Abstract
The aim of this study was to determine whether an intraarticular administration of a vanilloid agonist resiniferatoxin (RTX) produces an analgesic effect in experimental arthritis. Knee-joint inflammation was induced in rats by intraarticular carrageenan (2%, 30 μL). Pain score and left/right hind leg weight distribution ratio were used to assess pain behavior. Changes in the knee dimensions were evaluated by measuring its external circumference and intraarticular area (ultrasound scanning). The intraarticular administration of RTX (0.0003% or 0.003%, 30 μL) provided a significant analgesic effect. Twenty-four hours after RTX, the pain score was reduced from 15.1 ± 4.7 to 6.9 ± 4.4 (P<0.01) with 0.0003% and was abolished (P<0.0001) with 0.003%. The improvement in weight-distribution ratio lasted for several days after the RTX administration. Reduction in knee circumference demonstrated that intraarticular RTX suppressed the carrageenan-induced edema by at least one-third. Ultrasound scanning revealed no RTX-induced decrease of the intraarticular area. The experiments demonstrated that intraarticular RTX inhibits pain behavior in knee-joint arthritis and that this effect is dose-dependent. They indicate a new direction for peripheral analgesia.
Keywords: Analgesia, Arthritis, Inflammation, Pain, TRPV1 receptors, Vanilloids
Introduction
Vanilloids bind to the transient receptor potential type V1 (TRPV1) channels, nonselective cation ionophores that play an important role in integration of afferent noxious signals generated by inflammatory mediators (1). Vanilloid agonists (capsaicin and its analogs) can produce selective and long-lasting blockade of afferent C- and some Aδ- fibers (2). The cloning of vanilloid receptors (3, 4) heralded the rapid advances in vanilloid pharmacology and has begun to play an important role in the development of new compounds with analgesic properties. This new development put a new emphasis on the results of previous studies with capsaicin. Resiniferatoxin (RTX) is an ultrapotent capsaicin analog with a unique spectrum of activities (2). Its initial excitatory effect relative to its inactivating effect is far less pronounced compared with capsaicin (2). Several studies (5-7) convincingly demonstrated that capsaicin selectively suppresses the conduction of impulses in the C- fibers and some Aδ- fibers.
Using the ability of vanilloid agonists to selectively suppress the conduction of noxious impulses, we demonstrated that RTX-induced blockade of the sciatic and saphenous nerves completely prevents the development of hyperalgesia caused by intraplantar injection of carrageenan (8). Otsuki et al. (9), using the less potent capsaicin, showed that its application to the sciatic and femoral nerves reduces pain behavior induced by the injection of monosodium urate crystals into a knee-joint.
The main aim of this study was to determine whether localized, intraarticular administration of RTX produces an analgesic effect in experimental arthritis. Changes in pain behavior were assessed with indices based on two basic signs in acute knee inflammation – limp (10, 9) and decrease in weight load on the affected leg (9, 11).
Methods
Male Sprague-Dawley rats weighing 225-275 g were used for the experiments. The rats were housed with a 12-h light/dark cycle and provided with food and water ad libitum. The protocol for this study was approved by the Institutional Panel on Laboratory Animal Care.
Joint inflammation was induced by carrageenan (Sigma Chemical, St. Louis, MO) injected percutaneously through the infrapatellar ligament (30G needle) into the left knee-joint cavity (2%, 30μL) with the animal under brief halothane (2%) anesthesia. The changes in pain behavior were determined during a period of approximately 10 days after the carrageenan injection. Pain score and weight-bearing index were used to assess pain behavior. Pain-score determination was based on walking pattern. Each rat was placed on an open bench. The scoring was similar to that used by Otsuki et al. (9). A score of 0 was assigned if the rat walked without a limp; a score of 1, if the rat used the injected paw with a limp; a score of 2, if the rat walked on three legs. If an animal walked both with a limp and on three legs the highest score was assigned. Some of the animals needed to be prodded to begin walking. To initiate ambulation, gentle backward tugging on the tail was provided. The walking patterns were observed every 5 min for 1 hour. The sum of the 12 scores (0-24) obtained during the 1-h session was recorded as a cumulative pain score. Weight-bearing index was determined by measuring weight distribution between left (inflamed) and right (contralateral control) hind legs. An Incapacitance Analgesia Meter® (Stoelting Co., Wood Dale, IL) was used for this aim (11). The rat was placed into the plastic box of the device where it was kept sitting during a 2-min period. The integrated paw pressure during this period was displayed separately for the right and left leg. The ratio between the pressures of the right and left leg was calculated as left/right hind leg weight distribution ratio. The measurement was repeated with a 1-h interval between them. Average value of these two measurements was used as an index of joint discomfort.
