Abstract
The hypothesis that ultrasound increases antibiotic transport through biofilms of Escherichia coli and Pseudomonas aeruginosa was investigated using colony biofilms. Biofilms grown on membrane filters were transferred to nutrient agar containing 50 μg/mL gentamicin. A smaller filter was placed on top of the biofilm and a blank concentration disk was situated atop the filter. Diffusion of antibiotic through the biofilms was allowed for 15, 30, or 45 min at 37°C. Some of these biofilms were exposed to 70 kHz ultrasound and others were not. Each concentration disk was then placed on a nutrient agar plate spread with a lawn of E. coli. The resulting zone of inhibition was used to calculate the amount of gentamicin that was transported through the biofilm into the disk. The E. coli and P. aeruginosa biofilms grown for 13 and 24 h were exposed to two different ultrasonic power densities. Ultrasonication significantly increased the transport of gentamicin through the biofilm. Insonation of biofilms of E. coli for 45 minutes more than doubled the amount of gentamicin compared to their non-insonated counterparts. For P. aeruginosa biofilms, no detectable gentamicin penetrated the biofilm within 45 min without ultrasound; however, when insonated (1.5 W/cm2) for 45 min, the disks collected more than 0.45 μg of antibiotic. Ultrasonication significantly increased transport of gentamicin across biofilms that normally blocked or slowed gentamicin transport when not exposed to ultrasound. This enhanced transport may be partially responsible for the increased killing of biofilm bacteria exposed to combinations of antibiotic and ultrasound.
Keywords: biofilm, gentamicin, antibiotic transport, ultrasound, P. aeruginosa, E. coli
INTRODUCTION
Bacteria within biofilms characteristically exhibit increased resistance to a wide range of antimicrobial agents compared to their planktonic counterparts. Costerton et al. speculated that this increased resistance to antimicrobials is due to changes in bacterial metabolism and genetic expression associated with sessile growth 1. In addition to phenotypic changes, biofilms may bind or slow the transport of antibiotics, protecting the enclosed bacteria from exposure to lethal levels of antimicrobials 2, 3. Furthermore, biofilms contain metabolically inactive cells and “persister” cells that are not responsive to conventional antibiotics 4. Whatever the cause, the decreased antimicrobial susceptibility renders normal antimicrobial chemotherapy ineffective in the treatment of biofilm-related implant infections. We are investigating a non-invasive means of enhancing antimicrobial agents in order to more easily treat clinical biofilm infections. Previously, our lab has reported that low frequency ultrasound effectively enhanced the action of certain antibiotics in killing bacterial biofilms in vitro and in vivo 5–11. The results of these previous experiments are promising; however, we have yet to identify the molecular mechanism of this phenomenon. Drawing upon previous research, we hypothesized that ultrasound increases the transport of certain antibiotics through biofilms of Escherichia coli and Pseudomonas aeruginosa 9, 12. Herein we report the results of experiments designed to test this hypothesis.
Previous research investigating the effect of biofilms on antimicrobial transport has shown that biofilms sometimes slow but seldom completely block the transport of antibiotics 4. Dunne et al. examined the diffusion of vancomycin and rifampin across a biofilm of Staphylococcus epidermidis using an equilibrium dialysis chamber 13. After 24 h, the concentration of both antimicrobials exceeded their respective minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) in the receiving side of the dialysis chamber. Similarly, Darouiche et al. found high concentrations of vancomycin in biofilms of S. epidermidis after exposure to the antimicrobial for 24 h 14. Yasuda et al. studied the effect of clarithromycin on the structure of biofilms formed by P. aeruginosa and on the transport of ofloxacin and gentamicin across the biofilms 15, 16. The biofilms that were formed by incubating filters with P. aeruginosa for 10 days exhibited reduced permeability of both ofloxacin and gentamicin. In addition, Anderl et al. found that ciprofloxacin reached the MIC throughout biofilms of wild-type Klebsiella pneumoniae in 20 min, but antibiotically active ampicillin failed to diffuse through the biofilm 17. However, ampicillin readily diffused through biofilms of mutant K. pneumoniae unable to express β-lactamase. Walters reported that both tobramycin and ciprofloxacin diffused across P. aeruginosa biofilms given enough time 18. Ciprofloxacin readily crossed the biofilm, but tobramycin took much longer. Nevertheless, it did reach a concentration five times the MIC after 36 h. Generally, it seems that antibiotics penetrate and cross biofilms. However, enzymatic degradation or ionic bonding may be responsible for inhibited transport across biofilms of K. pneumoniae and P. aeruginosa biofilms in the case of ampicillin and tobramycin respectively.
