Abstract
Notch signaling plays a critical role in T-cell differentiation and leukemogenesis. We previously demonstrated that, while pre-TCR is required for thymocytes proliferation and leukemogenesis, it is dispensable for thymocyte differentiation in Notch3-transgenic mice. Notch3-transgenic premalignant thymocytes and T lymphoma cells overexpress pTα/pre-TCR and display constitutive activation of NF-κB, providing survival signals for immature thymocytes. We provide genetic and biochemical evidence that Notch3 triggers multiple NF-κB activation pathways. A pre-TCR-dependent pathway preferentially activates NF-κB via IKKβ/IKKα/NIK complex, resulting in p50/p65 heterodimer nuclear entry and recruitment onto promoters of Cyclin D1, Bcl2-A1 and IL7-receptor-α genes. In contrast, upon pTα deletion, Notch3 binds IKKα and maintains NF-κB activation through an alternative pathway, depending on an NIK-independent IKKα homodimer activity. The consequent NF-κB2/p100 processing allows nuclear translocation of p52/RelB heterodimers, which only trigger transcription from Bcl2-A1 and IL7-receptor-α genes. Our data suggest that a finely tuned interplay between Notch3 and pre-TCR pathways converges on regulation of NF-κB activity, leading to differential NF-κB subunit dimerization that regulates distinct gene clusters involved in either cell differentiation or proliferation/leukemogenesis.
Keywords: IKKα, NF-κB pathways, Notch3, pre-TCR
Introduction
NF-κB transcription factors play an evolutionarily conserved and critical role in triggering the transcription of a variety of genes involved in inflammatory and immune responses and in the control of cell proliferation, differentiation and apoptosis (Karin et al, 2002; Karin and Lin, 2002). In mammalian cells, the NF-κB family is composed of five members, RelA (p65), RelB, c-Rel, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100), and exists as a heterogeneous collection of homodimers and heterodimers (Ghosh and Karin, 2002). The activity of NF-κB homo- and hetero-dimers is tightly controlled through the association with inhibitory proteins of the IκB family that sequester them into the cytoplasm. The pathways that lead to NF-κB activation depends on components of the IκB kinase (IKK) complex, which contains two catalytic subunits, IKKα and IKKβ and a regulatory IKKγ/NEMO (Hayden and Ghosh, 2004). NEMO, like IKKβ, is required for signaling through the canonical NF-κB pathway. The critical event in initiating this pathway is the IKKβ-dependent phosphorylation of IκBα to trigger its ubiquitin-dependent degradation and induce nuclear entry of a ubiquitous NF-κB dimer, p65:p50. Recent work has yielded some knowledge of an alternative pathway based on a regulated processing of the NF-κB2/p100 precursor protein, allowing the resulting p52 fragment to translocate to the nucleus in association with some other NF-κB proteins (mainly RelB) (Senftleben et al, 2001; Dejardin et al, 2002; Yilmaz et al, 2003). This pathway depends on NF-κB-induced kinase (NIK) that specifically phosphorylates and activates IKKα, and upon activation recruits IKKα into a multiprotein complex with p100. This alternative pathway plays a crucial role in controlling the development, organization and function of secondary lymphoid tissue (Senftleben et al, 2001; Dejardin et al, 2002; Derudder et al, 2003; Bonizzi et al, 2004). However, its specific role in thymus development has not been addressed, as yet. The relative role of the canonical and non-canonical NF-κB activation pathways in regulating the molecular mechanisms involved in T-cell differentiation, proliferation and/or neoplastic transformation are yet to be elucidated. In a previous report, we demonstrated that T-cell leukemia/lymphoma arising in transgenic mice overexpressing an lck-driven constitutively active intracellular domain of Notch3 (Notch3-IC), displays a persistent high expression of pre-T-cell receptor (pre-TCR) pTα chain and an IκBα-dependent constitutive activation of NF-κB (Bellavia et al, 2000). Pre-TCR signaling has recently been shown to lead to the activation of canonical NF-κB pathway, independently on ligand binding (Aifantis et al, 2001) and, more recently, we demonstrated that PKCθ might mediate such an activation, since deletion of PKCθ in Notch3-IC transgenic mice, decreases the IκBα-dependent activation of NF-κB while reducing the incidence of leukemia (Felli et al, 2005). Finally, we observed that deletion of pTα gene mostly abrogated the development of leukemia/lymphoma in Notch3 transgenic mice, while the constitutive activation of Notch3 was able to rescue the blockage of the pre-TCR check-point by promoting immature DN thymocytes to progress toward a more differentiated phenotype (e.g. acquisition of CD4, CD8 and TCRβ expression) in the absence of pTα/pre-TCR (Bellavia et al, 2002). We report here that activated Notch3 signaling triggers multiple NF-κB activation pathways in Notch3-IC transgenic mice. Abrogation of pTα/pre-TCR function drastically reduces the activation of the canonical pathway, by switching on a NIK-independent alternative pathway activation that is strictly dependent on IKKα activity, essential for p100 processing to p52 (Bonizzi and Karin, 2004). This allows Notch3 to keep NF-κB constitutively active despite the absence of membrane associated pre-TCR, leading to transcriptional regulation of different p52/RelB complex-dependent target genes specifically involved in thymocyte survival and differentiation.
Together, these observations suggest that a finely tuned interplay between Notch3 and pre-TCR signaling pathways converges on the regulation of NF-κB activity and may direct T cells toward either further differentiation or neoplastic transformation outcomes.