In addition to the behavioral indices, two measurements reflecting changes of the knee dimensions were done. With the animal under light halothane anesthesia, the circumference of the knee was determined with a flexible tape measure (12). Ultrasound (US) scanning was employed to assess intraarticular changes of the knee. Real-time B-mode US was performed using a Titan (SonoSite Inc., Bothel, WA.) ultrasound system with a 10 MHz probe (axial resolution of 0.76 mm at depth of 30 mm). US examination was performed under halothane (2%) anesthesia, with the knee shaved and flexed to 90°. The area between the patella and tibia was scanned in a midline longitudinal axis. A hypoechoic or anechoic area encompassed by the patellar ligament, the femur and the tibia was measured by fitting elliptical calipers between these structures. A representative image is given in Figure 1. The average of three measurements of the left (inflamed) and right (contralateral control) knee was used for the assessment of intraarticular changes.
Figure 1.
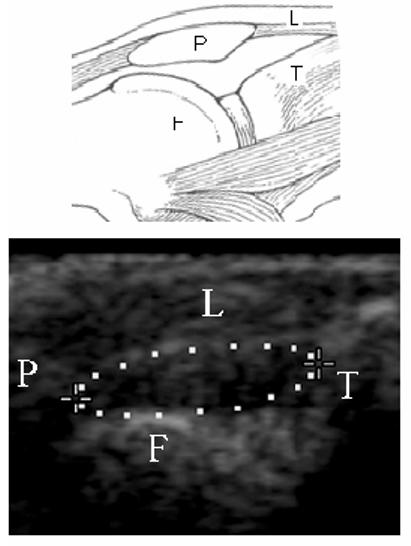
The longitudinal view of the left knee after the intraarticular injection of carrageenan. A sketch (upper part) and representative B-mode image of the knee joint. F-femur, T-tibia, P-patella, L-patellar ligament, ellipse – the measured area. In the sonogram, the knee is flexed at 90°,
RTX (Sigma) in a volume of 30 μL was injected into the knee-joint cavity percutaneously through the intrapatella ligament with the animal under brief halothane (2%) anesthesia. Two concentrations of RTX were used, 0.0003% (0.09 μg) and 0.003% (0.9 μg) (chosen on the basis of our previous experiments) [8]. Before an experiment, RTX (initially dissolved in dimethyl sulfoxide to a concentration of 1μg/μL and stored at −80° C under nitrogen) was diluted in 0.9% saline with 0.3% Tween 80 (to avoid precipitation). RTX was injected into the knee-joint with Indian ink to confirm the intraarticular site of the injection. Each animal was dissected at the end of the experiment to confirm the localization of the injection. To prevent the initial excitatory effect of RTX, intraarticular bupivacaine (0.5%, 10 μL) was administered (providing local anesthetic effect for approximately 1h) 10 min before RTX. After bupivacaine-RTX administration, the animals exhibited no behavioral signs associated with pain (flinching or licking of the paw).
Five groups of rats (n=8 per group) were used. In two groups that received carrageenanbupivacaine-RTX (CBR), RTX (0.0003% or 0.003%) was used against the background of the carrageenan-induced joint inflammation. After baseline measurements of the variables, carrageenan was administered in these two groups and variables were measured 3 h and 23 h later. Then bupivacaine was injected followed by RTX and the variables were measured 3 and 24 h, 2, 3, 6 and 8 days after the RTX injection. Three other groups of animals were used as controls. The carrageenan-bupivacaine-vehicle (CBV) group received the injection of carrageenan and the injection of bupivacaine 24 h later followed by an injection of the vehicle for RTX (dimethyl sulfoxide in saline with Tween 80). The measurements of variables were the same as in the CBR groups. The animals in the bupivacaine-RTX (BR) group, did not receive an injection of carrageenan before both of the agents were administered. In the SV group, saline was used instead of bupivacaine and vehicle was used instead of RTX. Before each of the measurements the rat was placed on a bench and carefully observed for a minute or two. Notes were made of the animal's gait, the posture of the affected hind paw, the condition of the knee.