In an attempt to model the diffusion of antibiotics through the alginate produced by P. aeruginosa in patients with cystic fibrosis, Williams et al. 19 used a diffusion assay test with alginate that had been exposed to 50-kHz ultrasound at fairly high intensities. Increasing the duration and the intensity of insonation increased the diffusion of tobramycin, piperacillin and ciprofloxacin through the alginate. The increase in diffusion was attributed to a decrease in the viscosity of the alginate as the alginate was fragmented by the intense insonation.
Interaction of gentamicin with the outer membrane of distal cells in the biofilm, as well as uptake of the antibiotic by the cells or binding by exopolysaccharides in the matrix, could significantly increase the amount of time required for cells in the center of biofilm microcolonies to be exposed to inhibitory or bactericidal levels of gentamicin. This is significant because P. aeruginosa and several other aerobic and facultative Gram-negative bacilli exhibit transient aminoglycoside resistance in response to exposure to extremely low concentrations of aminoglycosides 20, 21. It is possible that transient antibiotic resistance exhibited by sessile bacteria could result from early exposure to sub-MIC concentrations of the antibiotic that subsequently upregulates resistance mechanisms. Ultrasound is known to enhance the transport of small molecules across polymer membranes and gels 22, 23. If similar transport enhancements occur in biofilms, then increased transport might saturate available binding sites more rapidly, increase the concentration of gentamicin in proximity to sequestered bacteria, and thus reduce exposure of bacteria to sub-MIC concentrations.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics
Cultures of E. coli (ATCC 10798) and P. aeruginosa (ATCC 27853) were grown on nutrient agar (NA). Planktonic cultures were grown in tryptic soy broth without dextrose (TSB, Difco, Detroit, MI). Gentamicin sulfate (Sigma, St. Louis, MO) was reconstituted in distilled water, filter-sterilized, and added to molten agar that had been cooled to 49°C. Physiological saline solution (PSS) was made by dissolving 9 g of NaCl in 1 L of distilled water followed by autoclave sterilization. The gentamicin MIC values for planktonic suspensions of the E. coli and P. aeruginosa are 6 and 3 μg/mL respectively. These values are very similar to values reported elsewhere 10, 24–29.
Standard concentration disks
Standard concentration disks were made by loading blank concentration disks (Becton Dickinson, Sparks, MD) with 0.5, 1, 2, 4, 6, 8, 10, and 12 μg of gentamicin as follows. Gentamicin was diluted in sterile distilled water to the desired concentration. Blank concentration disks were placed in sterile polystyrene petri dishes and wetted by pipette with 20 μL of the prepared gentamicin solution. The petri dishes were refrigerated for 48 h to dry the disks, afterwhich the disks were placed in sterile vials and refrigerated until use.
Diffusion experiments
These experiments employed an adaptation of the experimental setup described by Anderl et al. 17. GN-6 Metricel membrane filters of 25 mm diameter (Pall Gelman, Ann Arbor, MI) were placed on NA and inoculated in the center with 10 μL of inoculation culture. This culture was created by diluting 10 μL of overnight culture in 990 μL of TSB. The filters were then incubated for 13 or 24 h at 37°C.
After incubation, the filters and their accompanying biofilm were transferred to different NA plates containing 50 μg/mL gentamicin. A 13-mm GN-6 Metricel filter was placed over the biofilm and a blank concentration disk was positioned on top of the upper filter of the sandwiched biofilm. The concentration disk was wetted with 40 μL of TSB and the plates were exposed to ultrasound or sham (no ultrasound) at 37°C for 15, 30, and 45 min. To apply ultrasound, the gentamicin plates were floated on the surface of water in an ultrasonic bath (SC-100, Sonicor, Copiaque, NY) in which a fixture was constructed to maintain a constant position throughout the experiment. E. coli biofilms were insonated at 0 W/cm2 (sham) and approximately 1.9 W/cm2 and 2.9 W/cm2; P. aeruginosa biofilms were insonated at 0 W/cm2 and approximately 1.5 W/cm2 and 2.5 W/cm2. After the designated time, the concentration disks were removed from the upper filter, placed in sterile polystyrene petri dishes, and refrigerated. Exposure of the biofilm to ultrasound produced no visible change in the biofilm.
The power densities were measured in separate experiments by placing a calibrated hydrophone (#8103, Bruel & Kjaer, Naerum, DK) in a Petri dish placed in the same position as the experimental Petri dishes. The Petri dish was filled with water to the same height as the agar to produce a similar acoustic situation.
