Abstract
The role of activated microglia (MG) in demyelinating neurodegenerative diseases such as multiple sclerosis is controversial. Here we show that high, but not low, levels of IFN-γ (a cytokine associated with inflammatory autoimmune diseases) conferred on rodent MG a phenotype that impeded oligodendrogenesis from adult neural stem/progenitor cells. IL-4 reversed the impediment, attenuated TNF-α production, and overcame blockage of IGF-I production caused by IFN-γ. In rodents with acute or chronic EAE, injection of IL-4–activated MG into the cerebrospinal fluid resulted in increased oligodendrogenesis in the spinal cord and improved clinical symptoms. The newly formed oligodendrocytes were spatially associated with MG expressing MHC class II proteins and IGF-I. These results point to what we believe to be a novel role for MG in oligodendrogenesis from the endogenous stem cell pool.
Introduction
Recovery from acute insults or chronic inflammatory and noninflammatory degenerative disorders in the CNS has been attributed to a limited capacity for neurogenesis and oligodendrogenesis, poor regeneration of injured nerves, and extreme vulnerability to degenerative conditions. Studies have demonstrated that the adult CNS contains stem cells that can give rise, albeit to a limited extent, both to neurons (1) and to oligodendrocytes (2) throughout life. Knowledge of the factors allowing such stem cells to exist, proliferate, and differentiate in the adult individual is a prerequisite for understanding and promoting the conditions conducive to CNS repair. This in turn can be expected to lead to the development of interventions aimed at boosting neural cell renewal from the endogenous stem cell pool or from exogenously applied stem cells.
Studies have shown that inflammation within the CNS blocks neurogenesis (3, 4) and causes structural damage to myelin (5, 6). Moreover, “paralysis” of microglia (MG) and/or macrophages arrests progression of the transient monophasic disease EAE (7, 8). All of those findings were interpreted as evidence in support of the traditional view that the effect of local immune cells in the CNS is detrimental, and hence that recovery would require blockage, arrest, or elimination of local immune responses. Likewise, the limited regeneration and excessive vulnerability of CNS neurons under inflammatory conditions or after an acute insult were put down to the poor ability of the CNS to tolerate the immune-derived defensive activity that is often associated with local inflammation and cytotoxicity mediated, for example, by TNF-α (reviewed in refs. 5, 9) or nitric oxide (10). More recent studies have shown, however, that although an uncontrolled local immune response indeed impairs survival of neurons (11) and oligodendrocytes (10) and interferes with repair processes (12), a local immune response that is properly controlled can support survival and promote remyelination (13) and recovery (14, 15). It was further shown that after an injury to the CNS, a local immune response that is well controlled in time, space, and intensity by peripheral adaptive immune processes (mediated by CD4+ helper T cells directed against autoantigens residing at the site of the lesion) is a critical requirement for post-traumatic neuronal survival and repair (14, 16–19). These and other results led our group to formulate the concept of “protective autoimmunity” (16).
The positive effect of the helper T cells is mediated, at least in part, through their dialog with MG, key players in the immune system’s innate arm. Among the compounds produced by these autoimmune T cells in their role as key players in the adaptive arm of the immune system is IFN-γ, a characteristic Th1 (proinflammatory) cytokine which, when present in moderate amounts, has a beneficial effect on neural tissues (19–21). Thus, whereas survival of neurons is supported by MG that encounter IFN-γ in well-controlled amounts, uncontrolled amounts of IFN-γ interfere with neural survival (19). These positive microglial effects do not negate the existence of destructive MG, whose inactivation might have a beneficial effect on the diseased brain (7). They do, however, argue in favor of the need for MG whose activity is well controlled and is thus supportive of CNS tissue maintenance and repair.
On the basis of our findings, we suggest that 1 of 2 possible fates awaits MG encountering IFN-γ: MG exposed to low concentrations of this cytokine exhibit an immune-mediated healing response, whereas those exposed to high IFN-γ concentrations respond by causing an immune-mediated demyelinating disease (5, 6); or autoimmune T cells that express the characteristic Th2 (antiinflammatory) cytokine IL-4 over a wide range of concentrations activate MG to support neuronal survival (19).
Here we examined whether IL-4, via modulation of MG both in vitro and in vivo, could overcome the destructive effects of high-dose IFN-γ, known to be associated with EAE. In vitro, a high dose of IFN-γ, but not a low dose, impaired the ability of MG to support oligodendrogenesis from adult neural stem cells and neural progenitor cells (NPCs). IL-4 counteracted the interference with oligodendrogenesis caused by high IFN-γ concentrations. When IL-4–activated MG were stereotaxically injected through the cerebral ventricles into the cerebrospinal fluid (CSF) of rats with acute EAE or of mice with a remitting-relapsing autoimmune disease, the animals demonstrated significantly more oligodendrogenesis and significantly less neurological deficit than did their vehicle-injected diseased controls.
Results
Effects of MG on oligodendrogenesis in vitro: the dual effect of IFN-γ.
Recent studies from our laboratory showed that IL-4 induces MG to secrete IGF-I and suppresses their production of TNF-α (19). In the present study we first examined whether MG that encounter a high dose of IFN-γ adopt a phenotype that interferes with oligodendrogenesis from NPCs, and if so, whether IL-4 can counteract this negative effect.
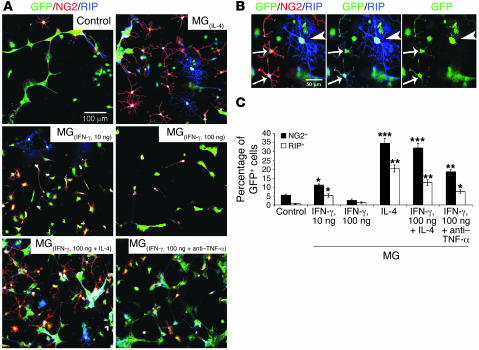
We first examined the effects on NPCs of MG that were preincubated for 24 hours in their optimal growth media (19), in the presence or absence of the cytokines IL-4 (10 ng/ml), IFN-γ (10 or 100 ng/ml), or IL-4 (10 ng/ml) together with IFN-γ (100 ng/ml). After the growth media and cytokine residues were washed off, each of the treated microglial preparations was freshly cocultured with dissociated NPC spheres on coverslips coated with Matrigel in the presence of differentiation medium. We used NPCs expressing GFP to verify that any differentiation of oligodendrocytes seen in the culture was derived from the NPCs rather than from contamination of the primary microglial culture. After 10 days we could discern both GFP-expressing NPCs colabeled with the oligodendrocyte progenitor marker NG2 and RIP-stained mature oligodendrocytes at the pre-ensheathing stage (22) (Figure 1). A few GFP+/NG2+ and GFP+/RIP+ cells were seen in control NPCs cultured without MG and in NPCs cocultured with MG pretreated with the low dose of IFN-γ [MG(IFN-γ, 10 ng)]. In cocultures of NPCs with MG(IL-4), however, the increase in number of GFP+/NG2+ and GFP+/RIP+ cells was dramatic. In contrast to the low dose of IFN-γ, treatment with 100 ng/ml IFN-γ caused MG to block oligodendrogenesis from the cocultured NPCs. Interestingly, the addition of IL-4 to MG treated with 100 ng/ml IFN-γ overcame the adverse effect of high-dose IFN-γ, with the result that these MG were able to induce NPCs to differentiate into oligodendrocytes. When we added neutralizing anti–TNF-α antibodies to MG(IFN-γ, 100 ng/ml), we observed a significant increase in the numbers of NG2+ and (to a lesser extent) RIP+ cells in NPCs cocultured with those MG. Anti–TNF-α had no effect on cocultures of NPCs with MG(IL-4) (Figure 1A). In a previous study by our group, addition of anti–IGF-I antibodies to cocultures of NPCs with MG(IL-4) blocked oligodendrogenesis (23), indicating that oligodendrogenesis from NPCs induced by MG(IL-4) is mediated by IGF-I. Notably, the newly differentiated oligodendrocytes were more branched in the presence of MG(IL-4) than in the presence of MG(IFN-γ, 10 ng) (Figure 1A). In each of the cocultures, all NG2+ and RIP+ cells were also found to be colabeled with GFP (Figure 1B). Some GFP+/NG2+ cells coexpressed RIP+, whereas well-branched mature oligodendrocytes (GFP+/RIP+) did not express NG2 at this stage (Figure 1B). Quantitative analysis verified that IL-4 and (to a lesser extent) anti–TNF-α had overcome the inhibitory effect of MG(IFN-γ, 100 ng) on oligodendrogenesis induction (Figure 1C). The effect of nonactivated MG in these experiments was neither destructive nor supportive.