The rats were assigned to the groups randomly (blocked randomization) and the persons responsible for the measurements of the effects were blinded to the type of treatment. Each animal was housed individually and identified only with a sequential number assigned prior to the beginning of the experiment. Since the control solution and the RTX solution were contained in identical vials and the solutions were both clear, they were indistinguishable to the examiner.
Data were analyzed with a two-way (group and time) analysis of variance, with time treated as a repeated-measures factor. Comparisons among groups at each time were performed with one-way analysis of variance. Multiple comparisons among means were made with Fisher's protected least-squares difference test. The results were declared significant if the P value was <0.05. Data are reported as the mean ± SD.
Results
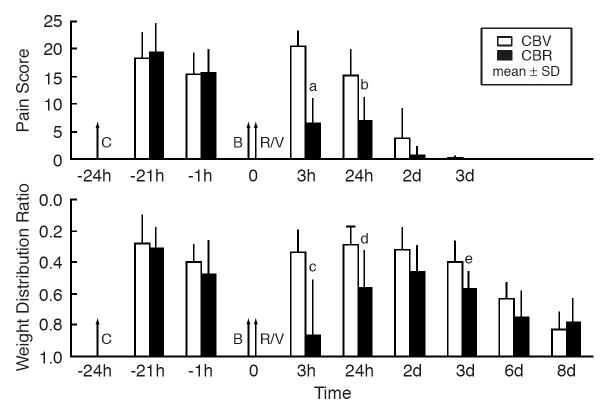
The comparison of two groups of animals (CBV and CBR) illustrating the magnitude and time course of changes in pain score and weight-distribution ratio after the injections of carrageenan and RTX is presented in Figure 2. In the CBV group, 3 h after the vehicle injection pain score was 20.5 ± 2.6 (P<0.0001). It lasted for several days, and on day 2 (3 days after carrageenan injection), the pain score was 3.8 ± 5.4 (P<0.05). Changes in the weight-distribution ratio lasted much longer, approximately 1 week.
Figure 2.
The magnitude and time course of changes in pain score and weight distribution ratio after injections of carrageenan and resiniferatoxin. Pain score: – a cumulative score based on walking patterns observed during an 1-hour period. Weight distribution ratio: – a ratio of weight distribution between left (inflamed) and right (contralateral control) hind legs of the sitting rat. Each column represents the mean ± SD for the carrageenan-bupivacaine-resiniferatoxin (CBR) group and the carrageenan-bupivacaine-vehicle (CBV) group (n=8 per group). Carrageenan (C: −2%, 30μL), bupivacaine (B: −0.5%, 10μL), resiniferatoxin (R: −0.0003%, 30 μL), or vehicle (V: −30 μL) were injected into the left knee-joint cavity. Time: − h (hours) or d (days) before (−) or after the injection of resiniferatoxin. Statistical significance: aP < 0.0001 vs. CBV at 3 h, bP<0.01 vs. CBV at 24 h, cP< 0.02 vs. CBV at 3 h, dP<0.02 vs. CBV at 24 h, eP< 0.05 vs. CBV at 3d.
In the CBR group (reflecting the effect of 0.0003% RTX), the effects of RTX on both pain score and weight-distribution ratio continued for a long period after its intraarticular injection. The difference between the CBV and CBR groups with pain score was significant at 3 h (decrease by 68%, P<0.0001), and 24 h (by 54%, P<0.01). Relative to the maximum pain score (seen at −21h, Figure 2) in the same group (CBR) the decrease in pain score was also significant at 3h, and 24h (P<0.0001 for both). With weight-distribution ratio, the difference between the CBV and CBR was statistically significant at 3 h (P<0.002), 24 h (P<0.02), and 3 days (P<0.05).
The RTX-induced changes in pain behavior were dose-dependent (Figure 3). Three hours after the RTX injection, the pain score was reduced from 20.5 ± 2.6 to 6.5 ± 4.3 (P<0.0001) with a concentration of 0.0003% and was abolished (P<0.0001) with a concentration of 0.003%. Twenty-four hours after RTX, the pain score was reduced from 15.1 ± 4.7 to 6.9 ± 4.4 (P<0.01) with 0.0003% and was abolished (P<0.0001) with 0.003%. The difference between pain scores in the two RTX groups was statistically significant at 3 h (P<0.005) and 24 h (P<0.01). In contrast to the pain score, the weight-distribution ratio at 24 h was not reversed by 0.003% RTX. Although the reversal at 24 h had a tendency to be greater with 0.003% (0.72 ± 0.19) than with 0.0003% (0.56±0.23), the difference was not statistically significant.
