Abstract
In a previous study, we identified an interesting mutant form of the Tax protein of bovine leukemia virus (BLV), designated D247G. This mutant protein strongly transactivated the long terminal repeat of BLV and was also able to transactivate the cellular proto-oncogene c-fos. This finding suggested that BLV that encode the mutant protein might propagate and induce lymphoma more efficiently than wild-type BLV. To characterize the effects of the strong transactivation activity of the mutant Tax protein, we constructed an infectious molecular clone of BLV that encoded D247G and examined the replication and propagation of the virus in vitro and in vivo. Cultured cells were transfected with the wild-type and mutant BLV, and then levels of viral proteins and particles and the propagation of viruses were compared. As expected, in vitro, mutant BLV produced more viral proteins and particles and was transmitted very effectively. We injected the wild-type and mutant BLV into sheep, which are easily infected with BLV, and monitored the proportion of BLV-positive cells in the blood and the expression of BLV RNA for 28 weeks. By contrast to the results of our analyses in vitro, we found no significant difference in the viral load or the expression of viral RNA between sheep inoculated with wild-type or mutant BLV. Our observations indicate that the mutant D247G Tax protein does not enhance the expansion of BLV and that there might be a dominant mechanism for regulation of the expression of BLV in vivo.
Bovine leukemia virus (BLV), a retrovirus related to human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2), causes enzootic bovine leukosis, a disease characterized by a very extended course that often involves persistent lymphocytosis and culminates in B-cell lymphoma (5). Under experimental conditions, sheep can easily be infected with BLV and tend to develop B-cell leukemia/lymphoma at higher frequencies and after shorter latent periods than cattle (3, 8).
In addition to the involvement of the classical structural genes of retroviruses, BLV has a region, designated pX and located between the env gene and the 3′ long terminal repeat (LTR), that contains at least seven open reading frames (21). Two well-characterized proteins encoded by the pX region, Tax and Rex, are involved in regulation of the expression and replication of BLV (6, 7). The Tax protein acts on a triplicate 21-bp enhancer motif, known as the Tax-responsive element, in the U3 region of the 5′ LTR and stimulates transactivation of the viral genome (6, 29). The Tax-responsive element consists of a sequence that resembles a cyclic AMP response element, and it has been suggested that Tax binds indirectly to this element through a member of the CREB/ATF family (1, 2, 4, 31). Furthermore, the Tax protein can cooperate with the Ha-Ras oncoprotein to induce full transformation of primary rat embryo fibroblasts (30), an observation that suggests that Tax not only activates the replication of BLV but also contributes to the induction of lymphoma. The Tax protein of HTLV-I, which is a structural and functional homolog of BLV Tax, modulates the expression and function of many cellular proteins that are involved in the regulation of cell growth (35). By contrast, little is known about the target genes that are activated by BLV Tax and the cellular factors that interact with BLV Tax.
Our group has identified various mutants of BLV Tax that stimulate viral LTR-directed transcription more effectively than wild-type Tax (25). Each of the mutants has at least one missense mutation between residues 240 and 265, and a single missense mutation in this region of Tax is sufficient for enhanced activity of the mutant Tax protein. In addition to the LTR of BLV, other retroviral enhancers, such as the enhancers of HTLV-I and of mouse mammary tumor virus, which cannot be activated by wild-type BLV Tax protein, are activated by one of these mutant Tax proteins (25). Furthermore, we demonstrated recently that a mutant Tax protein but not wild-type Tax activated the upstream sequence of the human c-fos gene, and the mutant Tax also raised levels of endogenous c-fos mRNA considerably in both human and bovine cell lines, whereas wild-type Tax did not activate the expression of human c-fos (26). The enhanced activity of such a mutant Tax protein might be due to alterations in the interactions between Tax and cellular factors as a result of the change in the amino acid sequence between amino acids 240 and 265. However, the mechanism by which such mutations increase the activity of Tax remains unknown.
Our findings raise the possibility that BLV that encodes a mutant form of Tax might produce more viral proteins and particles, activating the cellular factors that are involved in regulation of cell growth and inducing lymphoma more rapidly and more frequently than the wild-type virus. However, no mutant forms of Tax with elevated transactivation activity have been isolated from naturally BLV-infected but clinically healthy cattle or cattle with enzootic bovine leukosis (25). Thus, it is possible that mutant Tax proteins with elevated transactivation activity might be disadvantageous for the survival and expansion of BLV during immunological attack by the host's immune system.
To clarify the effects of a mutant Tax protein with increased transactivation activity on the replication and propagation of BLV in vivo and in vitro, we constructed an infectious molecular clone of BLV (pBLV-IFD247G) that encoded the mutant Tax D247G, which has elevated transactivation activity. We observed elevated levels of viral proteins and viral particles and increased rates of cell-to-cell transmission in the case of cells transfected with pBLV-IFD247G in vitro. By contrast to the results in vitro, we found no differences in the proportion of BLV-positive cells and the expression of viral RNA between sheep inoculated with pBLV-IFWT and those inoculated with pBLV-IFD247G. Thus, it appears that Tax with elevated transactivation activity is not advantageous for the expansion of BLV in vivo. Our results also suggest that there might be a mechanism for regulating BLV LTR-directed transcription whose effects dominate the activation of LTR by Tax and play an important role in viral silencing in vivo.
MATERIALS AND METHODS
Construction of plasmids.
The wild-type and mutant infectious molecular clones of BLV, pBLV-IFWT and pBLV-IFS240P, respectively. have been described previously (10, 25). To construct the pBLV-IFD247G mutant infectious molecular clone of BLV which encoded a mutant Tax protein with elevated transactivation activity, we replaced the ClaI-Eco47III sequence in the Tax coding region (amino acids 26 to 272) of the wild-type clone pBLV-IFWT with the ClaI-Eco47III sequence in pME18Neo that encoded the D247G mutant of BLV Tax (25) (Fig. 1A). pGV-BLTR (25), which included the firefly gene for luciferase driven by a BLV LTR, was used for the analysis of transactivation activity. pRL-SV40 (Promega, Madison, Wis.) includes a gene for luciferase from Renilla and was used for normalization of efficiencies of transfection (25). pME18Neo and pBluescript II SK(+) (Stratagene, La Jolla, Calif.) were used as negative control plasmids.
