvement of this gene in PAA activation and in penicillin biosynthesis to clarify the molecular mechanism of this important step of β-lactam antibiotic biosynthesis.
MATERIALS AND METHODS
Strains and culture conditions
P. chrysogenum Wis 54-1255, a low-penicillin-producing strain with a single copy of the penicillin gene cluster [21], was used as the parental strain. P. chrysogenum Wis 54-1255 pyrG, a uridine auxotroph blocked in the pyrG gene [22], was used as the recipient strain in transformation procedures. P. chrysogenum npe10 pyrG, a deletion strain containing multiple copies of the entire penicillin gene cluster [23,24], and P. chrysogenum AS-P-99, a strain containing multiple copies of the penicillin gene cluster [21], were used to study the genome localization of the phl gene.
Competent cells of Escherichia coli DH5α strain were used for high-efficiency transformation, amplification and isolation of plasmid DNA. It was grown in LB (Luria–Bertani) medium with ampicillin (100 μg/ml), chloramphenicol (30 μg/ml) or kanamycin (50 μg/ml).
P. chrysogenum spores were obtained from plates of Power medium [23] (n=18) grown for 5 days at 28 °C. Seed cultures were initiated by inoculating fresh spores from one sporulated Petri dish into CI (complex inoculation) medium (20 g/l corn steep solids, 10 g/l yeast extract, 58 mM sucrose, 50 mM calcium carbonate, pH 5.7) or DI (defined inoculation) medium [18] after incubation for 24 h. Penicillin production was studied in cultures in CP (complex production) medium [4 g/l potassium phenylacetate, 20 g/l Pharmamedia (Traders Protein, Memphis, TN, U.S.A.), 50 g/l lactose, 0.03 M ammonium sulphate and 0.05 M calcium carbonate, pH 6.6] or DP (defined production) medium [18]. Cultures (100 ml in 500 ml flasks) were inoculated with 5% seed cultures and incubated in an orbital shaker (at 250 rev./min for up to 96 h at 25 °C). The mycelia were harvested by filtration, washed with 1 vol. of 0.9 g/l NaCl, and stored at −80 °C until use. The resistance to PAA was studied in DP medium with 0.5, 1.0, 1.5, 2.0, 2.5 and 3 g/l potassium phenylacetate or potassium phenoxyacetate. In all experiments, PAA or phenoxyacetic acid were neutralized to pH 8.0 with concentrated KOH before use.
Transformation of P. chrysogenum protoplasts
Protoplasts of P. chrysogenum Wis 54-1255 pyrG were obtained as described by Díez et al. [22] and were transformed according to the procedure of Cantoral et al. [25]. Transformant clones were selected by complementation of the uridine auxotrophy or phleomycin resistance. Uridine and phleomycin were added at final concentrations of 100 μg/ml and 30 μg/ml respectively when necessary. The plasmids that were used are listed in Table 1.
Table 1. Plasmids used in the present study.
| Plasmid | Characteristics | Origin |
|---|---|---|
| pULJL43 | Integrative vector. Contains the ble resistance marker under control of the fungal pcbC promoter. | [43] |
| pAMF9L | Autonomous replicating plasmid (AMA1 sequence). Contains the P. chrysogenum pyrG gene as selective marker. | [44] |
| pAMF9E | Derivative of pAMF9L containing the phl gene in a 6.6 kb EcoRI DNA fragment. | Present study |
| pZE1.2 | Derivative of the E. coli vector pZero carrying the 6.6 kb EcoRI DNA fragment containing the phl gene. | Present study |
| pBle3 | Integrative plasmid designed for phl gene disruption by double recombination. | Present study |
| pZphl6 | Integrative plasmid carrying a 4 kb StuI/SacII fragment that includes the phl gene. Used for complementation studies. | Present study |
Isolation of genomic DNA of P. chrysogenum strains and phage library constructions
Genomic DNA was isolated from P. chrysogenum cultures in MPPY (40 g/l glucose, 3 g/l NaNO3, 2 g/l yeast extract, 0.5 g/l KCl, 0.5 g/l MgSO4·7H2O, 0.01 g/l FeSO4·7H2O, adjusted to pH 6.0) [26], supplemented with 100 μg/ml uridine when necessary, as described previously [26a]. A P. chrysogenum AS-P-78 genomic library in the vector LambdaGEM-12 (Promega) [27] was used to clone the phl gene. The oligonucleotides used as primers in different experiments are listed in Table 2.
Table 2. Oligonucleotides used in this work.
| Oligonucleotides | Nucleotide sequence | Use |
|---|---|---|
| Ocla5 | 5′-TATTACCCCGAGGATTTG-3′ | Intron 1, spore PCR analysis, probe |
| Oaa14 | 5′-AACACCGCCCAGTCTGTGAAC-3′ | Intron 1, spore PCR analysis |
| Oaa5 | 5′-GCCCGGCGAACAGCATTTG-3′ | Intron 2, intron 3 |
| Oaa11 | 5′-GGAAGGAGACCAAGCGCAAC-3′ | Intron 2 |
| Oaa2 | 5′-AACAGAGACGTCACGGAATCT-3′ | Intron 3 |
| Oaa10 | 5′-GAGACAGCTGCCGATTTCCTC-3′ | Intron 4 |
| Oaa9 | 5′-CACAATTCGTGCCTCGACT-3′ | Intron 4 |
| Oaa12 | 5′-GGCATCGAGCACGTGTTTAT-3′ | Intron 5 |
| Ocla3 | 5′-TACCCCCGACATTTTT-3′ | Intron 5, probe |
| QC1 | 5′-GGTCAGAATGGGATAAGCTTTGGCCGTGTTG-3′ | Site-directed mutagenesis |
| QC2 | 5′-CAAACACGGCCAAAGCTTATCCCATTCTGACC-3′ | Site-directed mutagenesis |
Southern hybridization and nucleic acid manipulations
Genomic DNA (2–4 μg) was digested and the fragments were separated on a 0.7% agarose gel and blotted on to Hybond-N+ membranes (Amersham Biosciences) using a vacuum system (Pharmacia VacuGene). Digoxigenin labelling, hybridization and detection were performed with the Genius kit (Boehringer Mannheim, Germany) according to the manufacturer's instructions. Hybridizations were done by standard procedures [27b] at 42 °C using 5×SSC (1×SSC is 0.15 M NaCl/0.015 M sodium citrate), 0.1% (w/v) dodecylsarcosine, 0.02% (w/v) SDS, 40% (w/v) formamide and 2% (w/v) blocking reagent. The hybridization signals were visualized with BCIP (5-bromo-4-chloroindol-3-yl phosphate) and NBT (Nitro Blue Tetrazolium) following standard procedures [27a]. PstI-digested λ bacteriophage DNA labelled with digoxigenin was used as size marker.
cDNA synthesis and intron analysis
cDNA was obtained from RNA extracted with the RNeasy kit (Qiagen) from mycelia grown for 48 h in CP medium. To elucidate the presence of putative introns in the DNA sequence, the region containing the expected intron sites was amplified by RT (reverse transcriptase)-PCR (Invitrogen) using the appropriate primers listed in Table 2. The amplified cDNA region was sequenced to confirm the presence of the introns. Automatic sequencing was performed with the AutoRead™ System (Pharmacia).
Site-directed mutagenesis and PCR analysis of spore DNA
In vitro mutagenesis was performed with the QuikChange® site-directed mutagenesis kit (Stratagene). Primers QC1 and QC2 (Table 2) were used to delete one nucleotide, creating a HindIII site.
PCR amplification of DNA from spores was used for analysis of the transformants. Spores were broken in distilled water with glass beads (425–600 μm diameter, Sigma) by vortex-mixing for 15 s. The beads were removed by centrifugation at 8000 g for 10 min, and 20 μl of the supernatant was used as DNA template solution for PCR.