Results
Persistence of Notch3-triggered NF-B activity in the absence of a functional pTα/pre-TCR
We previously observed that thymocytes from Notch3-IC transgenic mice display dysregulated high expression of pTα/pre-TCR chain and constitutive NF-κB activity (Bellavia et al, 2000). Furthermore, we demonstrated that combined overexpression of pTα and Notch3 in transgenic mice, triggers important molecular events, some of which are NF-κB-mediated and cooperate in sustaining Notch3-induced lymphomagenesis (Bellavia et al, 2002; Talora et al, 2003; Felli et al, 2005). In order to investigate the possible role of pTα/pre-TCR in mediating Notch3-dependent NF-κB constitutive activity, we monitored the ability of Notch3 to regulate NF-κB activity in the absence of pTα, by utilizing previously established Notch3-IC/pTα−/− double-mutant mice (Bellavia et al, 2002). Electrophoretic mobility shift assay (EMSA) was performed with nuclear extracts from freshly isolated unfractionated thymocytes obtained from 4-week-old wild type (wt) and Notch3-IC transgenic (N3-IC) and 6–8 weeks old Notch3-IC/pTα−/− double-mutant (N3-IC/pTα−/−) mice, ages at which the thymocyte subset distribution is similar with respect to CD4 and/or CD8 expression in all the mice (Figure 1A). As shown in Figure 1B, NF-κB DNA-binding activity is significantly increased in Notch3 mice, but, interestingly, it is still higher in Notch3/pTα−/− double mutant, with respect to wt mice, suggesting that pre-TCR is dispensable for Notch3-induced NF-κB activation. However, Figure 1C shows that while in Notch3-IC thymocytes most of DNA-bound NF-κB complexes are p50/p65, as previously described (Bellavia et al, 2000), in double-mutant thymocytes we observed a decreased supershift of p65, accompanied by an increased supershift of p50 and p52 and a displaced RelB binding, that suggest the increased generation of p50/p50 inhibitory homodimers and p52/RelB heterodimers. Figure 2 further support this hypothesis, showing a decrease of p50 nuclear translocation accompanied by a sustained higher nuclear translocation of p52 in Notch3-IC/pTα−/− with respect to Notch3-IC transgenic and wild-type thymocytes (Figure 2A). While Notch3-IC/pTα−/− and Notch3-IC thymocytes display a higher nuclear traslocation of RelB with respect to wt (Figure 2A). The immunofluorescence staining directly shows the increased nuclear translocation of p52 (Figure 2B). Furthermore, a decreased amount of phosphorylated p65 is observed in nuclear and total extracts of Notch3/pTα−/− double mutant with respect to Notch3-IC transgenic thymocytes (Figure 2C), leading to the generation of a decreased amount of functionally active heterodimers composed of phosphorylated p65 bound to p50 in Notch3/pTα−/− double-mutant thymocytes (Figure 2C lower right panel). Interestingly, we also observed a significant increase of the p52 precursor, p100, nuclear translocation in Notch3/pTα−/− double mutant with respect to Notch3-IC transgenic thymocytes (Figure 2A). p100 and p52 increased translocation could also be supported by an increased p100 synthesis, since increased p100 mRNA levels were observed in double-mutant thymocytes (Figure 2D). Finally, a pulse-chase assay indicated that p100 processing was also increased in Notch3/pTα−/− double mutant with respect to Notch3-IC transgenic thymocytes (Figure 2E), which further supports the increased generation of p52.
Figure 1.
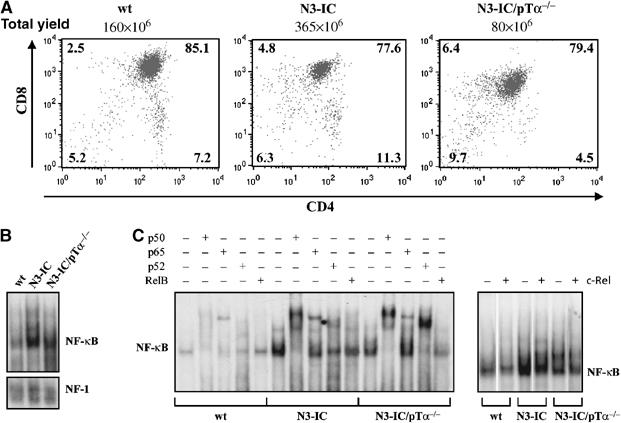
Notch3 constitutively activates NF-κB and the balance between different dimeric complexes depends on the presence of pre-TCR. (A) CD4+ and/or CD8+ subset distribution (detected by CD4 versus CD8 two-color FCA) of thymocytes from 4-week-old wt and Notch3-IC transgenic mice (N3-IC), and 8-week-old Notch3-IC/pTα−/− mice (N3-IC/pTα−/−); (B) EMSA of NF-κB complex in nuclear extracts from freshly isolated thymocytes of wt, N3-IC transgenic and N3-IC/pTα−/− mice. (C) The nuclear extracts were also incubated in the absence (−) or presence (+) of antibodies against individual NF-κB proteins (p50, p65, p52 and RelB, left panel; c-Rel, right panel) to characterize the complexes. An unrelated antibody was used as a negative control (not shown).
Figure 2.
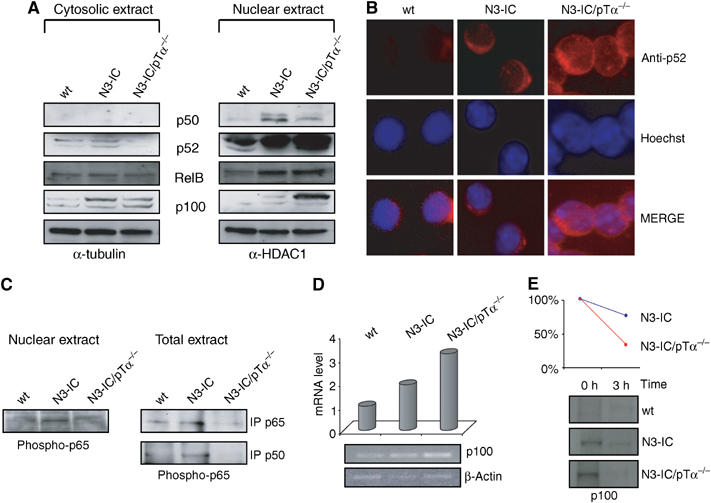
Nuclear translocation of different NF-κB complexes in Notch3 transgenic and Notch3/pTα−/− mice. (A) Western blot analysis of p52, p100, p50 and RelB in cytoplasmic and nuclear extracts from thymocytes of wt, N3-IC transgenic and N3-IC/pTα−/− double-mutant mice. In total, 50 μg of protein was loaded into each lane. (B) Immunofluorescence with anti-p52 antibody (red) and Hoechst staining (blue) of freshly isolated thymocytes from wt, Notch3 transgenic and N3-IC/pTα−/− mice. (C) Nuclear extracts from freshly isolated thymocytes from 4-week-old wt and Notch3 transgenic mice, and 8-week-old N3-IC/pTα−/− mice were revealed by Western blot with an anti-phospho-p65 (left panel). Whole-cell extracts (400 μg) of freshly isolated thymocytes was immunoprecipitated with mouse monoclonal anti-p65 or rabbit polyclonal anti-p50 antibodies and revealed by Western blot with anti-phospho-p65 (right panels). (D) p52/p100 mRNA expression was assayed by RT–PCR from freshly thymocytes derived from wt, Notch3 transgenic and N3-IC/pTα−/− double-mutant mice, β-actin was used as loading control. (E) p100 processing in thymocytes derived from wt, Notch3 transgenic and N3-IC/pTα−/−. Upper panel, graphic representation of p100 processing as determined by densitometry; lower panel, pulse-labeled cells chased for 3 h.
