Abstract
Neurotransmitter receptor clustering is thought to represent a critical parameter for neuronal transmission. Little is known about the mechanisms that anchor and concentrate inhibitory neurotransmitter receptors in neurons. GABAA receptor (GABAAR) α5 subunits mainly locate at extrasynaptic sites and are thought to mediate tonic inhibition. Notably, similar as synaptic GABAARs, these receptor subtypes also appear in cluster formations at neuronal surface membranes and are of particular interest in cognitive processing. GABAAR α5 mutation or depletion facilitates trace fear conditioning or improves spatial learning in mice, respectively. Here, we identified the actin-binding protein radixin, a member of the ERM family, as the first directly interacting molecule that anchors GABAARs at cytoskeletal elements. Intramolecular activation of radixin is a functional prerequisite for GABAAR α5 subunit binding and both depletion of radixin expression as well as replacement of the radixin F-actin binding motif interferes with GABAAR α5 cluster formation. Our data suggest radixin to represent a critical factor in receptor localization and/or downstream signaling.
Keywords: actin, extrasynaptic, GABAA receptor, radixin, α5 subunit
Introduction
Neurotransmitter receptors are involved in fast neuronal transmission at chemical synapses. In processes of neurotransmitter receptor targeting (Kneussel, 2005), receptors are thought to enter the neuronal surface membrane at extrasynaptic sites, followed by subsequent lateral membrane diffusion. Submembrane scaffold molecules, which locate at postsynaptic specializations, bind and cluster surface membrane receptors opposite to axon terminals that release the appropriate neurotransmitter (Kneussel and Betz, 2000; Moss and Smart, 2001; Kim and Sheng, 2004). In contrast to the localization at synapses, neurotransmitter receptors also locate and concentrate at extrasynaptic surface membrane sites (Hanchar et al, 2004). NMDA-type glutamate receptors at synaptic or extrasynaptic sites activate alternative signaling cascades, leading to either activation or deactivation of the transcription factor CREB, respectively (Hardingham et al, 2002). Inhibitory GABAA receptor (GABAAR) subtypes containing α5 subunits assemble with β and γ subunits (McKernan and Whiting, 1996) and are predominantly expressed in hippocampus (Wisden et al, 1992). Here, they form clusters (Hutcheon et al, 2004) that are mainly found at extrasynaptic locations (Brunig et al, 2002), but to a lesser extent, are also found at synapses (Christie and de Blas, 2002). Extrasynaptic α5-subunit-containing GABAARs are thought to mediate tonic inhibition (Caraiscos et al, 2004). Notably, mutant mice revealed that this GABAAR subpopulation is critically involved in hippocampus-dependent cognitive processes including spatial learning (Collinson et al, 2002) and trace fear conditioning (Crestani et al, 2002). Although GABAAR function has been extensively characterized, the submembrane interactions of this receptor family, including the anchoring at membrane specializations, are largely unknown. Gene depletion of the scaffold protein gephyrin, a main component at inhibitory postsynaptic sites (Kneussel and Betz, 2000), alters the clustering of many GABAAR subtypes (Essrich et al, 1998; Kneussel et al, 1999). However, a direct interaction of gephyrin with any GABAAR subunit has not been reported. It is therefore likely that currently unknown factors exist which anchor GABAARs at gephyrin scaffolds and/or cytoskeletal elements. Notably, GABAAR α5 subunit clusters are among the few subtypes that are completely unaltered upon gephyrin scaffold depletion (Kneussel et al, 2001), although gephyrin has been described at both synaptic and extrasynaptic sites (Danglot et al, 2003). We therefore particularly screened for α5 subunit interacting proteins to shed light on the synaptic/extrasynaptic anchoring of this hippocampal GABAAR subpopulation. Our data identified the ERM-family protein radixin as an essential clustering factor for α5-subunit-containing GABAARs and suggest that a regulated mechanism activates radixin and subsequently bridges respective receptor subtypes with the actin cytoskeleton, which underlies the plasma membrane.
Results
Radixin interacts with GABAAR α5 subunits
To identify novel GABAAR α5 subunit-binding proteins, we performed a yeast two-hybrid screen using the GABAAR α5 subunit intracellular TMIII-TMIV loop (amino acids 342–429) as bait. From 2.8 million clones of an adult rat brain library, three clones were isolated that coded for amino acids 89–185 of radixin, a member of the ERM (ezrin, radixin, moesin) protein family, known to interact with transmembrane proteins (Tsukita et al, 1994) (Figure 1A–C). We mapped the radixin-binding site of GABAAR α5 by generating N-terminal TIII-TMIV-loop deletion mutants based on the bait construct. All mutants lacking residues 342–352 did not interact with radixin (Figure 1A), suggesting for a necessary requirement of this sequence in radixin binding. Consistently, a construct containing residues 342–357 was sufficient to bind radixin. Notably, intracellular TMIII-TMIV loop sequences of GABAAR α1, α3, β2 or γ2 subunits did not interact with radixin in this assay (Figure 1D). The radixin-binding motif of GABAAR α5 is identical in other mammals (man, mouse, cow, dog, monkey) and shares 93% amino acid identity with bird and fish. Moreover, the binding motif is not present in the extrasynaptic GABAAR δ subunit.
Figure 1.

Identification of radixin as an interactor of GABAAR α5 subunits in the yeast two-hybrid screen. (A) Mapping of the radixin binding site on the large intracellular loop of GABAAR α5 (bait). (B) Radixin domain structure. The fragment isolated from the yeast two-hybrid screen is located in the FERM domain (black bar). (C) Alignment of the amino-acid sequence of rat radixin (NM_001005889) with the sequence encoded by the positive clone from the screen. (D) Intracellular TMIII-TMIV loops of the GABAA receptor subunits α1, α3, β2 and γ2 were tested for radixin interaction.
Radixin–GABAAR α5 interaction requires radixin activation
To substantiate these interaction data biochemically, we performed co-immunoprecipitation experiments using rat brain extracts. As detected with a radixin-specific antibody, endogenous radixin specifically co-precipitated with endogenous GABAAR α5 subunit protein (Figure 2A). Co-precipitation resulted in a 2.5-fold increase in radixin band intensity, as compared to the input material, indicating a physiological relevance of the interaction. Radixin can exist in both an inactive closed conformation (Ishikawa et al, 2001), due to intramolecular interaction, and an active open conformation, known to depend on Rho-family GTPase signaling-mediated phosphorylation (Matsui et al, 1998) (Figure 2B). To investigate whether active radixin could bind to the GABAAR α5 intracellular TMIII–TMIV loop, we performed GST-pulldown assays using rat brain extracts (Figure 2C), and probed them with radixin-specific or anti-ERM C-terminal phospho-threonine-specific antibodies. Radixin displayed specific binding to immobilized GST-GABAAR α5 loop fusion protein but not to GST alone. Also, the phospho-ERM antibody recognized a band at this size in the pulldown fraction. Together with the data from the yeast two-hybrid system, these results indicate that radixin, including its active conformation, is a direct GABAAR α5 subunit interactor.
Figure 2.