Zones of inhibition and quantitation of gentamicin
The concentration disks were placed on NA plates spread with a lawn of E. coli (ATCC 10798). The calibration disks containing standard amounts of gentamicin were also placed on NA plates spread with E. coli. A lawn was assured by spreading the plate with 100 μL of planktonic E. coli culture created by diluting 10 μL of overnight culture in 990 μL of TSB. The plates were incubated for 24 h at 37°C. The zones of inhibition created by the gentamicin in the disks were measured with a 150 mm stainless dial caliper with 0.02 mm graduations (Cole Palmer, Vernon Hills, IL). The sizes of the zones of inhibition caused by the calibration concentration disks were correlated with the amount of gentamicin within the disk that created it. A plot of average zones of inhibition versus mass of gentamicin was created using the calibration disks (Fig. 1). The following correlation was used to convert the measured experimental zones of inhibition to micrograms of gentamicin in the disk,
Figure 1.
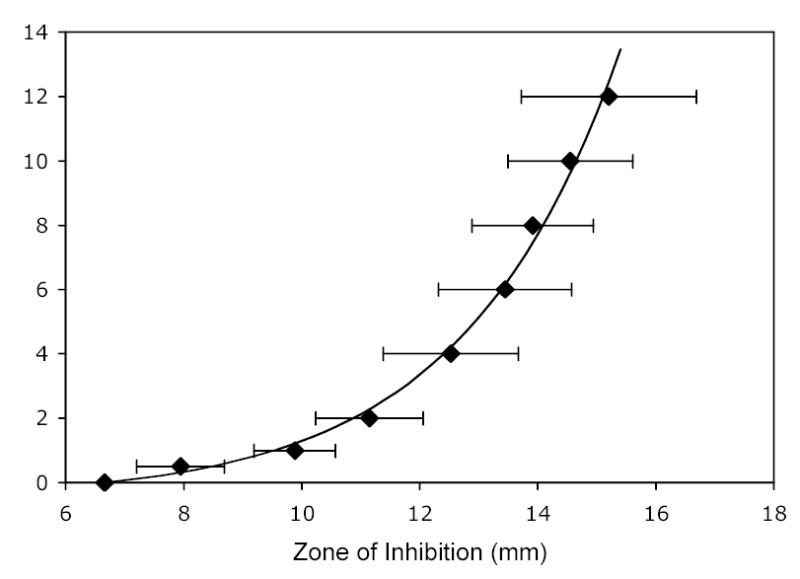
The correlation between the amount of gentamicin contained in a concentration disk and the resulting size of the zone of inhibition of E. coli. The mean and standard deviation (n=8) are represented by the diamonds and error bars; the line is the correlation given in Equation 1.
| (1) |
in which G is the mass of calibration gentamicin applied to the disk, and ZI is the diameter of the zone of inhibition, and 6.68 mm is the diameter of the concentration disk.
Biofilm thickness
Biofilms grown on the 25 mm membranes were stained with SYTO 9 and propidium iodide (LIVE/DEAD BacLight, Molecular Probes, Eugene, OR) 30, 31 and examined under a laser scanning confocal microscope (Zeiss LSM10, Oberkochen, Germany). The vertical thickness was measured in several places on each biofilm and ranged from 55 to 133 microns, depending on the species of bacteria.
Statistical analysis and graphic presentation
The data were analyzed using a heterogeneous variance ANOVA procedure in which the variances as well as the means are modeled as functions of the factors. The Kenward-Roger method 32 was used to approximate the degrees of freedom for the tests. Statistical tests were performed for treatment length, biofilm age, and presence and intensity of ultrasound. Additional tests to identify any interactions of these effects were performed. The data is presented in the form of box plots, which depict the interquartile range (25th and 75th percentile), the median, adjacent values, and outliers in the data, represented respectively by the top and bottom of the box, the central line, the error bars, and the dots.
RESULTS
The present experiments were designed to test the hypothesis that ultrasound increases the rate of transport of antibiotic through the biofilms into concentrations disks. Parallel experiments were constructed to directly compare the amount of gentamicin transport with and without application of ultrasound. Other factors that were studied included the species of biofilm and the age of the biofilm.
The results in general showed the presence of any biofilm significantly decreased the transport of antibiotic into the disk and the amount of accumulated gentamicin increased with time (see Tables 1 and 2 and Figures 2 and 3). Most importantly, the disks atop ultrasonicated biofilms contained more gentamicin than the non-insonated disks.
Table 1.
Average amount of gentamicin (μg) accumulated in disks (n=6) atop biofilms of E. coli.
| Power density | 0 W/cm2 | 1.9 W/cm2 | 2.9 W/cm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure time | 15 min | 30 min | 45 min | 15 min | 30 min | 45 min | 15 min | 30 min | 45 min |
| No Biofilm* | 1.38† | 1.95 | 1.79 | 1.76 | 3.24 | 3.48 | 1.30 | 2.76 | 3.08 |
| 13-hr biofilm | 0.03 | 0.06 | 0.07 | 0.01 | 0.38 | 0.84 | 0.08 | 0.25 | 0.60 |
| 24-hr biofilm | 0.00 | 0.02 | 0.05 | 0.00 | 0.26 | 0.47 | 0.04 | 0.25 | 0.46 |
The disk was place atop 2 filter membranes on the gentamicin plate with no biofilm present.