Figure 3.
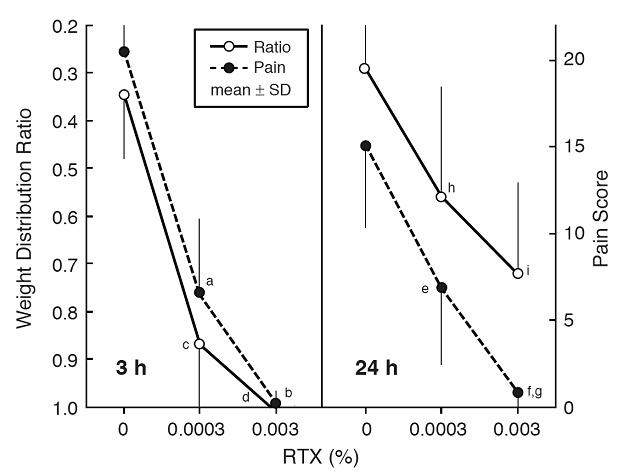
The dose-dependence of the effects of resiniferatoxin on pain score and weight distribution ratio 3 h (left side) and 24 h (right side) after its injection into the knee-joint cavity. Each point represents the mean ± SD for three groups (n=8 per group) with 0% (vehicle only), 0.0003%, and 0.003% of RTX injected into the left knee-joint cavity in a volume of 30 μL 24h after the induction of carrageenan-induced inflammation. Statistical significance: aP < 0.0001 vs. 0% RTX, bP<0.005 vs. 0.0003% RTX, cP< 0.002 vs. 0% RTX, dP<0.001 vs. 0% RTX, eP< 0.0005 vs. 0% RTX, fP <0.0001 vs. 0% RTX, gP<0.01 vs. 0.0003% RTX, hP< 0.02 vs. 0% RTX, iP<0.0005 vs. 0% RTX.
RTX clearly decreased the knee swelling induced by carrageenan (Figure 4). At 24 h (Box A) the increases in the circumference from baseline was 8.2 ± 1.9 mm in the CBV group and 5.0 ± 2.3 mm in the CBR group (P<0.02 for the difference between groups). At 3 d (Box B) the difference between the groups continued to be statistically significant (P<0.01).
Figure 4.
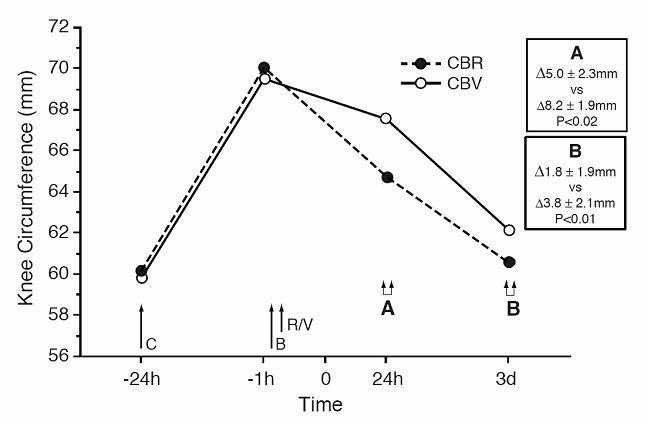
The effect of resiniferatoxin on the carrageenan-induced increase in the knee circumference. CBR: -- carrageenan-bupivacaine-resiniferatoxin group; CBV: -- carrageenan-bupivacaine-vehicle group (n=8 per group). Carrageenan (C: −2%, 30μL), bupivacaine (B: −0.5%, 10μL), resiniferatoxin (R: −0.0003%, 30μL) or vehicle (V: −30μL) was injected into the knee-joint cavity. Boxes display differences in the increases (Δ) of the knee circumference (mean ± SD) from baseline level in the CBR group (upper line) vs. the CBV group (lower line) at 24h (A) and 3d (B).
The US comparison of the left (inflamed) and right (contralateral control) knee demonstrated a profound effect of carrageenan. Three hours after carrageenan injection the area of the knee-joint chosen for the comparison was 6.0 ± 1.1 mm2 on the inflamed side and 3.7 ± 0.5 mm2 on the contralateral side (P<0.001). Three days later (CBV group), the difference was almost the same: 6.9 ± 2.7 mm2 vs. 3.3 ± 0.7 mm2 (P<0.001). In contrast to the knee circumference (Figure 4), there was no RTX-induced decrease of the hypoechoic/anechoic area on day 3, when limping had completely disappeared: The values in the CBV and CBR groups were identical.