The possible participation of c-Rel to the NF-κB complex in thymocytes of different mice was also analyzed by EMSA. The EMSA shown in Figure 1C (right panel) reveals that NF-κB complexes do not contain significant amount of c-Rel subunit bound to DNA. This observation is in agreement with a previous report showing that c-Rel DNA-binding activity is very weak in thymocyte extracts (Weih et al, 1994).
Together, these data suggest that Notch3 signaling is able to constitutively activate both the canonical and alternative NF-κB activation pathways in thymocytes, and that the relative balance between the two pathways depends on the presence or absence of a funtional pre-TCR.
Notch3 is able to trigger an NIK-independent IKKα-dependent alternative pathway of NF-κB activation in the absence of pTα/pre-TCR
Stimuli that lead to activation, nuclear translocation and DNA binding of NF-κB heterodimers impact on IKK complex, by regulating IKK assembling and activation. In the canonical NF-κB signaling pathway, the activated IKK complex, predominantly acting through IKKβ in an IKKγ-dependent manner, catalyzes phosphorylation, polyubiquitination and subsequent proteosomal degradation of IκBα. We previously showed that IκBα degradation is a critical event in the activation of NF-κB in Notch3-IC transgenic thymocytes (Bellavia et al, 2000). Here we show that IκBα degradation is absent in Notch3-IC/pTα−/− mice, since its protein levels are similar to wild-type thymocytes and significantly higher than in Notch3-IC thymocytes (Figure 3A). The reduced IκBα degradation observed in Notch3IC/pTα−/− thymocytes does not depend on protein resynthesis but possibly to an increased stability. Indeed, blocking protein synthesis with cycloheximide (CHX) for 3 and 6 h, IκBα was more rapidly degraded in Notch3-IC transgenic with respect to double-mutant thymocyte extracts in which, however, a lower degradation rate is appreciated (Figure 3G).
Figure 3.
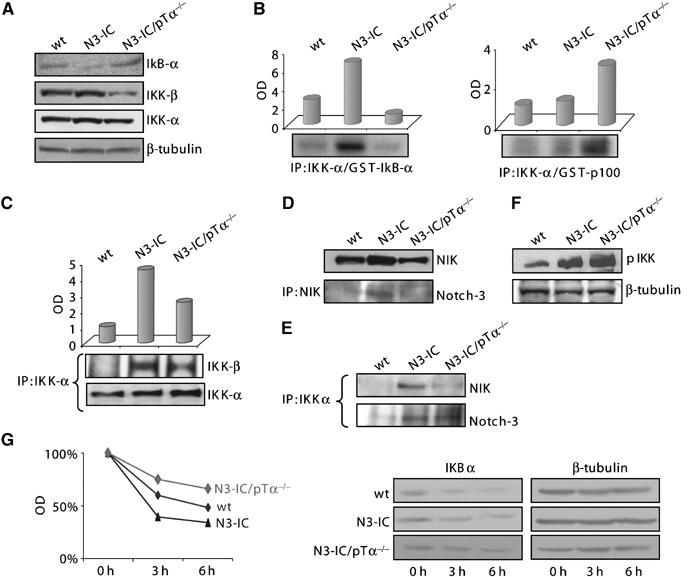
Notch3 regulates the assembling and activation of different IKK complexes, depending on the presence of pre-TCR. (A) Western blot analysis of whole-cell extracts (50 μg) from freshly isolated thymocytes probed with anti-IκBα, IKKβ and anti-IKKα. The β-tubulin expression was used as loading control. (B) In vitro kinase assay using both GST-IκBα (left panel) and GST-p100 (right panel) as exogenous substrates performed on IKKα immunoprecipitation from cell lysates of thymocytes from 4-week-old wt and Notch3 transgenic mice, and 8-week-old N3-IC/pTα−/− mice. The upper panels show a graphic representation of both IκBα and p100 phospho-substrates amount as determined by densitometry. (C) Total cell extracts (400 μg) derived from wt, N3-IC and N3-IC/pTα−/− thymocytes were immunoprecipitated with mouse monoclonal anti-IKKα antibody and revealed in Western Blot with both anti-IKKα and anti- IKKβ antibodies. The upper panel shows a graphic representation of the IKKα/IKKβ complex amount as determined by densitometry. (D) Upper panel, Western blot analysis of whole-cell extracts (50 μg) from freshly isolated thymocytes probed with an anti-NIK antibody. Lower panel, total cell extracts (400 μg) derived from wt, N3-IC and N3-IC/pTα−/− thymocytes, immunoprecipitated with anti-NIK and revealed in Western blot with anti-Notch3 antibody. (E) Total cell extracts (400 μg) derived from wt, Notch3 and N3-IC/pTα−/− thymocytes were immunoprecipitated with mouse monoclonal anti-IKKα antibody and revealed in Western blot with both anti-NIK antibody (upper panel) and anti-Notch3 antibody (lower panel). (F) Western blot analysis of whole-cell extracts (50 μg) from freshly isolated thymocytes probed with anti-phospho-IKKα. The β-tubulin expression was used as loading control. (G) IκBα stability analysis in thymocytes derived from wt, Notch3 transgenic and N3-IC/pTα−/− by cycloheximide (CHX) treatment (right panel). The β-tubulin expression was used as loading control. Graphic representation of IκBα protein half-lives as determined by densitometry (left panel).
In keeping with these observations, the levels of IKKβ are significantly decreased in thymocytes from Notch3-IC/pTα−/− with respect to Notch3-IC mice (Figure 3A, middle panel). In contrast, the lower panel of Figure 3A shows that the levels of IKKα are similar in both Notch3-IC transgenic and Notch3-IC/pTα−/− double-mutant thymocytes. Together, these observations prompted us to study the composition of IKK complex in different mice. Thymocyte extracts were immunoprecipitated with anti-IKKα, followed by immunoblotting with IKKβ and IKKα. Figure 3C shows a decrease of IKKα/IKKβ complex formation in Notch3-IC/pTα−/− thymocytes with respect to Notch3-IC cells, while showing a slight increase of IKKα revealed by immunoblotting with IKKα itself, in the same extracts. The IKKα-mediated phosphorylation of specific IKK complex substrates, IκBα and p100, were also assessed and the panel B of Figure 3 shows a decreased IκBα phosphorylation, associated to an increased p100 phosphorylation in Notch3-IC/pTα−/− thymocytes, further supporting the increased function of an IKKα-dependent alternative NF-κB pathway in the absence of a functional pre-TCR (Figure 3B).