The concentrations were determined by zones of inhibition.
Table 2.
Average amount of gentamicin (μg) accumulated in disks (n=6) atop biofilms of P. aeruginosa.
| Power density | 0 W/cm2 | 1.5 W/cm2 | 2.5 W/cm2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure time | 15 min | 30 min | 45 min | 15 min | 30 min | 45 min | 15 min | 30 min | 45 min |
| No Biofilm* | 0.94† | 1.70 | 1.38 | 1.35 | 2.38 | 2.37 | 1.74 | 2.81 | 3.25 |
| 13-hr biofilm | 0.00 | 0.00 | 0.00 | 0.04 | 0.23 | 0.79 | 0.09 | 0.76 | 1.45 |
| 24-hr biofilm | 0.00 | 0.00 | 0.00 | 0.03 | 0.20 | 0.34 | 0.00 | 0.22 | 0.80 |
The disk was place atop 2 filter membranes on the gentamicin plate with no biofilm present.
The concentrations were determined by zones of inhibition.
Figure 2.
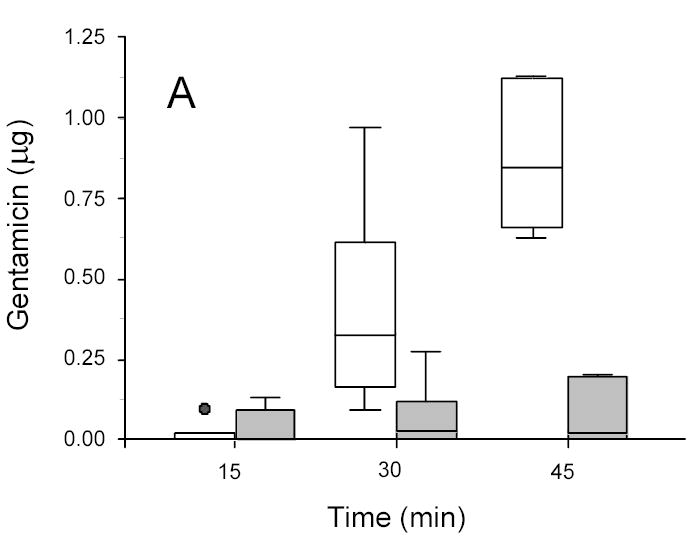
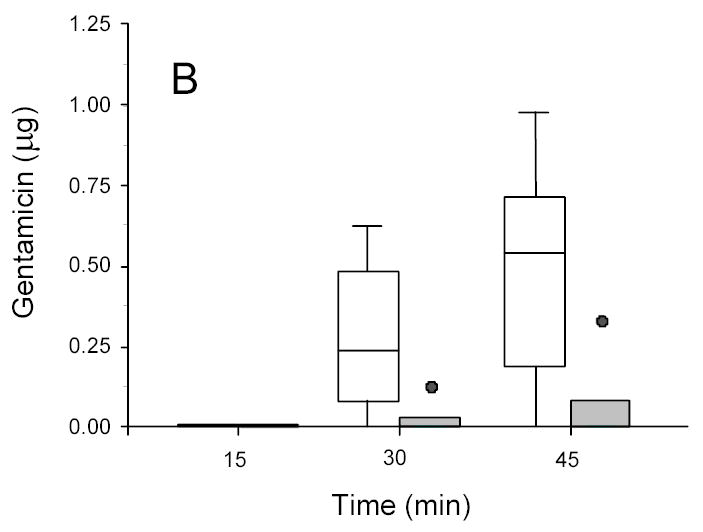
Gentamicin in disks recovered from ultrasonicated (1.9 W/cm2) (open box) and sham (non-insonated) (gray box) E. coli biofilms. A: 13-hr-old biofilms. B: 24-hr-old biofilms.
Figure 3.