In the BR and SV groups (control), no pain behavior was evident. Rats of both groups walked without a limp; therefore a score of 0 was recorded at all time points after the intraarticular injection. Injection of bupivacaine-RTX without the background of carrageenan-induced inflammation (BR group) did not cause any significant changes in the weight-distribution ratio. The index values were: 1.09 ± 0.28 (baseline), 1.15 ± 0.25 (3h after the injection), 1.01 ± 0.21 (24h), 1.02 ± 0.09 (2d), and 1.10 + 0.11 (3d).
Rat behavior did not indicate any sedative effect of RTX both at 3h and 24h after the agent injection.
Discussion
Our results demonstrated that a single intraarticular injection of RTX produces an analgesic effect in carrageenan-induced arthritis. With the limping index the effect lasts more than 24h; as long as this model provided possibility to observe the effect (due to limited duration of limping after the injection of carrageenan). This effect is dose-dependent and can be induced by relatively small doses of RTX starting from 0.09μg (0.0003%, 30μL). This dose is approximately 1000 times lower than the dose producing analgesia following systemic administration of RTX in rats (13). It is of interest that when intraarticular and systemic equianalgesic doses of morphine were compared (inflamed knee model in rats) [14] the intraarticular dose was 10 times lower than the systemic dose. This may indicate that compared to morphine, RTX has a much wider margin of safety regarding acute systemic toxicity.
The experiments demonstrate a difference in the time course of RTX effects between pain score and weight-distribution ratio. The changes induced by carrageenan injections disappeared by the third day (Figure 2) with pain score, at the same time, they lasted for 8-9 days with weight-distribution ratio. As a result, the RTX effect could be observed for a greater period of time when the weight-distribution ratio was used. This difference is difficult to explain. One of the possible explanations of the discrepancy between the tests is that limping score selectively reflects intensive pain on movement, while weight-distribution ratio (with the rat in the box of the device) may be able to reveal even mild pain in a sitting position. Another explanation is that the weight-distribution test may reflect not only pain but also a non-painful joint discomfort that could last much longer than pain.
It is of interest to compare the effects of RTX in arthritis and those in a rat model of postoperative pain (15). Equal concentrations of RTX (0.0003%) used in both models produce analgesia of similar duration. The incisional pain study also indicated that RTX does not suppress motor activity.
Localized analgesic effects of RTX have previously been demonstrated in carrageenan-induced foot models of hyperalgesia with percutaneous injection of the agent to the sciatic and saphenous nerves (8) or its direct administration into the plantar tissue (16). Neubert et al. reported that a peripheral injection of RTX produced the local analgesic effect by reversible inactivation of the nerve terminal endings. Otsuki et al. (9), using a monosodium urate model of knee-joint arthritis, demonstrated the analgesic effect of capsaicin administered systemically to the neonatal rat or locally to the sciatic and femoral nerves. Although the authors did not find complete analgesia with capsaicin, they did observe a significant effect. Topical capsaicin (cream) has been used to relieve pain of osteoarthritis, and several reviews confirmed its effectiveness using meta-analysis (17,18). However, the relatively weak effectiveness of capsaicin cream, the need for multiple applications, and skin irritation limits its use.
Changes in the knee circumference in our experiments clearly indicated that intraarticular RTX suppressed the carrageenan-induced edema by at least one-third (Figure 4). In a previous study (8), pretreatment with RTX, injected to the sciatic and saphenous nerves, reduced carrageenan-induced edema of the foot to a similar degree. Lam and Farrell (19), using Evans blue content method, demonstrated that knee inflammation induced by intraarticular carrageenan was reduced by 44% in the knees of rats that had previously been injected with capsaicin. They also observed that chronic joint denervation produced a 37% reduction in the inflammation. These observations indicate that at least one component the anti-inflammatory effect of vanilloid agonists is to counteract neurogenic inflammation. This confirms an earlier observation that local application of capsaicin to the sciatic nerve prevents neurogenic inflammation in the lateral part of the dorsal skin of the rat's paw (20).