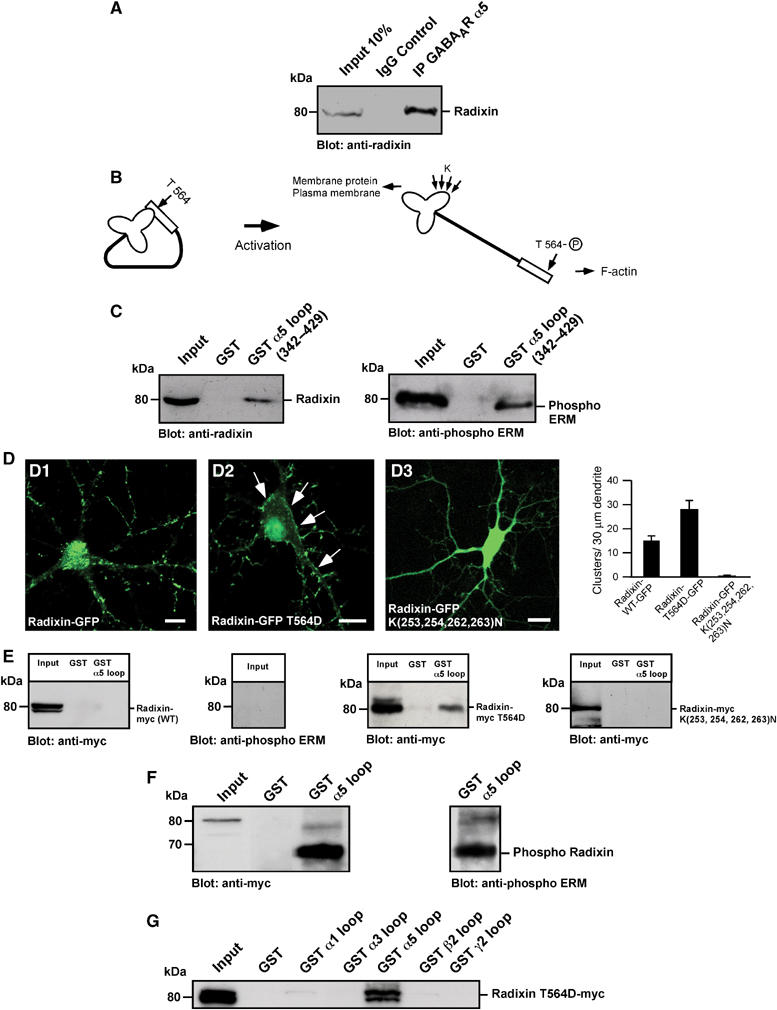
Intramolecular activation of radixin is required for GABAAR α5 subunit binding. (A) Co-immunoprecipitation of radixin and GABAAR α5 from rat brain extract. (B) Schematic representation of radixin intramolecular activation upon phosphorylation of threonine 564. Arrows: sites of mutagenesis; K: lysine; T: threonine. (C) Pulldown assays from rat brain extract with immobilized GABAAR α5 loop fused to GST. (D) Neuronal localization of wild-type and mutant radixin proteins and quantification of clusters per 30 μm dendrite. Arrows depict prominent membrane localization. (E) GST-pulldown assays with myc-tagged radixin wild-type and mutant proteins upon heterologous expression in HEK293 cells. (F) Combinatorial application of kinase activators and the phosphatase inhibitor Calyculin A leads to radixin activation in COS cells. The form of radixin, which binds to the GABAAR α5 loop is phosphorylated at threonine 564, as detected with a phospho ERM-specific antibody. (G) Intracellular TMIII-TMIV loops of the GABAAR subunits α1, α3, β2 and γ2 are not able to bind the active radixin mutant in GST-pulldown assays. Scale bars: 20 μm.
Since the open conformation of ERM proteins is able to bind both membrane proteins and/or F-actin (Tsukita et al, 1994; Simons et al, 1998; Vaiskunaite et al, 2000), we applied site-directed mutagenesis to functionally address whether the subcellular localization of radixin might depend on its activation state. The generation of radixin mutants was based on a wild-type radixin-GFP fusion protein, which displayed similar subcellular localization as endogenous radixin (Supplementary Figure 1A). First, we introduced a negative charge in the radixin polypeptide by replacing threonine 564 (Figure 2B and D), a modification known to mimic phosphorylation and to keep ERM proteins in the open and active conformation (Huang et al, 1999; Ishikawa et al, 2001). This mutant (Radixin-GFP T564D) should be able to bind both actin filaments and phosphatidylinositol-4,5-bisphosphate (PIP2) and consistently displays a strong tendency to locate at the plasma membrane. In fact, both its localization at the neuronal plasma membrane and the number of puncta per 30 μm dendrite were even more prominent, as compared to wild-type radixin-GFP (Figure 2D). We also generated a FERM-domain group mutant (Radixin-GFP (K253,254,262,263N)), in which surface membrane localization and subsequent intramolecular activation is prevented and which is restricted to the cytoplasm (Figure 2D and Supplementary Figure 5A and B). This finding is in line with a previously described corresponding ezrin mutant, deficient in PIP2 binding (Barret et al, 2000). In addition, respective myc-tagged radixin mutants were heterologously expressed in both HEK293 or COS cells and lysates were tested for interaction with the GABAAR α5 subunit intracellular loop in a GST-pulldown assay (Figure 2E and data not shown). Notably, wild-type radixin-myc, expressed in these cell lines, did not interact with α5 subunit loops. The antibody specific for the phosphorylated form of the C-terminal threonine residue did not detect phosphorylated ERM proteins in the cell lysate. In contrast, the active radixin T564D mutant displayed specific interaction, whereas the nonactivatable (K(253,254,262,263)N) mutant did not bind to α5 subunit loops (Figure 2E). These data show that, in contrast to neurons, HEK293 and COS cells are unable to sufficiently activate wild-type radixin and further indicate that an open and active conformation of radixin, as caused by T564D replacement, is a requirement for GABAAR α5 interaction. We then applied a combination of kinase activators and the phosphatase inhibitor Calyculin A to HEK293 and COS cells, expressing wild-type radixin-myc. Remarkably, under these conditions, specific interaction with the GABAAR α5 subunit intracellular loop was observed (Figure 2F and data not shown). However, in COS cells, the pulldown experiment revealed a myc-specific signal of lower molecular weight, as compared to the input band. As a control, detection with the phospho-ERM-specific antibody revealed that the band of lower molecular weight indeed represents the phosphorylated form of radixin-myc (Figure 2F), suggesting that slight differences in SDS–PAGE are due to posttranslational modifications of the phosphorylated protein in this cell type. These data show that radixin phosphorylation, leading to an open and active conformation of the molecule, is an essential requirement for GABAAR α5 interaction.
In addition to the analysis in the yeast system, we tested in a GST-pulldown assay whether the activated full-length radixin T564D mutant would bind other GABAAR subunit loops (Figure 2G). However, in accordance with our previous observations, experiments with different GABAAR subunit TMIII-TMIV loops indicated that the open conformation, represented by Radixin T564D-myc, exclusively bound GABAAR α5 but not GABAAR α1, α3, β2 or γ2 loops.
Radixin colocalizes with GABAAR α5 subunits in neurons
We then investigated whether both proteins colocalize in neurons. Immunostaining of mature hippocampal cultures revealed a significant number of colocalized puncta in neuronal dendrites (Figure 3), indicating that endogenous radixin is localized to positions that harbour endogenous GABAAR α5 subunit clusters (Figure 3, A3). A quantitative evaluation upon immunostaining of endogenous proteins revealed that approximately 57% of total α5-subunit-immunoreactive puncta merged with radixin immunoreactivity. Vice versa 63% of total radixin immunoreactive puncta merged with GABAAR α5 immunoreativity (Figure 3B).