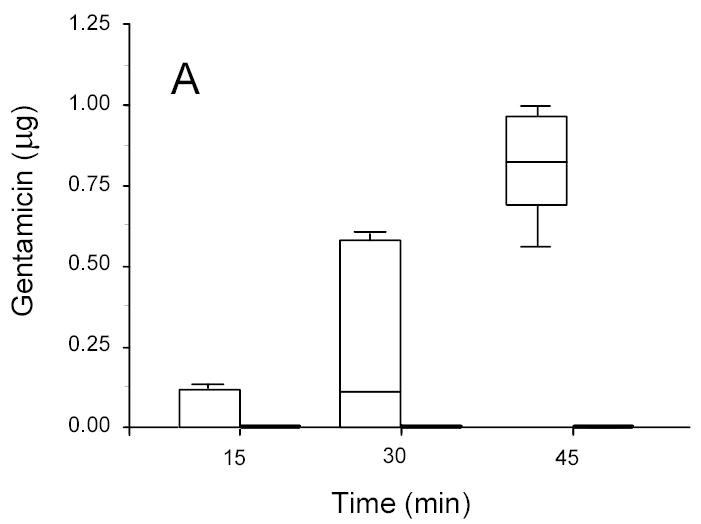
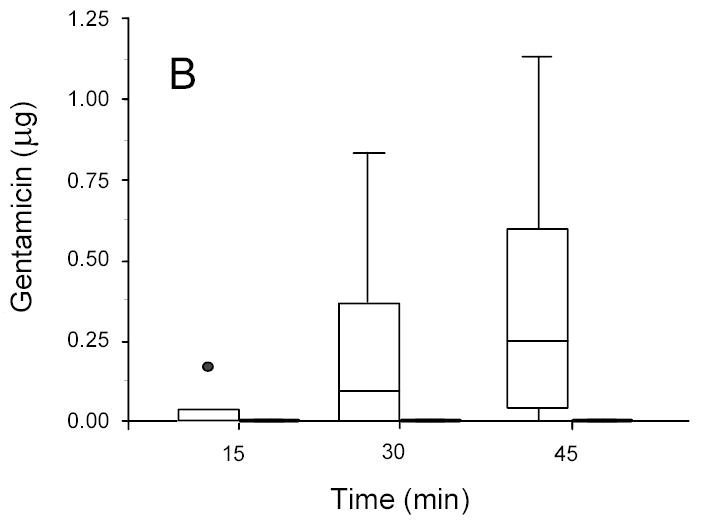
Gentamicin in concentration disks recovered from ultrasonicated (1.5 W/cm2) (open box) and sham (non-insonated) (gray box) P. aeruginosa biofilms. A: 13-hr-old biofilms. B: 24-hr-old biofilms.
For E. coli, 45 minutes of ultrasound more than doubled the amount of gentamicin transported through the biofilm; shorter lengths of insonation time also increased the amount of transport, but not to the same extent. The ANOVA on the complete data set showed that the amount of transport into the concentration disk increased with intensity of ultrasound (p < 0.0001), increased with length of treatment time (p < 0.0001) and decreased with the age of biofilm (p < 0.0001).
For P. aeruginosa it is noteworthy that colony biofilms of both age effectively blocked the transport of gentamicin within the time frame studied; however, significant transport through the same biofilm was observed upon application of ultrasound (Figure 3). The ANOVA on the complete data set showed that transport increased with intensity of ultrasound (p < 0.0001), with length of treatment time (p < 0.0001), and decreased with biofilm age (p < 0.0001).
DISCUSSION
Factors affecting transport
These results show that several factors influence the transport of antibiotic within or through a biofilm. In addition to ultrasound (which will be discussed below), the amount of transport is a strong function of the species of biofilm bacteria, the age of the biofilm, and the time allowed for transport. It is not surprising that the amount of antibiotic crossing the biofilm increased with time, since more time was allowed for the gentamicin to accumulate in the disk. However, it is noteworthy that the amount crossing the biofilm significantly decreased with the age of the biofilm, indicating that a 13-h biofilm is physically, chemically or biologically different than a 24-h biofilm. Measurements with the confocal microscope showed no significant difference in thicknesses of the 13-h 24-h biofilms (F > 0.3). With respect to biological differences, Sauer et al. have shown that there are several stages of biofilm development in which genetic expression changes during the course of biofilm development 33. Such transient changes in the biofilm genetic expression and resulting phenotype appear to impact the transport of antibiotics as the biofilm matures.
Stewart et al. have shown that many factors can retard the diffusion of antibacterial agents through biofilms 3, 34, 35. Such factors include the volume fraction of solids in the biofilm, reversible binding (adsorption/desorption) of agent to the biofilm components, irreversible binding to a fixed number of sites, and inactivation or enzymatic degradation of agent within the biofilm. Our observations that gentamicin transport through E. coli and P. aeruginosa biofilms is significantly influenced by biofilm age suggest that one or more of Stewart’s “retarding” factors (cited above) increase as the biofilm matures. Often older biofilms are observed to be more recalcitrant towards antimicrobial agents 36, and we posit that decreased transport due to binding, inactivation, or enzymatic degradation in older biofilms could contribute to their recalcitrance.