Isolation of disrupted mutants by phl gene replacement
The phl gene was disrupted by the double marker gene replacement technique [18,19] using the construction pBble3 in which the phl was inactivated by a double mutation: (i) a pyrG gene insertion (in the opposite orientation) within the 5′ region of the region of the gene, and (ii) a frameshift mutation downstream of the pyrG insertion site to avoid the possibility of the formation of a truncated Phl from some internal promoter.
HPLC determination of benzylpenicillin
Benzylpenicillin in the cultures was determined using a Waters Systems HPLC equipped with a 2487 dual absorbance detector, using a 4.6 mm×250 mm RPC18 Lichrospher® 100 (Merck) column with a flow rate of 0.8 ml/min. Samples were analysed using, as a mobile phase, buffer A [15 mM ammonium formate, pH 5.0, and 5% (v/v) acetonitrile] and buffer B [buffer A/acetonitrile, 1:4 (v/v)]. The benzylpenicillin was quantified by determining the absorbance at 214 nm.
In vitro assay for PAA-CoA ligase
Protein extract (500 μg; quantified by the Bradford method [27b]) diluted in extraction buffer [30 mM Tris/HCl, pH 8.5, 1 mM DTT (dithiothreitol) and 100 μg/ml Pefabloc (Sigma) in glycerol] was mixed with 10 mM PAA, 5 mM ATP, 10 mM magnesium chloride, 1 mM CoA sodium salt and 0.75 mM DTT at pH 8.0 in a total volume of 0.3 ml and was incubated at 28 °C for 30 min. The same reaction volume of methanol was added to precipitate the proteins. The PAA-CoA formed was determined using a Waters Systems HPLC, as described above, with a flow rate of 0.9 ml/min using, as a mobile phase, solvent A (10 mM ammonium phosphate and 5 mM tetrabutylammonium hydrogen sulphate, pH 5.5) and solvent B (100% acetonitrile). The PAA-CoA formed was quantified by determining the absorbance at 260 nm, using pure PAA-CoA (Sigma) as standard, and is given as μg per mg of protein extract.
Computer analyses
Computer analyses of nucleotide and amino acid sequences were performed with the Lasergene® software package (DNASTAR Madison, WI, U.S.A.), BLASTX2 and BLASTN (http://www.ncbi.nlm.nih.gov/BLAST/). Multiple alignments were made with CLUSTALW (http://www.ebi.ac.uk/clustalw/). GeneDoc (http://www.psc.edu/biomed/genedoc) was used for editing the multiple sequence alignments. Putative conserved domains were detected with the Protein Families Database of Aligments and Hidden Markov Models (PFAM; http://www.sanger.ac.uk/Software/Pfam/).
RESULTS
Isolation of bacteriophages containing the phl gene from a P. chrysogenum library
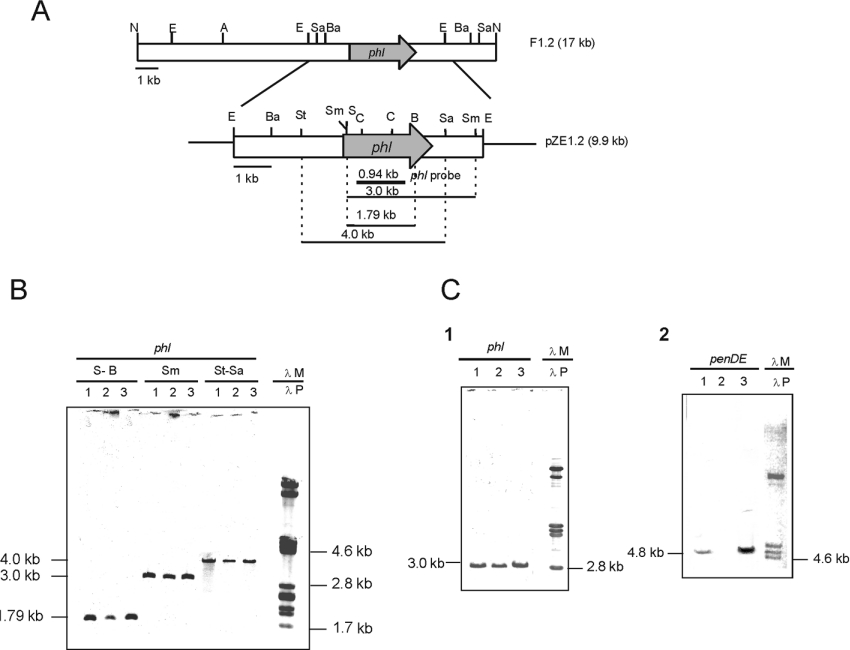
A 2105-bp DNA fragment was amplified by PCR from the genomic DNA of P. chrysogenum using oligonucleotides Ocla3 and Ocla5 as primers. The amplified DNA fragment was sequenced and was shown to be identical with that of P. chrysogenum BW1901 reported in the patent application [17]. This DNA fragment was used as a probe to screen a LambdaGEM-12 genomic library of P. chrysogenum AS-P-78. Two positive bacteriophages were isolated and purified by four consecutive rounds of hybridization. A 17-kb NotI-hybridizing DNA band of bacteriophage F2 was subcloned in the pZero vector; the resulting plasmid was named pZ1.2. Restriction endonuclease mapping and hybridization with the 2105-bp PCR probe allowed the insertion of the PCR-amplified fragment in the central region of the 17-kb fragment in this bacteriophage. A 6.6-kb EcoRI fragment was subcloned (new plasmid pZE1.2) and a region of 3770 bp was sequenced on both strands (Figure 1).
Figure 1. The phl gene is present as a single copy in different P. chrysogenum strains.
(A) P. chrysogenum DNA fragment cloned in phage F1.2 and in plasmid pZE1.2 (9.9 kb). The phl gene is indicated with a shaded arrow. The 0.9-kb phl fragment used as probe in hybridization experiments is indicated with a solid bar. Restriction endonuclease sites: A, ApaI; Ba, BamHI; C, ClaI; E, EcoRI; N, NotI; S, SalI; Sa, SacII; Sm, SmaI; St, StuI. (B) Hybridization of the DNA of three different strains of P. chrysogenum with the phl probe. Lane 1, parental Wis 54-1255 strain; lane 2, deletion mutant Wis npe10; lane 3, penicillin-overproducer AS-P-99 strain. The DNA was digested with different enzyme mixtures: S-B, SalI/BglII; Sm, SmaI; St-Sa, StuI/SacII. λM, size markers [λ bacteriophage digested with PstI hybridized with labelled λPst as probe (λP)]. (C) Hybridization of SmaI-digested DNA of the three P. chrysogenum strains with the phl (panel 1) or SalI-digested DNA of the three strains with the penDE (panel 2), showing that the phl gene is present in the three strains, whereas the penDE gene is absent in the deletion mutant Wis npe10 and amplified (thick band) in strain AS-P-99. Lanes 1, 2, and 3, DNA of the three P. chrysogenum strains as in (B).
An open reading frame of 2076 bp that contained five putative introns was identified. The presence of the five introns corresponding to positions 277–340 (64 bp), 723–822 (100 bp), 1093–1148 (56 bp), 1311–1377 (67 bp) and 1689–1743 (55 bp), numbered from the ATG of the gene, was confirmed by RT-PCR using RNA isolated from a 48 h culture of P. chrysogenum Wis 54-1255 grown in CP medium, and the specific oligonucleotides listed in Table 1 as primers. The gene was named phl (phenylacetyl-CoA ligase).
