Abstract
The immune system is capable of mounting robust responses against invading pathogens but refrains from attacking self. Many studies have focused on tolerance induction of Th1 cells, whose failure results in development of autoimmune diseases. However, the molecular mechanisms governing tolerance induction in Th2 cells and its relation to allergic responses remain unclear. Here we used both in vivo and in vitro protocols to demonstrate that Th2 cells either containing a mitogen and extracellular kinase kinase 1 (MEKK1) mutant or lacking JNK1 or the E3 ubiquitin ligase Itch cannot be tolerized. In a mouse allergic model, injection of high-dose tolerizing antigen failed to block the development of airway inflammation in Itch–/– mice. This study suggests that MEKK1-JNK signaling regulates Itch E3 ligase–mediated tolerogenic process in Th2 cells. These findings have therapeutic implications for allergic diseases.
Introduction
Pathogen invasion elicits an adaptive immune response through the generation of antigen-specific T cells. Antigenic stimulation of T cells drives naive CD4+ T cells into effector T cells of either Th1 or Th2 phenotypes, depending on the property of the antigenic peptide and the strength or duration of the stimulation (1). Th1 cells are characterized by production of proinflammatory IFN-γ, which is effective in counteracting viral infections and other intracellular pathogens, whereas Th2 cells produce IL-4 and are responsible for the immune elimination of extracellular helminthic pathogens (2). Although a robust immune response is critical for successful elimination of pathogens, the immune system is also equipped with a mechanism to prevent excessive damage to normal cells and tissues, termed as self. Failed self-tolerance may lead to adverse consequences: uncontrolled Th1 responses can result in autoimmune diseases such as type 1 diabetes or rheumatoid arthritis (3, 4), whereas abnormal Th2 cell activation can result in asthmatic or allergic symptoms (5).
Multiple mechanisms have been implicated in induction of peripheral immune tolerance, including peripheral deletion of autoreactive T cells, generation of Tregs, and T cell anergy (6, 7). It is well known that effective T cell activation requires both antigen-specific engagement of the T cell antigen receptor (TCR) and costimulation of accessory molecules. Stimulation of TCR alone in the absence of costimulation results in an inactive state that renders T cells hypoproliferative to secondary encounter with fully activating stimuli, a phenomenon called T cell anergy (8). The molecular mechanisms underling induction of T cell anergy have been an active area of investigation. A recent study suggested the involvement of a genetic program in which multiple genes are up- or downregulated in response to incomplete stimulation, which eventually affects the T cell responses to complete antigenic stimulation (9). It was found that several structurally and functionally diverse E3 ubiquitin ligases, such as Cbl-b, Itch, and Grail, are upregulated in anergic T cells (10–12), thus implicating the protein ubiquitination pathway in development of immune tolerance. However, whether Itch is involved in T cell tolerance in vivo remains unknown.
The E3 ligase Itch was originally discovered by mapping of the gene locus responsible for the mutation in itchy mice, which display abnormal immunological and inflammatory immune responses, and constant itching in the skin, particularly in older mice (13). Itch polypeptide contains an N-terminal PKC-related C2 domain, protein-interacting WW domains, and a C-terminal HECT (homologous to the E6-associated protein carboxy terminus) ligase domain, thus belonging to the HECT type of E3 ligases (14, 15). Itch associates with Jun family proteins c-Jun and JunB through its WW domains and promotes their ubiquitination (16). The upregulation of JunB in Itch–/– T cells drives a Th2-biased differentiation, which correlates with increased levels of IgG1 and IgE in the sera of itchy mice (16). This conclusion is supported by increased Th2 differentiation in JunB transgenic mice (17) and defective Th2 development in JunB-ablated mice (18). Importantly, Itch is the first E3 ligase found to be directly activated by phosphorylation (19). Itch activation in T cells requires signaling from the TCR via the mitogen and extracellular kinase kinase 1 (MEKK1) to JNK1. T cells that either carry a MEKK1 kinase-dead mutant (MEKK1ΔKD) or lack JNK1 activities display increased production of Th2 cytokines, just like Itch–/– T cells (19). It should be noted that earlier studies have documented that anergic T cells are defective in transactivation or transcription of Jun proteins (20, 21). It is not clear whether this JNK-Itch genetic pathway plays a role in T cell tolerance.
The majority of studies on T cell anergy have been focused on Th1 cells, with very few reports addressing intracellular events involved in induction of anergic Th2 cells (22). To further understand the immunological significance of JNK signaling and Itch in T cell anergy, we established an in vivo mouse model of antigen-induced tolerance. We demonstrate that MEKK1, JNK1, and Itch are involved in Th2 immune tolerance. A deficiency in any of these molecules results in resistance to either in vivo antigen-induced or in vitro–induced T cell anergy. Thus, the results provide a molecular mechanism explaining the generation of peripheral Th2 tolerance, which has implications in the prevention and treatment of allergic diseases.
Results
Loss of Itch results in resistance to in vivo tolerance induction.
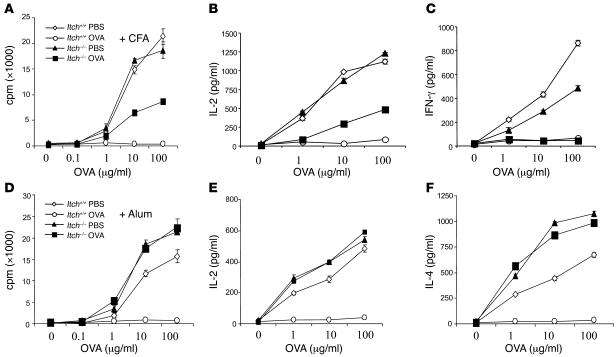
Itch was recently suggested to be involved in T cell anergy induction in vitro (10). To better understand the biological role of Itch in T cell tolerance, we established an in vivo mouse model of immune tolerance. In this model, mice were immunized s.c. with an OVA-derived antigenic peptide conjugated with either CFA to induce a Th1 response, or alum to elicit a Th2 reaction (23). In addition, the mice received concurrent i.p. injection of soluble high-dose OVA to induce tolerance, or PBS as control. Eight- to 10-week-old mice were used in these experiments, and at these ages, the mice did not exhibit any immunological abnormality. It should be mentioned that we established a protocol that allowed us to induce Th1 or Th2 tolerance in similar settings, in contrast to the classical method using inhaled low-dose antigen to induce only Th2 tolerance via the generation of regulatory γ/δ T cells as previously described (24). Ten days after immunization, we isolated splenic and popliteal lymph node T cells and measured their proliferation in response to OVA peptide plus irradiated APCs. WT T cells displayed reduced proliferation when the mice were tolerized with OVA, in comparison with T cells from PBS-treated mice (Figure 1A). By contrast, Itch–/– T cells from OVA-tolerized mice showed higher proliferative response compared with T cells from similarly treated WT mice, when both mice were immunized with OVA plus CFA. IL-2 production was also markedly reduced in WT T cells tolerized with soluble OVA as compared with PBS-treated cells (Figure 1B). Itch–/– T cells from OVA-tolerized mice showed modest IL-2 production in response to restimulation with OVA peptide, when the mice were immunized with OVA-CFA. However, Itch deficiency did not prevent the inhibition of IFN-γ production in T cells from OVA-tolerized mice (Figure 1C).