It has been previously shown that the activation of the alternative pathway mainly depends on NF-κB-inducing kinase (NIK) that specifically phosphorylates and activates IKKα, and, upon activation, recruits IKKα into a multiprotein complex, allowing p100 processing to p52 (Xiao et al, 2004). We therefore examined the protein expression levels of NIK and its binding to IKKα in thymocyte extracts obtained from different mice. The Figure 3 shows that thymocytes from Notch3-IC transgenic thymocytes display higher levels of NIK (Figure 3D) as well as increased IKKα/NIK complex (Figure 3E), when compared to wild-type and double-mutant thymocytes.
Thus, unexpectedly, in thymocytes from double-mutant mice, in which we observe a preferential activation of an IKKα-dependent alternative pathway, the protein levels of NIK, as well as its interaction with IKKα, are decreased (Figure 3E). On the other hand, we observed an increased nuclear translocation of p100 (Figure 2A), which has been shown to be also able, in cooperation with activated IKKα, to sustain the processing of p100 to generate p52 (Qing and Xiao, 2005).
The reduced interaction between IKKα and NIK, showed in double-mutant thymocytes extracts (Figure 3E), may also explain the reduction of IKKβ bound to IKKα in IP experiment shown in Figure 3C. Indeed, it has been previously shown that the activation of IKKβ, required to form a functional IKKα/IKKβ heterodimer, requires the recruitment of NIK by IKKα (O'Mahony et al, 2000).
It has been recently reported that Notch1 associates with p56lck and PI3K kinases and increases their phosphorylation in activated T cells (Sade et al, 2004). In order to investigate whether Notch3 was able to act similarly with respect to IKKα, we examined the possible interaction between Notch3 and the IKKα kinase. Figure 3E shows that Notch3 forms a complex with IKKα in the extracts of thymocytes obtained from both Notch3 and Notch3-IC/pTα−/− mice, suggesting that this unmodified contact is pTα/pre-TCR independent. Interestingly, the immunoblotting with an anti-phosphoIKK antibody of thymocyte extracts showed a significant increased phosphorylation in extracts from double-mutant thymocytes (Figure 3F). Given the decrease of IKKα/IKKβ complex formation in Notch3-IC/pTα−/− thymocytes with respect to Notch3-IC cells (Figure 3C), this observation further supports the possible increased activation/phosphorylation of IKKα in double-mutant thymocytes, despite a decreased IKKα/NIK interaction.
Altogether our observations suggest that Notch3 is able to activate canonical and alternative NF-κB pathways by regulating the assembling and activation of different IKK complexes. Both these pathways convey on constitutive NF-κB activation, but differ each other, the first one requiring the presence of a pTα/pre-TCR receptor, which leads mainly to heterodimer IKKα/IKKβ complex formation. The second one is instead pre-TCR-independent and possibly results in the formation of IKKα/IKKα homodimer. Importantly, IKKα forms a complex with Notch3 independently on the presence of pre-TCR (Figure 3E, lower panel), but only the presence of pre-TCR allows NIK to participate at the Notch3-IKKα complex (Figure 3E, upper panel and Figure 3D, lower panel).
The Notch3-induced recruitment of different NF-κB heterodimers on gene promoters depends on the presence of pre-TCR
It has been recently reported that the two NF-κB activation pathways differentially activate a number of genes (Bonizzi and Karin, 2004). The canonical pathway has been suggested to be mainly involved in the transcriptional control of genes involved in the innate immune response, but also in lymphoid cell proliferation and protection from apoptosis, while the alternative pathway plays a central role in the expression of genes involved in the development of secondary lymphoid organs.
The results reported above demonstrate that in the absence of pTα/pre-TCR, Notch3 specifically activates an IKKα-dependent alternative NF-κB activation pathway. Interestingly, we previously reported that enforced expression of Notch3 is effective in inducing T-cell leukemia only in the presence of a functional pre-TCR (Bellavia et al, 2002). On the other hand, in the absence of pTα, Notch3 is able to rescue the blockage of the pre-TCR check-point by forcing immature DN thymocytes to progress toward a more differentiated phenotype, while it is not able to restore the total thymocyte yield (Bellavia et al, 2002). The failure of Notch3-IC/pTα−/− double-mutant mice to drive sufficient thymocyte population expansion, despite a significant rescue of T-cell differentiation, supports a model in which Notch3 and pre-TCR signals cooperate in a nonredundant manner to regulate either proliferation or differentiation of thymocytes and allowed us to hypothesize that the Notch3-induced differential activation of these two pre-TCR-dependent and -independent NF-κB pathways may play a specific role in either thymocyte proliferation/leukemogenesis or differentiation.
To explore this hypothesis we compared the expression levels of genes known to be responsive to NF-κB transcriptional regulation and differentially involved in these processes in thymocytes obtained from wild type, Notch3-IC mutant and Notch3-IC/pTα−/− double-mutant mice. We considered cyclin D1, known for its growth-promoting function in several cancers, Bcl2-A1, an antiapoptotic gene previously described as a pre-TCR-induced and Notch3-increased regulator of thymocyte survival (Bellavia et al, 2000; Mandal et al, 2005); IL7Rα, known to play a critical role in early thymocyte development (Peschon et al, 1994). Figure 4 shows that all of these genes are significantly upregulated (from 3.5- up to 7.5-fold of increase) in thymocytes of transgenic with respect to wild-type mice. In contrast, such an effect was variably influenced by the absence of pre-TCR. Indeed, while the levels of cyclin D1 (Figure 4A) completely returned to the wild-type levels, the levels of IL7Rα (Figure 4C) were further increased whereas the levels of Bcl2A1 (Figure 4B) remained at an intermediate level in thymocytes from Notch3-IC/pTα−/− double mutant with respect to Notch3-IC transgenic and wild-type mice.
Figure 4.