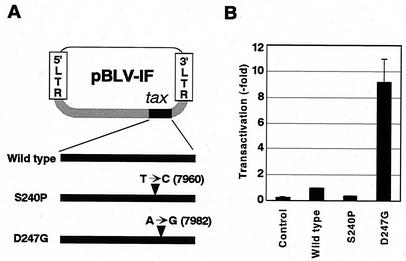
FIG. 1.
(A) Schematic representation of pBLV-IF encoding wild-type and mutant Tax. Positions of nucleotide substitutions are indicated by arrowheads. Number in parentheses correspond to positions in the sequence determined by Sagata et al. (22). (B) Transactivation activities of wild-type and mutant Tax proteins encoded by infectious clones of BLV. 293T cells were transfected with the reporter plasmid pGV-BLTR, the reference plasmid pRL-SV40, and individual BLV molecular clones or the control plasmid pBluescript II SK+. At 48 h after transfection, cells were recovered, and the activities of firefly and Renilla luciferases were measured in cell lysates. For each sample, the firefly luciferase activity (pGV-BLTR) was normalized by reference to the Renilla luciferase activity (pRL-SV40). The values are means and standard deviations (error bars) of results from three independent experiments.
To construct plasmids that expressed BLV Tax, we amplified tax genes from sheep inoculated with the infectious molecular clones by PCR with primers BtaxF and BtaxR and introduced the products of PCR into the XhoI and XbaI sites of pME18Neo, as described previously (25). Four clones from each sheep were used for luciferase assays, as described below, and were also sequenced with a BigDye Terminator cycle sequencing kit and a genetic analyzer (ABL Prism 310; PE Applied Biosystems, Norwalk, Conn.) as described previously (25).
Cells and transfection.
293T cells (human embryonic kidney cells that express the large T antigen of simian virus 40 [SV40]), FLK cells (fetal lamb kidney cells), FLK-BLV cells (FLK cells infected with BLV), and CC81 cells (cat cells transformed with mouse sarcoma virus) were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin, and streptomycin. For Western blotting, 293T cells (107) were transfected with 20 μg of a BLV molecular clone and 2 μg of pRL-SV40 by electroporation, as described previously (25). For analysis of luciferase activity, 293T cells (3 × 105/well) were transfected with 1 μg of pGV-BLTR, 1 μg of an infectious BLV clone, and 0.3 μg of pRL-SV40 as described elsewhere (23).
Luciferase assays.
At 60 h after transfection, 293T cells were harvested and subjected to assays of luciferase activity as described previously (23).
Western blot analysis.
Viral proteins of BLV in cells and growth medium were detected by Western blotting with serum from sheep that had been infected with BLV, as described previously (25).
RT assay.
Viral particles that had been secreted into the growth medium from cells transfected with BLV clones were concentrated and subjected to the reverse transcription (RT) reaction as described elsewhere (10).
Fluorescence microscopy.
Cell smears were fixed in a mixture of acetone and methanol (1:1, vol/vol) at −20°C for 15 min. For detection of BLV antigens, fixed cell smears were incubated for 30 min at room temperature with serum from a BLV-infected sheep (11) and then treated with fluorescein isothiocyanate-conjugated) rabbit antibodies against the F(ab′)2 fragment of sheep immunoglobulin G (Cappel, Cochranville, Pa.) for 30 min. Samples were mounted in phosphate-buffered saline that contained 90% glycerol and examined with a fluorescence microscope (Provis AX; Olympus, Tokyo, Japan).
Syncytium formation assay.
At 24 h after transfection, CC81 cells transfected were replated at 8 × 104 cells/well in 12-well culture plates and cultured for an additional 2 days in RPMI 1640 medium supplemented with 4 mg of Polybrene (Sigma, St. Louis, Mo.) per ml. The cells were fixed and stained, and then the number of syncytia and the number of nuclei in each syncytium were determined as described previously (10). A total of 2 × 105 peripheral blood mononuclear cells (PBMC) isolated from sheep blood were mixed with 105 indicator CC81 cells/well in 24-well culture plates and then cultured for 72 h in complete medium. The PBMC in the growth medium were removed. Then the CC81 cells were fixed and stained, and the syncytia were counted.
Serial passage of cells after transient transfection with infectious BLV clones.
Twenty-four hours after transfection, CC81 and FLK cells were replated in 10-cm-diameter dishes and cultured in RPMI 1640 medium supplemented with 4 mg of Polybrene per ml. Cultured cells were serially passaged at 4-day intervals. Aliquots of cells were examined for the formation of syncytia by indirect immunofluorescence microscopy.
Animals, preparation of liposomes, and injection of infectious BLV clones into sheep.
Fifteen sheep (as shown in Table 1) which were negative for BLV-specific antibodies were purchased from Japan Lamb Company (Hokkaido, Japan). For experimental infections in 1999 (experiment 1), 100 μg of plasmid DNA were entrapped by DOTAP liposomal transfection reagent (Roche Diagnostics, Mannheim, Germany) and injected at three separate sites in the back and shoulders, as described elsewhere (32). For injections in 2000 (experiment 2), 200 μg of plasmid DNA was entrapped by the mixture of N-(α-trimethylammonioacetyl)-didodecyl-d-glutaate chloride (1 μmol), dilauroyl phosphatidylcholine (2 μmol), and dioleoyl phosphatidylethanolamine (2 μmol), as described previously (27), and injected as described above.
TABLE 1.
Injection of sheep with pBLV-IF encoding wild-type or D247G Taxa
| Expt no. | Injected BLV clone | Sheep no. | Sex | WBC/mm3 | PBL/mm3 | Age at injection (yr) | Provirus detectedb (wk) | Antibodies to BLVc
|
|
|---|---|---|---|---|---|---|---|---|---|
| gp51 | p24 | ||||||||
| 1 | Wild type | G250 | M | 8,000 | 6,960 | 1 | − | − | |
| G279 | M | 5,000 | 3,900 | 1 | 4 | + | − | ||
| G296 | M | 5,500 | 4,015 | 1 | 8 | − | − | ||
| D247G | G245 | M | 8,200 | 6,478 | 1 | − | − | ||
| G287 | M | 5,000 | 3,600 | 1 | 6 | + | − | ||
| G298 | M | 4,900 | 4,312 | 1 | − | − | |||
| pME18Neo (control) | G267 | M | 5,500 | 3,245 | 1 | − | − | ||
| G274 | M | 4,000 | 2,360 | 1 | − | − | |||
| 2 | Wild type | W109 | M | 10,500 | 7,980 | 1 | − | − | |
| W196 | M | 9,000 | 7,560 | 1 | − | − | |||
| B.0249 | M | 13,600 | 8,432 | 1 | − | − | |||
| D247G | W195 | F | 11,100 | 7,437 | 1 | 4 | + | − | |
| B.0234 | F | 15,700 | 13,816 | 1 | 6 | + | − | ||
| B.0240 | M | 9,000 | 6,930 | 1 | − | − | |||
| pBluescript II (control) | Or.111 | M | 9,300 | 6,789 | 1 | − | − | ||
Experimental injections were made in 1999 (experiment 1) and 2000 (experiment 2). WBC, white blood cells; PBL, peripheral blood lymphocytes.