Figure 1. Itch deficient mice are resistant to tolerance induction.
(A) WT and Itch–/– mice were immunized with OVA in CFA, together with simultaneous injection of soluble OVA or PBS as control. Splenic and lymph node T cells (5 × 105 cells per well) from OVA-immunized Itch+/+ and Itch–/– mice tolerized with OVA or PBS as control were cultured in triplicate with increasing concentrations of OVA peptide plus irradiated splenocytes as APCs. After 48 hours of culture, the cell proliferation was measured by 3H-thymidine incorporation using a scintillation counter. (B and C) The supernatants obtained at 48 hours of culture as in A were collected and were measured for the concentrations of IL-2 (B) and IFN-γ (C) by ELISA. (D–F) Similar experiments were performed, but the mice were immunized with OVA in alum. The concentrations of IL-2 (E) and IL-4 (F) were measured.
Markedly, Itch deficiency almost completely prevented the OVA-induced inhibition of the proliferative response in T cells from mice immunized with OVA plus alum (Figure 1D). Correspondingly, Itch–/– T cells isolated from mice immunized with OVA plus alum continued to produce normal amounts of IL-2, despite tolerization with a high dose of soluble OVA (Figure 1E). Notably, with Th2-biased immunization, IL-4 production was not inhibited in Itch–/– T cells (Figure 1F). It should be noted that this protocol truly induced Th2 cell anergy, not T cell death, since this unresponsive state was reversed by the addition of exogenous IL-2 (see Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI26858DS1). Thus, Itch is involved in T cell tolerance induction, particularly under Th2-biased conditions.
Itch in T cell anergy induction in vitro.
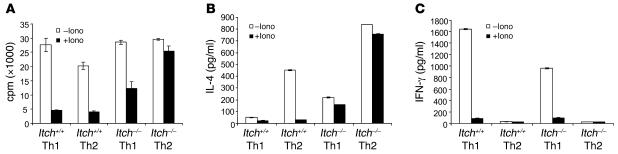
To further complement our observation on a functional role of Itch in T cell tolerance induction in the in vivo system, we adopted an in vitro T cell anergy system in which purified naive CD4+ T cells were first expanded under either Th1 or Th2 conditions, using a recently published protocol with ionomycin pretreatment (12). The cells were then treated with ionomycin for 16 hours and restimulated with anti-CD3 plus anti-CD28 for the measurement of cell proliferation and cytokine production. As in the in vivo antigen-induced tolerance, ionomycin pretreatment of both Th1 and Th2 WT T cells resulted in proliferative inhibition (Figure 2A). In the Th1-differentiated Itch–/– T cells, there was a partial restoration of cell proliferation. In contrast, the Itch–/– Th2 cells displayed almost equivalent thymidine incorporation with or without ionomycin treatment.
Figure 2. Th2 cells from Itch–/– mice fail to be anergized in vitro.
(A) Naive CD4+ T cells were purified using MACS beads. Cells were differentiated into either Th1 or Th2 for 6 days. The viable cells were rested overnight and were treated with ionomycin (50 ng/ml) for 16 hours and washed twice; cells (2 × 105) were then stimulated in triplicate with anti-CD3 plus anti-CD28 for 48 hours. The proliferative response was determined by the measurement of 3H-thymidine incorporation. (B and C) IL-4 (B) and IFN-γ (C) cytokines were measured by sandwich ELISA using the culture supernatant harvested after 48 hours as in A.
The cytokine production from those cells was also measured by ELISA. Consistent with our previous observations (16), the production of IL-4 was increased in Itch–/– Th2 cells (Figure 2B). Ionomycin pretreatment caused a reduction of IL-4 production in WT Th2 cells, and loss of Itch resulted in a marked resistance to ionomycin-induced suppression of IL-4 production in Th2 cells (Figure 2B). Compared with WT Th1 cells, the IFN-γ production was reduced in Itch–/– Th1 cells (Figure 2C). However, ablation of Itch did not rescue the reduced IFN-γ production in ionomycin-pretreated cells. To further complement the ionomycin-induced T cell anergy, we took an alternative approach to induce T cell tolerance with anti-CD3 in the absence or presence of B7.1 costimulation. Consistent with the ionomycin experiment, it was found that absence of costimulation resulted in T cell anergy in WT Th1 and Th2 cells (Supplemental Figure 2). However, Itch deficiency caused a marked rescue in anergized Th2 cells, with much less effect on Th1 cells. Therefore, as found in antigen-induced T cell tolerance in vivo, Itch is an important tolerogenic regulator for Th2 cells, while having only a modest effect on Th1 cells.
Loss of T cell tolerance in Itch–/– TCR transgenic mice.
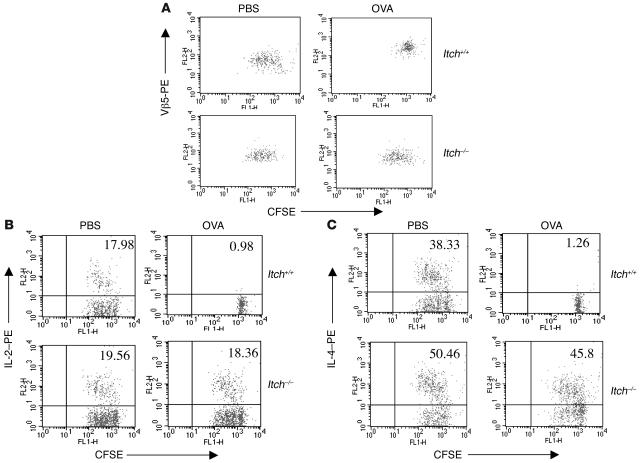
In addition to the in vivo and in vitro systems as described above, we further investigated whether the tolerized state of T cells can be maintained, following previously published protocols (12, 25, 26). Briefly, we performed adoptive transfer experiments by using PBS-treated or antigen-tolerized T cells from WT and Itch–/– mice containing an OVA-specific CD4-restricted Vβ5/Vα2 OTII TCR transgene. The isolated T cells were first labeled with CFSE and then adoptively transferred into syngeneic Thy1.1 mice. The recipient mice were then challenged with OVA peptide in alum to initiate a Th2 immune response. The division of adoptively transferred T cells was analyzed by FACS analysis. WT T cells (Vβ5+ and Thy1.2+) from OVA-tolerized mice exhibited markedly reduced division in comparison with WT T cells from PBS-treated mice (Figure 3A, top panels). However, Itch–/– T cells from OVA-tolerized mice exhibited almost the same level of division as cells from PBS-treated Itch–/– mice upon antigen restimulation (Figure 3A, bottom panels).