Figure 3.
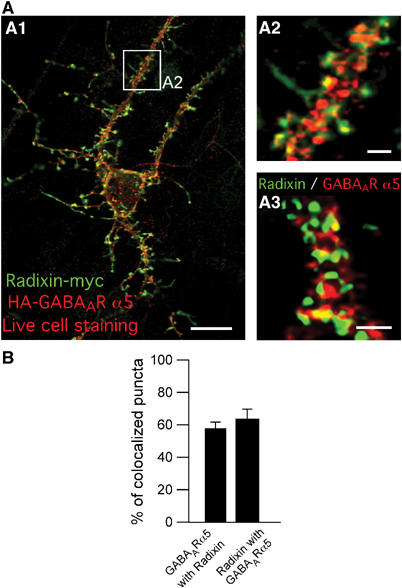
Colocalization of radixin (green) and GABAAR α5 (red) in neuronal dendrites. (A) Coimmunostaining of cultured hippocampal neurons expressing endogenous (A3) or tagged versions of radixin and GABAAR α5 (A1 and A2). As shown in A1 and A2, prior to fixation, neurons were incubated with anti-HA antibody to ensure surface staining of the receptor. (B) Quantification of endogenous GABAAR α5 subunit colocalization with endogenous radixin and vice versa. Scale bars: 20 μm (A1); 3 μm (A2, A3).
We also tested whether the observed clusters contain the active radixin conformation by application of coimmunostaining with the phospho-specific ERM antibody, indicative of active ERM proteins, and a cell membrane marker (Supplementary Figure 1D). The results indicate that indeed, in neurons radixin is activated and that radixin clusters actually locate at the plasma membrane. They are further in conclusion with the observation that active radixin (Radixin-GFP T564D) is detected at membrane locations (Figure 2, D2) and in the P2 plasma membrane fraction upon differential centrifugation (Supplementary Figure 5A). To control the colocalization of endogenous proteins, tagged constructs of both radixin and GABAAR α5 were generated (Supplementary Figure 1A, B and E), which enabled us to perform live cell staining of the receptor in order to visualize its surface localization. Upon neuronal expression of radixin-myc together with HA-GABAAR α5, similar staining patterns were observed (Figure 3A, A1 and A2), confirming that both interactors colocalize and further revealing that interaction mainly occurs at the cell surface. In contrast, radixin-GFP fluorescence did not colocalize with GluR2-containing AMPA receptors at excitatory synapses (Supplementary Figure 1C). Also, no significant colocalization was obtained upon neuronal coexpression of either ezrin-myc (8%) or moesin-myc (4%) together with HA-GABAAR α5 (Supplementary Figure 2). We therefore conclude that GABAAR α5 is a specific interactor of radixin within the ERM family.
In accordance with the literature (Brunig et al, 2002), synaptic clusters of GABAAR α5 were rarely detected. Quantitative analysis revealed that 25% of GABAAR α5 clusters locate at synapses with 75% remaining at extrasynaptic sites, as judged by triple immunostaining of endogenous receptor subunits together with radixin and the synaptic marker SV2 (Figure 4A and B). Immunoreactive puncta of endogenous radixin were found to localize at synapses with similar frequencies. Here, 87% of radixin puncta were extrasynaptic (Figure 4A and B). For coclusters of GABAAR α5 and radixin, we calculated a synaptic localization of 7.5%, a finding which indicates that more than 90% of total coclusters are found at extrasynaptic positions (Figure 4A and B).
Figure 4.
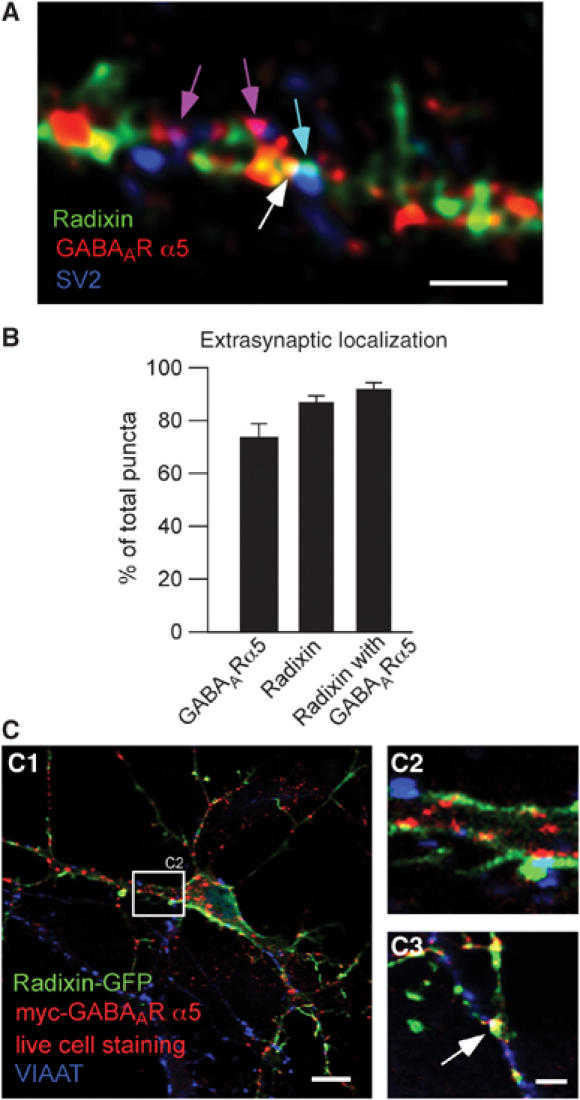
Synaptic and extrasynaptic localization of radixin, GABAAR α5 and radixin/GABAAR α5 coclusters. (A) Triple immunostaining of endogenous radixin (green), endogenous GABAAR α5 (red) and the synaptic marker SV2 (blue) on mature cultured hippocampal neurons. Colored arrows depict examples of colocalization. (B) Quantification of extrasynaptic radixin, GABAAR α5 and radixin/ GABAAR α5 coclusters. (C) Neuronal expression of radixin-GFP and myc-tagged GABAAR α5 (red). Prior to fixation, neurons were incubated with anti-myc antibody to ensure surface detection of the receptor. Radixin/GABAAR α5 coclusters are occasionally found at VIAAT-positive inhibitory synapses (white, arrow). Scale bars: 15 μm (C1); 4 μm (A, C3).
Since this experiment was performed upon fixation of neurons with subsequent immunostaining, our quantification also includes intracellular receptor molecules on their way to or from the surface membrane. We therefore performed live cell staining, labelling surface membrane receptors only. Neuronal expression of radixin-GFP (Supplementary Figure 1A and B) together with myc-GABAAR α5 also displayed synaptic coclusters, as detected by counterstaining against synaptophysin (data not shown) or the vesicular inhibitory amino-acid transporter VIAAT, selective for inhibitory synapses (Figure 4C). The ratio of synaptic/extrasynaptic coclusters was similar between live cell staining and analysis on fixed cells (data not shown), confirming our previous observation (Figure 3A) that radixin–receptor interactions mainly occur at the plasma membrane. Furthermore, these data show that radixin-GABAAR α5 coclusters occasionally occur at inhibitory synapses.