Blood was periodically collected from each sheep, and total DNA was extracted from the blood and used as the template for PCR for detection of BLV proviral genomes, as described in Materials and Methods. The week at which the BLV proviral genome was first detected is indicated.
The BLV antibody in serum from sheep at 15 weeks after injection was detected by use of an immunodiffusion test as described in Materials and Methods. −, negative; +, positive.
Extraction of total DNA and detection of BLV proviral DNA.
Total cellular DNA was extracted from blood with a Wizard genomic DNA purification kit (Promega) and diluted to 10 ng/ml in distilled water. Thirty nanograms of total DNA was used to amplify the full-length BLV LTR by PCR in a 30-μl reaction mixture that contained 1× PCR buffer, 0.2 mM each of the four deoxynucleoside triphosphates, 2.5 μM MgCl2, and 0.5 U of Taq polymerase (Promega) with primers (0.33 mM) BLTRF and BLTRR (25). Amplification was achieved by 35 cycles of incubation at 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. One microliter of the mixture after PCR was used as the template for nested PCR with primers 256 (5′-GAGCTCTCTTGCTCCCGAGAC-3′) and 453 (5′-GAAACAAACGCGGGTGCAAGCCAG-3′). The sequences of primers were based on data in the report by Sagata et al. (22). The amplification products were subjected to electrophoresis on a 2% agarose gel.
Immunodiffusion test.
For detection of antibodies against BLV, immunodiffusion was performed at 15 weeks after injection, using the BLV env glycoprotein gp51 and the gag protein p24 as antigens, as described previously (3, 15).
Quantification of BLV-positive cells.
To determine the percentages of white blood cells that were positive for BLV, we used a system for real-time quantitative PCR (LightCycler System; Roche Diagnostics). Thirty nanograms of total DNA from blood was amplified in the presence of LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics) and primers 256 and 453 for amplification of part of the BLV LTR. We also amplified the sheep gene for β-globin in each sample, using primers PC03 (5′-ACACAACTGTGTTCACTAGC-3′) and PC04 (5′-CAACTTCATCCACGTTCACC-3′) (34) to monitor the concentration of template DNA. Total DNA from FLK-BLV cells, which harbor six copies of the BLV genome, was serially diluted with total DNA from FLK cells, and these samples were used as standard templates for calculations of percentages of BLV-positive cells.
Detection of viral RNA by RT-PCR.
Total RNA was extracted from sheep PBMC with Trizol reagent (Invitrogen, Carlsbad, Calif.). Approximately 500 to 1,000 ng of total RNA were used, with a Superscript preamplification system (Invitrogen), for synthesis of cDNA. BLV tax/rex and cellular β-actin cDNAs were amplified by PCR as described above with primers 4758 (5′-AGGCGCTCTCCTGGCTACTG-3′) and 7333 (5′-GGCACCAGGCATCGATGGTG-3′) and actinF (5′-CGTCGCCTTGGACTTCGAGCAGGA-3′) and actinR (5′-GCTGGAAGGTGGACAGGGAGGCCAGGA-3′), respectively. The products of amplification were subjected to electrophoresis on a 2% agarose gel.
RESULTS
Production of viral proteins and particles from cultured cells transfected with pBLV-IF that encoded wild-type or mutant Tax.
To elucidate the effects of mutant BLV Tax with elevated (D247G) or reduced (S240P) transactivation activity on the replication and propagation of BLV, we constructed recombinant provirus clone, pBLV-IFD247G, by replacing part of the Tax coding region (amino acids 26 to 272) of the wild-type clone pBLV-IFWT (10) with the appropriate sequence for D247G (Fig. 1A).
We examined the transactivation activity of the Tax proteins encoded by each of the molecular clones (Fig. 1B). We transfected 293T cells with pBLV-IFS240P (25) or pBLV-IFD247G plus the reporter plasmid pGV-BLTR, which linked the full-length BLV LTR upstream of the luciferase gene, and then we analyzed luciferase activity. We detected significantly elevated transactivation activity in cells that had been transfected with pBLV-IFD247G. By contrast, no LTR-directed activation was seen in cells that had been transfected with pBLV-IFS240P. These results supported our previous finding that the transactivation activity of the mutant D247G protein was markedly higher than that of wild-type Tax (25).
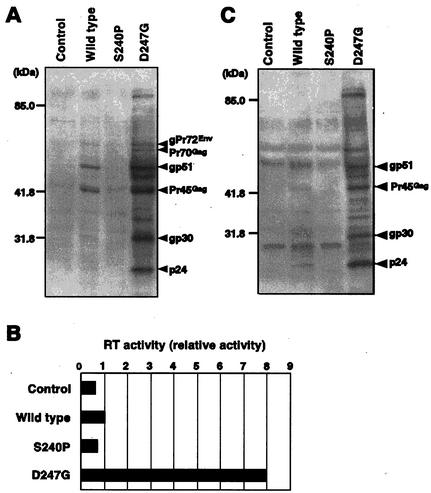
We next examined the expression of viral proteins in cells that had been transfected with the various forms of pBLV-IF by Western blotting (Fig. 2A). We observed strongly immunoreactive bands of proteins that could correspond to at least three precursor proteins (gPr72Env, Pr70Gag, and pr45Gag) and three mature proteins (gp51, gp30, and p24) in lysates of cells that had been transfected with pBLV-IFD247G. The intensities of these bands were markedly higher than those of bands from cells that had been transfected with pBLV-IFWT. In cells transfected with pBLV-IFS240P or the control plasmid, there were no detectable viral proteins. No specific bands of immunoreactive proteins were observed when the Western blot analysis was performed with serum from an uninfected sheep (data not shown).