Figure 1. High-dose IFN-γ, by inducing TNF-α, inhibits the ability of MG to support oligodendrogenesis, whereas MG activated by IL-4 can overcome the inhibition.
(A) GFP-expressing NPCs (green) were cultured for 10 days without MG (control) or cocultured for 10 days with MG(IL-4), MG(IFN-γ, 10 ng/ml), MG(IFN-γ, 100 ng/ml), MG activated by both IFN-γ (100 ng/ml) and IL-4 (10 ng/ml), or MG(IFN-γ, 100 ng/ml) in the presence of anti–TNF-α (1 ng/ml). (B) Colocalization of GFP, NG2, and pre-ensheathing marker of oligodendrocytes RIP. Arrows show the same cells expressing the indicated markers. Arrowhead shows a highly branched GFP+/RIP+ oligodendrocyte. Separate confocal channels are shown in 2 right panels. (C) Quantification of NG2+ or RIP+ cells (expressed as a percentage of GFP+ cells) obtained from confocal images. Data are from 2 independent experiments in replicate cultures; bars represent mean α SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control (2-tailed Student’s t test). The P values indicated in the figure represent a comparison of the groups as analyzed by ANOVA.
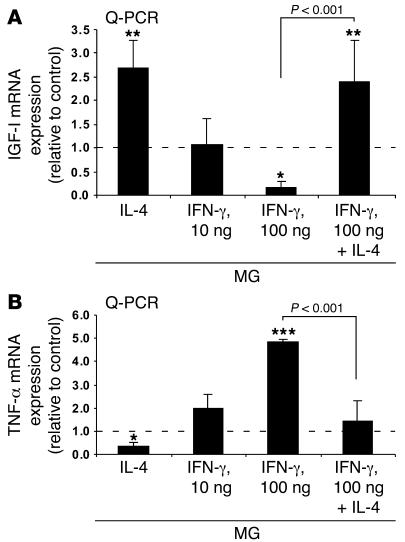
To examine the correlation between the production of IGF-I and the ability of MG(IL-4) to overcome the impediment to oligodendrogenesis, and to determine how this relationship is influenced by TNF-α production, we carried out quantitative real-time PCR (Q-PCR) analyses (Figure 2). The results showed that low-dose IFN-γ had no effect on the expression of IGF-I, whereas high-dose IFN-γ completely arrested it. In contrast, IL-4 had a robust effect on IGF-I expression and could overcome the negative effect of high-dose IFN-γ on IGF-I (Figure 2A). In addition, whereas high-dose IFN-γ induced TNF-α production, IL-4 arrested it (Figure 2B), possibly explaining the ability of IL-4 to overcome the negative effect of high-dose IFN-γ on IGF-I.
Figure 2. IL-4 partially reverses downregulation of IGF-I expression and upregulation of TNF-α expression in MG activated by 100 ng/ml IFN-γ.
(A) Q-PCR of MG identical to those described in Figure 1 24 hours after treatment. A significant increase in IGF-I was seen in MG activated by 10 ng/ml IL-4. (B) TNF-α transcripts in MG activated by 100 ng/ml IFN-γ concomitantly with 10 ng/ml IL-4 compared with IFN-γ–activated MG. Values represent the relative amounts of amplified mRNA normalized against β-actin and are expressed as fold of induction relative to untreated control MG (dashed line). Data are from 2 independent experiments in replicate cultures; bars represent mean α SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; ANOVA.
MG(IL-4) induce oligodendrogenesis from endogenous neural stem cells and NPCs in an acute EAE model.
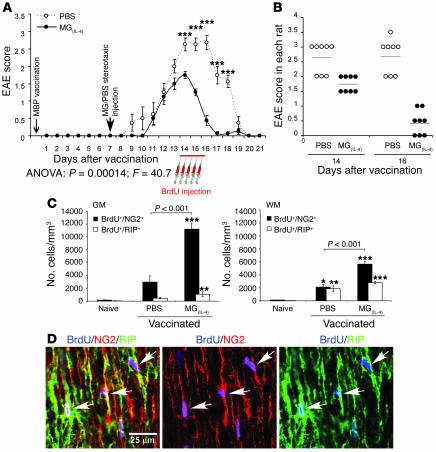
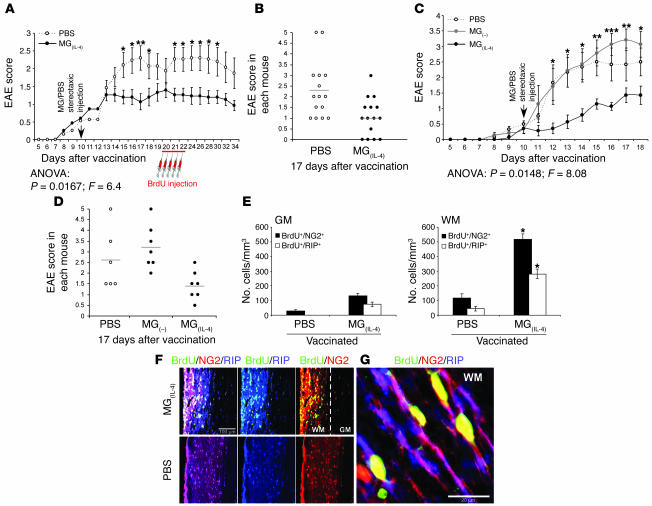
The finding that MG(IL-4) in vitro created conditions favoring differentiation of NPCs into oligodendrocytes and overcame the negative effect of MG(IFN-γ, 100 ng) on oligodendrogenesis encouraged us to investigate whether MG(IL-4) would promote oligodendrogenesis from endogenous adult NPCs (2, 24) in animals with acute or chronic EAE. To examine this possibility we first induced acute EAE in adult Lewis rats (n = 8) by immunizing them with myelin basic protein (MBP) emulsified in CFA. Seven days later we introduced 10 ng/ml MG(IL-4) into the CSF of these rats via stereotaxic bilateral injection into their cerebral ventricles. Control rats (n = 8) were similarly injected with PBS. Seven days after injection of MG(IL-4) or PBS, the rats were injected i.p. with BrdU every 12 hours for 2.5 days to identify proliferating cells, and 21 days after the MBP vaccination their spinal cords were examined for the appearance of newly formed oligodendrocytes.
