Abstract
Bone morphogenetic proteins (BMP) inhibit proliferation and induce apoptosis in pulmonary artery smooth muscle cells (PASMC) from normal subjects. Dysfunction of BMP signaling due to mutations in and/or downregulation of BMP receptors has been implicated in idiopathic pulmonary arterial hypertension (IPAH). We examined whether BMP differentially regulates gene expression in PASMC from normal subjects and IPAH patients using the Affymetrix microarray analysis. BMP-2 treatment (200 nM for 24 hrs) altered expression levels of 6,206 genes in normal and IPAH PASMC. 1,063 of these genes were regulated oppositely by BMP-2: 523 genes were downregulated by BMP-2 in normal PASMC but upregulated in IPAH PASMC, whereas 540 genes were upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC. The divergent effects of BMP-2 on gene expression profiles indicate that PASMC may undergo significant phenotypic changes in IPAH patients during development of the disease. The transition of the antiproliferative effect of BMP-2 in normal PASMC to its proliferative effect in IPAH patients is attributed potentially to its differential effect on expression patterns of various genes that are involved in cell proliferation and apoptosis. Among the 6,206 BMP-2-sensitive genes, there are more than 1800 genes whose expression levels were negatively (correlation coefficient, r, < −0.9) or positively (with r > +0.9) correlated with the pulmonary arterial pressure. These results suggest that BMP-mediated gene regulation is significantly altered in PASMC from IPAH patients and mRNA expression changes in BMP-regulated genes may be involved in the development of IPAH.
Keywords: DNA microarray, gene expression profile, transcription factor, bone morphogenetic protein receptor
Idiopathic pulmonary arterial hypertension (IPAH) is a fatal and progressive disease that dominantly affects women [1,2]. Sustained pulmonary vasoconstriction, excessive pulmonary vascular remodeling (which is characterized by intimal and medial hypertrophy in the vascular wall), lumen obliteration of the small arteries and arterioles, and in situ thrombosis are the major causes for the elevated pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) in patients with IPAH. Bone morphogenetic proteins (BMPs) regulate cell proliferation, apoptosis, and differentiation in many cell types [3-8]. Experiments in vitro have demonstrated that BMPs (e.g., BMP-2/-7) inhibit proliferation [9] and induce apoptosis [10] in pulmonary artery smooth muscle cells (PASMC) from normal subjects and patients without pulmonary hypertension. The antiproliferative and proapoptotic effects of BMPs on normal PASMC may be an important requisite for maintaining a thin pulmonary vascular wall and a low pulmonary vascular resistance under normal conditions [11].
Dysfunction of BMP receptors (BMP-R), including BMP-RIa, BMP-RIb, and BMP-RII, due potentially to mutations in the BMP-RII gene (BMPR2) [12] and downregulation of the BMP-RIa gene (BMPR1a) [13] and BMPR2 [10,14] expression, have been implicated in the development of pulmonary vascular intimal and medial hypertrophy in IPAH patients. Indeed, BMP-mediated antiproliferative and proapoptotic effects are both markedly inhibited in PASMC from IPAH patients (in comparison to PASMC from normal subjects and normotensive patients); the antiproliferative effect of BMPs on normal PASMC was actually reversed to be proliferative in IPAH-PASMC [9,10]. These observations suggest that PASMC from IPAH patients may undergo significant phenotypic changes and, accordingly, respond to BMPs differently in comparison with PASMC from normal subjects.
Signal transduction of BMP-mediated effects in PASMC involves homo- and/or hetero-meric dimerization of BMP receptors (BMP-RIa, -RIb, and -RII) and activation (i.e., phosphorylation) of the downstream signaling proteins, Smads (mothers against decapentaplegic proteins) [8]. The increased Smads in the nucleus, in addition to activating gene transcription, can also form heterogeneous polymers with co-repressors (e.g., TGIF, c-Ski, and SnoN) to induce repression of target gene transcription [8,15-19]. Pulmonary vascular remodeling in IPAH patients involves multiple functional and structural alterations in small pulmonary arteries. Increased PASMC proliferation and inhibited PASMC apoptosis are two of the major contributors to the thickening of the pulmonary vascular wall [11]. The purpose of this study was to determine potential differences in BMP-induced gene expression patterns in PASMC from normal subjects and IPAH patients as a result of the phenotypic changes in IPAH-PASMC.
Methods and Materials
Cell culture. Human PASMC from two normal subjects and two IPAH patients (diagnosed on the basis of the criteria used in the National Institutes of Health Registry for Primary Pulmonary Hypertension) were used at the 5th-6th passage in the study. Cells were cryopreserved at passage 3, replated onto flasks to amplify cell number for 2-3 passages, and then used for the proposed experiments. We used normal and IPAH PASMC at the same passage for the proposed experiments (e.g., treated with BMP-2). Approval to use these human tissues was granted by the UCSD Institutional Review Board. The mean pulmonary arterial pressure of the IPAH patients, a 57-yr-old woman and a 31-yr-old man were 51 and 53 mmHg, respectively. The procedure used to prepare cultured PASMC from transplant patients has been described previously [20]. For morphological experiments, the cells were stained with the membrane-permeable nucleic acid stain, 4′, 6′-diamidino-2-phenylindole (DAPI, 5 μM); the blue fluorescence emitted at 461 nm was used to visualize the cell nuclei. A smooth muscle α-actin antibody was used to evaluate expression of α -actin in DAPI-stained cells.