Figure 3. Tolerance induction of antigen-specific T cells.
(A) Itch+/+ and Itch –/– OTII mice were treated with PBS or with high-dose soluble OVA peptide. CD4+ T cells (Vβ5+Thy1.2+) isolated from those mice were labeled with CFSE and adoptively transferred into syngeneic Thy1.1 mice, followed by immunization with OVA in alum. The CFSE profile was analyzed in Thy1.2-gated Vβ5+ T cells. (B and C) T cells from the adoptively transferred mice were stimulated with OVA plus APCs in vitro, and the CFSE profile and intracellular IL-2 (B) and IL-4 (C) were examined by FACS analysis.
We next examined cell division and cytokine production of adoptively transferred cells in vitro by using a combination of intracellular staining and CFSE labeling. Antigen restimulation of purified CD4+ T cells from PBS-treated mice induced both proliferation and cytokine (IL-2 and IL-4) production in WT and Itch–/– T cells (Figure 3, B and C, top left panels). As expected, OVA-tolerized WT T cells exhibited proliferative arrest as well as reduced IL-2 and IL-4 production (top right panels). However, adoptively transferred T cells from OVA-tolerized Itch–/– mice proliferated in response to in vitro antigen stimulation and continued to produce both IL-2 and IL-4 (bottom right panels), although the percentages of IL-2– and IL-4–producing cells were slightly lower than in T cells from PBS-treated counterparts. These results further establish a role for Itch in maintenance of T cell tolerance.
Itch and Jun expression in tolerized Th2 cells.
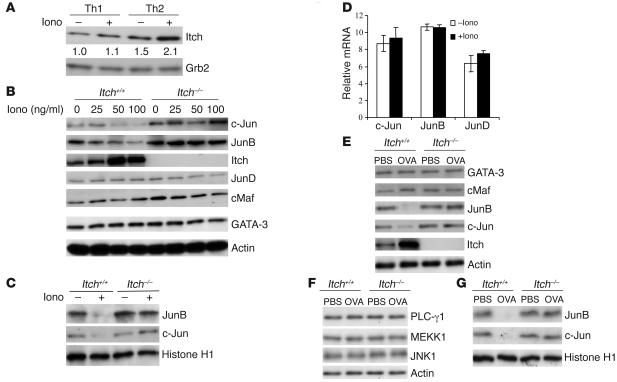
To obtain molecular insights into the role of Itch in anergy induction in Th2 cells, we examined Itch protein expression in differentiated Th1 or Th2 cells untreated or treated with ionomycin for 16 hours and found that Itch was elevated in Th2 cells after ionomycin treatment, whereas in Th1 cells, Itch levels remained largely unchanged (Figure 4A).
Figure 4. Intracellular events in anergic Th2 cells.
(A) Detection of Itch in Th1 and Th2 cells upon ionomycin (Iono) treatment. Differentiated Th1 or Th2 cells were left unstimulated or stimulated with ionomycin (50 ng/ml) for 16 hours. Cell lysates were probed with anti-Itch antibody. The intensity of the protein bands was quantitated, and the relative amount of Itch in untreated WT T cells was given an arbitrary value of 1.0. The same membrane was reprobed with anti-actin to assess equal loading of the samples. (B) Th2 cells were first treated with different concentrations of ionomycin for 16 hours, and the cell lysates were immunoblotted with antibodies as indicated. (C) Th2 cells were left untreated or treated with ionomycin, washed, and then stimulated with anti-CD3 plus anti-CD28. The nuclear fractions were isolated and immunoblotted with anti-JunB, –c-Jun, or –histone H1 antibody. (D) Th2 cells left untreated or treated with ionomycin were measured for the mRNA levels of c-Jun, JunB, or JunD by real-time PCR. (E) T cells from PBS-treated or OVA-tolerized WT or Itch–/– OTII mice were analyzed for the protein levels of the indicated molecules by immunoblotting. (F) Lysates from cells treated similarly to those described above were immunoblotted with the indicated antibodies. (G) T cells as in E were restimulated with OVA peptide plus irradiated splenocytes. The nuclear fractions were isolated and immunoblotted with the indicated antibodies.
We have previously found that loss of Itch leads to JunB accumulation in T cells (16). To assess whether Itch-regulated Th2 cell anergy induction involves changes in JunB or other transcription factors, we examined the intracellular protein levels of such molecules in untreated or ionomycin-anergized Th2 cells. There were no obvious changes in the protein levels of GATA-3 or c-Maf before or after ionomycin treatment in WT Th2 cells (Figure 4B). However, the levels of JunB and c-Jun, but not JunD, were markedly reduced after ionomycin treatment. Importantly, this reduction in JunB and c-Jun levels was not observed in Itch–/– Th2 cells even after ionomycin treatment. Notably, we observed a dose-dependent increase in Itch expression in WT cells upon ionomycin stimulation. We further examined the nuclear translocation of Jun proteins in ionomycin-treated cells upon restimulation. The amounts of nuclear JunB and c-Jun were also decreased in anergic WT T cells (Figure 4C). This reduction was not seen in Itch–/– T cells. To further confirm that Itch regulates Jun proteins through protein turnover, we examined the mRNA levels of the different Jun family members by real-time PCR and found no significant change upon ionomycin treatment (Figure 4D).
We also investigated whether Itch regulates JunB and c-Jun levels in T cells tolerized in vivo. There was an obvious reduction in the amounts of JunB and c-Jun in lysates of OVA-tolerized WT T cells, but not in Itch–/– T cells from similarly treated mice (Figure 4E), whereas other signaling molecules such as PLC-γ1, MEKK1, or JNK1 remained unchanged (Figure 4F). Similarly, the levels of JunB and c-Jun were reduced in nuclear extracts of OVA-tolerized WT T cells, and loss of Itch rescued such reduction to a large degree (Figure 4G).