Although the symptoms of paralysis observed in this model of acute EAE do not result from demyelination, they are beneficially affected by IL-4 (25–27). We therefore postulated that not only oligodendrogenesis but also functional integrity would benefit from MG(IL-4), possibly in part through the IGF-I that these MG produce (19, 23). Follow-up of the clinical manifestations of EAE with and without microglial treatment showed that after injection with MG(IL-4), the onset of EAE symptoms was delayed and the severity and duration of clinical paralysis were significantly reduced (Figure 3, A and B). Similar results were obtained in an independent experiment carried out in the absence of BrdU, indicating that BrdU did not affect the manifestation of disease or the treatment efficacy. Because the clinical symptoms of EAE in this model are manifested by tail and hind limb paralysis, we assessed oligodendrogenesis in the spinal cords of these rats. Spinal cords were excised, and longitudinal sections from the T8–T9 region were analyzed for newly formed oligodendrocytes and MG. In both the untreated [MG(–)] and the MG(IL-4)-treated groups of rats with EAE we detected cells that were triple-labeled for BrdU (proliferating cells), NG2, and RIP. Some of the proliferating cells in both the gray and the white matter of MG(IL-4)-treated rats were BrdU+/NG2+/RIP+, indicating that they had differentiated into committed oligodendrocytes. In those rats there were significantly more BrdU+/NG2+ cells in the gray matter and significantly more BrdU+/RIP+ cells in the white matter than in PBS-treated rats (Figure 3C). Some oligodendrogenesis was observed in rats with EAE that were injected with PBS, leading us to consider the possibility that such oligodendrogenesis represents a self-reparative mechanism of myelin renewal that is induced by the inflammatory conditions even in the absence of any intervention [such as injection of MG(IL-4)]. Examination showed that the amount of oligodendrogenesis occurring in naive rats was very small; thus in the absence of treatment, oligodendrogenesis in rats with EAE that were injected with PBS was more abundant than in naive rats and was further increased by MG(IL-4) (Figure 3C). Coexpression of NG2 and RIP in the BrdU+ cells confirmed that the newly formed cells were oligodendrocytes (Figure 3D). It should be noted that BrdU in these experiments was injected at the peak of the disease, a stage at which a spontaneous mechanism that limits the proinflammatory response is likely to be operating, with consequent reduction in the number of Th1 cells and/or the appearance of Th2 cells. It is therefore possible that the oligodendrogenesis seen in rats with EAE was triggered as a result of this decline in number of Th1 cells or a spontaneous increase in number of Th2 cells or both (28).
Figure 3. Intraventricularly injected MG(IL-4) significantly improves the clinical symptoms of acute EAE and induces oligodendrogenesis in rats.
On day 7 after MBP vaccination, rat brain lateral ventricles were stereotaxically injected bilaterally with PBS or with syngeneic MG(IL-4) (n = 8 per group). From day 14, BrdU was injected for 2.5 days. Naive rats received the same course of BrdU injection (n = 4). Spinal cords were excised 7 days after the first BrdU injection (21 days after immunization). (A) EAE scores in rats treated either with MG(IL-4) or with PBS (n = 8 per group). Data are mean α SEM. ***P < 0.001; Student’s t test. (B) EAE scores of individual rats 14 and 16 days after vaccination. (C) NG2+ or RIP+ cells colabeled with BrdU+ cells were quantitatively analyzed both in gray matter (GM) and in white matter (WM) at 300-μm intervals along longitudinal 30-μm sagittal sections of spinal cord (T8–T9; n = 6–8 per group). Data are mean α SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus naive; ANOVA. (D) Representative confocal images of an area taken from the white matter of an MG(IL-4)-treated rat. Newly formed oligodendrocytes were identifiable by colocalization of BrdU and NG2 or RIP (arrows). Separate confocal channels are shown in 2 right panels.
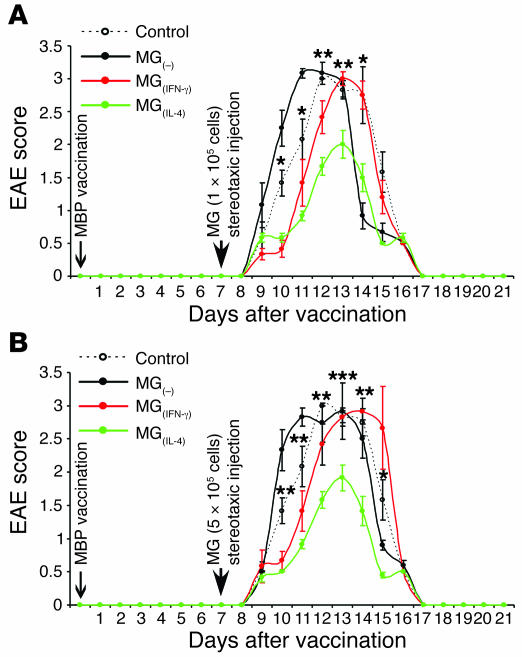
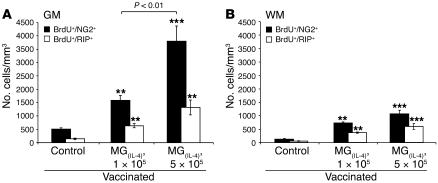
These findings prompted us to examine whether the observed effect of MG(IL-4) in vivo depends on the number of injected cells and whether IFN-γ–activated MG would have any effect. To examine the effects of different amounts of the differently activated MG on the clinical course of EAE, we carried out the experiment described in Figure 4. Included in that experiment were groups of rats with EAE that were injected with MG(–), MG(IFN-γ), or MG(IL-4) at 1 of the 2 tested cell dosages (1 × 105 or 5 × 105 cells) and a group of noninjected control rats with EAE. As shown, MG(IFN-γ) did not exacerbate the clinical manifestations at either dosage (Figure 4, A and B), and at the lower dosage it even had a small beneficial effect in that it delayed the onset of the disease relative to untreated (control) animals with EAE (P = 0.0045, F = 14.16; ANOVA; Figure 4A). MG(–) at both dosages had no significant effect either on symptom severity or on disease onset. Treatment with MG(IL-4) was equally beneficial at both tested dosages (Figure 4, A and B). With the object of utilizing the same animals for both clinical and cellular analysis, we injected all rats in the above experiments with BrdU. In the experiment described in Figure 4 we administered the first BrdU injection 10 days after the immunization with MBP/CFA, and not after 14 days as in the experiment described in Figure 3. This was done to enable us to detect a possible earlier effect of MG(IL-4) on oligodendrogenesis than that observed in the earlier experiment. As shown in Figure 5, A and B, treatment with MG(IL-4) yielded an increase in oligodendrogenesis even when the latter was assessed as early as 3 days after MG were administered (which coincided with the time point at which BrdU was injected). Moreover, a stronger effect was obtained with the higher MG(IL-4) dosage, indicating its dose dependence. Comparison of the effect of low-dose MG(IL-4) in this experiment to the effect shown with the same dose in Figure 3 revealed differences between them in the ratios of newly formed mature (BrdU+/RIP+) oligodendrocytes both in the white matter and in the gray matter. These differences might be attributable to differences in timing of the BrdU injection.
Figure 4. Effect of MG on the clinical course of EAE depends on their number and activity.
On day 7 after induction of EAE in rats as described in Figure 3, their brain lateral ventricles were stereotaxically injected bilaterally with syngeneic MG(–), MG(IFN-γ), or MG(IL-4) (n = 6 per group). One group of rats with EAE remained untreated and served as a control (n = 6). From day 10, BrdU was injected for 2.5 days. Spinal cords were excised 11 days after the first BrdU injection, by which time disease in the rats of all groups was resolved. (A) EAE scores in rats injected stereotaxically with low-dose (1 × 105 cells in 5 μl PBS for 5 minutes) of differently activated MG. (B) EAE scores in rats injected stereotaxically with high doses (5 × 105 cells in 5 μl PBS for 5 minutes). Data are mean α SEM. *P < 0.05, **P < 0.01, ***P < 0.001, MG(IL-4) versus control; Student’s t test. Significant differences (2-factor repeated measures ANOVA) were found between the MG(–) and MG(IL-4) groups: (A) P = 0.0001, F = 61.1; (B) P = 0.0001, F = 79.4.