Affymetrix GeneChip analysis. Hybridization probes for GeneChip analysis were prepared from total RNA isolated from normal and IPAH PASMC treated with vehicle or BMP-2 (200 nM for 24 hrs). The total RNA was converted to double-stranded cDNA using an oligo(dT) primer containing the T7 promoter. The cDNAs were used to prepare biotinylated cRNA using the Bioarray High Yield Kit (Enzo) according to the manufacturer's protocol. The biotinylated cRNA probes were fragmented and applied to individual oligonucleotide Human Genome U95Av2 GeneChip arrays (Affymetrix), which contain probe sets for more than 12,000 human genes. Two chips were used for each group (i.e., the cells treated or not with BMP-2). The signal intensity from hybridized cRNA was quantified and used for quantitating gene expression level. Affymetrix probe set number is the same as the GenBank accession number. A full complement of the microarray findings for all the tissues tested has been deposited to the NCBI GEO databank (No. GSE2559).
To identify and quantify genes that are regulated by BMP-2 in PASMC from normal subjects and IPAH patients, we employed the Gene Chip analysis software. Using the default parameters of the Affymetrix GeneChip analysis software, 30-36% of the 12,000 genes were scored as being present (or marginal) in the control and BMP-2-treated PASMC, respectively. The normalization factors used to compare the two sets of data (i.e., with or without BMP-2 treatment) indicated that the global levels of hybridization from the two-cRNA samples in each group (control and BMP-2-treated groups) were roughly equivalent.
The gene expression levels in normal and IPAH PASMC after treatment with BMP-2 (A BMP-2) were normalized to the gene expression levels in control (A Control) normal and IPAH PASMC (cells without treatment of BMP-2). The ratio (R) of the gene expression levels in BMP-2-treated cells to the gene expression levels in control cells was determined by the equation: R = (A BMP-2 − A Control)/(A Control), and used to indicate the BMP-2-mediated changes in gene expression. Therefore, a positive number of R indicates that BMP-2 upregulates expression of a gene, whereas a negative number of R denotes that BMP-2 downregulates the mRNA expression of a gene. The BMP-2-induced changes (R) in gene expression were compared between normal PASMC and IPAH PASMC to specify the genes that are oppositely regulated by BMP-2 in normal and IPAH PASMC.
Real-time PCR. Total RNA was extracted from human PASMC by using the RNeasy Mini Kit (Qiagen). Super-Script reverse transcriptase (Invitrogen) was used to synthesize cDNA. RNA (2 μg) was first incubated with oligo(dT) (1 μl at 0.5 μg/μl) at 70°C for 10 min. Then 8 μl of a solution that contained 10x buffer, 10 mM dNTP, 20 mM MgCl2, 0.1 M DTT, 40 U/μl RNaseOUT, and 50 U/μl SuperScript II reverse transcriptase were added to the samples and incubated for 10 min at 30°C, 60 min at 42°C, and 5 min at 95°C. RNase-H (1 μl at 2U/μl; GIBCO) was added to each reaction, and the samples were incubated for 20 min at 37°C. The sense and antisense primers were specifically designed from the coding regions of each gene (Table 1). Real-time PCR containing 1 μg of cDNA template, 0.5 μM each of forward and reverse primers, and SYBR Green PCR Kit (Qiagen) was performed in a total volume of 20 μl. Thermal cycling conditions were as follows: initial incubation of 10 min at 95°C followed by 45 cycles of 20 s at 94°C, 20 s at 55°C annealing temperature, and 30 s at72°C. Real-time PCR was performed using a Prism 7700 thermocycler (PE Applied Biosystems). Analysis of cycle threshold (CT) was performed using Opticon 2 Analysis Software; normalized values were obtained for each group by subtracting matched glyceraldehyde-3-phosphate dehyodrogenase (GAPDH) CT values.
Table 1.