A role for MEKK1-JNK1 signaling in Th2 tolerance.
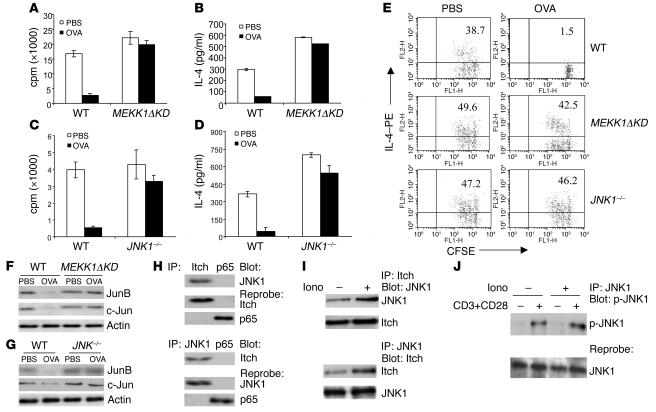
A recent study showed that T cells containing MEKK1ΔKD are biased toward Th2 cytokine production (19), a phenotype similar to that exhibited by Itch–/– T cells (16). Furthermore, MEKK1 activity is required for JNK1-dependent Itch activation and subsequent JunB and c-Jun degradation (19). Thus, if Itch-dependent JunB degradation is required for induction of Th2 tolerance, MEKK1ΔKD or JNK1–/– mice should be refractory to tolerance induction. To test this prediction, we examined in vivo tolerance induction of WT and MEKK1ΔKD T cells using the OVA-alum immunization protocol described above. Like Itch–/– T cells, MEKK1ΔKD T cells were refractory to the soluble antigen–induced proliferative inhibition (Figure 5A). We next examined the cytokine production by those cells. More IL-2 and IL-4 were produced in MEKK1ΔKD T cells in comparison with WT T cells upon restimulation with OVA peptide plus APCs (Figure 5B and Supplemental Figure 3A).
Figure 5. MEKK1 and JNK1 in Th2 tolerance.
(A) WT or MEKK1ΔKD mice were tolerized with soluble OVA or treated with PBS as control, plus simultaneous immunization with OVA in alum. T cells were stimulated for proliferation in vitro by OVA antigen peptide plus APCs. (B) The production of IL-4 from the in vitro–cultured cells was measured. (C and D) WT or JNK1–/– mice were subjected to tolerance induction. The cell proliferation and IL-4 production were determined. (E) Adoptive transfer of PBS-treated or OVA-tolerized T cells from WT, MEKK1ΔKD, or JNK1–/– mice. The purified CD4+ T cells were labeled with CFSE, and the recipient mice were immunized with OVA in alum. The intracellular IL-4 production and the CFSE profile of gated Thy1.2+ T cells were examined. (F and G) Lysates from T cells from PBS-treated or OVA-tolerized WT, MEKK1ΔKD, or JNK1–/– mice were immunoblotted with antibodies as indicated. (H) T cells from OVA-tolerized WT mice were subjected to immunoprecipitation with anti-Itch (top panel) or anti-JNK1 (bottom panel), with anti-p65 of NF-κB as a control. The immunoprecipitates were blotted with the indicated antibodies. (I) The interaction of JNK1 and Itch was analyzed from cells without or with ionomycin treatment. (J) T cells were left untreated or tolerized with ionomycin, and the cells were restimulated with anti-CD3 plus anti-CD28 for 10 minutes. Lysates were immunoprecipitated with anti-JNK1 antibody and immunoblotted with anti–phospho-JNK1 (p-JNK1).
In addition to MEKK1ΔKD mice, JNK1–/– mice also display increased IL-4 production (27, 28), and JNK1 directly activates Itch ligase activity through its phosphorylation (19). We therefore investigated whether JNK1 is also involved in tolerance induction of Th2 cells. Indeed, similarly to MEKK1ΔKD T cells, JNK1–/– T cells showed resistance to soluble OVA–induced inhibition of proliferation (Figure 5C), IL-4 (Figure 5D), and IL-2 production (Supplemental Figure 3B). In addition, we performed in vitro anergy studies using differentiated Th2 cells from MEKK1ΔKD or JNK1–/– mice and found that Th2 cells from those mutant mice were refractory to ionomycin-induced T cell anergy (data not shown). We further examined a potential role of MEKK1 or JNK1 in the tolerance induction of Th1 cells using an in vivo protocol, as described earlier. Loss of MEKK1 or JNK1 did not have much effect on the Th1 anergy induction (Supplemental Figure 4, A and B), whereas under the same conditions, Th2 tolerance was largely rescued in those mutant T cells (Supplemental Figure 4, C and D).
We also performed an adoptive transfer experiment by using CFSE-labeled cells from control or OVA-tolerized WT, MEKK1ΔKD, or JNK1–/– mice to examine the property of tolerance maintenance of the tolerized T cells. The recipient mice were immunized with OVA peptide in alum, and their T cells were isolated and examined for cell division and intracellular cytokine production. WT T cells from OVA-tolerized mice did not divide and failed to produce IL-2 or IL-4 (Figure 5E). However, T cells from OVA-injected MEKK1ΔKD or JNK1–/– mice continued to proliferate and produced IL-4 (Figure 5E) and IL-2 (Supplemental Figure 3C).
Since MEKK1ΔKD and JNK1–/– T cells also contain more JunB and c-Jun proteins (19), we also examined the levels of Jun proteins in the tolerized T cells derived from WT or mutant mice. The reduced levels of JunB and c-Jun in OVA-tolerized WT T cells were not observed in MEKK1ΔKD T cells (Figure 5F) or in JNK1–/– T cells (Figure 5G). To further link the Itch-mediated protein ubiquitination with JNK signaling, we examined the association between Itch and JNK1. We consistently observed interaction between the 2 molecules in different cell systems, including transiently transfected cells and cultured cell line cells, most likely via Itch WW domains (data not shown). In addition, we observed an interaction between Itch and JNK1 in tolerized T cells (Figure 5H). More importantly, this interaction between JNK1 and Itch was increased in tolerized T cells in comparison with nontolerized cells (Figure 5I). It should be noted that JNK1 phosphorylation was not altered before or after ionomycin tolerization (Figure 5J), implying that JNK1 is upstream of Itch-mediated JunB degradation.
Effect of reduced JunB expression on Th2 anergy.