Figure 5. Dose dependency of oligodendrogenesis induced in MG(IL-4) -injected rats.
NG2+ or RIP+ cells colabeled with BrdU+ cells were quantitatively analyzed in (A) gray matter and (B) white matter at 300-μm intervals along longitudinal 30-μm sagittal sections of spinal cord (T8–T9) from MBP-vaccinated rats injected with low- or high-dose MG(IL-4) (n = 5–6 per group) as indicated. Data are expressed as mean α SEM. **P < 0.01, ***P < 0.001 versus control; ANOVA).
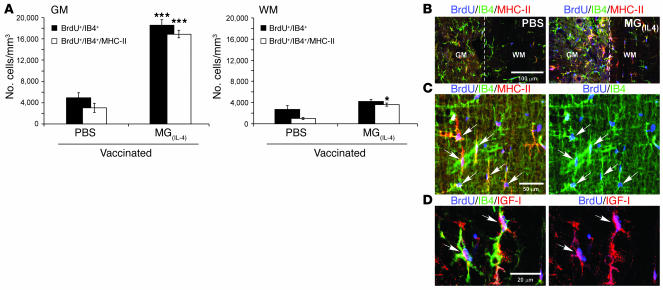
According to the traditional view, MG that express MHC class II molecules are the activated MG that are present in inflammation-associated diseases (29–31). Recent studies, however, by our group and by others, showed that not all MHC class II–expressing MG are destructive (19, 32). For example, MHC class II–expressing MG that are activated by low-dose IFN-γ or by IL-4 support cell survival (19). Analysis of consecutive sections obtained from the rats described in Figure 3 revealed the presence of newly formed MG in rats with EAE that were treated with both PBS and MG(IL–4), with the highest accumulation seen in the gray matter of the MG(IL-4)-treated rats (Figure 6A). No newly formed MG were seen in naive rats. Analysis of newly formed MG (BrdU- and isolectin B4–positive; BrdU+/IB4+) in the gray matter of both PBS-treated and MG(IL-4)-treated rats revealed that most of these new MG were MHC class II+, and that they were significantly more numerous in the rats treated with MG(IL-4) than in the PBS-treated rats (Figure 6, A and B). Confocal scanning microscopy confirmed the expression of MHC class II by the newly formed MG (BrdU+/IB4+) in the white matter of MG(IL-4)-treated rats (Figure 6C).
Figure 6. Rats injected intraventricularly with MG(IL-4) exhibit increased microglial proliferation and MHC class II (MHC-II) expression.
The spinal cords analyzed in Figure 3 were also examined for microgliogenesis. (A) Quantitative analysis of IB4+ and IB4+/MHC class II+ cells colabeled with BrdU+ (mean α SEM) from both gray matter and white matter (n = 8 per group). *P < 0.05, ***P < 0.001 versus PBS; 2-tailed Student’s t test. (B) Representative confocal microscopy of longitudinal sagittal sections of spinal cords (T8–T9) stained with BrdU and costained with IB4 for MG and MHC class II 21 days after injection with PBS or with MG(IL-4). The most abundant populations of BrdU+ cells were immunoreactive to IB4 in both control and MG(IL-4)-injected rats. Significantly more MHC class II+ cells were seen, especially in the gray matter, in slices from MG(IL-4)-injected rats than from those of PBS-injected control rats. Note that the images are from areas that include both gray matter and white matter; dashed line shows the border between the 2 areas. (C and D) Most of the newly formed MG (IB4+/BrdU+) in MG(IL-4)-treated rats coexpressed (C) MHC class II and (D) IGF-I (arrows).
Because MG can be induced to express IGF-I (19), and because the MG in this study induced oligodendrogenesis in vitro (Figure 1) and overcame the strongly proinflammatory conditions mediated by high-dose IFN-γ, we sought to identify IGF-I–expressing MG in the spinal cords of MG(IL-4)-treated rats. Newly formed BrdU+/IB4+ MG were found to express IGF-I (Figure 6D). Not all MHC class II+/IGF-I+ MG were BrdU+, however, suggesting that those cells might be either the injected MG or the newly formed ones.
MG (IL-4) induce oligodendrogenesis from endogenous neural stem cells and NPCs in a model of chronic EAE.
To determine whether MG(IL-4) is also beneficial in a mouse model of chronic EAE, we compared mice treated with MG(IL-4) and with PBS. EAE was induced in C57BL/6J mice by immunization with the encephalitogenic myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (MOG35–55) emulsified in incomplete Freund’s adjuvant containing Mycobacterium tuberculosis and Bordetella pertussis toxin (33). Ten days after EAE induction we injected MG(IL-4) or PBS into the CSF via bilateral stereotaxic injection into the cerebral ventricles. BrdU was injected for 2.5 days, starting at day 20. The 2 groups of injected mice differed significantly both in the severity of paralysis (Figure 7A) and in the number of mice expressing the disease (Figure 7B). To verify that the effect of MG(IL-4) was due to their activation, we repeated the experiment and tested an additional group of mice in which EAE was induced and MG(–) were injected into the cerebral ventricles as described for Figure 7. As in the case of the rats with EAE (Figure 4), the injected MG(–) did not have any beneficial effect; if at all, they had an adverse effect — albeit not statistically significant — compared with PBS-injected mice with EAE (Figure 7, C and D). In the white matter (but not in the gray matter) of mice with EAE, significantly more newly formed oligodendrocytes (BrdU+/NG2+/RIP+) were observed in the group treated with MG(IL-4) than in the PBS-treated group (Figure 7, E and F). In contrast to PBS-treated rats with monophasic (acute) EAE, hardly any oligodendrogenesis could be seen in their counterparts in which EAE was chronic or in naive mice (data not shown), suggesting that chronic conditions do not favor oligodendrogenesis. With the aid of scanning confocal microscopy, coexpression of NG2 and RIP by the newly formed oligodendrocytes was confirmed in the white matter of the MG(IL-4)-treated mice with chronic EAE (Figure 7G).
Figure 7. In mice with chronic EAE, intraventricularly injected MG(IL-4) significantly improves clinical features and induces oligodendrogenesis.
Spinal cords were excised 12 days after the last BrdU injection. (A and B) EAE scores in mice injected with either MG(IL-4) or PBS (n = 15 per group). *P < 0.05, **P < 0.01; Student’s t test. (C and D) Lack of beneficial effect of MG(–). In an independent experiment, mice with EAE were injected with MG(IL-4) (n = 7), MG(–) (n = 7), or PBS (n = 6) on day 10 after MOG vaccination. *P < 0.05, **P < 0.01, ***P < 0.001, MG(–) versus MG(IL-4); Student’s t test. ANOVA revealed no significant effect of MG(–) injection relative to PBS injection, whereas MG(IL-4) had a significant effect. Shown are (A and C) mean α SEM and (B and D) individual EAE scores on day 17. (E) NG2+ and RIP+ cells colabeled with BrdU+ cells were quantitatively analyzed at 300-μm intervals in both gray matter and white matter of the spinal cord (n = 4 per group). Data are mean α SEM. *P < 0.05 versus PBS; 2-tailed Student’s t test. (F) Representative confocal images of longitudinal sections of spinal cords stained with BrdU and costained for NG2 and RIP 34 days after immunization in MG(IL-4)- and PBS-treated mice. Separate confocal channels are shown in 2 right panels. (G) Newly formed oligodendrocytes were identifiable by colocalization of BrdU, NG2, and RIP in the white matter of MG(IL-4)-treated mice.