With respect to the influence of the biofilm species, in the absence of ultrasound, gentamicin penetrated the E. coli biofilm in 30 min. However, no detectable amount of gentamicin traversed the colony biofilms of P. aeruginosa in 45 min. Subsequent experiments revealed that gentamicin penetrated both our 13-h and 24-h biofilm within 180 min (data not shown). Anderl et al., using a wild type and a β-lactamase deficient mutant of K. pneumoniae showed that detectable amounts of ampicillin could not penetrate wild-type biofilms in 240 minutes, whereas measurable amounts of antibiotic penetrated the mutant biofilm in only 10 minutes 17. They attributed the lack of measurable penetration to the enzymatic degradation of ampicillin by the wild-type biofilm. In our experiments neither of the two species is considered to have any specific gentamicin-degrading enzymes expressed, at least in the planktonic phenotype. Walters et al. 18 showed that a similar aminoglycoside, tobramycin, penetrated a P. aeruginosa colony biofilm after about 13 hrs, much longer than the 45 minutes examined herein.
At present we have no explanation as to why gentamicin easily penetrates biofilms of E. coli, but not P. aeruginosa. Both biofilms contain negatively charged polysaccharides that can bind the positively charged aminoglycoside. However, it is possible that there are more binding sites or tighter binding sites in the P. aeruginosa biofilm.
Another factor that influences the rate of antibiotic transport is the chemistry of the antibiotic itself. Anderl et al. have shown that in wild-type K. pneumoniae biofilm, the rates of ampicillin and ciprofloxacin penetration were vastly different, particularly in the wild-type biofilm wherein ampicillin failed to penetrate, but ciprofloxacin penetrated easily 17. However, in the mutant biofilm (in which β-lactamase activity would not be present to interfere with ampicillin transport) the initial rate of ampicillin transport was much more rapid than that of ciprofloxacin.
Effect of Ultrasound
Ultrasound is known to enhance the transport of small molecules across porous membranes 22, 37 and increase transport of larger molecules such as DNA through agarose 23 or insulin through skin 38. In general, the precise mechanisms of such increased transport have not been identified and may be different in each situation; but the enhancement is often speculatively attributed to increased microconvection from ultrasonic heating 37, to ultrasonic vibrational interactions with bubbles (cavitation events) 39, to reduction in boundary layer thickness due to of turbulence or microconvection 37, or to “oscillatory-enhanced dispersion” caused by oscillatory flow in channels 6. In fact, the data of Tables 1 and 2 show that there is a large amount of gentamicin flux across the porous filter membranes in the absence of the biofilm, and that the flux is increased with application of ultrasound. Because the amount of gentamicin transported into the disk without biofilm is much larger than with biofilm, the biofilm (with or without ultrasound) is much less permeable to gentamicin than the micro-porous polymer membrane, and thus the biofilm is controlling the overall rate of transport.
A large amount of data was collected concerning the effect of ultrasound on the transport of gentamicin through biofilms, most of which could not be presented in this paper 40. When all of the data was considered statistically, a relationship between intensity and transport emerges. Compared to when using non-insonated biofilms, the amount of gentamicin contained in the disks increased significantly when the biofilms were exposed to ultrasound for 30 to 45 min. For E. coli biofilms, exposure to 2.9 W/cm2 resulted in the transport of larger amounts of gentamicin than exposure to 1.9 W/cm2. Similarly for P. aeruginosa biofilms, higher insonation intensity increased the amount of transport. Thus there appears to be a dose-response relationship between the intensity of ultrasound and the amount of trans-biofilm transport. Such an intensity-dependent transport is consistent with transport mechanisms based upon cavitation events, microconvection, and oscillatory-enhanced diffusion.
A very interesting set of data is shown in Figure 3 in which insonation at 1.5 W/cm2 rendered a previously impenetrable P. aeruginosa biofilm permeable to gentamicin transport. As discussed previously, we do not attribute the lack of penetration in the absence of ultrasound to inactivation of gentamicin by the biofilm. This data indicates that gentamicin can be successfully transported through the biofilm within 15 minutes given the additional “boost” by the ultrasound.
Even in the presence of ultrasound the age of the biofilm has an effect of upon the transport. More antibiotic was transported across the younger biofilms than the older ones (compare Figs 2A and 3A to 2B and 3B). Thus physiologic or phenotypic differences within a single species of biofilm (as well as differences due to species) still contribute to the transport in the presence of ultrasound.
Our data does not allow us to differentiate or identify which of the possible mechanisms for ultrasonic-enhanced transport are operational within the biofilm. We tend to discount the possible role of cavitation because micrographs of P. aeruginosa biofilms 18 do not show many voids that could serve as nuclei for cavitation bubbles. Rather, we posit that vibrations in the biofilm may increase convection in pores and channels by oscillatory-enhanced dispersion, and thus increase the overall rate of transport beyond that due to diffusion alone. The enhanced transport does not negate the normal adsorption nor the binding interactions between the antibiotic and the components of the biofilm; these probably occur as usual. But we postulate that binding sites are filled more quickly as ultrasound enhances the transport, and thus the breakthrough time (when antibiotic first enters the disk) decreases, and a larger amount of antibiotic eventually enters the disk in the allotted time.