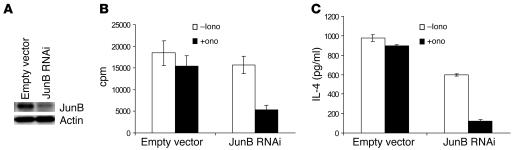
The above-described results clearly suggested that MEKK1-JNK1 signaling is involved in the tolerance induction of Th2 cells, most likely via Itch-mediated ubiquitination and degradation of JunB. To further determine a critical role of JunB in Th2 anergy induction, we investigated whether knock down of JunB in Itch–/– Th2 cells can reestablish an anergic state. Retroviral vector carrying JunB interfering RNA sequences (29) was transduced into Itch–/– Th2 cells, which led to a decrease in JunB protein levels (Figure 6A). These cells were further subjected to ionomycin-induced anergy induction. Although Itch–/– Th2 cells were not anergized by ionomycin treatment, as described earlier, reduction of JunB expression resulted in marked decreases in T cell proliferation (Figure 6B) and in IL-4 production (Figure 6C) upon restimulation with anti-CD3 plus anti-CD28. The results clearly suggest that JunB is the substrate for Itch during Th2 anergy induction.
Figure 6. Suppression of JunB expression reverses anergy induction in Itch–/– T cells.
(A) Naive CD4+ T cells were isolated from Itch–/– mice using MACS beads, and the cells were cultured under Th2-differentiating conditions for 2 days. The cells were then infected with retrovirus derived from pSuper interfering RNA (RNAi) empty vector or pSuper JunB RNAi. Aliquots of cell lysates were immunoblotted with anti-JunB. The same membrane was reprobed with anti-actin. (B and C) The cells were then incubated with ionomycin for 16 hours, washed, and restimulated with anti-CD3 and anti-CD28. The cell proliferation (B) and IL-4 production (C) were measured.
Th2 tolerance in the development of airway inflammation.
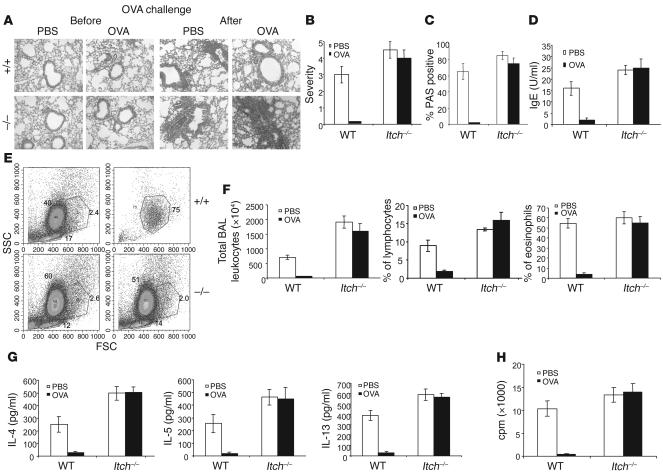
To further examine the biological significance of Itch-mediated Th2 tolerance, we established a protocol to combine soluble antigen treatment with a murine model of lung inflammation. It was found that preinjection of a high dose of OVA peptide into the WT mice blocked airway inflammation, as revealed by absence of alveolar lymphocyte infiltration in the lung (Figure 7A, top panels). In sharp contrast, Itch–/– mice displayed similar levels of lung inflammation and lymphocyte accumulation with PBS treatment or OVA tolerization (Figure 7A). It should be noted that high-dose exposure of either WT or Itch–/– mice to OVA did not lead to the development of lung inflammation. We further performed statistical analysis of lung inflammation by counting pathological lesions. High-dose OVA exposure almost completely abrogated the lung inflammatory lesions, whereas Itch–/– mice showed almost similar severity with or without OVA tolerance induction (Figure 7B). Similarly, we counted the numbers of mucus-secreting bronchial epithelial cells, and the OVA-tolerized Itch–/– mice displayed levels of PAS+ mucus-secreting cells in the lung airway that were similar to the levels in PBS-treated Itch–/– mice (Figure 7C). The serum concentrations of OVA-specific IgE were also measured, and the reduced IgE production in OVA-tolerized WT mice was not detected in Itch–/– mice (Figure 7D). Thus, loss of Itch results in excessive Th2 response and development of elevated airway inflammation.
Figure 7. Breakdown of Th2 tolerance leads to airway inflammation.
(A) WT or Itch–/– mice (4 mice for each group) were injected with high-dose soluble OVA or PBS as control on day 0 and day 3. On day 9, the mice were sensitized with OVA in alum, followed by aerosol OVA exposure for 4 consecutive days from day 19. A portion of lung tissues before or after OVA aerosol challenge was processed for H&E staining. (B) The lung sections stained with H&E were scored for severity of inflammation on a scale of 0–5; at least 500 fields were scored per mouse. The data represent the means of 4 mice per group. (C) Lung sections were stained with PAS, and PAS+ cells were counted; 1,000 goblet cells were counted. The results represent the mean percentage of positive cells from 4 mice. (D) Sera collected from the mice were analyzed for OVA-specific IgE by ELISA. (E) BAL fluid was analyzed for granulocyte and lymphocyte infiltration by flow cytometry. FSC, forward scatter; SSC, side scatter. (F) Total cell numbers and the percentage of lymphocytes and eosinophils were determined. (G) The cytokine concentrations for IL-4, IL-5, and IL-13 in BAL fluid were measured by ELISA. (H) T cells from bronchial lymph nodes of WT and Itch–/– mice were stimulated in vitro with OVA plus APCs, and the cell proliferation was measured by thymidine incorporation. A–D are representatives of 3 repeated experiments with 4 mice per group.
We further examined the lymphocyte infiltration in the bronchoalveolar lavage (BAL) fluid and found that, in OVA-tolerized WT mice, the total numbers of leukocytes and the numbers of lymphocytes and eosinophils in BAL fluid were reduced (Figure 7, E and F). Cytokine concentrations in BAL fluid were also measured. Consistent with the more severe allergic phenotype, there were higher amounts of IL-4, IL-5, and IL-13 in BAL fluid of Itch–/– mice than in that of nontolerized mice (Figure 7G). The amounts of those cytokines were markedly reduced in OVA-tolerized WT mice, whereas higher levels of Th2 cytokines were detected in BAL samples of Itch–/– mice even after tolerization. In addition, we examined the tolerance induction of T cells from bronchial draining lymph nodes. The proliferative response of WT T cells from OVA-tolerized mice was completely inhibited (Figure 7H). In sharp contrast, Itch–/– T cells from PBS-treated and OVA-treated mice showed similar levels of proliferation. These results further support an important role for Itch in Th2-mediated allergic responses.