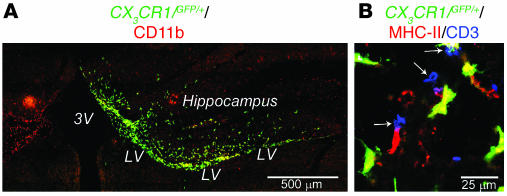
To follow the fate of the injected MG and trace their local interaction with T cells, we prepared MG from newborn knock-in mice in which 1 copy of the gene encoding the chemokine receptor CX3CR1 had been replaced with GFP reporter cDNA (34). GFP expression in these mice is under the control of the CX3CR1 promoter, and consequently heterozygous (CX3CR1/GFP/+) mice express both the receptor and GFP on CNS MG, peripheral monocytes, and a subset of mononuclear phagocytes that includes macrophages and dendritic cells (35, 36). Seven days after the vaccination, C57BL/6J mice with chronic EAE received unilateral stereotaxic injections of MG(–) or MG(IL-4) from syngeneic CX3CR1/GFP/+mice (1 × 105 cells in 3 μl PBS for 3 minutes) into the CSF via the right brain lateral ventricles (Bregma, –0.4; L, 0.8; V, 2.5). On examination GFP+ cells coexpressing MHC class II were detected adjacent to T cells (CD3+) along the ventricle approaching the spinal cord. Figure 8 depicts the distribution of MG(IL-4)-GFP+ MG in the injected lateral ventricle (Figure 8A) and in the spinal cord at segment T8–T9 adjacent to the ependyma of the central canal associated with CD3+ cells (Figure 8B). There were no detectable differences in the migration of MG(–) and MG(IL-4).
Figure 8. Distribution of CX3 CR1/GFP/+ MG 9 days after stereotaxic injection.
(A) Localization of GFP+ cells colabeled with the microglial marker CD11b in the right lateral ventricle (LV) after injection of MG(IL-4) from CX3CR1/GFP/+ mice into mice with EAE. Note the hippocampal area in the MG(IL-4)-injected mice was heavily populated by MG(IL-4)-GFP+ cells. (B) MG(IL-4)-GFP+ cells coexpressing MHC class II populated the spinal cord at level T8–T9 adjacent to the ependyma of the central canal associated with CD3+ cells (arrows). 3V, third ventricle.
Discussion
The results of this study lead us to attribute a novel role to MG as both supporters and blockers of oligodendrocyte renewal from the endogenous NPC pool in the adult CNS. The in vitro findings showed that MG(IL-4), in part via production of IGF-I and downregulation of TNF-α, were remarkably potent in counteracting the impediment to oligodendrogenesis induced by high-dose IFN-γ. In vivo, MG(IL-4) supported oligodendrogenesis and clinical recovery in rats and mice in which severe inflammatory conditions are known to evoke clinical symptoms of transient or chronic EAE.
Defense mechanisms in the form of activated MG commonly operate in acute and chronic neurodegenerative conditions, but often the CNS is unable to tolerate them (10, 37). As a result, activated MG have generally been viewed as a uniformly hostile cell population that causes inflammation, interferes with cell survival (38), and blocks neurogenesis (3, 4). Recent studies have shown, however, that whether the effect of activated MG on the injured or inflamed CNS will be positive or negative is determined by the type of activation, and that just as activated MG can be inimical to cell survival in some instances, they can be protective in others (14, 19, 20, 39).
In the present study we showed that injection of MG(IL-4) into the CSF of rats and mice suffering from acute or chronic EAE caused an increase in the number of newly formed MG. Most of the new MG expressed MHC class II and IGF-I. Recent evidence supports the active participation of IGF-I in maintenance of the integrity and homeostasis of the CNS. This growth factor was shown, for example, to play an important role in the differentiation (22, 40) and survival (41, 42) of oligodendrocytes and to be beneficial in the treatment of EAE (43, 44). It seems reasonable to assume that the IGF-I produced by MG(IL-4) is responsible, at least in part, for the shift to a Th2 phenotype (45) and thus for the increased number of MHC class II+ MG as well as of the newly formed BrdU+/MHC class II+ MG expressing IGF-I. The increased oligodendrogenesis was found to correlate with a higher incidence of newly formed MHC class II+ MG. In view of these findings, it might be worth considering noninvasive therapeutic intervention with IGF-I (46) or with some of the growth factors (47) that have a similar effect to that of IL-4 but are able to pass through the blood-brain barrier, rather than — or in addition to — activated MG. Delivering drugs directly to the CNS along the olfactory and trigeminal nerves could also potentially be used to target IL-4 to the CNS. The intranasal method has already been utilized for delivery of other cytokines to the CNS (48).
The finding that newly formed MG express MHC class II in vivo suggests that these MG exert their effects on oligodendrogenesis by acting as antigen-presenting cells for CD4+ helper T cells (13, 49). Alternatively, it is possible that the signal transduction pathway activated by IL-4 for IGF-I production is the same pathway as the one needed for MHC class II expression. This would mean that MHC class II expression by MG is not a requirement for oligodendrogenesis but is nevertheless correlated with it. In either case, our results contradict the traditional belief that parenchymal MHC class II–bearing MG are associated only with pathology (29, 30). Support for our contention comes from a recent suggestion that parenchymal MG expressing MHC class II are not required for induction of EAE (50). Our preliminary data from experiments in transgenic mice with conditional expression of diphtheria toxin receptor on CD11c+ promoter (51) suggest that MG are responsible for the induced oligodendrogenesis observed after vaccination (data not shown).
Given the rapid rate of improvement in rats with clinical symptoms of acute EAE, it seems unlikely that the observed recovery in this model is an outcome of the induced oligodendrogenesis. It is more likely that the phenomena of improved recovery and enhanced oligodendrogenesis are unrelated and that both can be attributed in part to growth factors (such as IGF-I) produced locally by MG(IL-4). IGF-I has been shown to be beneficial for neural tissue survival and renewal (19, 22, 23, 52–56). It should be borne in mind, however, that any growth factor or cytokine may exhibit a broad spectrum of activity even for the same indication, depending on the context and dosage. As an example, treatment with IGF-I fails to enhance CNS myelin repair during autoimmune demyelination (57). Moreover, the effect of MG(IL-4) on oligodendrogenesis under in vitro conditions was completely blocked by anti–IGF-I (23). A similar finding was reported for TNF-α, and the detrimental effect of this cytokine might also be dependent on context and dosing (58, 59).
Previous studies of rats and mice with EAE showed that the inflammatory response not only induces proliferation and mobilization of endogenous progenitors (60) but also attracts exogenously delivered adult NPCs (33), implying that autoimmune brain inflammation, even in the presence of a large proportion of Th1 cells and hence of abundant IFN-γ, leads to conditions that promote cell renewal. This suggestion is further supported by the present finding that even in the absence of treatment, rats with monophasic EAE exhibited a dramatic increase in the number of newly formed oligodendrocytes compared with naive rats. This oligodendrogenesis was further increased after injection of MG(IL-4). It thus appears that encephalitogenic T cells, meaning T cells that recognize self antigens in the CNS even if the quantity and affinity of the T cells are such that they cause transient paralysis, can promote oligodendrogenesis from the endogenous NPCs found along the ependymal layer lining the central canal of the spinal cord (2). Our contention that oligodendrogenesis requires MG/macrophages activated in a certain way is supported by a recent report that minocycline, in addition to downregulating MHC class II expression by MG/macrophages (32), also impairs remyelination.