The phl gene encodes a protein similar to other aryl-CoA ligases
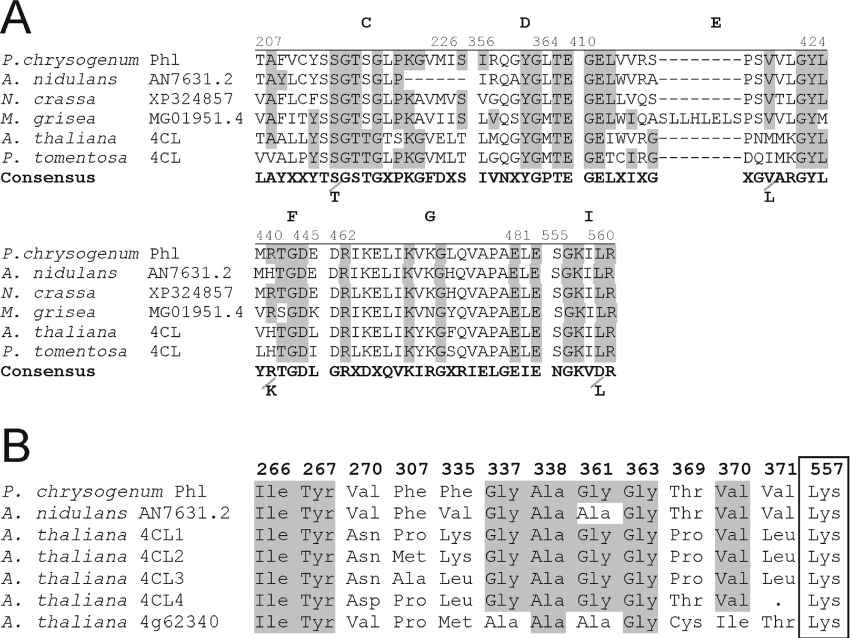
The protein encoded by the phl gene consisted of 578 amino acids and showed a molecular mass of 62.6 kDa. Computer analysis of the amino acid sequence revealed that it corresponded to an aryl-CoA ligase of the family of acyladenylate-forming enzymes (see the Discussion). The Phl protein showed approx. 30% identical amino acids with four different 4-coumaroyl-CoA ligases of Arabidopsis thaliana and other plants. These enzymes catalyse the activation of 4-coumaric acid to 4-coumaroyl-CoA, the starter unit of phenylpropanoid metabolism in plants [28]. The highest similarity of the Phl protein was with proteins of unknown function deduced from the genomes of Aspergillus nidulans (65% identity), Neurospora crassa (54% identity), Aspergillus fumigatus (30% identity), Gibberella zea and Magnaporthe grisea (approx. 30% identity) (Figure 2). The presence of Phl protein in Asp. nidulans is consistent with the ability of that fungus to produce penicillin. The Phl-like protein in the other fungi is probably related to the detoxifying activity of this protein (see below).
Figure 2. Conserved motifs and amino acids involved in the substrate-specificity code of aryl-CoA ligases.
(A) Conserved motifs in phl and the acyladenylate protein family. The consensus motifs C, D, E, F, G, I were as proposed by Martín [8], Marahiel et al. [30] and Turgay et al. [31]. (B) Amino acids involved in the substrate-specificity code of aryl-CoA ligases [37] numbered according to the positions in the P. chrysogenum Phl protein. Note the amino acid conservation (shaded) in the P. chrysogenum Phl protein in comparison with the four coumaroyl-CoA ligases (4CL1–4CL4) of A. thaliana. Protein At4g62340 (bottom line) is an A. thaliana acyladenylate enzyme but not a true 4-coumaroyl-CoA ligase, and is shown for comparative purposes. P. tomentosa, Populus tomentosa.
Interestingly, the Phl protein showed no significant similarity to PAA-CoA ligases described in several PAA-degrading bacteria (see the Discussion).
Aryl-capping enzymes: the encoded PAA-CoA ligase lacks a PCP domain and contains a C-terminal peroxisome-targeting motif
Aryl-capping enzymes have been shown to be involved in the biosynthesis of peptide siderophores and other secondary metabolites starting with an aryl group [29]. However, it was unclear if biosynthesis of phenylacetylpenicillin responds to this model. The Phl protein contained all the consensus motifs of acyladenylating enzymes of type A, including the five motifs involved in substrate recognition, designated as: C (adenylate-forming motif); D; E; F (ATP-binding motif); and G, H and I (putative CoA binding) [30,31]. These motifs also occur in the amino-acid-activating modules of the NRPSs [3]. The C and E motifs in Phl are partially modified with respect to the consensus motif of NRPS, suggesting that the Phl protein has a different substrate specificity.
In contrast with the modules of NRPSs, the Phl protein lacks a PCP domain, i.e. the phosphopantothenic acid attachment site [32] that is present in other acyladenylate-forming enzymes.
The Phl protein shows a C-terminal SKI (Ser-Lys-Ile) motif that adjusts to the consensus microbody (peroxisome) targeting sequence (see the Discussion). Targeting of the Phl to peroxisomes (microbodies) is very interesting because the last enzyme of the penicillin pathway, the IAT, is also targeted to microbodies [33].
The phl gene is present in a single copy and is not located in the 50-kb amplified region that contains the penicillin gene cluster
The presence and the number of copies of the phl gene was tested by Southern hybridization with a 0.94-kb probe internal to the phl gene in three relevant strains: P. chrysogenum Wis 54-1255 that contained a single copy of the penicillin gene cluster Wis 54-1255, npe10 (a deletion derivative of P. chrysogenum Wis 54-1255 lacking the 50-kb region containing the pen cluster) and a high-penicillin-producing strain, P. chrysogenum AS-P-99, that contains multiple copies of the 50-kb region, including the entire penicillin gene cluster.
Results showed that the three strains contained a single 3.0-kb SmaI-hybridizing band of the same intensity (Figure 1B), indicating that the phl gene is not located in the 50-kb region deleted in strain npe10 and that it is not amplified in strain AS-P-99, in contrast with the other penicillin biosynthesis genes pcbAB, pcbC and penDE, as shown by comparative hybridization with a probe internal to the penDE gene (Figure 1C). As observed in Figure 1(C), strain npe10 lacks the penDE gene; moreover, this gene is amplified in tandem repeats in strain AS-P-99 (as shown by the thick band of the same size as for Wis 54-1255; see the Discussion).
However, a second phl-related gene was shown by hybridization with the entire phl gene under low-stringency conditions (see below).
Amplification of phl leads to increased penicillin production
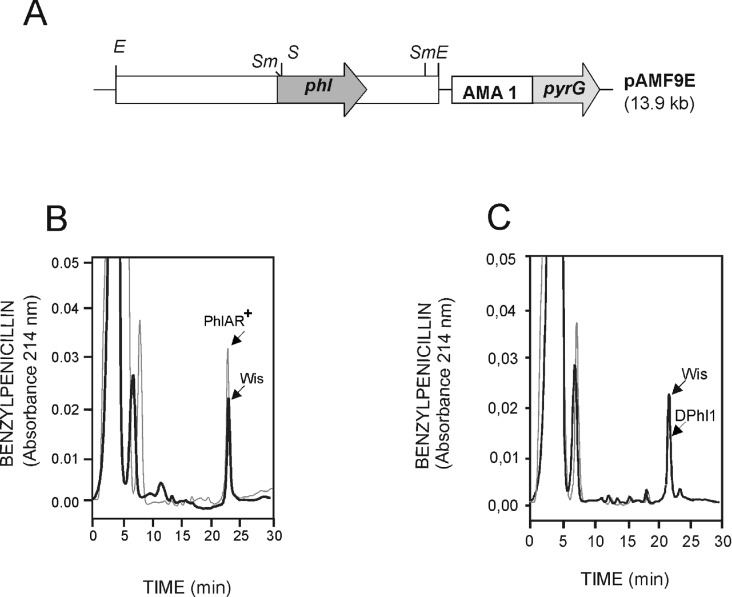
The role of the phl gene in relation to penicillin biosynthesis and PAA resistance was studied by gene amplification and gene deletion. For amplification studies, plasmid pAMF9E, carrying the phl gene expressed from its own promoter and the pyrG marker in an autonomous replicating plasmid (AMA1 replicon), was used to transform P. chrysogenum Wis 54-1255 pyrG−, and transformants were selected by complementation of the pyrG auxotrophy (Figure 3A). Eight randomly selected transformants were analysed by Southern hybridization using a probe internal to the phl gene. They all contained the transforming phl gene without rearrangement.