Our US measurements of the knee-joint cavity demonstrated that US makes it possible to measure very small areas (3-6 mm2) in the knee cavity. This is comparable with the reported ability of US to measure a knee bursa with a short axis of ≤ 2 mm (21). We found that carrageenan injection significantly increases the hypoechoic/anechoic area representing the joint cavity, probably reflecting changes due to joint effusion. This increase did not regress in either the control (CBV) or the RTX-treated (CBR) group even 4 days after carrageenan administration, when the knee circumference had almost completely recovered. This may be explained by the slow process of knee-exudate resorption as compared with the recovery of knee inflammation. The absence of any difference between the CBR and CBV groups indicates that RTX does not have any significant effect on the changes measured by US, which probably results from the absence of the effect on exudate resorption. The knee exudate may be a factor responsible for the discrepancy between the difference in the duration of the changes in pain score (shorter) and weight-distribution ratio (longer) after the injection of carrageenan (Figure 2). If this discrepancy indicates that the weight-bearing test reflects not only pain but also a non-painful joint discomfort (see above) the joint effusion might be responsible for this discomfort.
TRVP1 is activated not only by vanilloid ligands but also by noxious heat and low pH and thus can be viewed as a molecular integrator of noxious stimuli in peripheral terminals of primary sensory neurons (22). An increase in TRPV1 expression in peripheral nociceptors is critical for the maintenance of inflammatory hyperalgesia (23). TRPV1 exhibits a time- and Ca2± dependent desensitization, a long lasting refractory state during which the receptor does not respond to vanilloids or other stimuli (24). Several studies have demonstrated that perineural administration of capsaicin can selectively block the conduction of impulses in the C- fibers and Aδ- fibers at the site of capsaicin application (5-7). For example, Chung et al. (6) found that capsaicin applied to the sciatic nerve completely eliminated responses to noxious heat stimuli and decreased responses to noxious mechanical stimuli. At the same time, they observed that responses to innocuous mechanical stimuli were increased. Xin et al. (25) reported that capsaicin markedly attenuated voltage-gated Na+ currents in DRG cells. They suggested that this effect may account for the transient blockade of the nerve conduction seen with capsaicin and may explain its analgesic properties.
The molecular mechanism of TRPV1 receptor desensitization has remained elusive. In the last few years, involvement of calmodulin (CaM) and CaM kinase II (binding of Ca2+/CaM complex to the TRPV1 and phosphorylation by CaM kinase II, respectively) in the phenomenon of TRPV1 receptor regulation has been shown (26, 27). These results indicate that the influx of Ca2+ through TRPV1 may feed back on the channels, inhibiting their gating. This type of feedback inhibition could play a role in the desensitization of the TRVP1 receptor produced by capsaicin (27). However, the molecular mechanism of TRPV1 receptor desensitization and the mechanism of transient blockade of nerve conduction seen with administration of vanilloid agonists might be quite different.
It is well established that vanilloid agonists are able to kill adult sensory neurons in culture and that this effect is most likely mediated by calcium influx (28). There is evidence that the increase in intracellular calcium produced by vanilloids can result in levels of calcium in sensory neurons in vivo that are high enough to cause irreversible neuronal damage. A significant loss of DRG neurons induced by systemic capsaicin has been reported (29); however, the dose of the agent was extremely high: 100 mg/kg, sc. Systemic RTX administered in a single dose of 150 μg/kg to 500 μg/kg caused a loss of C-fibers sensory functions that lasted more than 4 weeks (30). The long duration of this effect was the basis for the suggestion that the local treatment of peripheral nerves with vanilloid agonists results in a permanent impairment of the function of vanilloid-sensitive afferent nerve fibers. Whether it is a result of the degeneration of the fibers or a long-lasting but reversible loss of their functions is a matter of controversy.
Initial electron microscopic studies did not find any axonal degeneration with perineural (31) or topical (32) application of capsaicin even at high (up to 1.5%) concentrations. In more recent studies of the neurotoxic effects of capsaicin applied to the skin (33, 34), administered perineurally (35), or into the urinary bladder (36), the authors visualized nerve fibers by immunohistochemical methods based on the determination of substance P (SP), calcitonin gene-related peptide (CGRP), and/or the pan-neuronal marker protein gene product (PGP 9.5). These studies demonstrated a profound loss of nerve-fiber staining in the epidermis (or urinary bladder wall) leading some of these authors to conclude that capsaicin induced axonal degeneration. However, none of these studies used electron microscopy to confirm this conclusion. When Avelino and Cruz (37) re-examined the problem of capsaicin-induced axonal degeneration in the rat bladder with the parallel use of immunohistochemical and electron microscopy methods, they found that both capsaicin and RTX caused desensitization and a profound reduction in SP and CGRP immunoreactive fibers without causing significant nerve changes demonstrated by electron microscopy. The authors concluded that vanilloids applied intravesically (urinary bladder) in full desensitizing concentrations exert their effect on the bladder (including depletion of SP and CGRP) lasting for 8 to 12 weeks without producing nerve-fiber degeneration. They suggested that the PGP immunoreactivity that was used in several capsaicin studies as an index of axon destruction could be lost because vanilloids cause an axonal transport blockade that slows the arrival of PGP to peripheral axons, a process that occurs without nerve degeneration.