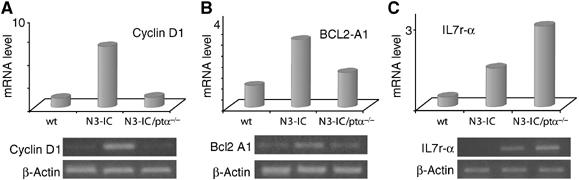
NF-κB target genes are differentially regulated by Notch3 depending on the presence or absence of pre-TCR. (A) Cyclin D1, (B) Bcl2 A1 and (C) IL7 receptor alpha expression was analyzed by RT–PCR from primary thymocytes derived from wt, Notch3 transgenic and N3-IC/pTα−/− double-mutant mice. β-Actin was used as loading control.
To address whether the different genes are direct targets for different pre-TCR-dependent or -independent NF-κB dimers, we performed chromatin immunoprecipitation (ChIP) experiments (Saccani and Natoli, 2002).
Figure 5 shows that the recruitment of p65, responsible for the strongest transcriptional potential of canonical NF-κB complex (Chan et al, 2005), to the promoters of those different genes is readily detected in chromatin extracts of Notch3 transgenic thymocytes, further supporting the hypothesis that in the presence of pre-TCR Notch3 preferentially activates the canonical NF-κB pathway. In contrast, in the absence of pre-TCR, p65 is never recruited to the different gene promoters, once again supporting the hypothesis that p65 does not bind DNA in the absence of pre-TCR, possibly because it cannot be phosphorylated (Figure 2C). Under these conditions, p52 appears to be the main NF-κB transcriptional complex component, since this protein is always recruited to the promoter of different genes in thymocytes from Notch3/pTα−/− double-mutant mice, confirming the constitutive processing of p100 induced by the activation of Notch3 in the absence of pre-TCR. However, while RelB is also recruited on the promoter of IL7rα and Bcl2A1 genes in thymocytes from double-mutant mice, it is not recruited onto the cyclin D1 promoter (Figure 5A). This suggests an increased formation of activatory RelB/p52 heterodimers, thus justifying the increased IL7rα and Bcl2A1 mRNA levels with respect to the wild-type samples. On the other hand, p52, in the absence of RelB, might form only inhibitory homodimers in cyclin D1 promoter justifying the lack of increase of cyclin D1 mRNA levels in double-mutant thymocytes. Interestingly, the fact that the IL7rα mRNA levels are higher in thymocytes from double-mutant mice in which only RelB/p52 dimers are recruited to the promoter, with respect to transgenic thymocytes in which both p50/p65 and RelB/p52 are recruited may suggest some antagonism between the different heterodimers. However, we cannot rule out that the final transcriptional effect on different gene expression depends from the interaction with transcription factors other than NF-κB.
Figure 5.
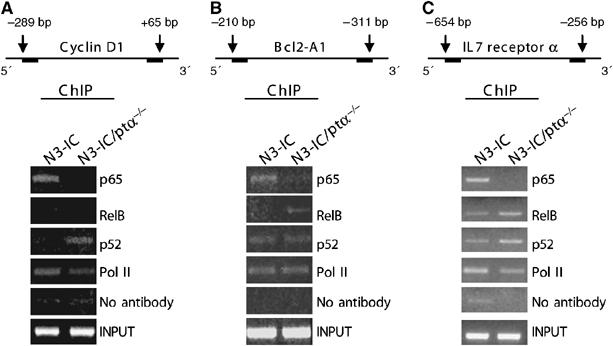
Notch3 induces the recruitment of different NF-κB complexes on gene promoters, depending on the presence of pre-TCR. Schematic representation of (A) cyclin D1, (B) Bcl2 A1 and (C) IL7 receptor α promoter regions assessed by chromatin immunoprecipitation are indicated. Primary T cells derived from Notch3 and N3-IC/pTα−/− mice were processed by using antibodies directed against p65, RelB, p52, and RNA polymerase II (pol II) or no antibody as control. Immunoprecipitated DNA was analyzed by PCR with (A) cyclin D1, (B) Bcl2 A1 and (C) IL-7 receptor α promoter-specific primers. The amount of input promoter DNA is also shown. The results are representative of three similar experiments.
Discussion
Our findings show that Notch3 is able to trigger both canonical and alternative NF-κB activation pathways, known to determine selective activation of different NF-κB dimers, dependent on IKKβ or IKKα kinase activity respectively. We demonstrate that the ability of Notch3 to trigger either NF-κB pathways depends on the presence or the absence of pTα/pre-TCR function, respectively. Therefore, we hypotesize that each pathway, by differentially regulating several genes, could mediate both the T-cell oncogenic (Bellavia et al, 2000) and/or differentiative (Bellavia et al, 2002) potential of Notch3.
The Notch3-induced pre-TCR-dependent NF-κB pathway might be mediated by PKCθ since our previously reported observations showed that PKCθ is a downstream target of Notch3-signaling and that its Notch-dependent membrane translocation and activation, via Lck, require a functional pre-TCR in order to trigger the IKKβ-dependent NF-κB canonical pathway (Felli et al, 2005). Most importantly, deletion of PKCθ decreases such an activation as well as the incidence of leukemia (Felli et al, 2005).
In the present report, we provide genetic and biochemical evidence that Notch3, when devoided of the functional cooperation with pTα/pre-TCR signaling, is able to induce constitutive activation of an alternative NF-κB pathway and suggest that this may have biological consequences. Indeed, we previously reported that deletion of pTα gene was able to abrogate tumor development in Notch3-IC transgenic mice (Bellavia et al, 2002), but, in contrast, Notch3 was able to overcome the differentiative blockade of thymocytes due to the absence of pre-TCR.
Overall, these data, together with our previous observation that Notch3 is able to transcriptionally activate pTα (Talora et al, 2003), allow us to speculate that Notch3 may sequentially activate firstly the alternative pathway of NF-κB to sustain thymocyte survival and early intrathymic differentiation steps before the appearance of pTα, then, being able to trigger the transcription of pTα, it may cooperate with pre-TCR to activate the canonical pathway of NF-κB, mediate the thymocyte expansion and allow further differentiation.