Radixin and gephyrin represent independent systems
Immunostaining detected very little colocalization of radixin with postsynaptic gephyrin, which is known to be critical for the clustering of GABAARs other than those containing the α5 subunit (Kneussel et al, 1999, 2001) (Figure 5A). Furthermore, triple expression of YFP-gephyrin together with radixin-myc and HA-GABAAR α5 revealed that GABAAR α5 does not simultaneously colocalize with both overexpressed gephyrin and overexpressed radixin. However, HA-GABAAR α5 subunit immunoreactivity either merged with radixin-myc or, to a lesser extent, with YFP-gephyrin fluorescence (Figure 5B, B2). Quantification revealed that about 60% of HA-GABAAR α5 colocalize with radixin-myc, whereas only 34% of HA-GABAAR α5 clusters were positive for YFP-gephyrin (Figure 5B), although co-immunoprecipitation of endogenous gephyrin and endogenous GABAAR α5 failed under our experimental conditions (Figure 5B). Triple detection against endogenous radixin, endogenous gephyrin and the inhibitory synapse marker VIAAT consistently showed that gephyrin mainly locates at synaptic sites, while radixin is rarely found in association with inhibitory presynaptic terminals (Figure 5C). In fact, only about 13% of radixin puncta colocalized with VIAAT, representing similar values as obtained for counterstaining with SV2 (Figure 4B). Furthermore, we employed biochemical approaches to investigate whether any association of radixin with gephyrin could be detected. Immunoprecipitation with a gephyrin-specific antibody displayed no specific association with radixin (Figure 5D). Vice versa, immunoprecipitation with a radixin-specific antibody did not lead to co-precipitation of gephyrin (Figure 5E). We therefore conclude that radixin and gephyrin represent independent systems.
Figure 5.
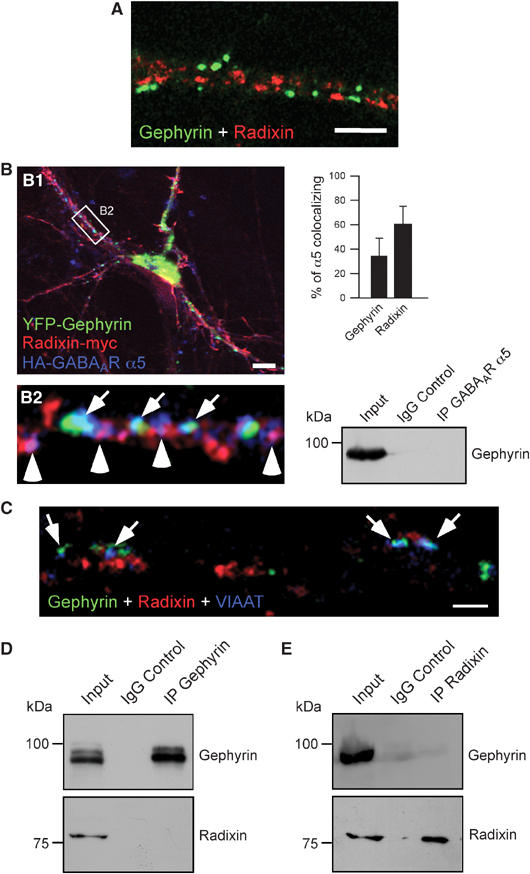
Radixin and gephyrin represent independent systems. (A) Immunostaining of endogenous radixin (red) and endogenous gephyrin (green) on a dendritic region. (B) Triple expression of YFP-gephyrin (green), radixin-myc (red) and HA-GABAAR α5 (blue) in cultured hippocampal neurons. Note that GABAAR α5 subunits colocalize either with radixin (arrowheads) or with gephyrin (arrows). Quantification of HA-GABAAR α5 colocalization with radixin-myc and YFP-gephyrin (upper right). Endogenous gephyrin does not co-immunoprecipitate with endogenous GABAAR α5 under our experimental conditions (lower right). (C) Triple immunostaining of endogenous gephyrin, radixin and VIAAT. Only about 14% of radixin clusters are found at VIAAT-positive inhibitory synapses. Arrows: gephyrin-positive synapses. (D) Immunoprecipitation of gephyrin from rat brain lysate. (E) Immunoprecipitation of radixin from rat brain lysate. Scale bars: 2 μm (A), 12 μm (B1) 1 μm (C).
Radixin is an essential clustering factor for α5 subunit-containing GABAARs
To investigate the role of radixin–GABAAR α5 subunit interactions, we performed loss-of-function experiments. We interfered with radixin expression as well as with radixin binding either to actin or the GABAAR α5 subunit.
Based on a previously described antisense oligonucleotide protocol against radixin (Takeuchi et al, 1994; Paglini et al, 1998) known to significantly knockdown radixin expression, a mixture of two oligonucleotide probes was microinjected into mature cultured hippocampal neurons. To visualize injected neurons, either GFP vector or lucifer yellow were added. Notably, fluorescent neurons displayed a drastic decrease in α5 subunit cluster number with remaining diffuse background immunoreactivity, as compared to untreated control neurons in the same culture or to sense-treated neurons (Figure 6A). In fact, the average number of clusters per 30 μm of dendrite dropped from 18.6 in control cells to 1.5 in antisense-treated cells (P<0.001; Figure 6B). The same treatment led to a reduction in radixin immunoreactivity but did not affect clustering of GABAAR α1 subunits (Supplementary Figure 3A and B).
Figure 6.
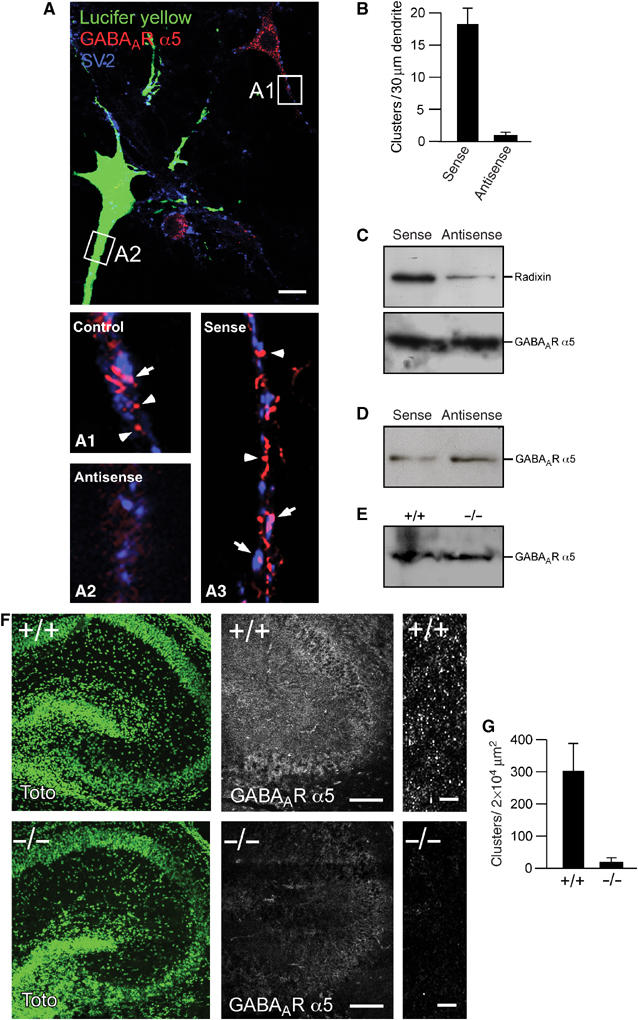
Knockdown or depletion of radixin expression leads to loss of GABAAR α5 clustering. (A) Top: An antisense oligonucleotide injected (green) and a noninjected cell are compared. Synapses are represented by SV2 (blue). The immunoreactivity for GABAAR α5 is diffuse in antisense-treated cells (A2), as compared to normal receptor clusters in untreated (A1) or sense oligonucleotide-treated cells (A3). Arrowheads and arrows depict extrasynaptic and synaptic clusters, respectively. (B) Quantification of GABAAR α5 cluster density in sense- and antisense-injected cells. (C) Whole-cell extracts from cultured hippocampal neurons confirm that radixin expression is efficiently downregulated upon antisense treatment, while total GABAAR α5 expression is unaltered. (D) Western Blot analysis of biotinylated surface GABAAR α5 in sense- or antisense-transfected neurons. (E) Western Blot analysis of P2 plasma membrane preparations from wild-type or radixin knockout mice. (F) Immunohistochemical analysis of wild-type and radixin knockout hippocampal slices. Left: TOTO staining to visualize overall tissue structure. Middle and right: GABAAR α5 staining reveals loss of GABAAR α5 receptor clusters with remaining diffuse signals upon radixin deficiency. (G) Quantification of GABAAR α5 subunit clusters between radixin +/+ and −/− genotypes (P<0.05). Scale bars: 10 μm in (A), 200 μm in (F) (middle) and 10 μm in (F) (right).