The controversy regarding nerve fiber degeneration vs. long-lasting loss of function without degeneration is possibly relevant to the high concentrations of vanilloid agonists, which are far in excess of the concentrations that produce analgesia lasting only several days. Even if RTX is used at very high concentrations that cause selective degeneration of nerve endings (C-fibers and Aδ-fibers) of a joint, nerve function will be able to eventually recover due to the process of fiber regeneration. This process should be faster and more complete after intraarticular RTX administration than after RTX application to the sciatic nerve because of the involvement of relatively short nerve terminals with i.a. RTX. Karai et al. (38) suggested the nociceptive neuronal or nerve terminal deletion as an effective and broadly applicable strategy for pain management. Such an approach is an acceptable alternative to a reversible interruption of the nerve fibers functions without their destruction that is achievable at lower concentrations of vanilloid agonists.
In conclusion, the experiments demonstrated that intraarticular RTX inhibits pain behavior in carrageenan-induced knee-joint arthritis and that this effect is dose-dependent.
Acknowledgement
The authors thank Edwin L. Bradley, Jr., Professor of Biostatistics at University of Alabama at Birmingham, for statistical analysis.
Footnotes
Supported by National Institutes of Health Grant GM065834
Implication Statement
The intraarticular administration of a vanilloid agonist resiniferatoxin inhibits pain behavior in knee-joint arthritis, this effect is dose-dependent.
References
- 1.Caterina MJ, Julius D. The Vanilloid receptor: a molecular gateway to the pain pathology. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 2.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel on the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Hayes P, Meadows HJ, Gunthrope MJ, et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–15. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 5.Petsche U, Fleischer E, Lembeck F, Handwerker HO. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983;265:233–40. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- 6.Chung JM, Lee KH, Hori Y, Willis WD. Effects of capsaicin applied to a peripheral nerve on the responses of primate spinothalamic tract cells. Brain Res. 1985;329:27–38. doi: 10.1016/0006-8993(85)90509-8. [DOI] [PubMed] [Google Scholar]
- 7.Pini A, Lynn B. C-fibre function during the 6 weeks following brief application of capsaicin to a cutaneous nerve in the rat. Eur J Neurosci. 1990;3:274–84. doi: 10.1111/j.1460-9568.1991.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 8.Kissin I, Bright CA, Bradley EL. Selective and long-lasting neural blockade with resiniferatoxin prevents inflammatory pain and hypersensitivity. Anesth Analg. 2002;94:1253–8. doi: 10.1097/00000539-200205000-00038. [DOI] [PubMed] [Google Scholar]
- 9.Otsuki T, Nakahama H, Niizuma H, Suzuki J. Evaluation of the analgesic effects of capsaicin using a new rat model for tonic pain. Brain Res. 1986;365:235–40. doi: 10.1016/0006-8993(86)91634-3. [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Nakahama H, Miyakawa H, Shima K. Arthritis induced in cat by sodium urate: a possible animal model for tonic pain. Pain. 1984;18:287–97. doi: 10.1016/0304-3959(84)90823-6. [DOI] [PubMed] [Google Scholar]
- 11.Bove SE, Calcaterra SL, Brooker RM, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarth Cart. 2003;11:821–30. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 12.Yu CY, Koo ST, Kim CH, et al. Two variables that can be used as pain indices in experimental animal models of arthritis. Neurosci Meth. 2002;115:107–13. doi: 10.1016/s0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 13.Xu XJ, Farkas-Szallasi T, Lundberg JM, et al. Effects of the capsaicin analogue resiniferatoxin on spinal nociceptive mechanisms in the rat: behavioral, electrophysiological, and in situ hybridization studies. Brain Res. 1997;752:52–60. doi: 10.1016/s0006-8993(96)01444-8. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaka H, Awad H, Yaksh TL. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology. 1996;85:808–16. doi: 10.1097/00000542-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kissin I, Davison N, Bradley EL., Jr Perineural resiniferatoxin prevents hyperalgesia in a rat model of postoperative pain. Anesth Analg. 2005;100:774–80. doi: 10.1213/01.ANE.0000143570.75908.7F. [DOI] [PubMed] [Google Scholar]