Discussion
The present study establishes a novel role for JNK signaling to Itch in peripheral tolerance induction by using both in vivo and in vitro models. The JNK-Itch pathway is primarily involved in anergy induction in Th2 cells, with only a modest contribution to tolerance induction in Th1 cells. Although previous studies have established the role of JNK1-dependent Itch activation in Th2 cytokine expression (19), this is the first time to our knowledge that this pathway has been shown to be involved in induction of Th2 tolerance. Consistent with Itch-mediated JunB ubiquitination, successfully anergized T cells contain lower levels of JunB and c-Jun than nontolerized cells, thereby preventing transcriptional activation of JunB-dependent IL-4 genes. Importantly, Itch-mediated tolerance induction of Th2 cells is relevant to the development of allergic airway inflammation. Thus, the present study provides solid genetic and biochemical evidence suggesting that JNK-induced Itch activation plays an important role in the tolerogenic process of Th2 cells.
Dysregulated Th2 responses result in increased allergic responses characterized by airway inflammation, eosinophilia, mucus production, and hypersensitivity via excessive secretion of Th2 cytokines including IL-4, IL-5, and IL-13 (2). It remains controversial, however, whether T cell anergy is involved in airway inflammation. It was hypothesized that a strong Th1 response can dampen Th2 cytokine production, and that reduced exposure to infectious pathogens diminishes the Th1 response, which leads to an increased Th2 response, and thus development of allergic asthma (i.e., the hygiene hypothesis) (5). Another mechanism involves generation of antigen-specific Tregs, via ICOS–ICOS ligand interaction, which inhibits allergen-induced pulmonary inflammation (30). In this study, we demonstrate the existence of a T cell intrinsic mechanism by which Th2 cells can be induced to enter a nonresponsive anergized state. Defective operation of this mechanism brought about by deficiencies in Itch, JNK1, or MEKK1 activities prevents Th2 tolerance induction and potentiates allergic inflammation.
Although the phenomenon of T cell anergy has been known for more than a decade, the majority of the experimental evidence was obtained with Th1 cells, with few examples documenting Th2 tolerance and addressing its underlying mechanisms. In fact, anergized Th0 cells produce IL-4 and IL-5, thus resembling Th2 cells (31). Injection of high-dose aqueous antigen was found to induce only Th1 tolerance without much of an effect on Th2 cells (32). However, partial activation of Th2 cells through chemically fixed APCs or altered peptide ligands was found to cause proliferative suppression in a manner similar to that of Th1 cells, but without an effect on IL-4 production (33). In another study, both cell proliferation and IL-4 production were reduced in anergic Th2 cells (22). These discrepancies could have arisen from the use of different experimental protocols, or the selection of specific T cell clones or antigens. Here we used both in vivo and in vitro protocols to conclusively demonstrate that MEKK1, JNK1, and Itch are important in induction of Th2 cell anergy, characterized by reduced proliferation and diminished IL-4 (and IL-2) production.
We have demonstrated that Itch is involved in peripheral tolerance via anergy induction of Th2 cells. At the same time, we have obtained evidence excluding other potential mechanisms such as the generation of Tregs, the cell surface expression of costimulatory molecules such as OX-40 or CTLA-4, and the superantigen-induced deletion of peripheral T cells (Supplemental Figure 5). However, it cannot be ruled out that Tregs from WT and Itch–/– mice may have different functionality. Particularly, recent studies have demonstrated that tolerance induction by inhaled antigen or oral antigen exposure involves the generation of Tregs and that TGF-β plays an important role in inhibiting normal T cell function by Tregs (34–36). Indeed, using fibroblasts, we have previously shown that Itch plays a role in regulating TGF-β signaling (37). This may raise a possibility that TGF-β is involved in the resistance of Itch–/– T cells to anergy induction. We tried to address this issue by performing coculture cell proliferation assay and found that Tregs from WT and Itch–/– mice show similar inhibiting activity (Supplemental Figure 7A), and that blocking of TGF-β function does not affect the T cell anergy induction, at least in our system (Supplemental Figure 7, B and C). In addition, incubation of CD4+CD25– responder cells with TGF-β or with Tregs from PBS- or OVA-treated mice does not induce Itch expression (Supplemental Figure 7D). It is possible that the tolerance induction using high-dose antigen through nonrespiratory routes as presented in this study, in which Itch plays an important role in Th2 tolerance, is different from that using the low-dose inhaled antigen protocol in which Tregs are induced and TGF-β is involved in preventing the generation of Th2 cells (34, 35). Nevertheless, further detailed studies using in vivo models are needed to clarify the potential involvement of Tregs, TGF-β, and Itch-mediated T cell anergy induction.
It should be noted that both IL-4 and IL-2 production was suppressed in anergized Th2 cells. We went further to show that exogenous IL-2 can overcome the suppressed proliferative phenotype of anergized Th2 cells (Supplemental Figure 6). By contrast, exogenous IL-4 had little effect on the proliferative nonresponsive state of tolerized Th2 cells. The data may suggest that, like Th1 cells, anergic Th2 cells also require IL-2 to recover from a hyporesponsive state. Indeed, a recent study showed that IL-4 is mostly important during early T cell expansion and that it fails to stimulate T cell division at the late stage of Th2 polarization (38). Therefore, the hyporesponsiveness of anergic and differentiated Th2 cells to exogenous IL-4 may be due to the timing of IL-4 addition. In support of this, we found that addition of IL-4 during the early phase of anergy induction could prevent proliferative nonresponsiveness of anergic Th2 cells (Supplemental Figure 6). Thus, IL-4 may prevent Th2 tolerance during the initiation phase of anergy induction, but not once the tolerance has been established. A cooperative function of IL-4 and IL-2 rescues the T cell anergy in Th2 cells deficient in Itch or JNK1 or containing mutant MEKK1.
Recently, Itch was suggested to be involved in Th1 anergy (10), but this was solely based on in vitro studies using a T cell line or cultured primary T cells. Our results based on the use of a relevant in vivo system as well as an in vitro system that uses normal primary T cells demonstrate that Itch is mainly involved in Th2 tolerance and makes only a modest contribution to Th1 tolerance. Most likely, this selectivity of Itch function is due to its ability to induce the Ubiquitin-dependent degradation of JunB, a transcription factor that is involved in Th2 cytokine production and differentiation but has no demonstrated role in Th1 function or differentiation. Furthermore, loss of JNK1, MEKK1, or Itch results in the accumulation of JunB and excessive Th2 response. Thus, JunB is likely to be involved in Th2 tolerance induction, in addition to its established role in IL-4 gene activation and Th2 differentiation. Indeed, suppression of JunB expression in Itch–/– T cells restored the anergy induction of Th2 cells (Figure 6). Future work should address the role of the JNK1-Itch-JunB pathway in development of airway inflammation in humans, which may help identify new targets for therapeutic intervention in allergic diseases.