Figure 3. Amplification of the phl gene results in increased benzylpenicillin production.
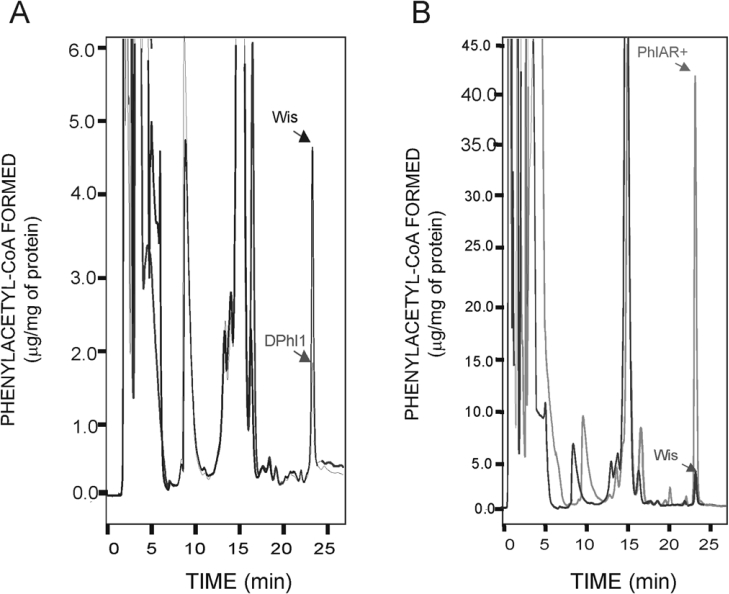
(A) Construction (pAMF9E) used to amplify the phl gene in an autonomous replicating plasmid containing the AMA1 sequence (ars) from Asp. nidulans. Restriction endonuclease sites: E, EcoRI; S, SalI; and Sm, SmaI. (B) HPLC of benzylpenicillin measured directly in culture broths of the parental strain (Wis, solid line) and the PhlAR+ transformant (broken line). (C) Direct HPLC determination of benzylpenicillin in culture broths of the parental Wis 54-1255 strain (solid line) and the disrupted Dphl1 mutant (broken line). Benzylpenicillin was eluted at 23 min. Note the significant decrease in penicillin production in the disrupted mutant. The second mutant DPhl2 showed the same reduction in penicillin production as DPhl1 (results not shown).
Results showed that all transformants produced increased penicillin levels (between 20 and 60% higher than the untransformed strain at 48 h, and between 35 and 80% at 72 h of incubation) in CP medium. One of the transformants, named PhlAR+, was used for further analysis. This transformant consistently overproduced penicillin, as shown by HPLC and bioassay analysis (Table 3 and Figure 3B). Results of three different fermentations in defined DP medium with increasing PAA concentrations (1.5–2.5 g/l) showed that the transformant overproduced penicillin (30–35% increase), particularly at high PAA concentrations.
Table 3. Benzylpenicillin production at 72 h and 96 h in defined DF medium by the parental strain Wis 54-1255 and transformant PhlAR+ at PAA concentrations of 1.5, 2.0 and 2.5 g/l.
Data for penicillin concentrations are means±S.D. for three separate flasks.
| Benzylpenicillin production (μg/mg dry weight) | |||
|---|---|---|---|
| Strain | PAA concentration (g/l) | 72 h | 96 h |
| Wis 54-1255 | 1.5 | 3.8±0.20 | 5.2±0.40 |
| PhlAR+ | 1.5 | 4.2±0.25 | 5.4±0.35 |
| Wis 54-1555 | 2.0 | 3.3±0.25 | 5.1±0.15 |
| PhlAR+ | 2.0 | 4.1±0.25 | 5.8±0.18 |
| Wis 54-1255 | 2.5 | 2.8±0.18 | 4.0±0.18 |
| PhlAR+ | 2.5 | 4.7±0.20 | 6.1±0.20 |
Disruption of the phl gene results in decreased penicillin production
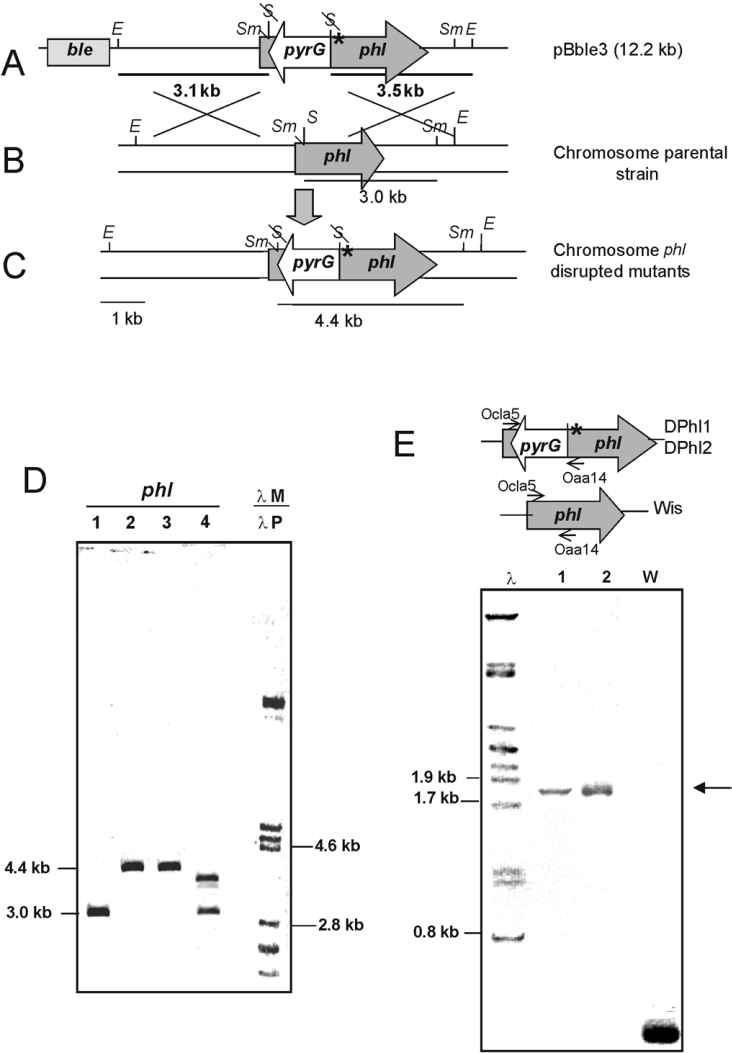
Targeted inactivation of the phl gene in P. chrysogenum Wis 54-1255 was performed by double crossing-over (gene replacement) between the endogenous copy and the pBble3 construction in which the phl gene was disrupted by inserting the pyrG in a SalI site in opposite orientation to the phl gene (Figure 4). Furthermore, a frameshift mutation was introduced at nucleotide position 257 in pBble3, to prevent translation of the 3′ region of phl if expressed from any putative promoter located in the pyrG insert.