The alternative pathway, independent of IKKβ and IKKγ, acts in a way strictly dependent on IKKα homodimer, which represents the direct target of Notch3 in the absence of pre-TCR. Indeed, in a pTα/pre-TCR deprived environment, Notch3 forms a complex with IKKα kinase, independently on NIK. This interaction results in enhanced IKKα kinase activity followed by a higher p100 substrate processing, and release of p52/RelB complex. Moreover, a high nuclear translocation of p52/RelB is observed, which was also supported by DNA-binding assay showing that NF-κB complex is mainly composed of p52 and RelB subunits in Notch3-IC/pTα−/− double-mutant thymocytes. This specific NF-κB composition may be responsible for the restored T-cell development, observed in Notch3-IC/pTα−/− double-mutant mice, beyond the DN developmental stage at which thymocytes are usually blocked in pTα−/− mice not overexpressing Notch3 (Fehling et al, 1995; Bellavia et al, 2002). In keeping with this hypothesis, many reports describe that the heterodimer p52/RelB is selectively recruited to the IKKα-dependent gene promoters of genes playing a crucial role in the organization of secondary lymphoid organs (Bonizzi et al, 2004; Bonizzi and Karin, 2004). Accordingly, we observed that in chromatin extracts of thymocytes of Notch3/pTα−/− double-mutant mice, p52/RelB heterodimer is recruited to the promoter of the prosurvival Bcl2A1 gene and of the IL7rα gene, which has been shown to play a crucial role in the early stages of intrathymic T-cell differentiation, while it is not recruited to the cyclin D1 promoter, which is more specifically associated to the triggering of cell proliferation in different cancers. RelB could also be complexed with p50 in Notch3-IC/pTα−/− thymocytes. However, it has been previously shown that in response to some stimuli (i.e. TNF-α), the association of p100 with RelB/p50 dimers in the nucleus increases, leading to the specific inhibition of RelB DNA binding (Derudder et al, 2003). The significant increase of p100 together with the decrease of p50 nuclear translocation, we observed in double-mutant cells, when compared to transgenic thymocytes, allowed us to exclude that RelB/p50 dimers are recruited to gene promoters in Notch3-IC/pTα−/− thymocytes. Upregulation of p100 might additionally display a proapoptotic and antioncogenic function (Wang et al, 2002) in Notch3/pTα−/− double-mutant mice and antagonize in this way tumorigenesis.
Overall, our previous (Bellavia et al, 2000; Felli et al, 2005) and present data suggest that pTα/pre-TCR can act as positive mediator in Notch3-induced canonical NF-κB activation pathway, leading to the generation of p50/p65 heterodimer. Indeed, we show here an increase of IκBα substrate phosphorylation as well as of phosphorylated p65 nuclear translocation in Notch3-IC transgenic with respect to wt and Notch3/pTα−/− double-mutant animals. The abrogation of pTα/pre-TCR function instead drastically reduces the activation of the canonical pathway, by switching on the activation of an alternative pathway that is strictly dependent on IKKα activity and independent on NIK. Therefore, we provide a model (Figure 6) by which Notch3 keeps NF-κB constitutively active both in the presence and in the absence of membrane associated pre-TCR. Either condition may result in the preferential generation of different IKK complexes, differentially requiring the cooperation of NIK and resulting in the activation of p50/p65 and/or p52/RelB complex followed by the transcriptional regulation of different NF-κB target genes, which may direct thymocytes toward further differentiation or neoplastic transformation outcomes.
Figure 6.
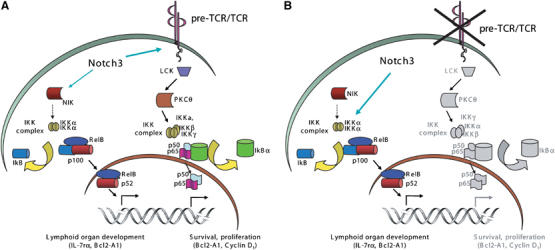
Speculative model of the mechanisms by which Notch3 induces the activation of different NF-κB pathways. (A) Outline of the molecular events triggered by Notch3 in the presence of a functional pre-TCR. Signals arising from Notch3 and pTα/pre-TCR cooperation mainly involve the β and γ subunits of the IKK complex, via PKCθ, resulting in degradation of IκBα, nuclear translocation and DNA binding of p50/RelA heterodimers. These molecular events are strictly associated to canonical NF-κB activation pathway and result in prosurvival/proliferative gene activation. In the presence of pre-TCR, Notch3 also increases the association between IKKα and NIK, possibly leading to a further increase of the overall NF-κB activation, via the non-canonical pathway. (B) In the absence of pre-TCR Notch3 switches on another pathway, directly interacting with and upregulating IKKα function, which enhances the phosphorylation level of p52 precursor, p100, and results in a higher p52/RelB heterodimer nuclear translocation. As a consequence, p52/RelB heterodimers accumulate in the nucleus and regulate genes that are crucial for the survival and differentiation of thymocytes.
Materials and methods
Mice
The generation and typing of Notch3-IC transgenic (Bellavia et al, 2000) and Notch3-IC/pTα−/− double-mutant (Bellavia et al, 2002) mice have been described.
Immunoprecipitation
Freshly isolated thymocytes were lysed in 20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA pH 8.0, 30 mM NaF, 2 mM Na-piro-Phosphate, 1 mM Na-orthovanadate (Na3VO4), Protease inhibitor cocktail tablets (Roche, Penzberg, Germany). In total, 300–500 μg of total proteins in lysates from each sample were immunoprecipitated with antibodies against p50 (sc-8414 Santa Cruz Biotechnology, Inc.), p65 (#3034, Cell Signaling), IKKα (IMG-136, IMGENEX) and NIK (sc-7211, Santa Cruz Biotechnology, Inc.) coniugated to A/G plus-agarose (sc-8014, Santa Cruz Biotechnology, Inc.).
Immunoblotting
For immunoblotting, proteins were resolved by 10% SDS–PAGE and blotted onto nitrocellulose membrane. Blots were incubated with the indicated primary antibodies, carefully washed and after incubation with horseradish peroxidase-labeled goat-antirabbit or goat-antimouse (Amersham, Arlinghton Heights, IL), developed with the ECL detection system (Amersham). Blots were incubated with antibodies directed against IKKα (IMG-136, IMGENEX), IKKβ (IMG-159, IMGENEX), p50 (#06–886, Upstate), p65 (#3034, Cell Signaling), p52/p100 (sc 298, SantaCruz Biotechnology Inc.), p-IKKα/β (Thr 23-R) (sc-21660-R, Santa Cruz Biotechnology, Inc.), RelB (sc-c19, Santa Cruz Biotechnology, Inc.), IκBα (sc-4094, Santa Cruz Biotechnology, Inc.), NIK (sc-7211, Santa Cruz Biotechnology, Inc.) and Notch3 (sc-20 Santa Cruz Biotechnology, Inc.). The levels of phospho-p65 in the immunoprecipitated samples were detected using an anti-phospho-p65 (#3031, Cell Signaling).