Interestingly, in the rat model of acute EAE, significantly more new oligodendrocytes were observed in the gray matter than in the white matter. In the mouse model of chronic EAE, however, the white matter contained significantly more new oligodendrocytes than did the gray matter. The difference might be the result of the different times, in relation to the BrdU injection, at which the animals were killed. In the rat model BrdU injections were started on day 14 after immunization, and the rats were killed 7 days later. In the mouse model BrdU was injected on day 20 after immunization, and the mice were killed 12 days later. Thus it is possible that in the rat model, proliferating progenitors (probably originating in the central canal; refs. 2, 61) began differentiating into oligodendrocytes while still in the gray matter, i.e., before migrating to the white matter. This might explain why in the mouse model examination of newly formed oligodendrocytes at later stages showed most of them in the white matter, having migrated there from the gray matter. We do not rule out the possibility, albeit less likely, that mice and rats have different progenitor cell populations originating at different locations of the spinal cord (2). Examination of the primary location of these progenitors is beyond the scope of this work.
The results of this study suggest what we believe to be a novel role for MG in ameliorating EAE and promoting differentiation of oligodendrocytes from adult NPCs (22). They also point to a link within the known beneficial effect of IL-4 in ameliorating EAE (6, 25–27), the role of IGF-I derived from MG(IL-4), and the requirement of viable MG for remyelination (62). Our findings thus support a key role for MG in promoting cell renewal from endogenous progenitors under pathological conditions (63). This notion is in line with the proposal that autoantibodies are needed for remyelination (64). On the basis of the present findings, as well as our previously reported results, we suggest that the cross-talk between T cells and MG lays the foundation for protection and repair in the adult CNS. We further claim that although administration of immunosuppressive treatment alone (with the aim of paralyzing inflammation-associated MG; refs. 7, 8; or T cells; ref. 65) is likely to ameliorate clinical signs at an early stage, it could yield adverse effects in the longer term. This argument is supported by both experimental and clinical data (66). We therefore suggest that rather than suppression, immunomodulation aimed at appropriate and well-controlled activation of MG might be the approach to adopt in designing ways to promote cell renewal under neurodegenerative conditions.
Methods
Animals.
Inbred adult male Lewis rats (12 weeks old), neonatal Lewis rats (P0–P1), adult male C57BL/6J mice (8–10 weeks old), neonatal C57BL/6J mice (P0–P1), and C57BL/6-CX3CR1-GFP heterozygous (CX3CR1/GFP/+) knock-in mice (34) were used. All animals were supplied by the Animal Breeding Center of The Weizmann Institute of Science, and all experiments and procedures were approved by the Weizmann Institute’s Animal Care and Use Committee.
Reagents.
Recombinant rat and mouse IFN-γ and IL-4 (both containing endotoxin at a concentration below 0.1 ng/μg cytokine) and goat anti-mouse neutralizing anti–TNF-α antibodies (containing endotoxin at a concentration below 0.001 EU/μg Ab) were obtained from R&D Systems.
NPC culture.
Coronal sections (2 mm thick) of tissue containing the subventricular zone of the lateral ventricle were obtained from the brains of adult C57BL/6J mice. The tissue was minced and then incubated for digestion at 37°C, 5% CO2 for 45 minutes in Earle’s balanced salt solution containing 0.94 mg/ml papain (Worthington Biochemical Corp.) and 0.18 mg/ml l-cysteine and EDTA. After centrifugation at 110 g for 15 minutes at room temperature, the tissue was mechanically dissociated by pipette trituration. Cells obtained from single-cell suspensions were plated (3,500 cells/cm2) in 75-cm2 Falcon tissue-culture flasks (BD Biosciences) in NPC-culturing medium (DMEM/F12 medium; Invitrogen Corp.; containing 2 mM l-glutamine, 0.6% glucose, 9.6 μg/ml putrescine, 6.3 ng/ml progesterone, 5.2 ng/ml sodium selenite, 0.02 mg/ml insulin, 0.1 mg/ml transferrin, and 2 μg/ml heparin; all from Sigma-Aldrich; and fibroblast growth factor-2 [human recombinant, 20 ng/ml] and epidermal growth factor [human recombinant, 20 ng/ml]; both from PeproTech). Spheres were passaged every 4–6 days and replated as single cells. GFP-expressing NPCs were obtained as previously described (33).
Primary microglial culture.
Brains from neonatal (P0–P1) C57BL/6J mice or Lewis rats were stripped of their meninges and minced with scissors under a dissecting microscope (Stemi DV4; Zeiss) in Leibovitz-15 medium (Biological Industries). After trypsinization (0.5% trypsin for 10 minutes at 37°C, 5% CO2), the tissue was triturated. The cell suspension was washed in culture medium for glial cells (DMEM supplemented with 10% FCS [Sigma-Aldrich], 1 mM l-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 mg/ml streptomycin) and cultured at 37°C, 5% CO2 in 75-cm2 Falcon tissue-culture flasks (BD Biosciences) coated with poly-d-lysine (PDL; 10 mg/ml; Sigma-Aldrich) in borate buffer (2.37 g borax and 1.55 g boric acid dissolved in 500 ml sterile water, pH 8.4) for 1 hour, then rinsed thoroughly with sterile, glass-distilled water. Half of the medium was changed after 6 hours in culture and every second day thereafter, starting on day 2, for a total culture time of 10–14 days. MG were shaken off the primary mixed brain glial cell cultures (150 rpm, 37°C, 6 hours) with maximum yields between days 10 and 14, seeded (105 cells/ml) onto PDL-pretreated 24-well plates (1 ml/well; Corning Incorporated), and grown in culture medium for MG (RPMI-1640 medium; Sigma-Aldrich; supplemented with 10% FCS, 1 mM l-glutamine, 1 mM sodium pyruvate, 50 mM β-mercaptoethanol, 100 U/ml penicillin, and 100 mg/ml streptomycin). The cells were allowed to adhere to the surface of a PDL-coated culture flask for 1 hour at 37°C, 5% CO2, and nonadherent cells were rinsed off.
Coculturing of mouse NPCs and mouse MG.
Mouse MG were treated for 24 hours with cytokines (IFN-γ, 10 ng/ml or 100 ng/ml, and IL-4, 10 ng/ml). Cultures of treated or untreated MG were washed twice with fresh NPC differentiation medium (same as the culture medium for NPCs but without growth factors and with 2.5% FCS) to remove all traces of the tested reagents, then incubated on ice for 15 minutes and shaken at 350 rpm for 20 minutes at room temperature. MG were removed from the flasks and immediately cocultured with NPCs (5 × 104 cells/well for both types of cells) for 10 days on cover slips coated with Matrigel (BD Biosciences) in 24-well plates in the presence of NPC differentiation medium. The cultures were then fixed with 2.5% paraformaldehyde in PBS for 30 minutes at room temperature and stained for neuronal and glial markers.
Induction and evaluation of acute and chronic EAE.
To induce chronic EAE we injected adult male C57BL/6J mice s.c. with 200 μg (300 μl) of MOG35–55 (Sigma-Aldrich) in incomplete Freund’s adjuvant containing 2.5 mg/ml M. tuberculosis (strain H37Ra; BD Diagnostics). Pertussis toxin (500 ng; Sigma-Aldrich) was injected on the day of the immunization and again 2 days later.