A loss of receptor clusters raises the question whether this effect is due to a defect in trafficking or anchoring. Since unclustered, diffuse receptors are barely, if at all detectable using light microscopy (Feng et al, 1998), we also treated neuron cultures with antisense oligonucleotides and performed biochemical analysis on total and surface membrane proteins to check whether unclustered receptors remain at the cell surface. Western blots of the respective samples showed that the total amount of GABAA receptor α5 was unchanged in cultures in which radixin was downregulated (Figure 6C). This observation could be confirmed applying biotinylation of total surface protein, followed by streptavidin precipitation and GABAAR α5-specific Western blot detection (Figure 6D).
Consistent with results obtained upon antisense oligonucleotide knockdown of radixin expression, hippocampal slices derived from radixin knockout mice (Kikuchi et al, 2002) genetically confirmed that radixin is an essential clustering factor for α5 subunit containing GABAARs. Here, receptor clusters were reduced to background levels in radixin-deficient tissue, as compared to wild-type tissue (P<0.05; Figure 6F and G). Specificity of the GABAAR α5-specific signal was controlled in a competition experiment using the GST-GABAAR α5-loop as blocking peptide (Supplementary Figure 4A). Staining of nuclei with the fluorescent dye TOTO confirmed that the anatomy of the hippocampal formation was unaltered between the genotypes (Figure 6F). Furthermore, control stainings confirmed the absence of radixin protein in slices derived from −/− mice, whereas immunostaining against the unrelated GABAAR α3 subunit was unaltered between the genotypes (Supplementary Figure 4B and C) In addition, P2 plasma membrane fractions of brain lysates derived from radixin knockout mice did not display major differences in the amount of surface GABAAR α5 (Figure 6E), indicating that unclustered receptors remain at the neuronal surface membrane upon the loss of radixin.
Radixin and its ERM-family homologs ezrin and moesin display a number of structural and functional similarities (Tsukita and Yonemura, 1997; Bretscher et al, 2002). Based on a described ezrin mutant (Allenspach et al, 2001), we also generated a GFP-fused deletion construct (Radixin-(1–468)-GFP), in which the C-terminal F-actin-binding domain of radixin is replaced by GFP (Figure 7A). Over-expression of Radixin-(1–468)-GFP induced severe loss of GABAAR α5 subunit clustering, while control cells within the same field of vision (Figure 7B) and cells expressing the noninterfering cytoplasmic mutant Radixin-GFP (K253,254,262,263N) (Supplementary Figure 5B) displayed normal GABAAR α5 subunit clusters. The average cluster number per 30 μm of dendrite was reduced to 6.4 in cells expressing the truncated radixin construct, as compared to 26.4 in control cells (P<0.001; Figure 7C). Consistently, this C-terminal deletion mutant Radixin-(1–468)-GFP does not interact with F-actin in a cosedimentation assay, as judged from its absence in the pellet fraction. In contrast, the active full-length form represented by Radixin-GFP T564D cosediments with F-actin and is therefore enriched in the pellet (Figure 7D). Also, wild-type radixin-GFP, expressed in neurons, highly colocalizes with rhodamine phalloidine-stained actin (Supplementary Figure 1F). However, since the N-terminal FERM domain is intact, Radixin-(1–468)-GFP is still able to associate with the plasma membrane as suggested by its prominent abundance in the P2 pellet upon differential centrifugation (Supplementary Figure 5A). Consequently, this mutant is likely to compete with endogenous radixin for GABAAR α5 interaction at the cell surface and thereby shields the receptor from its anchor to the actin cytoskeleton.
Figure 7.
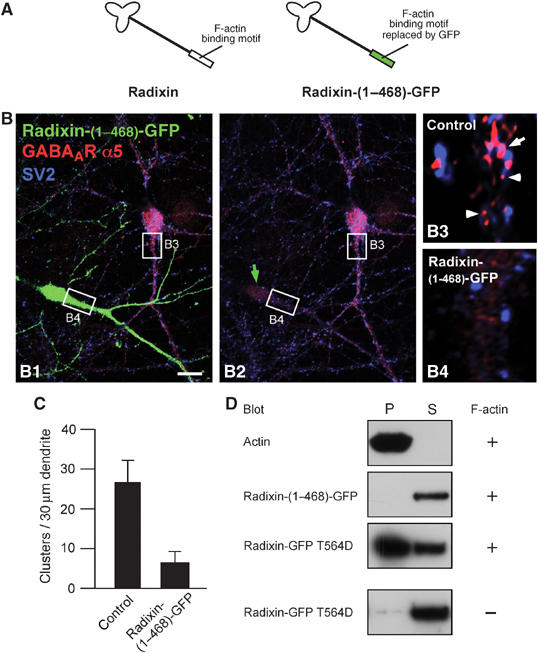
Deletion of the F-actin-binding site in radixin leads to loss of GABAAR α5 clustering. (A) Schematic drawing of the truncated molecule. The F-actin-binding motif is replaced by GFP. (B) Hippocampal neurons expressing Radixin-(1–468)-GFP. A transfected (green) and a nontransfected control cell are shown (B1). B2: Same image without the green channel. Green arrow: transfected cell; arrow: synaptic; arrowheads: extrasynaptic. Scale bar (B): 20 μm. (C) Quantification of GABAAR α5 cluster density in control and transfected cells. (D) Characterization of F-actin-binding capacity of different radixin constructs in a cosedimentation assay. Plus and minus indicates the presence or absence of F-actin in the experiment. P: pellet, S: supernatant.