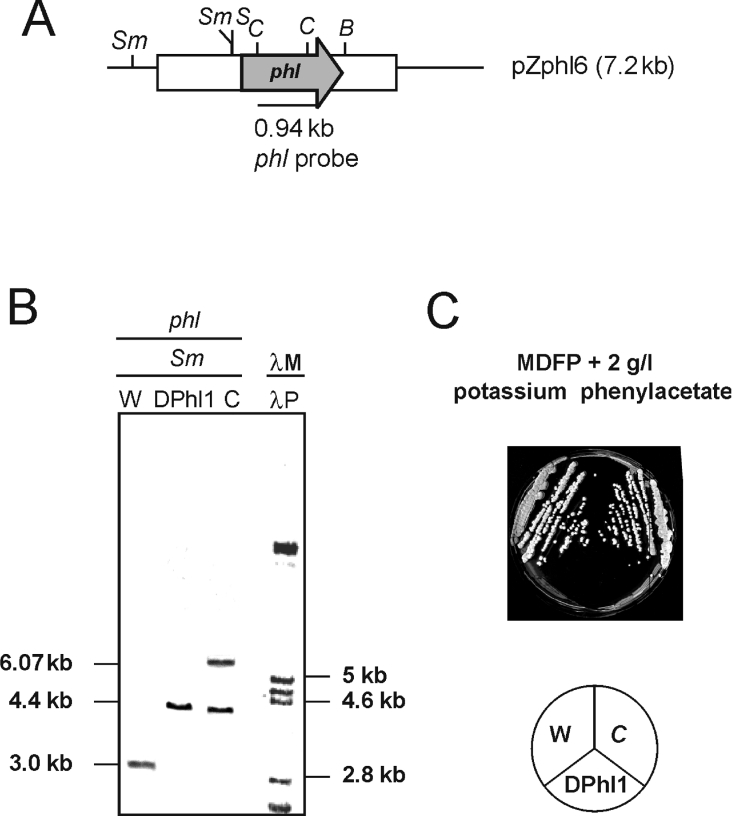
Figure 4. Disruption strategy and Southern hybridization showing the inactivation of phl in two different transformants.
(A) Construction pBble3 in which the phl gene (shaded arrow) was disrupted in vitro by insertion of the pyrG gene (white arrow) and by creating a frameshift mutation (*) (see text). Restriction endonuclease sites: E, EcoRI; S, SalI; Sm, SmaI. (B) Recombination (double crossing-over with the genome); letter codes as in (A). (C) Organization of the phl region in the disrupted mutants; letter codes as in (A). (D) Southern hybridization of the DNA of the parental strain Wis 54-1255 (lane 1), two disrupted mutants DPhl1 and DPhl2 (lanes 2 and 3) and an abnormal non-disrupted transformant (lane 4) showing ectopic integration. Lanes 1–4 were hybridized with the phl probe. Lane λM, control size markers (λ digested with PstI), was hybridized with a labelled λ probe (λP). The sizes (in kb) of the hybridizing bands are indicated. (E) PCR amplification using primers Ocla5 and Oaa14, showing that the two disrupted mutants contain the same amplification band (lanes 1 and 2, arrow), different from that of the non-disrupted Wis 54-1255 (lane W).
A total of 689 clones of the 1858 pyrG+ transformants (selected by complementation of the uridine auxotrophy) tested were phleomycin-sensitive, indicating that a double cross-over had occurred. All 689 clones were tested by PCR amplification of the phl gene region using primers Ocla5 and Oaa14 to detect whether the phl gene had been disrupted (by introduction of the pyrG insert present in the exogenous construction).
Two independent clones containing the disrupted phl gene, named DPhl1 (disrupted in phl 1) and DPhl2, were isolated. These two transformants were confirmed by Southern hybridizations using probes internal to the phl (Figure 4D) and the pyrG genes. The phl-hybridizing band (4.4 kb SmaI) was 1.4 kb larger than the band in the control Wis 54-1255 strain, a size increase that corresponded exactly to the size of the pyrG insert (Figure 4D, lanes 2 and 3). A large number of non-disrupted transformants that contained the intact original 3.0-kb band and the pBble3 cassette integrated in a non-homologous site were also observed (Figure 4D, lane 4). The two disrupted transformants showed the same hybridization pattern and an identical PCR product (Figure 4E), although the double recombination may have ocurred in different places within the homologous region.
The nucleotide sequences of the PCR products showed the presence of the frameshift mutation in the phl gene and confirmed the introduction of the pyrG without rearrangement of the con-struction.
The two disrupted mutants DPhl1 and DPhl2 produced 40% less penicillin than the parental strain at PAA concentrations of 1.5 and 1.0 g/l (Table 4 and Figure 3C). The effect of phl-gene disruption on penicillin production was dependent upon the concentration of PAA used because the phl disruption affected significantly the resistance of the strains to PAA (see below).
Table 4. Benzylpenicillin production at 72 and 96 h in culture broths in DF medium of the parental strain Wis 54-1255 and the DPhl1 transformant disrupted in the phl gene.
Data for penicillin concentrations are means±S.D. for three separate flasks.
| Benzylpenicillin production (μg/mg dry weight) | |||
|---|---|---|---|
| Strain | PAA concentration (g/l) | 72 h | 96 h |
| Wis 54-1255 | 0.5 | 3.2±0.32 | 4.9±0.30 |
| DPhl1 | 0.5 | 2.8±0.25 | 4.0±0.22 |
| Wis 54-1255 | 1 | 3.7±0.20 | 5.1±0.15 |
| DPhl1 | 1 | 2.3±0.25 | 3.6±0.35 |
| Wis 54-1255 | 1.5 | 3.8±0.28 | 5.4±0.4 |
| DPhl1 | 1.5 | 2.3±0.20 | 3.4±0.25 |
Amplification of phl leads to high resistance to PAA, and disruption of this gene results in PAA-supersensitive strains
The resistance to PAA of the DPhl1 and DPhl2 mutants and the PhlAR+ transformant containing the amplified phl gene was tested at PAA concentrations of 1.0, 1.5, 2.0, 2.5 and 3.0 g/l in DP medium.
Under these conditions, the parental Wis 54-1255 was resistant up to 2.0 g/l PAA. This strain showed very poor growth at 2.5 g/l PAA and failed to grow at concentrations of 3 g/l PAA. By contrast, the PhlAR+ strain grew well at 3 g/l PAA.
On the other hand, the two phl-disrupted mutants were susceptible to PAA concentrations of 1.5 g/l and failed to grow at 2.0 g/l. These results indicate that the PAA-CoA ligase encoded by the phl gene is a PAA-detoxifying enzyme (see the Discussion). Similar results were obtained when phenoxyacetic acid was used instead of PAA, suggesting that the PAA-CoA ligase was able to detoxify P. chrysogenum from the accumulation of both PAA and phenoxyacetic acid.
PAA-CoA ligase activity in the disrupted mutants: evidence for a second phl-related gene
Since the phl-disrupted mutants were still able to produce reduced levels of benzylpenicillin, the PAA-CoA ligase was measured in the different strains using an HPLC analysis that quantified the PAA-dependent and CoA-dependent conversion of PAA into PAA-CoA. Results showed that both the DPhl1 and DPhl2 mutants had 39.1% less PAA-CoA ligase activity (average of five determinations) than the undisrupted Wis 54-1255 control strain (Figure 5A). These results confirm that the phl gene encodes PAA-CoA ligase activity, and, at the same time, indicate that there is a second gene in P. chrysogenum that encodes the remaining PAA-CoA ligase activity.
Figure 5. PAA-CoA formed in vitro by the PAA-CoA ligase.