In addition to Itch, 2 other E3 ligases, Cbl-b and Grail, have been implicated in T cell anergy induction (10–12). E3 ligases have substrate specificity via direct binding to the target proteins. Cbl-b has been shown to associate with PLC-γ1 and promote ubiquitination and subsequent modification of its biological function (12), in contrast to the Itch-mediated ubiquitination of JunB via the direct interaction of Itch WW domains with the PPXY motifs of JunB (16). Another layer of regulation involves the E3 ligase itself, such as the JNK signaling–induced phosphorylation of Itch and subsequent activation of its ligase activity (19). Therefore, the E3 ligases do have their specificity, which determines the nonredundancy in different aspects of T cell anergy induction. Nevertheless, these E3 ligases may also cooperate with each other to play a synergistic and perhaps concerted role during T cell tolerance. Obviously, study of mice deficient in 2 or more E3 ligases will give a clearer answer.
Methods
Mice.
WT and Itch–/– mice on a C57BL/6 background were described previously (16). The genotyping of mice was performed by PCR. Mice 8–10 weeks old were used for the experiments. The Itch–/– mice did not develop any abnormality in our facility until 5 or 6 months of age. OVA-specific OTII TCR transgenic mice were bred with Itch–/– mice. B6.PL-Thy1a (Thy1.1) mice were from Jackson Laboratory. MEKK1ΔKD mutant mice and JNK1–/– mice were previously described (19). Animal experiments were approved by the Institutional Animal Care and Use Committee of La Jolla Institute for Allergy and Immunology.
T cell isolation.
CD4+ T cells were isolated from spleen and lymph nodes using MACS beads (Miltenyi Biotec) by negative selection following the manufacturer’s instructions. For isolation of naive CD4+ T cells, CD4+ T cells were further subjected to positive selection of CD62L+ cells using MACS beads. Purity of the isolated cells was determined by FACS analysis.
Flow cytometry.
CD4+ T cells isolated as described above were stained with FITC- or PE-conjugated antibodies against murine CD4, CD25, CTLA-4, and OX-40 (BD Biosciences — Pharmingen) for 30 minutes. The cells were washed twice in FACS buffer (PBS plus 1% FCS plus 0.01% sodium azide). Live cells were analyzed on a FACScan flow cytometer (BD) and CellQuest software (BD).
Cell proliferation.
T cells were plated in 96-well plates with immobilized anti-CD3 (0.5 μg/ml) and anti-CD28 (1 μg/ml), or in combination with anti–CTLA-4 antibodies (5 μg/ml). Cells were cultured for 48 hours, during the last 10 hours of which the cells were pulsed with 1 μCi/ml of 3H-thymidine. 3H-Thymidine incorporation was enumerated using a β plate counter. Data were presented as the mean value from triplicate wells.
In vivo tolerance induction.
The mice were tolerized to OVA peptide as described (39), with minor modifications. Mice were immunized s.c. with 50 μg OVA323–339 peptide either in CFA or in alum and concurrently given an i.p. injection of 500 μg soluble OVA peptide or PBS as control to induce tolerance. Mice were killed on day 10, and CD4+ T cells were isolated from spleen and popliteal lymph nodes as described above. CD4+ T cells were plated in 96-well round-bottom plates with OVA peptide added at concentrations of 0–100 μg/ml plus irradiated splenocytes as APCs for 48 hours, and the plates were pulsed for 12 hours with 3H-thymidine to examine the proliferative response. The culture supernatants were also assayed for cytokine concentrations.
Adoptive transfer.
The mice were injected i.v. with 500 μg of OVA peptide or PBS on day 0 and day 3. On day 10, CD4+ T cells were isolated and labeled with CFSE. Labeled cells (5 × 105) were injected i.v. into Thy1.1 mice. The recipient mice were immunized with 50 μg of OVA peptide in alum. On day 3, draining lymph node cells were collected, and cell suspension was stained with Thy1.2-APCs and Vβ5-PE. Thy1.2+ cells were gated, and CFSE profile was checked in Vβ5+ cells. The lymph node cells were also stimulated in vitro with OVA peptide (2 μg/ml) with irradiated APCs and cultured for 8 hours, during the last 2 hours of which brefeldin A was added. Cells were washed and stained with Thy1.2-APCs, followed by intracellular staining with PE-labeled anti–IL-2 or anti–IL-4.
Induction of in vitro T cell anergy.
For the Th cell differentiation, naive CD4+ T cells (1 × 105) isolated from the spleen and lymph nodes were stimulated with immobilized anti-CD3 (1 μg/ml) and anti-CD28 (5 μg/ml) in 24-well plates for 2 days. For Th1 differentiation, the cells were then stimulated in the presence of 5 ng/ml of recombinant IL-12 and 10 μg/ml of anti–IL-4 antibody (BD Biosciences — Pharmingen). For Th2 differentiation, cells were stimulated in the presence of 100 U/ml of recombinant IL-4 and 10 μg/ml anti–IFN-γ antibody (BD Biosciences — Pharmingen) and 5 μg/ml of anti–IL-12 antibody (BD Biosciences — Pharmingen). Cells were allowed to differentiate for another 4 days. Naive CD4+ T cells differentiated as described above were washed twice and rested for 1 day. Then they were stimulated with ionomycin (50 ng/ml; Sigma-Aldrich) for 16 hours, washed twice, and restimulated with anti-CD3 and anti-CD28 for 48 hours to check the proliferation and cytokine profile.
Cytokine measurement.
Cytokine secretion was measured for IL-2, IL-4, and IFN-γ by sandwich ELISA. Purified anti–mouse IL-2, IL-4, and IFN-γ antibodies (BD Biosciences — Pharmingen) were used to coat ELISA plates. Recombinant mouse cytokines (BD Biosciences — Pharmingen) were used to construct a standard curve. For intracellular staining, the cells were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) for 8 hours, during the last 2 hours of which brefeldin A (5 μg/ml) was added. Cells were then fixed with 4% paraformaldehyde for 20 minutes, washed, and then surface-labeled with FITC–anti-CD4 antibody. The cells were then permeabilized with 0.5% saponin for 10 minutes, stained with PE-conjugated anti–IL-2, –IL-4, or –IFN-γ, and analyzed on a FACSCalibur (BD Biosciences).
Immunoblotting.