If radixin represents a GABAAR α5 subunit clustering factor, specific interference with radixin-receptor binding should also affect receptor clustering. We therefore mutated the radixin-binding motif in α5 subunits. Based on data from our deletion constructs, we generated group mutants restricted in radixin binding, by alanine replacement of critical residues in the α5 subunit intracellular loop region (Supplementary Figure 6A). Both mutants failed to interact with radixin in the yeast two-hybrid system, however, they displayed about 30% of radixin binding capacity in a GST-pulldown assay as compared to the wild-type loop, although equal amounts of proteins were offered (Supplementary Figure 6A–C). The combination of both group mutants should therefore display dramatically compromised radixin binding. As expected, the combined loop mutant myc-GABAAR α5 (Y343A,F344A,T345A,W349A,W351A) displayed a drastic reduction in receptor cluster number upon neuronal overexpression, as compared with expression of a myc-tagged wild-type receptor subunit (Supplementary Figure 6D), thereby confirming previous loss-of-function results.
To address the question if extrasynaptic α5-containing receptors uncoupled from the radixin–actin complex remain functional, we performed whole-cell patch-clamp recordings from hippocampal neurons overexpressing the F-actin binding-deficient mutant Radixin-(1–468)-GFP and studied tonic GABAergic currents. The application of 100 μM bicuculline methiodite caused a reversible reduction in tonic inward current in both GFP (n=24; Figure 8A and C) and Radixin-(1–468)-GFP-expressing neurons (n=31; Figure 8B and C). This indicates that α5 subunit-containing GABAARs which lose radixin-dependent submembrane anchoring indeed remain functional within the neuronal plasma membrane. Since loss of extrasynaptic anchoring is likely to induce diffusional motility of α5 subunit-containing GABAARs across the neuronal plasma membrane, we asked whether uncoupled receptors might contribute to and modulate synaptic responses. To test this, we studied GABAergic miniature inhibitory postsynaptic currents (mIPSCs), which could be readily recorded in both GFP and Radixin-(1–468)-GFP-expressing neurons (Figure 8D and E). Averaged mIPSCs displayed a small but significant difference with respect to their 10–90% rise-times in GFP and Radixin-(1–468)-GFP-expressing neurons (GFP: 1.68±0.09 ms, n=17; Radixin-(1–468)-GFP: 1.92±0.07 ms, n=18; P=0.0417; Figure 8F). However, no significant differences were observed with respect to mIPSC size (P=0.7974; Figure 8G) and decay time constants (P=0.9278; Figure 8H), indicating that our experimental conditions had no major effect on quantal GABAergic synaptic transmission.
Figure 8.
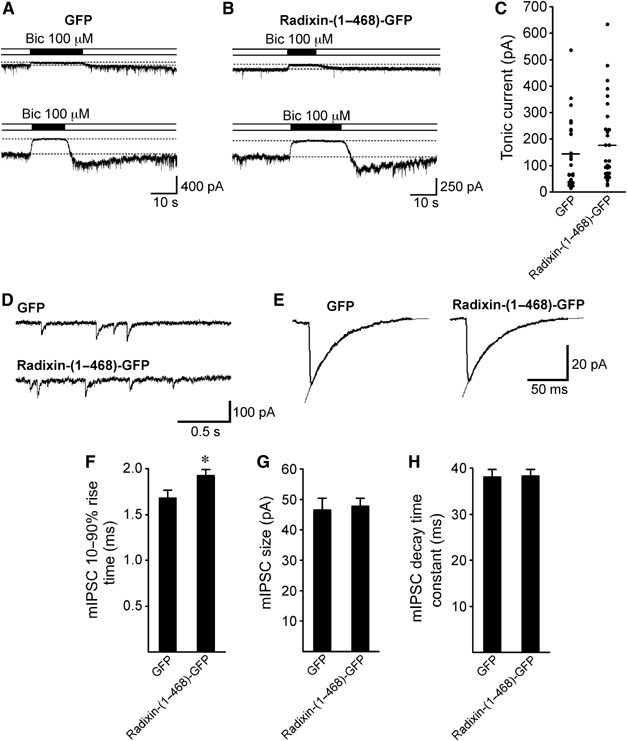
Tonic and quantal synaptic GABAergic currents in the presence of the F-actin binding-deficient mutant Radixin-(1–468)-GFP. Tonic GABAergic currents were determined by the application of 100 μM bicuculline methiodite (Bic) as the difference in holding current (dashed lines in A and B). Owing to cell variabilities, representative experiments are shown for two GFP-expressing (A) and two Radixin-(1–468)-GFP-expressing neurons (B) with small (upper traces) and large tonic current components (lower traces). (C) Summary of tonic current measurements derived from GFP-expressing (n=24) and Radixin-(1–468)-GFP (n=31)-expressing neurons. Horizontal lines represent mean values (GFP: 145 pA; Radixin-(1–468)-GFP: 176 pA). (D) Representative GABAergic miniature inhibitory postsynaptic currents (mIPSCs) recorded from a GFP- and a Radixin-(1–468)-GFP-expressing neuron. (E) Averaged mIPSCs from representative GFP (left trace, 379 events averaged)- and Radixin-(1–468)-GFP (right trace, 368 events averaged)-expressing neurons (lines represent single-exponential fits). Bar graphs show mean 10–90% rise times (F), sizes (G) and decay time constants (H) for mIPSCs from GFP (n=17)- and Radixin-(1–468)-GFP-expressing neurons (n=18).
In summary, based on the independent loss-of-function assays described, we conclude that radixin represents an essential clustering factor for GABAAR α5 subunits but is not involved in receptor trafficking.
Discussion
We identified a novel interaction between the GABAAR α5 subunit and the actin-binding protein radixin. The detailed study of this interaction leads to the following conclusions: (i) functional activation of radixin is a necessary requirement for GABAAR α5 subunit binding; (ii) radixin is an essential clustering factor for GABAAR α5 subunits and (iii) radixin and gephyrin represent independent receptor clustering systems.
The ERM-family protein radixin was originally isolated as an actin-binding protein (Tsukita and Hieda, 1989). Together with its closest homologues ezrin and moesin it shares an N-terminal globular FERM domain and a C-terminal F-actin-binding site (Tsukita and Yonemura, 1997; Vaiskunaite et al, 2000). Radixin binds transmembrane proteins such as CD44 (Tsukita et al, 1994). Knockout of radixin in mice leads to deafness (Kitajiri et al, 2004) and a loss of surface membrane localization of the protein MRP2 (Kikuchi et al, 2002).
We show that endogenous GABAAR α5 subunits specifically bind radixin derived from brain lysate. This interaction is likely to be direct, as it was not only observed by immunoprecipitation or in GST-pulldown assays from native tissue, but also obtained in the yeast two-hybrid system, which requires complementation of a transcriptional regulator in the yeast nucleus.
Our data further reveal that phosphorylation-dependent activation of radixin is required for GABAAR α5 binding. According to previous reports, activation of ERM proteins is a multistep process (Fievet et al, 2004), which requires conformational changes (Bretscher et al, 2002). It was shown that the N-terminal domain of ERM proteins binds to C-terminal sequences via an intramolecular association, thereby masking the F-actin-binding site (Simons et al, 1998; Ishikawa et al, 2001). During activation, PIP2 binding of the N-terminal FERM domain is followed by phosphorylation of the C-terminus (Fievet et al, 2004). Group mutation of four lysine residues in the ezrin FERM domain (analogous to the mutant radixin-GFP K(253,254,262,263)N in this study) blocks PIP2 binding and therefore membrane association (Barret et al, 2000). Together, these data lead to an activation model in which PIP2 binding is a necessary requirement for the subsequent phosphorylation of ERM proteins, which in turn opens the intramolecular association, thereby exposing the F-actin-binding site (Simons et al, 1998).