RT–PCR analysis
Total RNA was isolated from thymocytes in guanidine isothiocyanate and further processed for reverse transcriptase (RT)–PCR as previously described (Bellavia et al, 2000). PCR was performed using the following primers: IL7-rα Forward, 5′ CGAGTGAAATGCCTAACTC 3′; IL7-rα Reverse, 5′ GCGTCCAGTTGCTTTCAC 3′; p100 Forward, 5′ GCCTGGATGGCATCCCCG 3′; p100 Reverse, 5′ CTTCTCACTGGAGGCACCT 3′; Bcl2-A1 Forward, 5′ ATTCCAACAGCCTCCAGATATG 3′; Bcl2-A1 Reverse, 5′ GAAACAAAATATCTGCAACTCTGG 3′; Cyclin D1 Forward, 5′ CGTGCAGAAGGAGATTGTGC 3′; Cyclin D1 Reverse, 5′ GTCTGCTTGTTCTCATCCGC 3′.
Cell labelling and pulse-chase assay
Freshly isolated thymocytes were starved for 1 h in DMEM lacking methionine and cysteine and followed by metabolically labelling for 45 min with 350 μCi 35S-labeled methionine/35S-labeled cysteine. The pulse-labeled cells were chased for 3 h in complete DMEM supplemented with 1 mg/ml cold methionine/cysteine and lysed in HNTG buffer (50 mM HEPES pH 7.5, 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 10% glycerol, supplemented with a protease inhibitor cocktail). The radiolabeled p100 and p52 were isolated by immunoprecipitation using anti-p52 (sc-298, Santa Cruz Biotechnology Inc.), fractionated by SDS–PAGE and visualized by autoradiography.
Cycloheximide assay
To inhibit protein synthesis freshly isolated thymocytes were cultured in RPMI in the presence of 10 μg/ml CHX for various time lengths. Total cell lysates were fractionated by SDS–PAGE and subjected to immunoblot analysis with anti-IκBα (sc- 847, Santa Cruz Biotechnology Inc.).
Generation of recombinant pGEX plasmids
Glutathione S-transferase (GST)-NF-κB2 (p100) fusion protein was created by insertion of p100 nucleotides encoding amino acids 1–899 into the PGEX-4T vector (Pharmacia, Uppsala, Sweden) between EcoR1 sites in frame with GST. The fusion protein construct uses the p100 stop codon.
Purification of GST-p100 proteins from Escherichia coli
GST fusion protein expression and purification were performed essentially as described by Smith and Johnson (1988). Briefly, overnight cultures of E.coli (BL21strain) trasformed with either pGEX-4T or pGEX-4T recombinants were diluted 1/10 in LB medium with ampicillin (100 μg/ml Sigma Sigma Chemical Co., Poole, Dorset, UK) and incubated a 37°C. After 4 h of growth isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma Chemical Co., Poole, Dorset, UK) was added at a final concentration 0.5 μmol/l, and the cultures were incubated for 4 h a 30°C. The bacterial cultures were pelleted by centrifugation at 5000 g for 5 min at 4°C and resuspended in 1/10 volume of NTEN (20 μmol/l Tris pH 8, 100 μmol/l NaCl, 1 μmol EDTA, 0.5% NP-40). The bacteria were then lysed by mild sonication and centrifuged at 10 000 g for 10 min at 4°C. An aliquot of 25 μl of glutathione-Sepharose 4B (1:1 vol/vol in NTEN containing 1% powdered milk; Pharmacia) was added to each milliliter of the bacterial supernatant, and the suspension was gently rocked for 30 min at 4°C. The glutathione-Sepharose beads were then washed three times with NETN and GST-fusion proteins were eluted with 15 μmol/l of reduced glutathione (Sigma) in 50 μmol/l Tris–HCl. pH 8. For analysis eluted proteins were loaded onto SDS–polyacrylamide gels and visualized by Coomassie blue staining.
IKK-α kinase assay and immunoblotting analysis
Thymocytes were lysed in buffer containing 20 mM Tris pH 7.6, and 0.5% NP-40, 0.25 M NaCl, 3 mM EDTA, 3 mM EGTA, 20 mM NaF, 2 mM Na3VO4, 1 mM dithiothreitol, 100 μg/ml leupeptin, 20 μg/ml apronitin and 1 mM phenylmethylsulfonyl fluoride (PMFS) and immunoprecipitated with anti-IKKα (IMG136 IMGENEX) coniugated to protein A/G plus-agarose (sc-8014, Santa Cruz Biotechnology, Inc.). The immunopellets were incubated with a kinase buffer master mix supplemented with 20 μM ATP, 5 μCi of [γ-32P]ATP and 3 μg of GST-IκBα substrate (sc-4094, Santa Cruz Biotechnology, Inc.) or 3 μg GST-p100 substrate at 30°C for 30 min. The samples were analysed by 10% SDS–PAGE and the phosphorylation status of GST-IκBα and GST-p100 substrates was detected by autoradiography.
Electrophoretic mobility shift assay
Nuclear extracts were prepared as previously described (Bellavia et al, 2000). Unfractionated thymocytes were derived from 5 weeks old wt and Notch3-IC transgenic mice and from 6 to 8 weeks old Notch-IC/pTα−/−. α 32P dATP-labeled double-stranded oligonucleotide spanning the NF-κB site (5′-GATCCAACGGCAGGGGAATTCCCCTCTCCTTA-3′) was incubated with 5 μg of nuclear extract at RT for 20 min with, 2 μg of poly(dI-dC) in 50 mM NaCl, 10 mM Tris pH 7.5, 1 mM DTT and 20% glycerol. Band shifts were resolved on non-denaturing 4% polyacrylamide gel. Antibodies against p50 (sc-114X), p65 (sc-7151), RelB (sc-226), c-Rel (N sc-70) (Santa Cruz Biotechnology, Inc.), and p52 (#413, Upstate Biotechnology, Lake Placid) were incubated for 20 min at RT before the binding reaction.