- 16.Neubert JK, Karai L, Jun JH, et al. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104:219–28. doi: 10.1016/s0304-3959(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WY, Li Wan Po A. The effectiveness of topically applied capsaicin. A meta-analysis. Eur Clin Pharmacol. 1994;46:517–22. doi: 10.1007/BF00196108. [DOI] [PubMed] [Google Scholar]
- 18.McQuay HJ, Moore RA. Oxford University Press; Oxford: 1998. An evidence based resource for pain relief. [Google Scholar]
- 19.Lam FY, Ferrell WR. Inhibition of carrageenan induced inflammation in the rat knee joint by substance P antagonist. Ann Rheum Dis. 1989;48:928–32. doi: 10.1136/ard.48.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jancso G, Kiraly E, Jancso-Gabor A. Direct evidence for an axonal site of action of capsaicin. Naunyn-Schiedeberg's Arch Pharmacol. 1980;313:91–4. doi: 10.1007/BF00505809. [DOI] [PubMed] [Google Scholar]
- 21.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966–71. [PubMed] [Google Scholar]
- 22.DiMarzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol. 202(12):372–9. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 23.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38-MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 24.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 25.Xin S, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol. 1999;277:G1180–8. doi: 10.1152/ajpgi.1999.277.6.G1180. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung J, Shin J, Lee SY, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–54. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 28.Chard PS, Bleakman D, Saviage JR, Miller RJ. Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: Involvement of calcium-activated proteases. Neuroscience. 1995;65:1099–108. doi: 10.1016/0306-4522(94)00548-j. [DOI] [PubMed] [Google Scholar]
- 29.Jancso G, Kiraly E, Joo F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurones in the adult rat. Neurosci Lett. 1985;59:209–14. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- 30.Szallasi A, Blumberg PM. Vanilloid receptor loss in rat sensory neurona associated with long term desensitization to resiniferatoxin. Neurosci Lett. 1992;136:51–4. doi: 10.1016/0304-3940(92)90679-2. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth A, Hall P, Wall PD, et al. Effects of capsaicin applied locally to adult peripheral nerve. II Anatomy and enzyme and peptide chemistry of peripheral nerve and spinal cord. Pain. 1981;11:379–88. doi: 10.1016/0304-3959(81)90637-0. [DOI] [PubMed] [Google Scholar]
- 32.Szolcsanyi J, Jancso-Gabor A, Joo F, et al. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn-Schiedeberg's Arch Pharmacol. 1975;287:157–69. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- 33.Reilly DM, Ferdinando D, Johnston C, et al. The epidermal nerve fibre network: characterization of nerve fibers in human skin by confocal microscopy and assessment of racial variations. Br J Derm. 1997;137:163–70. doi: 10.1046/j.1365-2133.1997.18001893.x. [DOI] [PubMed] [Google Scholar]
- 34.Simone DA, Nolano M, Johnson T, Wendelshafer-Crabb G, Kennedy WR. Ontradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–59. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dux M, Sann H, Schemann M, Jancso G. Changes in fibre populations of the rat hairy skin following selective chemodenervation by capsaicin. Cell Tissue Res. 1999;296:471–7. doi: 10.1007/s004410051307. [DOI] [PubMed] [Google Scholar]
- 36.Dasgupta P, Chandiramani VA, Beckett A, Scaravilli F, Fowler CJ. The effect of intravesical capsaicin on the suburothelial innervation in patients with detrusor hyperreflexia. BJU International. 2000;85:238–45. doi: 10.1046/j.1464-410x.2000.00427.x. [DOI] [PubMed] [Google Scholar]
- 37.Avelino A, Cruz F. Peptide immunoreactivity and ultrastructure of rat urinary bladder nerve fibers after topical desensitization by capsaicin or resiniferatoxin. Auto Neurosci. 86:37–46. doi: 10.1016/S1566-0702(00)00204-6. [DOI] [PubMed] [Google Scholar]
- 38.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]