Recent observations by many investigators indicate that the recalcitrance of biofilms cannot be attributed entirely to the lack of eventual penetration of antibiotic into the biofilm 4, 17, 18, 35, 41. The lack of oxygen penetration may also be involved 18. We propose that just as ultrasound can increase the transport of gentamicin within biofilms, it can also increase the transport of oxygen and other small molecules that may increase the metabolic state and render the cells more susceptible to the antibiotic. Ultrasound does increase the effectiveness of gentamicin in killing cells in E. coli and P. aeruginosa biofilms 5–8. Although we have yet to identify the exact mechanism(s), the observations reported herein support the role of two possible processes. First, ultrasound may enhance diffusion of oxygen into the biofilm and render metabolically inactive bacteria sufficiently active so that the antibiotics are effective. Secondly, the enhanced rate of transport of antibiotic into the biofilm may kill the cells before they can respond genetically to resist the antimicrobial attack. More work remains to be done to identify and take advantage of the role of ultrasound in enhancing the action of antibiotics against biofilms.
Acknowledgments
Funding for this research was provided by NIH grant HL59923 and by Brigham Young University. Brian Daniels did the confocal microscopy and Angela Dunford measured the MIC values.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS. Biofilm Accumulation Model that Predicts Antibiotic Resistance of Pseudomonas aeruginosa Biofilms. Antimicrob Agents Chemother. 1994;38:1052–1058. doi: 10.1128/aac.38.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart PS. Theoretical Aspects of Antibiotic Diffusion into Microbial Biofilms. Antimicrob Agents Chem. 1996;40:2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LL, Peterson RV, Pitt WG. Treatment of bacterial biofilms on polymeric implants using antibiotics and ultrasound. J Biomat Sci Polymer Ed. 1998;9:1177–1185. doi: 10.1163/156856298x00712. [DOI] [PubMed] [Google Scholar]
- 6.Peterson RV, Pitt WG. The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli. Colloids and Surfaces B: Biointerfaces. 2000;17:219–227. [Google Scholar]
- 7.Qian Z, Sagers RD, Pitt WG. The Effect of Ultrasonic Frequency upon Enhanced Killing of P. aeruginosa Biofilms. Annals Biomed Eng. 1997;25:69–76. doi: 10.1007/BF02738539. [DOI] [PubMed] [Google Scholar]
- 8.Qian Z, Sagers RD, Pitt WG. The role of insonation intensity in acoustic-enhanced antibiotic treatment of bacterial biofilms. Colloids and Surfaces B: Biointerfaces. 1997;9:239–245. [Google Scholar]
- 9.Qian Z, Sagers RD, Pitt WG. Investigation of the mechanism of the bioacoustic effect. J Biomed Mater Res. 1999;44:198–205. doi: 10.1002/(sici)1097-4636(199902)44:2<198::aid-jbm10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Rediske AM, Roeder BL, Brown MK, Nelson JL, Robison RL, Draper DO, et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob Agents Chemother. 1999;43:1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rediske AM, Roeder BL, Nelson JL, Robison RL, Schaalje GB, Robison RA, et al. Pulsed ultrasound enhances the killing of E. coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44:771–772. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitt WG, Ross SA. Ultrasound increases the rate of bacterial cell growth. Biotechnol Prog. 2003;19:1038–1044. doi: 10.1021/bp0340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunne WM, Jr, Mason EO, Jr, Kaplan S. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrobial Agents and Chemotherapy. 1993;37:2522–2526. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darouiche RO, Dhir A, Miller AJ, Landon GC, Raad II, Musher DM. Vancomycin Penetration into Biofilm Covering Infected Prostheses and Effect on Bacteria. J Infect Diseases. 1994;170:720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. Interactions between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrobial Agents and Chemotherapy. 1993;37:1749–1755. doi: 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda H, Ajiki Y, Koga T, Yokota T. Interaction betwee clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrobial Agents and Chemotherapy. 1994;38:138–141. doi: 10.1128/aac.38.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrobial Agents and Chemotherapy. 2000;44:1818–1824. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrob Agents Chemother. 2003;47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KA, Clark HA, Allison DG. Use of ultrasound to facilitate antibiotic diffusion through Pseudomonas aeruginosa alginate. J Antimicrob Chemother. 1995;36:463–473. doi: 10.1093/jac/36.3.463. [DOI] [PubMed] [Google Scholar]