(A) PAA-CoA formed in vitro by the PAA-CoA ligase of the parental strain Wis 54-1255 and the disrupted DPhl1. (B) PAA-CoA formed in vitro by the PAA-CoA ligase of the parental strain Wis 54-1255 and the PhlAR+ transformant containing multiple copies of the phl gene. Pure PAA-CoA was used as standard (elution time 23.5 min). A stock solution of 100 μg/ml PAA-CoA was used to determine the standard curve. The reactions were performed with 500 μg of protein (crude extract) per reaction (0.3 ml volume) in all cases. Note the different scales between panels (A) and (B).
A search was then made for a phl-like gene by hybridization with the 0.62-kb phl probe. Under very-low-stringency conditions, a second 6.0-kb hybridizing band was detected (results not shown), which probably encodes a second aryl-CoA ligase.
On the other hand, amplification of the phl gene in the PhlAR+ strain resulted in an 8-fold increase in the PAA-CoA formed (Figure 5B). This large increase indicates that several copies of the autonomous replicating plasmid were expressed. However, the increase in penicillin production was not proportional to this increase in PAA-CoA ligase (Figure 3), suggesting that there is a limitation in other biosynthetic steps (e.g. enzymes involved in isopenicillin N formation, or IAT) in the Wis 54-1255 strain that contains a single copy of the penicillin gene cluster.
Complementation of the phlD1 mutant restores full penicillin production and PAA resistance
The DPhl1 strain was complemented with the pZphl6 plasmid. The introduction of the phl construction in the plasmid without rearrangement was confirmed by Southern hybridization (Figure 6). Three transformants with the correct hybridization pattern were tested for PAA resistance and penicillin production. Complemented transformants regained resistance to 2 g/l PAA (Figure 6), and the levels of penicillin were restored to those of the parental Wis 54-1255 cultures.
Figure 6. Complementation of the disrupted mutants with the phl gene restores PAA resistance.
(A) Complementation of the PhlD1 mutant with the phl gene (construction pZphl6). Restriction endonuclease sites: B, BgIII; C, ClaI; S, SalI; and Sm, SmaI. (B) Hybridization of DNA of strains Wis 54-1255 (lane W), DPhl1 (lane DPhl1) and one complemented clone (lane C) with the phl probe. λM, size markers (λ bacteriophage digested with PstI) hybridizing with labelled λ phase (λP). (C) The complemented clone (C) recovered full resistance to PAA. The penicillin production of the complemented clone was restored to the level of that of Wis 54-1255 (W).
DISCUSSION
The penicillin gene cluster consists of three genes (pcbAB, pcbC and penDE) with the same arrangement in different penicillin-producing fungi [7,34,35]. A 50-kb DNA region encoding the entire cluster is deleted in the P. chrysogenum npe10 mutant, and the same region is amplified in tandem copies in the strains that produce high levels of penicillin [21,23]. As shown in the present paper, hybridization studies demonstrated that phl is present in a single copy in DNA digested with different restriction enzymes; the hybridizing bands were, in all cases, the same size, suggesting the presence of an identical phl gene and adjacent regions in all strains. The phl gene is present in P. chrysogenum npe10 that lacks the 50-kb amplified region, including the penicillin gene cluster. This finding indicates that the PAA-CoA ligase gene has a different evolutionary origin from the other penicillin genes and does not appear to have been transferred horizontally, as has been proposed for the penicillin gene cluster [1].
The PAA-CoA ligase belongs to the acyladenylate protein family [31]. These proteins activate the acyl or aryl acids to acyl-AMP or aryl-AMP respectively using ATP. After activation, the carboxy group is transferred to the thiol group of CoA, forming a thioester and releasing AMP.
All members of the acyladenylate family show a highly conserved AMP-binding domain [PROSITE (http://www.expasy.org/prosite/) ID PS00455]. However, they differ in their substrate specificity (e.g. chain length and presence of an aromatic ring) and cellular localization. The most well-characterized aryl-CoA ligases are the 4-coumaroyl-CoA ligases [36,37]. The substrate specificity of two of the four putative 4-coumaroyl-CoA ligases of A. thaliana has been studied in detail by Schneider et al. [38]. These authors have defined a substrate-specificity code (binding pocket) for 4-coumaroyl-CoA ligases based on the amino acids occurring in positions 252–256, 293, 320, 322, 323, 346, 348, 354–356 and 540 of these proteins. The catalytic Lys540 is always invariant [38]. The P. chrysogenum Phl protein and the highly conserved Asp. nidulans homologous protein belong to the aryl-CoA ligases of the 4-coumaroyl-CoA ligase type (Figure 2B). The other Asp. nidulans Phl-related proteins show some differences in the binding pocket and may serve to activate other substrates.
Aryl-capping enzymes that add aryl side chains to non-ribosomal peptides such as siderophores (e.g. enterobactin, pyochelin and yersiniabactin) and antitumour drugs (e.g. bleomycin and epothilone) have been reported previously [29]. The acyladenylate enzymes share several motifs (ATP-binding motifs) with the A domains (amino-acid-activating) of NRPS but, in contrast with the latter enzymes, the PAA-CoA ligase and other acyladenylate enzymes lack the phosphopantetheine-binding PCP domain. Thus the Phl protein is essentially an aryl-capping enzyme, because it lacks the PCP domain.
In some cases (e.g. EntE, involved in dihydroxybenzoic acid activation for enterobactin biosynthesis), the aryl-capping enzyme transfers the activated aromatic acid to a separate PCP [named ArCP (aromatic-acid-carrier protein)] domain (in this case the EntB protein). In other cases, the activated aryl group is transferred to a specific ArCP domain that forms part of a larger NRPS, e.g. the salicylic-acid-activating aryl-capping protein of the PchD (pyochelin cluster) transfers the activated salicylic acid to an ArCP domain of pyochelin synthetase I [29]. In P. chrysogenum, the first acceptor of the activated PAA-CoA is the IAT.
The IAT contains a PAA-CoA-acceptor site with a thioesterase active centre (GKS309RGSTL) that is likely to be involved in cleavage of the thioester bond of PAA-CoA [10] to transfer the PAA to the non-ribosomal-peptide-derived 6-APA [2]. Indeed, a Ser309→Ala mutation proved that Ser309 in the thioesterase centre is essential for IAT activity [39]. Transfer of the PAA molecule to the IAT has been confirmed by electrospray MS [40].
The C-terminal sequence of the Phl protein is characteristic of peroxisomal enzymes. The IAT of P. chrysogenum is also known to be located in microbodies (peroxisomes) [33]. This enzyme and Phl may therefore form a functional complex located in peroxisomes. In summary, the Phl protein acts as a peptide aryl-capping enzyme that transfers the activated PAA to the IAT in the peroxisomes.
Disruption of the phl gene confers increased sensitivity to PAA or phenoxyacetic acid, and increasing the phl copy number results in higher levels of resistance to these compounds. This result supports observations of the role of other PAA-degrading enzymes on the detoxification of PAA [41]. Increasing the phl gene expression level is therefore a useful tool to enhance PAA resistance and penicillin biosynthesis. Indeed, selection of PAA-resistant clones has been reported as a method to isolate increased-penicillin-producing strains [42].
Disruption of the phl gene reduced benzylpenicillin production but did not block completely penicillin biosynthesis. Since we measured benzylpenicillin specifically using HPLC, this result could not be explained by the accumulation of isopenicillin N or other hydrophilic penicillins. The remaining benzylpenicillin production suggests that a second Phl-like protein exists in P. chrysogenum. The gene encoding this second protein was detected by hybridization at reduced stringency. In the genome of Asp. nidulans there is a phl gene (65% identity) and three other putative acyl-CoA ligase genes, and similar genes might be present in P. chrysogenum, although the substrate specificity and affinity for PAA of those acyl-CoA ligases remains to be studied in this organism.