FIG. 2.
Biological features of infectious clones of BLV that encoded wild-type and mutant Tax proteins. (A) Detection by Western blotting of BLV proteins in cells transfected with the BLV clones. 293T cells were transfected with the infectious clones of BLV or the control plasmid and pRL-SV40. At 60 h after transfection, the growth medium was recovered for experiments for which results are shown (B and C), and cells were used for preparation of cell lysates. Cell lysates were fractionated by SDS-10% PAGE, and viral proteins were detected by Western blot analysis with serum from a BLV-infected sheep. The amounts of cell lysate subjected to Western blotting were varied by reference to the efficiency of transfection, which was assessed by monitoring Renilla luciferase activity. Positions of protein markers and of BLV structural proteins are indicated. (B and C) Detection of virus particles secreted into the growth medium by cells transfected with the BLV clones by RT assay (B) and Western blotting (C). Virus particles in the growth medium were concentrated by ultracentrifugation and resuspended in 100 μl of RPMI 1640. Ten microliters of the concentrated preparation of virus was used for the RT assay. RT activity is presented relative to the activity of the concentrated preparation of virus released from cells transfected with pBLV-IFWT (B). Viral proteins in the concentrated preparations were also analyzed by Western blotting (C) with serum from a BLV-infected sheep, as described above.
To examine the ability of the Tax proteins encoded by the various forms of pBLV-IF to support the production and secretion of viral particles in vitro, we transfected 293T cells with each plasmid and then concentrated the viral particles in the growth medium by ultracentrifugation. Then we analyzed a portion of the solution of virus by a RT assay (Fig. 2B). Potent RT activity was detected in the growth medium of cells transfected with pBLV-IFD247G, suggesting that there were more viral particles in the growth medium of pBLV-IFD247G-transfected cells than in that of pBLV-IFWT-transfected cells. The remainder of each solution of virus was subjected to Western blotting for the detection of viral proteins in virions with serum from BLV-infected sheep (Fig. 2C). The intensities of bands that corresponded to BLV proteins (gp51, Pr45Gag, gp30, and p24) obtained from the growth medium of pBLV-IFD247G-infected cells were higher than the intensities obtained from that of pBLV-IFWT-infected cells. No detectable virus particles were released from cells that had been transfected with pBLV-IFS240P or the control plasmid. No specific bands of proteins were detected in the control analysis with serum from an uninfected sheep (data not shown).
Our results demonstrated that, in vitro, the infectious molecular clone of BLV that encoded a mutant Tax with elevated transactivation activity supported the production of more viral proteins and particles than the wild-type infectious clone.
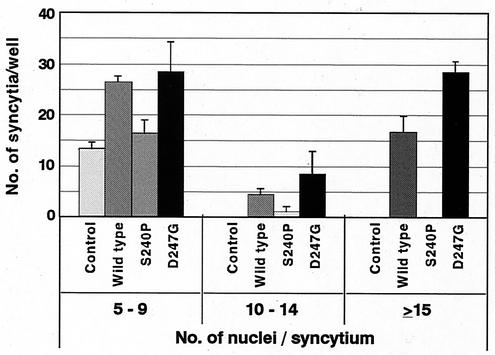
Formation of syncytia in cells transfected with the various forms of pBLV-IF.
We compared pBLV-IFWT and pBLV-IFD247G for their ability to induce the formation of syncytia, which is one of the cytopathic effects of BLV (Fig. 3). Expression of the Env protein of BLV is a prerequisite for the formation of syncytia. Thus, it is likely that the number of syncytia and the number of nuclei in each syncytium are dependent on the amount of Env on the cell surface. We transfected CC81 cells with the various forms of pBLV-IF and counted syncytia and nuclei in each syncytium 3 days later. Syncytia were formed in cultures of cells that had been transfected with pBLV-IFWT and pBLV-IFD247G; however, more syncytia and more nuclei per syncytium were observed in the case of pBLV-IFD247G. This result, together with the results shown in Fig. 1 and 2, demonstrated that the Tax mutant D247G encoded by pBLV-IFD247G induced the production of more viral proteins from the genome than did wild-type Tax.
FIG. 3.
Formation of syncytia by CC81 cells transfected with infectious BLV clones. CC81 cells were transfected with infectious clones of BLV or the control plasmid. Then, 24 h after transfection, transfected cells were replated at 8 × 104 cells/well in 12-well culture plates and cultured for 2 days in complete medium with 4 mg of Polybrene per ml. The cells were fixed and stained, and the syncytia and nuclei in each syncytium were counted. Syncytia were classified into three groups: syncytia with 5 to 9 nuclei (left), 10 to 14 nuclei (center), and 15 or more nuclei (right). Average values from three wells with standard error (error bars) are shown.
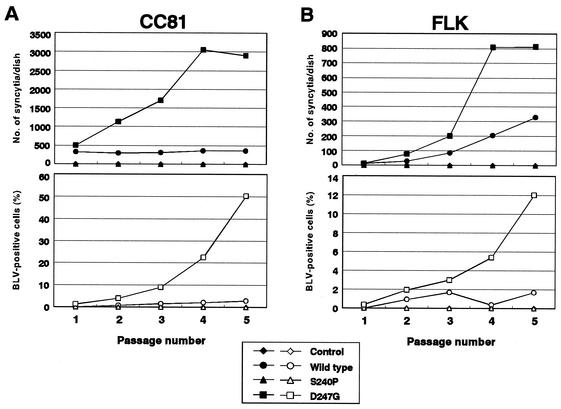
Propagation of mutant BLV in CC81 and FLK cells after transient transfection with the various forms of pBLV-IF.
To examine the ability of BLV that encoded wild-type and mutant Tax to infect adjacent cells, we transfected CC81 and FLK cells with the various forms of pBLV-IF and then monitored the kinetics of viral infection by counting the number of syncytia in passaged cells and determining the percentages of cells that expressed viral proteins at four-day intervals (Fig. 4). There were markedly and rapid increases in the number of syncytia and BLV-positive cells in the case of both CC81 and FLK cells that had been transfected with pBLV-IFD247G compared with pBLV-IFWT. The difference in transfection efficiency between cells transfected with pBLV-IFWT and cells transfected with pBLV-IFD247G was less than twofold in both cell lines (pBLV-IFWT-pBLV-IFD247G ratio, 1.2:1 in CC81 cells and 1.9:1 in FLK cells), indicating that the results in Fig. 4 were not attributable to variations in transfection efficiency. No syncytia and no BLV-positive cells were detected in cultures of cells that had been transfected with the control plasmid or with pBLV-IFS240P.