To induce monophasic EAE, we immunized adult male Lewis rats s.c. in the hind footpad with 25 μg MBP 68–86, emulsified (1:1 dilution) in 100 μl of complete Freund’s adjuvant containing 2 mg M. tuberculosis (strain H37Ra, BD Diagnostics). Clinical signs were evaluated in a blinded fashion by at least 2 investigators. Body weight and clinical score were recorded daily (0, healthy; 1, limb tail paralysis; 2, ataxia and/or paresis of hind limbs; 3, paralysis of hind limbs and/or paresis of forelimbs; 4, tetraparalysis; 5, moribund state or death).
Stereotaxic injection of activated MG.
Seven days after the vaccination, Lewis rats with monophasic EAE were injected bilaterally with syngeneic MG(–), 10 ng/ml MG(IFN-γ), 10 ng/ml MG(IL-4), or PBS stereotaxically (1 × 105 cells in 5 μl PBS for 5 minutes) into the CSF via the brain lateral ventricles (Bregma, –0.8; L, 1.2; V, 4.5). Ten days after the vaccination, C57BL/6J mice with chronic EAE received bilateral stereotaxic injections of syngeneic MG(IL-4) or PBS (1 × 105 cells in 3 μl PBS for 3 minutes) into the CSF via the brain lateral ventricles (Bregma, –0.4; L, 0.8; V, 2.5).
Administration of BrdU and tissue preparation.
The cell-proliferation marker BrdU was dissolved by sonication in PBS and injected i.p. (50 mg/kg body weight) every 12 hours for 2.5 days starting on day 14 after MBP vaccination in adult male Lewis rats or on day 20 after MOG vaccination in adult male C57BL/6J mice. One week (for rats) or 2 weeks (for mice) after the first BrdU injection, the animals were deeply anesthetized and perfused transcardially, first with PBS and then with 4% paraformaldehyde. Their spinal cords were removed, postfixed overnight, and then equilibrated in phosphate-buffered 30% sucrose. Free-floating 30-μm longitudinal sections were collected on a freezing microtome (SM2000R; Leica Microsystems) and stored at 4°C prior to immunohistochemistry.
Immunocytochemistry and immunohistochemistry.
Cover slips from cocultures of NPCs and mouse MG were washed with PBS; fixed as described above; treated with a permeabilization/blocking solution containing 10% FCS, 2% bovine serum albumin, 1% glycine, and 0.1% Triton X-100 (Sigma-Aldrich); and stained with a combination of the rabbit anti-NG2 chondroitin sulfate proteoglycan (1:500 dilution) and mouse anti-RIP (1:2,000 dilution). To capture the MG we used FITC-conjugated Bandeiraea simplicifolia IB4 (1:50 dilution; Sigma-Aldrich). Expression of IGF-I was detected by goat anti–IGF-I (1:20 dilution; R&D Systems).
For immunohistochemistry, longitudinal sections of the spinal cord or coronal sections of the brain (30 μm) were treated with a permeabilization/blocking solution containing 10% FCS, 2% bovine serum albumin, 1% glycine, and 0.05% Triton X-100 (Sigma-Aldrich). Primary antibodies were applied for 1 hour in a humidified chamber at room temperature. For BrdU staining, sections were washed with PBS and incubated in 2 N HCl at 37°C for 30 minutes. Sections were blocked for 1 hour with blocking solution. The tissue was then stained with rat anti-BrdU (1:200 dilution; Oxford Biotechnology Ltd.) in combination with rabbit anti-NG2 (1:300 dilution) and mouse anti-RIP (1:1,000 dilution) antibodies diluted in PBS containing 0.05% Triton X-100, 0.1% Tween 20, and 2% horse serum. For labeling of MG we used IB4 (1:50 dilution). To detect expression of cell-surface MHC class II proteins we used mouse anti–MHC class II Abs (1:50 dilution; IQ Products). Expression of IGF-I was detected by goat anti–IGF-I Abs (1:10–1:100 dilution; R&D Systems). T cells were detected with anti-CD3 Abs (1:200 dilution; SouthernBiotech). Sections were incubated with the primary antibody for 24 hours at 4°C, washed with PBS, and incubated with the secondary antibodies in PBS for 1 hour at room temperature while protected from light. Secondary antibodies used for both immunocytochemistry and immunohistochemistry were Cy-3–conjugated donkey anti-mouse, Cy-3–conjugated goat anti-rabbit, Cy-5–conjugated goat anti-rat, Cy-2–conjugated goat anti-rat, and Cy-5–conjugated donkey anti-goat. All antibodies were purchased from Jackson ImmunoResearch Laboratories Inc. and used at a dilution of 1:250–1:500. Control sections (not treated with primary antibody) were used to distinguish specific staining from staining of nonspecific antibodies or autofluorescent components. Sections were then washed with PBS and coverslipped in polyvinyl alcohol with diazabicylo-octane as anti-fading agent.
Q-PCR analysis.
Total cellular RNA purification and cDNA synthesis was performed as described previously (19). We assayed the expression of specific mRNAs using Q-PCR with selected gene-specific primer pairs. Q-PCR reactions were performed with a high-speed thermal cycler (LightCycler; Roche Diagnostics Corp.), and the product was detected by FastStart Master SYBR Green I (Roche Molecular Biochemicals) according to the manufacturer’s instructions. The amplification cycle was 95°C for 10 seconds, 60°C for 5 seconds, and 72°C for 10 seconds. The primers used for IGF-I were sense, 5′-CCGGACCAGAGACCCTTTG-3′; antisense, 5′-CCTGTGGGCTTGTTGAAGTAAAA-3′; and for TNF-α were sense 5′-ACAAGGCTGCCCCGACTAT-3′; antisense, 5′-CTCCTGGTATGAAGTGGCAAATC-3′. Melting curve analysis confirmed that only 1 product was amplified.
Quantification and stereological counting procedure.
For microscopic analysis we used a Zeiss LSM 510 confocal laser scanning microscope (magnification, ×40). For experiments in vitro we scanned fields of 0.053 mm2 (n = 8–16 from at least 2 different coverslips) for each experimental group. For each marker, 500–1,000 cells were sampled. Cells coexpressing GFP, NG2, and RIP were counted.
For in vivo experiments with rats and mice with EAE, oligodendrogenesis and proliferation of MG in the spinal cord were evaluated by counting cells that were double or triple labeled with BrdU and markers of premature oligodendrocytes (NG2), MG (IB4), antigen-presenting cells (MHC class II), or a pre-ensheathing marker of oligodendrocytes (RIP) from sagittal longitudinal sections at segment T8–T9 of the spinal cord. The number of cells per cubic millimeter were counted at 300-μm intervals in gray and white matter in each rat (n = 5–8 per group) or mouse (n = 4 per group). Specificity of BrdU+/NG2+, BrdU+/RIP+, BrdU+/IB4+, or BrdU+/IB4+/MHC class II+ coexpression was assayed using the confocal microscope (LSM 510; Zeiss) in optical sections at 1-μm intervals. Counting was evaluated automatically using Image-Pro Plus 4.5 software (MediaCybernetics).
Statistics.
The in vitro results were analyzed by Tukey-Kramer multiple comparison ANOVA and are expressed as mean α SD. In vivo results were analyzed by 2-tailed Student’s t test or 1-way ANOVA and are expressed as mean α SEM. Significance of the EAE score was analyzed by Mann-Whitney test, 2-factor repeated measures ANOVA.