- 20.Karlowsky JA, Hoban DJ, Zelenitsky SA, Zhanel GG. Altered denA and anr gene expression in aminoglycoside adaptive resistance in Pseudomonas aeruginosa. Journal of Antimicrobial Chemotherapy. 1997;40:371–376. doi: 10.1093/jac/40.3.371. [DOI] [PubMed] [Google Scholar]
- 21.Karlowsky JA, Saunders MH, Harding GAJ, Hoban DJ, Zhanel GG. In vitro characterization of aminoglycoside adaptive resistance in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 1996;40:1387–1393. doi: 10.1128/aac.40.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Ohdaira E, Ide M. Enhancement in Diffusion of Electrolyte through Membrane Using Ultrasonic Dialysis Equipment with Plane Membrane. Jpn J Appl Phys. 1995;34:2725–2729. [Google Scholar]
- 23.Ma Y, Yeung ES. Effect of Ultrasound on the Separation of DNA Fragments in Agarose Gel Electrophoresis. Anal Chem. 1990;62:1194–1196. doi: 10.1021/ac00210a019. [DOI] [PubMed] [Google Scholar]
- 24.Staneck JL, Glenn S, Dipersio JR, Leist PA. Wide Variability in Pseudomonas-Aeruginosa Aminoglycoside Results among 7 Susceptibility Testing Procedures. Journal of Clinical Microbiology. 1989;27:2277–2285. doi: 10.1128/jcm.27.10.2277-2285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic Enhancement of Antibiotic Action on Gram-Negative Bacteria. Antimicrob Agents and Chemother. 1994;38:2577–2582. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrews J, Walker R, King A. Evaluation of media available for testing the susceptibility of Pseudomonas aeruginosa by BSAC methodology. Journal of Antimicrobial Chemotherapy. 2002;50:479–486. doi: 10.1093/jac/dkf181. [DOI] [PubMed] [Google Scholar]
- 27.Ioannides-Demos LL, Liolios L, Wood P, Spicer WJ, McLean AJ. Changes in MIC alter responses of Pseudomonas aeruginosa to tobramycin exposure. Antimicrobial Agents and Chemotherapy. 1998;42:1365–1369. doi: 10.1128/aac.42.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronvall G. Analysis of a Single Reference Strain for Determination of Gentamicin Regression Line Constants and Inhibition Zone Diameter Breakpoints in Quality Control of Disk Diffusion Antibiotic Susceptibility Testing. J Clin Microbiol. 1982;16:784–793. doi: 10.1128/jcm.16.5.784-793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landrygan J, James PA, Brooks D, Kubiak EM. Reproducibility of control organism zone diameters for batches of IsoSensitest agar manufactured from 1996 to 2000 using the BSAC disc susceptibility test method. Journal of Antimicrobial Chemotherapy. 2002;49:391–394. doi: 10.1093/jac/49.2.391. [DOI] [PubMed] [Google Scholar]
- 30.Takenaka S, Iwaku M, Hoshino E. Artificial Pseudomonas aeruginosa biofilms and confocal laser scanning microscopic analysis. J Infect Chemother. 2001;7:87–93. doi: 10.1007/s101560100014. [DOI] [PubMed] [Google Scholar]
- 31.Heersink J, Costerton WJ, Stoodley P. Influence of the Sonicare toothbrush on the structure and thickness of laboratory grown Streptococcus mutans biofilms. Am J Dent. 2003;16:79–83. [PubMed] [Google Scholar]
- 32.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 33.Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart PS, Grab L, Diemer JA. Analysis of biocide transport limitation in an artificial biofilm system. J Appl Microbiol. 1998;85:495–500. doi: 10.1046/j.1365-2672.1998.853529.x. [DOI] [PubMed] [Google Scholar]
- 35.Stewart PS, Raquepas JB. Implications of reaction-diffusion theory for the disinfection of microbial biofilms by reactive antimicrobial agents. Chem Engr Sci. 1995;50:3099–3104. [Google Scholar]
- 36.Anwar H, Strap JL, Costerton JW. Establishment of Aging Biofilms: Possible Mechanism of Bacterial Resistance to Antimicrobial Therapy. Antimicrob Agents and Chemother. 1992;36:1347–1351. doi: 10.1128/aac.36.7.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floros JD, Liang H. Acoustically Assisted Diffusion Through Membranes and Biomaterials. Food Tech. 1994;48:79–84. [Google Scholar]
- 38.Mitragotri S, Blankschtein D, Langer R. Ultrasound-Mediated Transdermal Protein Delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 39.Nyborg WL. Biological Effects of Ultrasound: Development of Safety Guidelines. Part II: General Review. Ultrasound Med Biol. 2001;27:301–333. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 40.Carmen J. An Investigation of the Mechanism of the Action of Ultrasound and Antibiotics on Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus epidermidisM.S. Thesis;Brigham Young University. 2001; pp. 78.
- 41.Dodds MG, Grobe KJ, Stewart PS. Modeling Biofilm Antimicrobial Resistance. Biotech Bioeng. 2000;68:456–465. [PubMed] [Google Scholar]