FIG. 4.
Propagation of mutant BLV in CC81 cells (A) and FLK cells (B) after transient transfection with infectious clones of BLV. Twenty-four hours after transfection, transfected cells were replated at 8 × 104 (CC81) cells or 5 × 104 (FLK) cells per 10-cm-diameter dish and serially passaged at 4-day intervals in the presence of 4 mg of Polybrene per ml. Aliquots of passaged cells were monitored for viral replication, at the indicated times, by indirect immunofluorescence microscopy of fixed cell smears with serum from a BLV-infected sheep (upper panels) and for syncytium formation (under panels).
Thus, our data obtained from analyses in vitro demonstrated that the infectious BLV clone that encoded a mutant Tax protein with increased transactivation activity supported enhanced replication and transmission of the virus.
Transfection of sheep with molecular clones of BLV.
Under experimental conditions, sheep can easily be infected with BLV via blood from BLV-infected cattle or sheep and they tend to develop B-cell leukemia/lymphoma at higher frequencies and after shorter latent periods than cattle (3, 8). Willems et al. established a method for the direct injection of cloned BLV provirus into sheep, and their method appears to be useful for investigation of the nature of mutant infectious clones in vivo (32). Therefore, we attempted to stably infect sheep with the various forms of pBLV-IF to determine the effects of the mutant Tax proteins on the expression and propagation of BLV in vivo. Table 1 shows the sheep used for in vivo injection.
We demonstrated previously that ovine major histocompatibility complex (MHC) class II (OLA) DRB1 alleles are associated with resistance or susceptibility to the development of BLV-induced ovine lymphoma (16). Therefore, we selected sheep that had the same genetic background with respect to OLA-DRB1. Experimental infections in 1999 (experiment 1) and 2000 (experiment 2) were performed with different lipofection reagents, as described in Materials and Methods. Fifteen sheep were injected with 100 to 200 μg of one of the various forms of pBLV-IF or control plasmid DNA that had been mixed with lipofection reagents. Blood was collected periodically, and total DNA was isolated from the blood and used for analysis by PCR with BLV LTR-specific primers. Within 4 to 8 weeks after inoculation, BLV proviral DNA had been detected in two of six sheep injected with pBLV-IFWT and three of six sheep injected with pBLV-IFD247G, indicating that these sheep had been persistently infected with BLV. When we detected antibodies against BLV by the immunodiffusion test at 15 weeks after inoculation, all the BLV-positive animals except sheep G296 were also seropositive.
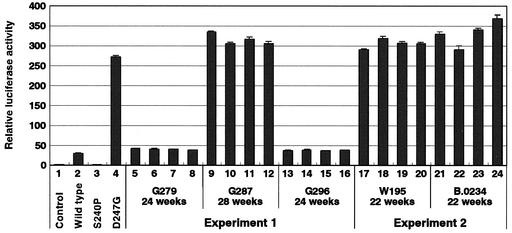
To confirm whether the tax gene encoded the proviral genome in persistently infected sheep, we subcloned this gene from sheep 22 to 28 weeks after injection into pME18Neo. We selected four resultant clones for each sheep and then measured the BLV LTR-directed transactivation activity of each Tax protein (Fig. 5). In all five sheep that had been persistently infected with BLV, Tax activity had been maintained. We also determined the nucleotide sequence of each tax clones and found no nucleotide changes (data not shown). These results indicated that persistent infection with BLV in sheep that had been injected with pBLV-IFD247G was not due to reversion of the mutant gene for Tax D247G to the wild-type gene in the proviral genome.
FIG. 5.
Transactivation of BLV LTR by Tax clones from sheep that had been persistently infected with pBLV-IFWT (G279 and G296) or pBLV-IFD247G (G287, W195, and B.0234). After 22 to 28 weeks, tax genes from BLV-positive animals were subcloned, and four tax clones per animal were picked at random for transactivation analysis. 293T cells were transfected with pGV-BLTR, pRL-SV40, and individual Tax-expressing plasmids. At 60 h after transfection, luciferase activity was monitored. Results are presented as described in the legend to Fig. 1B. Transactivation activity of wild-type (lane 2) and mutant (S240P, lane 3; and D247G, lane 4) Tax is also indicated. Average values from triplicate transfections with standard deviations (error bars) are shown.
Proviral loads in sheep injected with the various forms of pBLV-IF.
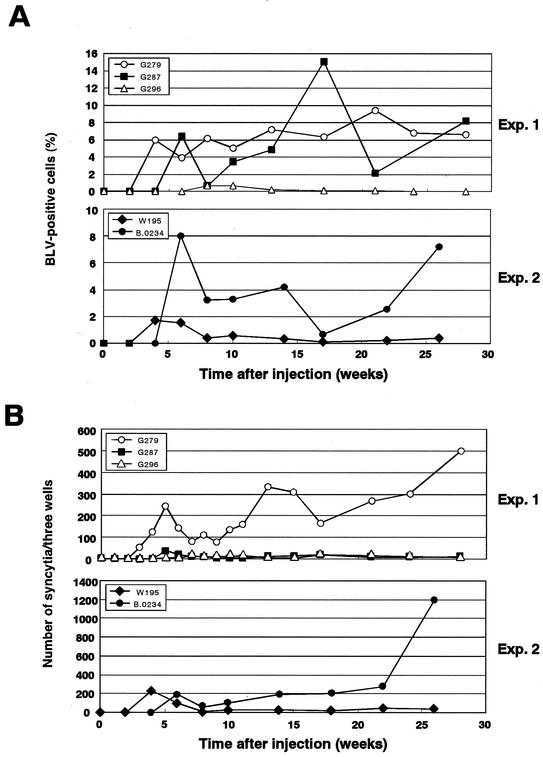
To evaluate viral propagation, we examined the percentages of BLV-infected cells in white blood cells from sheep injected with the various forms of pBLV-IF (Fig. 6). We amplified part of the BLV LTR region in the proviral genome and determined the amounts of products by a real-time quantitative PCR, as described in Materials and Methods (Fig. 6A). The results indicated that sheep could be divided into two groups. In the first group (G279, G287, and B.0234), the level of BLV-positive white blood cells remained between 2% and 15%; in the second group (G296 and W195), the level of BLV-positive white blood cells was lower than 2%. However, there was no significant difference in the percentage of BLV-positive cells between sheep that had been inoculated with pBLV-IFWT and sheep that had been inoculated with pBLV-IFD247G.