Chromatin immunoprecipitation assay
Protein complexes were crosslinked to DNA in living nuclei by adding formaldehyde (Sigma, Inc.) directly to freshly isolated thymocytes to a final concentration of 1%. Crosslinking was allowed to proceed for 10 min at 37°C and was then stopped by the addition of glycine to a final concentration of 0.125 M. Crosslinked cells were washed twice with phosphate-buffered saline containing PMSF 1 mM and pelletted. Nuclei were extract with a 20 mM Tris pH 8, 3 mM MgCl2, 20 mM KCl buffer containing protease inhibitors, pelleted by microcentrifugation and lysed by incubation in SDS lysis buffer (1% sodium dodecyl sulfate, 10 mM EDTA, 50 mM Trischloride pH 8.1), containing protease inhibitors. The resulting chromatin solution was sonicated for 15 pulses of 15 s at 80% power to generate 300–1000 bp DNA fragments. After microcentrifugation, the supernatant was diluted 1:10 with a dilution buffer (0.01% sodium dodecyl sulfate, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Trischloride pH 8.1, 167 mM NaCl, containing protease inhibitors), precleared with Salmon Sperm DNA/Protein A agarose (#157, Upstate Biotechnology), and divided into aliquots. In total, 5 μg of antibody (anti-p52 sc-298, anti-p65 sc-h286, anti-RelB sc-226, anti-Pol II sc-899, Santa Cruz Biotechnology Inc.) was added to each aliquot of chromatin and incubated on a rotating platform for 12–16 h at 4°C. Antibody–protein–DNA complexes were isolated by immunoprecipitation with Salmon Sperm DNA/Protein A agarose (#157, Upstate Biotechnology). Following extensive washing, bound DNA fragments were eluted and analyzed by subsequent PCR using primers specific for the cyclin D1, Bcl2 A1 and IL7rα mouse promoters.
Immunofluorescence
Cytospin preparations of thymocytes from wt, Notch3-IC transgenic and double-mutant Notch3-IC/pTα−/− mice were fixed in 4% paraformaldehyde for 20 min at room temperature, incubated in 0.2% Triton X-100 to permeabilize cells and then incubated in blocking buffer (PBS with 3% BSA). Primary antibody was a mouse monoclonal anti-p52 (sc-7386, Santa Cruz Biotechnology Inc.). Secondary antiboby was Texas-RED-conjugated anti-mouse Ig (Jackson Immuno Research Laboratories). All slides were examined by confocal microscopy.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), the Ministero della Salute and the Biologia e Medicina Molecolare (BEMM) Center of Excellence.
References
- Aifantis I, Gounari F, Scorrano L, Borowski C, von Boehmer H (2001) Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-kappaB and NFAT. Nat Immunol 2: 403–409 [DOI] [PubMed] [Google Scholar]
- Bellavia D, Campese AF, Alesse E, Vacca A, Felli MP, Balestri A, Stoppacciaro A, Tiveron C, Tatangelo L, Giovarelli M, Gaetano C, Ruco L, Hoffman ES, Hayday AC, Lendahl U, Frati L, Gulino A, Screpanti I (2000) Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J 19: 3337–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia D, Campese AF, Checquolo S, Balestri A, Biondi A, Cazzaniga G, Lendahl U, Fehling HJ, Hayday AC, Frati L, von Boehmer H, Gulino A, Screpanti I (2002) Combined expression of pTalpha and Notch3 in T cell leukemia identifies the requirement of preTCR for leukemogenesis. Proc Natl Acad Sci USA 99: 3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M (2004) Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J 23: 4202–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288 [DOI] [PubMed] [Google Scholar]
- Chan C, Li L, McCall CE, Yoza BK (2005) Endotoxin tolerance disrupts chromatin remodeling and NF-kappaB transactivation at the IL-1 beta promoter. J. Immunol 175: 461–468 [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR (2002) The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17: 525–535 [DOI] [PubMed] [Google Scholar]
- Derudder E, Dejardin E, Pritchard LL, Green DR, Korner M, Baud V (2003) RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. J Biol Chem 278: 23278–23284 [DOI] [PubMed] [Google Scholar]
- Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H (1995) Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature 375: 795–798 [DOI] [PubMed] [Google Scholar]
- Felli MP, Vacca A, Calce A, Bellavia D, Campese AF, Grillo R, Di Giovine M, Checquolo S, Talora C, Palermo R, Di Mario G, Frati L, Gulino A, Screpanti I (2005) PKC theta mediates pre-TCR signaling and contributes to Notch3-induced T-cell leukemia. Oncogene 24: 992–1000 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18: 2195–2224 [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2: 301–310 [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3: 221–227 [DOI] [PubMed] [Google Scholar]
- Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, Ruiz-Vela A, Ciofani M, Zuniga-Pflucker JC, Screpanti I, Look AT, Korsmeyer SJ, Rajewsky K, von Boehmer H, Aifantis I (2005) The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med 201: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony A, Lin X, Geleziunas R, Greene WC (2000) Activation of the heterodimeric IkappaB kinase alpha (IKKalpha)-IKKbeta complex is directional: IKKalpha regulates IKKbeta under both basal and stimulated conditions. Mol Cell Biol 20: 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL (1994) Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180: 1955–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing G, Xiao G (2005) Essential role of IkappaB kinase alpha in the constitutive processing of NF-kappaB2 p100. J Biol Chem 280: 9765–9768 [DOI] [PubMed] [Google Scholar]
- Saccani S, Natoli G (2002) Dynamic changes in histone H3 Lys 9 methylation occurring at tightly regulated inducible inflammatory genes. Genes Dev 16: 2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade H, Krishna S, Sarin A (2004) The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem 279: 2937–2944 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293: 1495–1499 [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67: 31–40 [DOI] [PubMed] [Google Scholar]
- Talora C, Campese AF, Bellavia D, Pascucci M, Checquolo S, Groppioni M, Frati L, von Boehmer H, Gulino A, Screpanti I (2003) Pre-TCR-triggered ERK signalling-dependent downregulation of E2A activity in Notch3-induced T-cell lymphoma. EMBO Rep 4: 1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui H, Schroering A, Ding JL, Lane WS, McGill G, Fisher DE, Ding HF (2002) NF-kappa B2 p100 is a pro-apoptotic protein with anti-oncogenic function. Nat Cell Biol 4: 888–893 [DOI] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Bravo R (1994) Constitutive and inducible Rel/NF-kappaB activities in mouse thymus and spleen. Oncogene 9: 3289–3297 [PubMed] [Google Scholar]
- Xiao G, Fong A, Sun SC (2004) Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase α (IKKα) to p100 and IKKα-mediated phosphorylation. J Biol Chem 279: 30099–30105 [DOI] [PubMed] [Google Scholar]
- Yilmaz ZB, Weih DS, Sivakumar V, Weih F (2003) RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J 22: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]