Acknowledgments
We thank S.R. Smith for editing the manuscript. M. Schwartz holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. S. Jung is the incumbent of the Pauline Recanati Career Development Chair and a Scholar of the Benoziyo Center for Molecular Medicine. The work was supported by Proneuron Ltd. and by The Erwin Green Alzheimer’s Research Fund.
Footnotes
Nonstandard abbreviations used: CSF, cerebrospinal fluid; IB4, isolectin B4; MBP, myelin basic protein; MG, microglia; MOG, myelin oligodendrocyte glycoprotein; NPC, neural progenitor cell; Q-PCR, quantitative real-time PCR.
Conflict of interest: The authors have declared that no conflict of interest exist
References
- 1.Eriksson P.S., et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Horner P.J., et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 4.Ekdahl C.T., Claasen J.H., Bonde S., Kokaia Z., Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartung H.P., et al. Inflammatory mediators in demyelinating disorders of the CNS and PNS. . J. Neuroimmunol. 1992;40:197–210. doi: 10.1016/0165-5728(92)90134-7. [DOI] [PubMed] [Google Scholar]
- 6.Olsson T. Critical influences of the cytokine orchestration on the outcome of myelin antigen-specific T-cell autoimmunity in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Rev. 1995;144:245–268. doi: 10.1111/j.1600-065x.1995.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Heppner F.L., et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 8.Huitinga I., van Rooijen N., de Groot C.J., Uitdehaag B.M., Dijkstra C.D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J. Exp. Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Probert L., et al. TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J. Neuroimmunol. 1997;72:137–141. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 10.Merrill J.E., Ignarro L.J., Sherman M.P., Melinek J., Lane T.E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. . J. Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 11.Chao C.C., Hu S., Molitor T.W., Shaskan E.G., Peterson P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. . J. Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 12.Stirling D.P., et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieber A.J., Kerr S., Rodriguez M. Efficient central nervous system remyelination requires T cells. Ann. Neurol. 2003;53:680–684. doi: 10.1002/ana.10578. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz M., Shaked I., Fisher J., Mizrahi T., Schori H. Protective autoimmunity against the enemy within: fighting glutamate toxicity. Trends Neurosci. 2003;26:297–302. doi: 10.1016/S0166-2236(03)00126-7. [DOI] [PubMed] [Google Scholar]
- 15.Hauben E., Schwartz M. Therapeutic vaccination for spinal cord injury: helping the body to cure itself. Trends Pharmacol. Sci. 2003;24:7–12. doi: 10.1016/s0165-6147(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 16.Moalem G., et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 17.Butovsky O., Hauben E., Schwartz M. Morphological aspects of spinal cord autoimmune neuroprotection: colocalization of T cells with B7-2 (CD86) and prevention of cyst formation. FASEB J. 2001;15:1065–1067. doi: 10.1096/fj.00-0550fje. [DOI] [PubMed] [Google Scholar]
- 18.Shaked I., Porat Z., Gersner R., Kipnis J., Schwartz M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J. Neuroimmunol. 2004;146:84–93. doi: 10.1016/j.jneuroim.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Butovsky O., Talpalar A.E., Ben-Yaakov K., Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol. Cell. Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Shaked I., et al. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. . J. Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- 21.Wildbaum G., Nahir M.A., Karin N. Beneficial autoimmunity to proinflammatory mediators restrains the consequences of self-destructive immunity. Immunity. 2003;19:679–688. doi: 10.1016/s1074-7613(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh J., et al. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butovsky O., et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2005;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Mothe A.J., Tator C.H. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Aharoni R., Teitelbaum D., Sela M., Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. . Proc. Natl. Acad. Sci. U. S. A. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L.Y., et al. Suppression of ongoing experimental allergic encephalomyelitis (EAE) in Lewis rats: synergistic effects of myelin basic protein (MBP) peptide 68-86 and IL-4. Clin. Exp. Immunol. 2000;120:526–531. doi: 10.1046/j.1365-2249.2000.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlan R., et al. Central nervous system gene therapy with interleukin-4 inhibits progression of ongoing relapsing-remitting autoimmune encephalomyelitis in Biozzi AB/H mice. Gene Ther. 2001;8:13–19. doi: 10.1038/sj.gt.3301357. [DOI] [PubMed] [Google Scholar]
- 28.Duda P.W., Schmied M.C., Cook S.L., Krieger J.I., Hafler D.A. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J. Clin. Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juedes A.E., Ruddle N.H. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J. Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 30.Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J. Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 31.Neumann H., Misgeld T., Matsumuro K., Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: involvement of the p75 neurotrophin receptor. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5779–5784. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W.W., Setzu A., Zhao C., Franklin R.J. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J. Neuroimmunol. 2005;158:58–66. doi: 10.1016/j.jneuroim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Pluchino S., et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 34.Jung S., et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davalos D., et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 36.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 37.Dijkstra C.D., De Groot C.J., Huitinga I. The role of macrophages in demyelination. J. Neuroimmunol. 1992;40:183–188. doi: 10.1016/0165-5728(92)90132-5. [DOI] [PubMed] [Google Scholar]
- 38.Popovich P.G., et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 39.Mitrasinovic O.M., et al. Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system. . J. Neurosci. 2005;25:4442–4451. doi: 10.1523/JNEUROSCI.0514-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason J.L., Goldman J.E. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol. Cell. Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- 41.Barres B.A., Schmid R., Sendnter M., Raff M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 42.Mason J.L., Ye P., Suzuki K., D’Ercole A.J., Matsushima G.K. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J. Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X., et al. Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol. Cell. Neurosci. 1994;5:418–430. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- 44.Hinks G.L., Franklin R.J. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol. Cell. Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- 45.Kooijman R., Coppens A. Insulin-like growth factor-I stimulates IL-10 production in human T cells. J. Leukoc. Biol. 2004;76:862–867. doi: 10.1189/jlb.0404248. [DOI] [PubMed] [Google Scholar]
- 46.Thorne R.G., Pronk G.J., Padmanabhan V., Frey W.H., 2nd. Science. 127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 47.Jin K., et al. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann. Neurol. 2003;53:405–409. doi: 10.1002/ana.10506. [DOI] [PubMed] [Google Scholar]
- 48.Ross T.M., et al. Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol. 2004;151:66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Arnett H.A., Wang Y., Matsushima G.K., Suzuki K., Ting J.P. Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration. J. Neurosci. 2003;23:9824–9832. doi: 10.1523/JNEUROSCI.23-30-09824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greter M., et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 51.Jung S., et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mason J.L., Xuan S., Dragatsis I., Efstratiadis A., Goldman J.E. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J. Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ness J.K., Wood T.L. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol. Cell. Neurosci. 2002;20:476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- 54.Aberg M.A., Aberg N.D., Hedbacker H., Oscarsson J., Eriksson P.S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trejo J.L., Carro E., Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudek H., et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 57.Cannella B., Pitt D., Capello E., Raine C.S. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. Am. J. Pathol. 2000;157:933–943. doi: 10.1016/S0002-9440(10)64606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruce A.J., et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 59.Frei K., et al. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J. Exp. Med. 1997;185:2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picard-Riera N., et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morshead C.M., et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 62.Kotter M.R., Setzu A., Sim F.J., Van Rooijen N., Franklin R.J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 63.Ziv Y., et al. T cells – immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 64.van Engelen B.G., Pavelko K.D., Rodriguez M. Enhancement of central nervous system remyelination in immune and non-immune experimental models of demyelination. Mult. Scler. 1997;3:76–79. doi: 10.1177/135245859700300203. [DOI] [PubMed] [Google Scholar]
- 65.Yednock T.A., et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 66.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat. Rev. Drug Discov. 2005;4:510–51. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]