FIG. 6.
Propagation of BLV in sheep that had been persistently infected with pBLV-IFWT (G279 and G296) or pBLV-IFD247G (G287, W195, and B.0234). (A) Blood from BLV-positive sheep was collected periodically for 6 months, and total DNA was isolated from the blood. Percentages of BLV-positive cells were monitored by real-time PCR as described in Materials and Methods. (B) PBMC were also prepared from blood, and 2 × 105 PBMC were mixed with 105 CC81 indicator cells and cultured for 72 h in complete medium. The CC81 cells in cocultures were fixed and stained, and then syncytia were counted.
We also examined the ability of sheep PBMC to induce formation of syncytia by indicator CC81 cells (Fig. 6B). The results of the syncytium formation assay were consistent with the results of real-time PCR for all BLV-positive sheep with the exception of G287. The results suggested that, in contrast to the results from analyses in vitro, the mutant Tax protein with elevated transactivation activity had no apparent effect on viral expansion of BLV-infected cells in vivo.
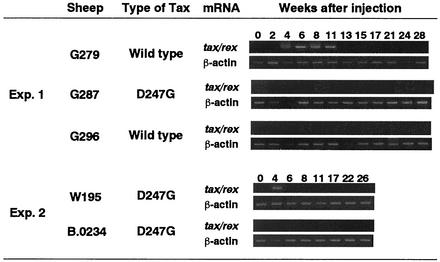
Expression of viral RNA in PBMC from sheep injected with the various forms of pBLV-IF.
To examine whether mutant Tax with increased transactivation activity could enhance the expression of viral RNA in vivo, we analyzed the expression of viral RNA that encoded Tax and Rex in freshly prepared PBMC from sheep by RT-PCR (Fig. 7). We detected viral RNA only in sheep G279 and W195 from 4 to 11 weeks and 4 weeks, respectively, after injection of BLV clones, and none was detected in the other four sheep by this method. However, we detected viral RNA in PBMC from all five BLV-positive sheep after isolation of PBMC 24 to 26 weeks after injection of BLV and incubation for 6 h at 37°C before extraction of RNA (data not shown).
FIG. 7.
Expression of BLV mRNA in sheep injected with infectious molecular clones of BLV. PBMC from BLV-positive sheep were recovered periodically for 6 months, and total RNA was isolated. RT-PCR was performed with primers for amplification of BLV tax/rex mRNA (204 bp) and cellular β-actin mRNA (407 bp), and products of PCR were subjected to 2% agarose gel electrophoresis.
Our results indicated that the expression of viral RNA in sheep inoculated with infectious BLV clones was independent of the ability of the Tax protein, encoded by the proviral genome, to transactivate the viral enhancer and, moreover, that silencing of BLV in vivo occurred even when the transactivation activity of the encoded mutant Tax was higher than that of the wild type.
DISCUSSION
The ability of the D247G mutant of the Tax protein of BLV to transactivate the BLV LTR is higher than that of wild-type Tax (25). The mutant protein can also transactivate the enhancer sequences of other retroviruses and the cellular proto-oncogene c-fos (25, 26). These findings suggested that the mutant Tax might increase the production of viral particles and the propagation of virus in the host animal and might cause the development of BLV-dependent lymphoma more frequently and more rapidly than wild-type Tax. However, no Tax mutants with elevated transactivation activity have been isolated from BLV-infected cattle, suggesting that, if anything, such Tax mutants might be disadvantageous for propagation of BLV in vivo.
To clarify the effects of the D247G mutant, we constructed an infectious molecular clone of BLV that encoded D247G Tax and use it to transfect cultured cells in vitro and to infect sheep. Then we examined the effects of D247G mutant on the replication and propagation of the virus. BLV that encoded D247G Tax produced more viral particles and was transmitted at an elevated rate in vitro (Table 2). However, there were no significant differences in the viral load and the expression of viral RNA between sheep injected with the molecular clone of BLV that encoded the wild-type and those injected with the clone that encoded mutant Tax, indicating that the higher activity of the mutant Tax did not influence the expansion of BLV in vivo (Table 2).
TABLE 2.
Effects of D247G mutant of BLV Tax on the characteristics of BLVa
| Experiment setting | Characteristic | Wild-type Tax | D247G Tax |
|---|---|---|---|
| In vitro | Transactivation of BLV LTR | + | +++ |
| Expression of viral proteins | + | +++ | |
| Secretion of viral particles | + | +++ | |
| Propagation of virus | + | +++ | |
| In vivo | Propagation of virus | + | + |
| Expression of viral RNA | + | + |
+, normal (wild type); +++, enhanced.
The results obtained in vitro were consistent with our previous finding that the mutant Tax protein can induce the production of BLV structural proteins and viral particles by the defective molecular clone pBLV-IFS240P, which encodes an inactive form of Tax (25). The result also demonstrate that when the mutant Tax protein with elevated activity was encoded by an infectious BLV clone, it increased the production of structural proteins and viral particles. When we examined the formation of syncytia by CC81 cells transfected with infectious clones, we observed only a slight difference between wild-type Tax and the D247G Tax with respect to the number of syncytia and the number of nuclei per syncytium. It is possible that cell fusion to form syncytia might occur at high frequency in cells transfected with pBLV-IFD247G because there were many more nuclei in the syncytia in cultures of cells that had been transfected with pBLV-IFD247G than in the syncytia of cells that had been transfected with pBLV-IFWT (data not shown).
The results of serial passaging indicated that, in the population of cells transfected with pBLV-IFD247G, the percentage of BLV-positive cells increased more rapidly than in the cells transfected with pBLV-IFWT. Almost all CC81 cells transfected with pBLV-IFD247G formed syncytia after eight passages (data not shown). Thus, the Tax mutant with elevated transactivation activity not only increased the production of viral proteins and particles but also appeared likely to accelerate the propagation of virus in vitro.