Polyclonal antibodies against c-Jun, JunB, JunD, GATA-3, c-Maf, actin, or Grb2 (Santa Cruz Biotechnology Inc.) were used for immunoblotting. Immunoblotting and membrane reprobing were performed as previously described (40).
Preparation of nuclear extracts.
Differentiated CD4+ T cells were either left unstimulated or stimulated with ionomycin (50 ng/ml) for 16 hours. Then the cells were washed and restimulated with anti-CD3 plus anti-CD28 for the indicated time periods. Nuclear extracts were prepared as previously described (16). The supernatants were used for immunoblotting.
Retroviral transduction.
Ectopic retroviruses to knock down mouse JunB were prepared using the pSuper interfering RNA vector as described recently (29). The vector was transfected into Plat-E packaging cells, and after 48 hours, the supernatant was collected and was used to infect differentiating Th2 cells from Itch–/– mice. The infection was repeated once after 24 hours. Forty-eight hours after the second infection, cells were then selected using puromycin (2 μg/ml) for 48 hours. The puromycin-resistant cells were cultured for another 4 days under Th2 conditions, rested overnight, and subjected to ionomycin-induced T cell anergy induction. The efficiency of JunB knock down was checked by immunoblotting.
Development of tolerance-based airway inflammation.
Mice were first tolerized as described earlier. Airway inflammation was induced on day 9 as described previously (41). Briefly, mice were sensitized by i.p. injection of 20 μg OVA protein adsorbed to 2 mg alum in PBS. On day 19, mice were challenged via the airways with OVA (5 mg/ml in 15 ml of PBS) for 30 minutes, once a day for 4 consecutive days, by ultrasonic nebulization. BAL was analyzed for the infiltration of granulocytes and lymphocytes. Differential counts of infiltrating cells were done by Giemsa staining of cytospin preparations. Lung histopathology, PAS staining, serum OVA-specific IgE concentration, and lung cytokine profiles were determined as described (41). All data were collected 3–24 hours after the final antigen challenge.
Supplementary Material
Acknowledgments
We thank R. Bai, K. Cooper, and A. Teng for technical assistance, R. Zuberi, M. Jeon, and M. Hundt for helpful discussion, and S. Schoenberger for providing MEC B7.1 cells. This work was supported by NIH grants and a Research Scholar grant from the American Cancer Society (ACS) to Y.-C. Liu, and by the Sandler Family Foundation to M. Karin, who is an ACS research professor.
Footnotes
Nonstandard abbreviations used: BAL, bronchoalveolar lavage; MEKK1, mitogen and extracellular kinase kinase 1; MEKK1ΔKD, MEKK1 kinase-dead mutant.
Conflict of interest: The authors have declared that no conflict of interest exist
References
- 1.Paul W.E., Seder R.A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Liblau R.S., Singer S.M., McDevitt H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 4.Szabo S.J., Sullivan B.M., Peng S.L., Glimcher L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 5.Umetsu D.T., McIntire J.J., Akbari O., Macaubas C., DeKruyff R.H. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 6.Kamradt T., Mitchison N.A. Tolerance and autoimmunity. N. Engl. J. Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 7.Walker L.S., Abbas A.K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz R.H. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 9.Macian F., et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 10.Heissmeyer V., et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 11.Anandasabapathy N., et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 12.Jeon M.S., et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Perry W.L., et al. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 14.Pickart C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 15.Weissman A.M. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 16.Fang D., et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 2002;3:281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 17.Li B., Tournier C., Davis R.J., Flavell R.A. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. . EMBO J. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartenstein B., et al. Th2 cell-specific cytokine expression and allergen-induced airway inflammation depend on JunB. EMBO J. 2002;21:6321–6329. doi: 10.1093/emboj/cdf648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao M., et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 20.Kang S.M., et al. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- 21.Mondino A., et al. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. . J. Immunol. 1996;157:2048–2057. [PubMed] [Google Scholar]
- 22.Faith A., Akdis C.A., Akdis M., Simon H.U., Blaser K. Defective TCR stimulation in anergized type 2 T helper cells correlates with abrogated p56(lck) and ZAP-70 tyrosine kinase activities. . J. Immunol. 1997;159:53–60. [PubMed] [Google Scholar]
- 23.Tobagus I.T., Thomas W.R., Holt P.G. Adjuvant costimulation during secondary antigen challenge directs qualitative aspects of oral tolerance induction, particularly during the neonatal period. . J. Immunol. 2004;172:2274–2285. doi: 10.4049/jimmunol.172.4.2274. [DOI] [PubMed] [Google Scholar]
- 24.McMenamin C., Pimm C., McKersey M., Holt P.G. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. . Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 25.Bansal-Pakala P., Jember A.G., Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat. Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 26.Kearney E.R., Pape K.A., Loh D.Y., Jenkins M.K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. . Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Dong C., et al. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 28.Dong C., et al. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 29.Sreeramaneni R., Chaudhry A., McMahon M., Sherr C.J., Inoue K. Ras-Raf-Arf signaling critically depends on the Dmp1 transcription factor. Mol. Cell. Biol. 2005;25:220–232. doi: 10.1128/MCB.25.1.220-232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbari O., et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. . Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 31.Gajewski T.F., Lancki D.W., Stack R., Fitch F.W. “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J. Exp. Med. 1994;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein H.J., Abbas A.K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J. Exp. Med. 1993;177:457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan-Lancaster J., Evavold B.D., Allen P.M. Th2 cell clonal anergy as a consequence of partial activation. J. Exp. Med. 1994;180:1195–1205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucida D., et al. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostroukhova M., et al. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-β and FOXP3. . J. Clin. Invest. 2004;114:28–38. doi: 10.1172/JCI200420509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson D.S., Larche M., Durham S.R. Tregs and allergic disease. J. Clin. Invest. 2004;114:1389–1397. doi: 10.1172/JCI200423595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai Y., Yang C., Hu K., Elly C., Liu Y.C. Itch E3 ligase-mediated regulation of TGF-beta signaling by modulating smad2 phosphorylation. Mol. Cell. 2004;15:825–831. doi: 10.1016/j.molcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Mohrs M., Lacy D.A., Locksley R.M. Stat signals release activated naive Th cells from an anergic checkpoint. J. Immunol. 2003;170:1870–1876. doi: 10.4049/jimmunol.170.4.1870. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Fueyo A., et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 40.Qiu L., et al. Recognition and ubiquitination of Notch by Itch, a hect-type E3 ubiquitin ligase. . J. Biol. Chem. 2000;275:35734–35737. doi: 10.1074/jbc.M007300200. [DOI] [PubMed] [Google Scholar]
- 41.Salek-Ardakani S., et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 2003;198:315–32. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.