In agreement with this activation model, deletion of the F-actin-binding motif in radixin (Radixin-(1–468)-GFP) also affects GABAAR α5 cluster formation in neurons, because this deletion mutant retains the ability to interact with the plasma membrane, as observed upon differential centrifugation. It is therefore plausible to conclude that GABAAR α5 clustering requires radixin binding to the submembrane actin cytoskeleton.
Immunocytochemistry revealed that GABAAR α5 cluster formations significantly colocalize with radixin, but not with ezrin or moesin in neurons. Although radixin and GABAAR α5 coclusters mainly localize extrasynaptically, they are occasionally detected at synaptic contacts. This observation is consistent with previous reports that GABAAR α5 subunits mainly localize at extrasynaptic sites (Brunig et al, 2002) but are, to a lesser extent, also found at GABAergic synapses (Christie and de Blas, 2002).
Applying different loss-of-function experiments, including analysis of radixin knockout tissue, we show that radixin is an essential requirement for the formation and/or maintenance of receptor clusters. Furthermore, we provide independent evidence that radixin is not involved in GABAAR α5 trafficking by application of surface biotinylation, analysis of radixin-deficient brain extract and electrophysiological approaches. Up to now, the glycine receptor clustering protein gephyrin has been the only known factor with a clustering function for GABAAR subtypes, although a direct interaction between gephyrin and any GABAAR subunit is currently not reported. In the present study, we show that both clustering factors, radixin and gephyrin, neither colocalize in neurons nor bind to each other and are therefore likely to function in separate systems. Gephyrin and ERM proteins, including radixin, multimerize and/or display head-to-tail self-interaction (Magendantz et al, 1995; Sola et al, 2004) and further bind to cytoskeletal elements (Tsukita and Yonemura, 1997; Kneussel and Betz, 2000; Vaiskunaite et al, 2000), features which are thought to be required for scaffold interactions of receptor clustering factors (Sola et al, 2004). In accordance with such structural requirements our data show that GABAAR α5 clustering relies on F-actin binding by radixin. Although it is apparent that ERM proteins can occur as oligomers (Berryman et al, 1995), the significance of the oligomeric species and the mechanisms that lead to oligomerization are currently barely understood. It is therefore also a possibility that radixin oligomers interact with GABAAR α5-containing receptor in vivo, thereby participating in the formation of receptor clusters. Together with the possibility that individual GABAARs may contain two α5 subunits, receptor–radixin–actin interactions may lead to complex scaffold formations at extrasynaptic and/or synaptic sites. Alternatively or in addition, other proteins may participate in the formation or stabilization of GABAAR α5-containing receptor–scaffold interactions.
Tonic inhibition is important for the regulation of excitability as it alters the probability for action potential generation and integrates excitatory signals, thereby also modulating rhythm generation in neuronal networks (Semyanov et al, 2004). However, why do extrasynaptic GABAAR α5 subunits assemble in cluster formations (Brunig et al, 2002; Hutcheon et al, 2004)? Notably, upon loss of clustering with our dominant-negative approach, bicuculline-sensitive tonic currents are still present, indicating that unclustered GABAAR α5 receptors remain functional in the neuronal surface. Different possibilities might explain the appearance of GABAAR α5 subunits in cluster formations rather than in an equal distribution across the neuronal surface: as synapses are not only pre- and postsynaptic appositions of neuronal cells, but are in close contact with glial cells, some of which are also known to release neurotransmitter molecules including GABA (Albrecht et al, 1998; Barakat and Bordey, 2002), extrasynaptic GABAAR α5 clusters might represent membrane specializations at neuron–glia contacts. Moreover, they might be involved in certain signaling cascades as known for extrasynaptic NMDA receptors (Hardingham et al, 2002).
Alternatively, preformed clusters at extrasynaptic sites may also fulfill a reserve pool function for the rapid recruitment of receptors or receptor clusters to and/or from synapses. Recruitment may occur by lateral diffusion of single receptor molecules following the fragmentation of clusters under certain conditions. Our data show that mIPSC amplitudes and decay kinetics remained unaffected upon dominant-negative loss of clustering, a finding which is in accordance with radixin being associated mainly with extrasynaptic receptors. However, mIPSC rise times were slightly increased in unclustered situations. A possible explanation for this observation might be that upon loss of extrasynaptic clustering, untrapped receptors start to diffuse and consequently distribute evenly across the neuronal surface. Hence, individual molecules may reach the coverage of presynaptic neurotransmitter release and therefore influence mIPSC rise times.
Under native conditions, it might also be a possible scenario that entire receptor clusters are subject of recruitment. Although in our experiments the majority of radixin associated GABAAR α5 subunit clusters were located at extrasynaptic sites, both proteins indeed occasionally localize at inhibitory synapses. Consistent with our observations, others also described a minority of GABAAR α5 subunits at synapses (Christie and de Blas, 2002) and mIPSC amplitudes have been shown to be slightly decreased in GABAAR α5 knockout mice (Collinson et al, 2002). Since binding of radixin to F-actin and α5-containing GABAARs is a phosphorylation-dependent process, GABAAR α5 clusters and/or radixin-GABAAR α5 coclusters might shuttle between extrasynaptic and synaptic sites upon regulatory processes, such as activation/inactivation of the radixin molecule. Although such mechanisms are currently speculative and certainly require further investigation, they would represent candidate scenarios for the regulation of synaptic strength and/or plasticity.
Materials and methods
Yeast two-hybrid assay
The Matchmaker LexA Yeast Two-Hybrid system (Clontech, Heidelberg, Germany) and a rat brain cDNA library (Origene, Rockville, MD) were used for interaction screening. 2.8 million independent clones were analyzed. Interaction of bait and prey fusions were assayed by activation of the LEU2 and lacZ reporters. Plasmid DNA of positive clones was recovered and inserts were analyzed by dideoxy sequencing.
Constructs
The GABAAR α5 TMIII-TMIV intracellular loop was subcloned as EcoRI–SalI fragment into pGILDA (Origene, Rockville, MD) and pGEX-5X1 (Amersham Biosciences, Freiburg, Germany). Group mutations were introduced using a site-directed mutagenesis kit (Stratagene, La Jolla, CA). Full-length GABAAR α5 subunit cDNA was amplified from mouse brain total RNA and cloned as a NotI–XhoI fragment into pBK-CMV (Stratagene, Amsterdam, Netherlands). Myc- or HA-tag sequences were inserted as BglII fragments in the GABAAR α5 subunit cDNA after the signal peptide sequence. Mouse radixin cDNA was cloned as an XhoI–KpnI fragment into pEGFP-N2 vector (BD Biosciences, Heidelberg, Germany). Mutant radixin cDNAs were generated using a site-directed mutagenesis kit (Stratagene, LaJolla, CA). For Radixin-(1–468)-GFP, a corresponding PCR amplified fragment was subcloned as a XhoI–KpnI fragment into pEGFP-N2 (BD Biosciences, Heidelberg, Germany). To generate myc-tagged radixin, a BamHI–NotI fragment was cloned into pBK-CMV. The myc-tag sequence was introduced as NotI–NotI insertion. All constructs were verified by dideoxy sequencing.