An interesting observation was that Phl is clearly different from bacterial PAA-degrading enzymes such as those of Pseudomonas putida, Azoarcus evasii, Bacillus halodurans, Stenophomonas sp. and E. coli (which all have identities of 7–10%). This finding indicates that, in contrast with bacterial aryl-CoA ligases, the Phl protein has probably evolved to serve a biosynthetic role, rather than a degradative function.
Acknowledgments
This work was supported by grants from the European Union (Eurofung QLK3-1999-00729) and the Junta of Castilla and León (LE-13/04). I.V. received a fellowship of the Diputación de León. We thank Dutch State Mines (Delft, Holland) for support and M. Mediavilla and M. Álvarez for excellent technical assistance.
References
- 1.Aharonowitz Y., Cohen G., Martín J. F. Penicillin and cephalosporin biosynthetic genes: structure, regulation and evolution. Annu. Rev. Microbiol. 1992;46:461–495. doi: 10.1146/annurev.mi.46.100192.002333. [DOI] [PubMed] [Google Scholar]
- 2.Martín J. F., Gutiérrez S., Demain A. L. β-Lactams. In: Anke T., editor. Fungal Biotechnology. Weinheim, Germany: Chapman & Hall; 1997. pp. 91–127. [Google Scholar]
- 3.Martín J. F. α-Aminoadipyl-cysteinyl-valine synthetases in β-lactam producing organisms. J. Antibiotics. 2000;53:1008–1021. doi: 10.7164/antibiotics.53.1008. [DOI] [PubMed] [Google Scholar]
- 4.Barredo J. L., Cantoral J. M., Álvarez E., Díez B., Martín J. F. Cloning, sequence analysis and transcriptional study of the isopenicillin N synthase of Penicillium chrysogenum AS-P-78. Mol. Gen. Genet. 1989;216:91–98. doi: 10.1007/BF00332235. [DOI] [PubMed] [Google Scholar]
- 5.Barredo J. L., van Solingen P., Díez B., Alvarez E., Cantoral J. M., Kattevilder A., Smaal E. B., Groenen M. A. M., Veenstra A. E., Martín J. F. Cloning and characterization of the acyl-coenzyme A: 6-aminopenicillanic-acid-acyltransferase gene of Penicillium chrysogenum. Gene. 1989;83:291–300. doi: 10.1016/0378-1119(89)90115-7. [DOI] [PubMed] [Google Scholar]
- 6.Díez B., Gutiérrez S., Barredo J. L., van Solingen P., van der Voort L. H. M., Martín J. F. The cluster of penicillin biosynthetic genes. J. Biol. Chem. 1990;265:16358–16365. [PubMed] [Google Scholar]
- 7.Montenegro E., Fierro F., Fernández F. J., Gutiérrez S., Martín J. F. Resolution of chromosomes III and VI of Aspergillus nidulans by pulsed-field gel electrophoresis shows that the penicillin biosynthetic pathway genes pcbAB, pcbC, and penDE are clustered on chromosome VI (3.0 megabases) J. Bacteriol. 1992;174:7063–7067. doi: 10.1128/jb.174.21.7063-7067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín J. F. Molecular control of expression of penicillin biosynthesis genes in fungi: regulatory proteins interact with a bidirectional promoter region. J. Bacteriol. 2000;182:2355–2362. doi: 10.1128/jb.182.9.2355-2362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin M. B., Fleming M. D., Skatrud P. L., Miller J. R. Molecular characterization of the acyl-coenzyme A:isopenicilllin N acyltransferase gene (penDE) from Penicillium chrysogenum and Aspergillus nidulans and activity of recombinant enzyme in Escherichia coli. J. Bacteriol. 1990;172:5908–5914. doi: 10.1128/jb.172.10.5908-5914.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Álvarez E., Meesschaert B., Montenegro E., Gutiérrez S., Díez B., Barredo J. L., Martín J. F. The isopenicillin N acyltransferase of Penicillium chrysogenum has isopenicillin N amidohydrolase, 6-aminopenicillanic acid acyltransferase and penicillin amidase activities, all of which are encoded by the single penDE gene. Eur. J. Biochem. 1993;215:323–332. doi: 10.1111/j.1432-1033.1993.tb18038.x. [DOI] [PubMed] [Google Scholar]
- 11.Fernández F. J., Cardoza R. E., Montenegro E., Velasco J., Gutiérrez S., Martín J. F. The isopenicillin N acyltransferase of Aspergillus nidulans and Penicillium chrysogenum differ in their ability to maintain the 40 kDa αβ heterodimer in an undissociated form. Eur. J. Biochem. 2003;270:1958–1968. doi: 10.1046/j.1432-1033.2003.03561.x. [DOI] [PubMed] [Google Scholar]
- 12.Brunner R., Rohr M. Phenacyl:coenzyme A ligase. Methods Enzymol. 1975;43:476–481. doi: 10.1016/0076-6879(75)43107-x. [DOI] [PubMed] [Google Scholar]
- 13.Kogekar R. G., Deshpande V. N. Biosynthesis of penicillin in vitro: part II – purification and properties of 6-aminopenicillanic acid–phenylacetyl-CoA/phenoxyacetyl-CoA transferase. Ind. J. Biochem. Biophys. 1983;20:208–212. [PubMed] [Google Scholar]
- 14.Martínez-Blanco H., Reglero A., Fernández-Valverde M., Ferrero M. A., Moreno M. A., Luengo J. M. Isolation and characterization of the acetyl-CoA synthetase from Penicillium chrysogenum. J. Biol. Chem. 1992;276:5475–5481. [PubMed] [Google Scholar]
- 15.Gouka R. J., van Hartingsveldt W., Bovenberg R. A., van Zeijl C. M., van den Hondel C. A., van Gorcom R. F. Development of a new transformant selection system for Penicillium chrysogenum: isolation and characterization of the P. chrysogenum acetyl-coenzyme A synthetase gene (facA) and its use as a homologous selection marker. Appl. Microbiol. Biotechnol. 1993;38:514–519. doi: 10.1007/BF00242947. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Blanco H., Orejas M., Reglero Á., Luengo J. M., Peñalva M. A. Characterisation of the gene encoding acetyl-CoA synthetase in Penicillium chrysogenum: conservation of intron position in plectomycetes. Gene. 1993;130:265–270. doi: 10.1016/0378-1119(93)90429-7. [DOI] [PubMed] [Google Scholar]
- 17.Gledhill L., Greaves P. A., Griffin J. P. A process for preparing an enzyme for Penicillium chrysogenum having phenylacetate-coenzyme A activity. WO 97/02349. International Patent. 1997
- 18.Casqueiro J., Gutiérrez S., Bañuelos Ó., Hijarrubia M. J., Martín J. F. Gene targeting in Penicillium chrysogenum: disruption of the lys2 gene leads to penicillin overproduction. J. Bacteriol. 1999;181:1181–1188. doi: 10.1128/jb.181.4.1181-1188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G., Casqueiro J., Bañuelos Ó., Cardoza R. E., Gutiérrez S., Martín J. F. Targeted inactivation of the mecB gene encoding cystathionine-γ-lyase shows that the transsulfuration pathway is required for high level cephalosporin biosynthesis in Acremonium chrysogenum C10 but not for methionine-induction of the cephalosporin genes. J. Bacteriol. 2001;183:1765–1772. doi: 10.1128/JB.183.5.1765-1772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mootz H. D., Marahiel M. Design and application of multimodular peptide synthetases. Curr. Opin. Biotechnol. 1999;10:341–348. doi: 10.1016/S0958-1669(99)80062-7. [DOI] [PubMed] [Google Scholar]