We examined the effects of mutant Tax during infection of sheep with BLV. We showed previously that ovine MHC class II DRB1 alleles are associated with resistance or susceptibility to the development of BLV-induced ovine lymphoma (16). Therefore, we checked the nucleotide sequences of the DRB1 gene in sheep and selected sheep with DRB1 alleles associated with susceptibility to lymphoma for inoculation experiments in vivo. We did not use the mutant clone pBLV-IFS240P for injection of sheep because a previous report demonstrated that a BLV clone with a mutation of tax that decreased its transcriptional activity by about 10-fold was not infectious (33).
BLV persistently infected two of six animals injected with pBLV-IFWT and three of six animals injected with pBLV-IFD247G. The five sheep that had been infected with the wild-type or the mutant BLV could be divided into two groups: (i) in sheep G279, G287, and B.0234, the white blood cells included ≥2% BLV-positive cells, while (ii) the white blood cells of sheep G296 and W195 included <2% BLV-positive cells. However, in sheep G287, the ability of PBMC to induce the formation of syncytia was considerably weaker than that in sheep G279 and B.0234. Proviral BLV DNA integrated into white blood cells in sheep G287 might have included a mutation that reduced of the ability to form syncytia, as described in a previous report (12).
In sera from sheep G296, we could not detect antibodies against BLV. This result might be due to a very low viral load in sheep G296. Contrary to the results from experiments in vitro, the viral loads of BLV-infected sheep were not correlated with the potential activity of the Tax protein encoded by the proviral genome, and in all BLV-positive sheep, the expression of BLV had been inhibited except at the early stages of infection. Thus, the mutant Tax protein with elevated transactivation activity had no apparent effect on the properties of BLV observed in vivo.
A previous study by Twizere and colleagues (28) showed the discordance between the nature of mutant BLV Tax in vitro and in vivo, and they also remarked that their results might be attributed to the different pathways involved in immortalization of fibroblast cells, which were used for the in vitro transformation assay, and oncogenesis of peripheral B lymphocytes.
In addition to the different target cells, the differences between the results of our in vitro and in vivo studies might also be attributable to the responses of host animals to the introduction of infectious BLV clones. Indeed, the mechanisms that regulate the expression of BLV in PBMC are likely to be much more complex than those that regulate the expression of BLV proteins in many cell lines. Viral RNA is maintained at a very low level and does not produce detectable viral proteins in host animals even though the full-length proviral genome is retained, and furthermore, loss-of-function mutations of Tax and LTR have not been observed (24, 25). The silencing mechanism allows BLV to persist even under immunological attack by the host's immune response. However, viral transcription in PBMC is activated and levels of viral RNA are increased considerably after 30 min of ex vivo culture (14, 17).
The silencing of BLV in host blood cells has been described as active repression of virus expression that is mediated by some unidentified factor(s) in cattle and human plasma (9, 36). Recent investigations by Pyeon et al. revealed that, in PBMC of BLV-infected cattle, the host cytokine interleukin-10 (IL-10) inhibits the expression of BLV (19, 20). Moreover, PBMC from BLV-infected animals with late-stage disease express considerably more IL-10 mRNA than animals that are not infected or are at the early stages of disease, suggesting that IL-10 might be a candidate for the factor that negatively regulates the expression of BLV (18).
Experiments with chemicals that interfere specifically with cellular signal transduction pathways showed that expression of BLV in cultures of freshly isolated cells is inhibited by cyclic AMP (13). Our experiments in vivo indicated that a repressive pathway might overcome the effects of Tax and that a Tax mutant with elevated transactivation activity does not allow bypass of such a pathway. Furthermore, no variant of Tax with markedly elevated transactivation activity has been identified in naturally infected cattle. Thus, it appears that Tax with particularly strong transactivation activity is not advantageous for the expansion and survival of BLV. Moreover, wild-type Tax with moderate transactivation activity might be appropriate for both the induction and the repression of the expression of BLV.
At the time of writing (October 2002), the sheep inoculated with infectious BLV clones are still at the asymptomatic stage. Tax is a weak oncoprotein and appears to be critical for the oncogenic potential of BLV. It is possible that an infectious clone of BLV encoding Tax with high transactivation activity might induce lymphoma more rapidly and more frequently than wild-type BLV. It will be interesting to monitor the effects of variations in the transactivation activity of Tax on BLV-induced leukemogenesis.
Acknowledgments
The first two authors contributed equally to this report.
This study was supported by grants from the Ministry of Education, Science, and Culture of Japan and by a Grant for Special Postdoctoral Researcher and a Special Grant for the Promotion of Research from RIKEN.