Co-immunoprecipitation/GST-pulldown assay
All buffers were supplemented with protease inhibitors (Roche, Mannheim, Germany) and processed in the cold. Brains of five adult rats were dissected in buffer 1 (50 mM glucose, 20 mM Tris, pH 8.0, 150 mM NaCl and 2.5 mM EDTA, homogenized and clarified at 1000 g for 10 min. Equal volumes of buffer 2 (20 mM Tris, pH 7.4, 150 mM NaCl, 24 mM sodium deoxycholate, 1% (v/v) Tween20 and 0.1% (w/v) SDS) were added followed by incubation for 30 min and sonication three times for 30 s. Extracts were aliquoted and frozen in liquid nitrogen. Protein content was determined by Bradford assay. Antibodies were coupled to protein G sepharose beads (Dynal Biotech, Oslo, Norway) overnight in buffer 1. Brain extracts were incubated with the beads over night, then washed and boiled in SDS sample buffer. For pulldown experiments, HEK293 cells were washed 30 h after transfection with PBS and harvested in 1 ml PBS, supplemented with 1% Triton and 1 mM PMSF. E. coli BL21 lysates were obtained by sonification and centrifugation at 10 000 g for 30 min. Bacterial lysates were coupled to glutathione-sepharose beads (Amersham, Freiburg, Germany) for 3 h. The HEK293 lysate was applied to the beads for 10–12 h. Beads were washed and boiled as described. For experiments with protein kinase activator/phosphatase inhibitor mix (all reagents from Calbiochem, Darmstadt, Germany), the membrane permeable adenosine 3′,5′-cyclic monophosphate, 8-(4-chlorophenylthio)-sodium salt and guanosine 3′,5′-cyclic monophosphate,N2,2′-O-dibutyryl-, sodium salt were added to the medium at 1 mM 3 h prior to harvesting the cells. In all, 0.1 mM 1,2-dioctanoyl-sn-glycerol and sphingosylphosphorylcholine were added to the extract. Calyculin A was applied to the culture 3 h prior to harvesting.
Electrophysiology
Hippocampal cultures were used for electrophysiological recordings 4–5 days after transfection with Radixin-(1–468)-GFP or GFP cDNA. Following a 24 h treatment with vigabatrin (100 μM), cultures were bathed with external solution containing (in mM): NaCl 140, KCl 5, CaCl2 2, HEPES 5, sucrose 10 and phenol red 0.01 mg ml−1; pH 7.4 (NaOH). Whole-cell patch-clamp recordings at −70 mV were performed on fluorescent neurons with a pipette solution containing (in mM): CsCl 140, CaCl2 1, MgCl2 1, EGTA 11, HEPES 5; pH 7.2 (CsOH). Recordings were done in the presence of 500 nM tetrodotoxin, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 50 μM DL-2-amino-5-phosphono-pentanoic acid. The amplitude of tonic GABAAR-mediated current was measured as the difference in the holding current before and during the application of 100 μM bicuculline methiodite. Analysis of mIPSCs was performed on averaged signals from individual cells. The mIPSC 10–90% rise-time and peak amplitude were determined and the mIPSC decay kinetics approximated with a single-exponential function. Data are presented as mean±s.e.m., and statistical analyses were performed with unpaired Student's t-tests.
Supplementary Material
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Acknowledgments
We thank B Gasnier for providing the VIAAT-specific antibody. We are grateful to T Voyno-Yasenetskaya for the radixin cDNA, to O El Far for advice with the yeast Two-Hybrid system and to S Plant for excellent technical assistance. This work was supported by the University of Hamburg and grants from the Deutsche Forschungsgemeinschaft (KN-556/1-1, KN-556/1-2 and SFB444/B7) to MK.
References
- Albrecht J, Bender AS, Norenberg MD (1998) Potassium-stimulated GABA release is a chloride-dependent but sodium- and calcium-independent process in cultured astrocytes. Acta Neurobiol Exp (Wars) 58: 169–175 [DOI] [PubMed] [Google Scholar]
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15: 739–750 [DOI] [PubMed] [Google Scholar]
- Barakat L, Bordey A (2002) GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol 88: 1407–1419 [DOI] [PubMed] [Google Scholar]
- Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V (2000) Mutagenesis of the phosphatidylinositol 4, 5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol 151: 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman M, Gary R, Bretscher A (1995) Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol 131: 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599 [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM (2002) Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol 443: 43–55 [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL (2002) Alpha5 subunit-containing GABA(A) receptors form clusters at GABAergic synapses in hippocampal cultures. NeuroReport 13: 2355–2358 [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci 22: 5572–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U (2002) Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA 99: 8980–8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A (2003) Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci 23: 264–278 [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci 1: 563–571 [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR (1998) Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282: 1321–1324 [DOI] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M (2004) Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164: 653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW (2004) Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci 76: 1–8 [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5: 405–414 [DOI] [PubMed] [Google Scholar]
- Huang L, Wong TY, Lin RC, Furthmayr H (1999) Replacement of threonine 558, a critical site of phosphorylation of moesin in vivo, with aspartate activates F-actin binding of moesin. Regulation by conformational change. J Biol Chem 274: 12803–12810 [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Fritschy JM, Poulter MO (2004) Organization of GABA receptor alpha-subunit clustering in the developing rat neocortex and hippocampus. Eur J Neurosci 19: 2475–2487 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tamura A, Matsui T, Sasaki H, Hakoshima T, Tsukita S (2001) Structural conversion between open and closed forms of radixin: low-angle shadowing electron microscopy. J Mol Biol 310: 973–978 [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S (2002) Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet 31: 320–325 [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781 [DOI] [PubMed] [Google Scholar]
- Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, Ito J, Tsukita S (2004) Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol 166: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M (2005) Postsynaptic scaffold proteins at non–synaptic sites. The role of postsynaptic scaffold proteins in motor–protein–receptor complexes. EMBO Rep 6: 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Betz H (2000) Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci 23: 429–435 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H (2001) Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci 17: 973–982 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H (1999) Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci 19: 9289–9297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magendantz M, Henry MD, Lander A, Solomon F (1995) Interdomain interactions of radixin in vitro. J Biol Chem 270: 25324–25327 [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143 [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG (2001) Constructing inhibitory synapses. Nat Rev Neurosci 2: 240–250 [DOI] [PubMed] [Google Scholar]
- Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A (1998) Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol 143: 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269 [DOI] [PubMed] [Google Scholar]
- Simons PC, Pietromonaco SF, Reczek D, Bretscher A, Elias L (1998) C-terminal threonine phosphorylation activates ERM proteins to link the cell's cortical lipid bilayer to the cytoskeleton. Biochem Biophys Res Commun 253: 561–565 [DOI] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O'Sullivan GA, Schmitt B, Betz H, Weissenhorn W (2004) Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J 23: 2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S (1994) Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol 125: 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Hieda Y (1989) A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: purification and characterization. J Cell Biol 108: 2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S (1997) ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci 22: 53–58 [DOI] [PubMed] [Google Scholar]
- Vaiskunaite R, Adarichev V, Furthmayr H, Kozasa T, Gudkov A, Voyno-Yasenetskaya TA (2000) Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem 275: 26206–26212 [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6