- 21.Fierro F., Barredo J. L., Díez B., Gutiérrez S., Fernández F. J., Martín J. F. The penicillin gene cluster is amplified in tandem repeats linked by conserved hexanucleotide sequences. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6200–6204. doi: 10.1073/pnas.92.13.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díez B., Álvarez E., Cantoral J. M., Barredo J. L., Martín J. F. Selection and characterization of pyrG mutants of Penicillium chrysogenum lacking orotidine-5-phosphate decarboxylase and complementation by the pyr4 gene of Neurospora crassa. Curr. Genet. 1987;12:277–282. [Google Scholar]
- 23.Fierro F., Montenegro E., Gutiérrez S., Martín J. F. Mutants blocked in penicillin biosynthesis show a deletion of the entire penicillin gene cluster at a specific site within a conserved hexanucleotide sequence. Appl. Microbiol. Biotechnol. 1996;44:597–604. doi: 10.1007/BF00172491. [DOI] [PubMed] [Google Scholar]
- 24.Cantoral J. M., Gutiérrez S., Fierro F., Gil-Espinosa S., van Liempt H., Martín J. F. Biochemical characterization and molecular genetics of nine mutants of Penicillium chrysogenum impaired in penicillin biosynthesis. J. Biol. Chem. 1993;268:737–744. [PubMed] [Google Scholar]
- 25.Cantoral J. M., Díez B., Barredo J. L., Alvarez E., Martín J. F. High-frequency transformation of Penicillium chrysogenum. Bio/Technology. 1987;5:494–497. [Google Scholar]
- 26.Anné J. Dissertationes de Agricultura 25. Belgium: KU Leuven; 1977. Somatic hybridization between Penicillium species after induced fusion of their protoplasts; pp. 39–117. PhD thesis. [Google Scholar]
- 26a.Fierro F., Gutiérrez S., Díez B., Martín J. F. Resolution of four chromosomes in penicillin-producing filamentous fungi: the penicillin gene cluster is located on chromosome II (9.6 Mb) in Penicillium notatum and chromosome I (10.4 Mb) in Penicillium chrysogenum. Mol. Gen. Genet. 1993;241:573–578. doi: 10.1007/BF00279899. [DOI] [PubMed] [Google Scholar]
- 27.Fernández F. J., Gutiérrez S., Velasco J., Montenegro E., Marcos A. T., Martín J. F. Molecular characterization of three loss-of-function mutations in the isopenicillin N acyltransferase gene (penDE) of Penicillium chrysogenum. J. Bacteriol. 1994;176:4941–4948. doi: 10.1128/jb.176.16.4941-4948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Sambrook J., Russel D. W. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. Molecular Cloning: a Laboratory Manual. [Google Scholar]
- 27b.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 28.Schröder J. Probing plant polyketide biosynthesis. Nat. Struct. Biol. 1999;6:714–716. doi: 10.1038/11472. [DOI] [PubMed] [Google Scholar]
- 29.Quadri L. Assembly of aryl-capped siderophores by modular peptide synthetases and polyketide synthases. Mol. Microbiol. 2000;37:1–12. doi: 10.1046/j.1365-2958.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 30.Marahiel M. A., Stachelhaus T., Mootz H. D. Modular peptide synthetases and polyketide synthases. Chem. Rev. 1997;97:2651–2673. doi: 10.1021/cr960029e. [DOI] [PubMed] [Google Scholar]
- 31.Turgay K., Krause M., Marahiel M. A. Four homologous domains in the primary structure of GrsB are related to domains in a superfamily of adenylate forming enzymes. Mol. Microbiol. 1992;6:529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 32.Lambalot R. H., Gehring A. M., Flugel R. S., Zuber P., Lacelle M., Marahiel M. A., Reid R., Khosla C., Walsh C. T. A new enzyme superfamily: the phosphopantetheinyl transferases. Chem. Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 33.Müller W. H., Bovenberg R. A. L., Groothuis M. H., Kattevilder F., Smaal E. B., van der Voort L. H. M., Verkleij A. J. Involvement of microbodies in penicillin biosynthesis. Biochim. Biophys. Acta. 1992;1116:210–213. doi: 10.1016/0304-4165(92)90118-e. [DOI] [PubMed] [Google Scholar]
- 34.Laich F., Fierro F., Cardoza R. E., Martín J. F. Organization of the gene cluster for biosynthesis of penicillin in Penicillium nalgiovense and antibiotic production in cured dry sausages. Appl. Environ. Microbiol. 1999;65:1236–1240. doi: 10.1128/aem.65.3.1236-1240.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laich F., Fierro F., Martín J. F. Production of penicillin by fungi growing on food products: identification of a complete penicillin gene cluster in Penicillium griseofulvum and a truncated cluster in Penicillium verrucosum. Appl. Environ. Microbiol. 2002;68:1211–1219. doi: 10.1128/AEM.68.3.1211-1219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozoya E., Hoffmann H., Douglas C., Schulz W., Schell D., Hahlbrock K. Primary structures and catalytic properties of isoenzymes encoded by the two 4-coumarate:CoA ligase genes in parsley. Eur. J. Biochem. 1988;176:661–667. doi: 10.1111/j.1432-1033.1988.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 37.Schneider K., Hövel K., Witzel K., Hamberger B., Schomburg D., Kombrink E., Stuible H. P. The substrate specificity-determining amino acid code of 4-coumarate:CoA ligase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8601–8606. doi: 10.1073/pnas.1430550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider K., Kienow L., Schmelzer E., Colby T., Bartsch M., Miersch O., Wasternack C., Kombrink E., Stuible H. P. A new type of peroxisomal acyl-coenzyme A synthetase from Arabidopsis thaliana has the catalytic capacity to activate biosynthetic precursors of jasmonic acid. J. Biol. Chem. 2005;280:13962–13972. doi: 10.1074/jbc.M413578200. [DOI] [PubMed] [Google Scholar]
- 39.Tobin M. B., Cole S. C. J., Kovacevic S., Miller J. R., Baldwin J. E., Sutherland J. D. Acyl-coenzyme A: isopenicillin N acyltransferase from Penicillium chrysogenum: effect of amino acid substitutions at Ser227, Ser230 and Ser309 on proenzyme cleavage and activity. FEMS Microbiol. Lett. 1994;121:39–46. doi: 10.1111/j.1574-6968.1994.tb07073.x. [DOI] [PubMed] [Google Scholar]
- 40.Aplin R. T., Baldwin J. E., Roach P. L., Robinson C. V., Schofield C. J. Investigations into the post-translational modification and mechanism of isopenicillin N:acyl-CoA acyltransferase using electrospray mass spectrometry. Biochem. J. 1993;294:357–363. doi: 10.1042/bj2940357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez-Sáiz M., Díez B., Barredo J. L. Why did the Fleming strain fail in penicillin industry? Fungal Genet. Biol. 2005;42:464–470. doi: 10.1016/j.fgb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Barrios-González J., Montenegro E., Martín J. F. Penicillin production by mutants resistant to phenylacetic acid. J. Ferm. Bioeng. 1993;76:455–458. [Google Scholar]
- 43.Gutiérrez S., Díez B., Montenegro E., Martín J. F. Characterization of the Cephalosporium acremonium pcbAB gene encoding α-aminoadiyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin-biosynthetic genes and evidence of multiple functional domains. J. Bacteriol. 1991;173:2354–2365. doi: 10.1128/jb.173.7.2354-2365.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fierro F., Kosalková K., Gutiérrez S., Martín J. F. Autonomously replicating plasmids carrying the AMA1 region in Penicillium chrysogenum. Curr. Genet. 1996;29:482–489. doi: 10.1007/BF02221518. [DOI] [PubMed] [Google Scholar]