REFERENCES
- 1.Adam, E., P. Kerkhofs, M. Mammerickx, R. Kettmann, A. Burny, L. Droogmans, and L. Willems. 1994. Involvement of the cyclic AMP-responsive element binding protein in bovine leukemia virus expression in vivo. J. Virol. 68:5845-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, E., P. Kerkhofs, M. Mammerickx, A. Burny, R. Kettman, and L. Willems. 1996. The CREB, ATF-1, and ATF-2 transcription factors from bovine leukemia virus-infected B lymphocytes activate viral expression. J. Virol. 70:1990-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aida, Y., M. Miyasaka, K. Okada, M. Onuma, S. Kogure, M. Suzuki, P. Minoprio, D. Levy, and Y. Ikawa. 1989. Further phenotypic characterization of target cells for bovine leukemia virus experimental infection in sheep. Am. J. Vet. Res. 50:1946-1951. [PubMed] [Google Scholar]
- 4.Boros, I. M., F. Tie, and C. Z. Giam. 1995. Interaction of bovine leukemia virus transactivator Tax with bZip proteins. Virology 214:207-214. [DOI] [PubMed] [Google Scholar]
- 5.Burny, A., Y. Cleuter, R. Kettmann, M. Mammerickx, G. Marbaix, D. Portetelle, A. van den Broeke, L. Willems, and R. Thomas. 1988. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Vet. Microbiol. 17:197-218. [DOI] [PubMed] [Google Scholar]
- 6.Derse, D. 1987. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J. Virol. 61:2462-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derse, D. 1988. trans-acting regulation of bovine leukemia virus mRNA processing. J. Virol. 62:1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djilali, S., A. L. Parodi, D. Levy, and G. L. Cockerell. 1987. Development of leukemia and lymphosarcoma induced by bovine leukemia virus in sheep: a hematopathological study. Leukemia 1:777-781. [PubMed] [Google Scholar]
- 9.Ferrer, J. F., and P. Gupta. 1989. Notification to readers. Expression of bovine leukemia virus genome is blocked by a nonimmunoglobulin protein in plasma from infected cattle. Science 244:632.. [DOI] [PubMed] [Google Scholar]
- 10.Inabe, K., K. Ikuta, and Y. Aida. 1998. Transmission and propagation in cell culture of virus produced by cells transfected with an infectious molecular clone of bovine leukemia virus. Virology 245:53-64. [DOI] [PubMed] [Google Scholar]
- 11.Inabe, K., M. Nishizawa, S. Tajima, K. Ikuta, and Y. Aida. 1999. The YXXL sequences of a transmembrane protein of bovine leukemia virus are required for viral entry and incorporation of viral envelope protein into virions. J. Virol. 73:1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston, E. R., M. A. Powers, L. C. Kidd, and K. Radke. 1996. Peripheral blood mononuclear cells from sheep infected with a variant of bovine leukemia virus synthesize envelope glycoproteins but fail to induce syncytia in culture. J. Virol. 70:6296-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerkhofs, P., E. Adam, L. Droogmans, D. Portetelle, M. Mammerickx, A. Burny, R. Kettmann, and L. Willems. 1996. Cellular pathways involved in the ex vivo expression of bovine leukemia virus. J. Virol. 70:2170-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagarias, D. M., and K. Radke. 1989. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J. Virol. 63:2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami, K., Aida, Y., Kageyama, R., Numakunai, S., Ohshima, K., Okada, K., and Ikawa, Y. 1994. Immunopathologic study and characterization of the phenotype of transformed cells in sheep with bovine leukemia virus-induced lymphosarcoma. Am. J. Vet. Res. 55:72-80. [PubMed] [Google Scholar]
- 16.Nagaoka, Y., H. Kabeya, M. Onuma, N. Kasai, K. Okada, and Y. Aida. 1999. Ovine MHC class II DRB1 alleles associated with resistance or susceptibility to development of bovine leukemia virus-induced ovine lymphoma. Cancer Res. 59:975-981. [PubMed] [Google Scholar]
- 17.Powers, M. A., and K. Radke. 1992. Activation of bovine leukemia virus transcription in lymphocytes from infected sheep: rapid transition through early to late gene expression. J. Virol. 66:4769-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyeon, D., K. L. O'Reilly, and G. A. Splitter. 1996. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J. Virol. 70:5706-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyeon, D., and G. A. Splitter. 1999. Regulation of bovine leukemia virus tax and pol mRNA levels by interleukin-2 and -10. J. Virol. 73:8427-8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyeon, D., F. J. Diaz, and G. A. Splitter. 2000. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovnak, J., and J. W. Casey. 1999. Assessment of bovine leukemia virus transcripts in vivo. J. Virol. 73:8890-8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagata, N., T. Yasunaga, J. Tsuzuku-Kawamura, K. Ohishi, Y. Ogawa, and Y. Ikawa. 1985. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc. Natl. Acad. Sci. USA 82:677-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajima, S., W. Z. Zhuang, M. V. Kato, K. Okada, Y. Ikawa, and Y. Aida. 1998. Function and conformation of wild-type p53 protein are influenced by mutations in bovine leukemia virus-induced B-cell lymphosarcoma. Virology. 243:735-746. [PubMed] [Google Scholar]
- 24.Tajima, S., Y. Ikawa, and Y. Aida. 1998. Complete bovine leukemia virus (BLV) provirus is conserved in BLV-infected cattle throughout the course of B-cell lymphosarcoma development. J. Virol. 72:7569-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajima, S., and Y. Aida. 2000. The region between amino acids 245 and 265 of the bovine leukemia virus (BLV) tax protein restricts transactivation not only via the BLV enhancer but also via other retrovirus enhancers. J. Virol. 74:10939-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajima, S., and Y. Aida. 2002. Mutant tax protein from bovine leukemia virus with enhanced ability to activate the expression of c-fos. J. Virol. 76:2557-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tana, S. Watarai, Y. Aida, S. Tajima, H. Kakidani, M. Onuma, and H. Kodama. 2001. Growth inhibition of cancer cells by co-transfection of diphtheria toxin A-chain gene plasmid with bovine leukemia virus tax expression vector. Microbiol. Immunol. 45:447-455. [DOI] [PubMed] [Google Scholar]
- 28.Twizere, J. C., P. Kerkhofs, A. Burny, D. Portetelle, R. Kettmann, and L. Willems. 2000. Discordance between bovine leukemia virus Tax immortalization in vitro and oncogenicity in vivo. J. Virol. 74:9895-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willems, L., A. Gegonne, G. Chen, A. Burny, R. Kettmann, and J. Ghysdael. 1987. The bovine leukemia virus p34 is a transactivator protein. EMBO J. 6:3385-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willems, L., H. Heremans, G. Chen, D. Portetelle, A. Billiau, A. Burny, and R. Kettmann. 1990. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 9:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willems, L., R. Kettmann, G. Chen, D. Portetelle, A. Burny, and D. Derse. 1992. A cyclic AMP-responsive DNA-binding protein (CREB2) is a cellular transactivator of the bovine leukemia virus long terminal repeat. J. Virol. 66:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willems, L., D. Portetelle, P. Kerkhofs, G. Chen, A. Burny, M. Mammerickx, and R. Kettmann. 1992. In vivo transfection of bovine leukemia provirus into sheep. Virology 189:775-777. [DOI] [PubMed] [Google Scholar]
- 33.Willems, L., R. Kettmann, F. Dequiedt, et al. 1993. In vivo infection of sheep by bovine leukemia virus mutants. J. Virol. 67:4078-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittwer, C. T., G. C. Fillmore, and D. R. Hillyard. 1989. Automated polymerase chain reaction in capillary tubes with hot air. Nucleic Acids Res. 17:4353-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 36.Zandomeni, R. O., E. Esteban, M. Carrera-Zandomeni, and J. F. Ferrer. 1994. Host soluble factors with blocking and stimulating activity on the expression of the bovine leukemia virus. J. Infect. Dis. 170:787-794. [DOI] [PubMed] [Google Scholar]