Oligonucleotide sequences of primers used for real-time PCR
| Standard Name | Accession Number* | Predicted Size (bp) | Sense/Antisense | Location, nt. |
|---|---|---|---|---|
| TGF- β receptor Type I (TβRIa) | L17075 | 214 | 5′-ATTACCTGGACATCGGCAAC-3′/5′-TTGGGCACCACATCATAGAA-3′ | 1170 1383 |
| TGF- β receptor Type IIα (TβRIIα) | D50683 | 155 | 5′-CCATGTCTCACAGCCAGCTA-3′/5′-CCAGGAGAAATAAGGGCACA-3′ | 3813 3967 |
| Apoptosis associated protein GADD34 | U83981 | 218 | 5′-TCCTGGGAGTATCGTTCAGG-3′/5′-CAGGGAGGACACTCAGCTTC-3′ | 997 1214 |
| Annexin V (ANX5) | U05770 | 196 | 5′-TTCAGCACCTTTAGCTGCATT-3′/5′-GAAAATGGCCAGGCATTAAA-3′ | 201 396 |
| Cytochrome c-1 (Cyt-c1) | J04444 | 205 | 5′-TGTCACGGCAACAGAGAGAC-3′/5′-AGAGTCAAAACCTCGCGAAA-3′ | 1151 1355 |
| GATA-binding protein (GATA2) | M68891 | 232 | 5′-GTCACTGACGGAGAGCATGA-3′/5′-GCCTTCTGAACAGGAACGAG-3′ | 925 1156 |
| Caveolin-2 | AF035752 | 193 | 5′-AGTTCCTGACGGTGTTCCTG-3′/5′-CGTCCTACGCTCGTACACAA-3′ | 277 469 |
| c-Myc binding protein | D89667 | 223 | 5′-TCCACTAGAGGCAAGGTGCT-3′/5′-ACCCTGGCCTTACACTCCTT-3′ | 92 314 |
| c-Fos | K00650 | 199 | 5′-TTTATAGTGGGCGGAAGTGG-3′/5′-ACGTCCTGGACAAAGGTCAC-3′ | 5115 5313 |
| Serotonin receptor (5-HT2B) | X77307 | 199 | 5′-GCCTTCTTCACACCTCTTGC-3′/5′-TGTCCTTTCGAGAACCATCC-3′ | 728 926 |
| Thrombin receptor | M62424 | 178 | 5′-GTGATTGGCAGTTTGGGTCT-3′/5′-GCCAGACAAGTGAAGGAAGC-3′ | 721 898 |
| Inositol 1,4,5-trisphosphate receptor type 1 (IP3R1) | D26070 | 200 | 5′-CTGATTCACCCACGAAGGTT-3′/5′-TGCAAATCAGGTGCTTTCTG-3′ | 8413 8612 |
Accession numbers are GenBank accession numbers for the sequences used in designing the primers.
Statistical analysis. Protocols for data analysis and documentation for the sensitivity and quantitative determination of Affymetrix microarrays have been described [21-23]. We used linear regression equations to calculate correlation of differential gene expressions between normal and IPAH PASMC. For real-time RT-PCR experiments, mRNA expression levels between normal and IPAH PASMC are expressed as means±SE. Statistical analysis was performed using unpaired and paired Student's t tests or ANOVA as indicated. Differences were considered to be significant when P < 0.05.
Results
Genes that are regulated by BMP-2 in PASMC from normal subjects and IPAH patients. PASMC from normal subjects and IPAH patients, cultured and passaged for the same time before experimentation, exhibited similar morphologies and expression of smooth muscle α-actin (Fig. 1). In BMP-2 (200 nM for 24 hrs) treated cells (normal and IPAH PASMC), 6,206 genes had altered expression levels as compared to control cells; the ratio of the gene expression level in BMP-2-treated cells to that in control cells was either greater (increased) or less (decreased) than zero (Fig. 2). Among the 6,206 genes, BMP-2 treatment upregulated 3,040 genes and downregulated 3,166 genes in normal PASMC, while BMP-2 upregulated 2,958 genes and downregulated 3,248 genes in IPAH PASMC. The changes in gene expression induced by BMP-2 treatment ranged from −8.98-fold (decreased by BMP-2) to +63.45-fold (increased by BMP-2) in normal PASMC, and from −10.35-fold to +61.24-fold in IPAH PASMC (Fig. 2).
Fig. 1.
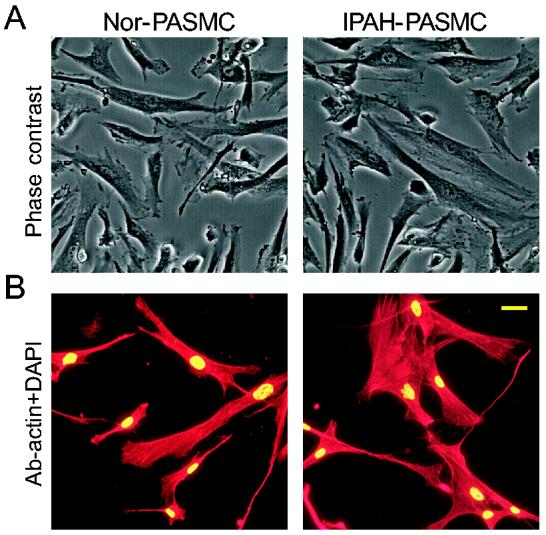
Morphology and purity of PASMC from normal subjects and IPAH patients. A: Phase-contrast photomicrographs show cultured normal and IPAH PASMC. B: Cell cultures, stained with the α-actin antibody (red) and DAPI (green), show that all DAPI-positive cells cross-react with the α-actin antibody in normal and IPAH PASMC. Bar, 20 μm.
Fig. 2.
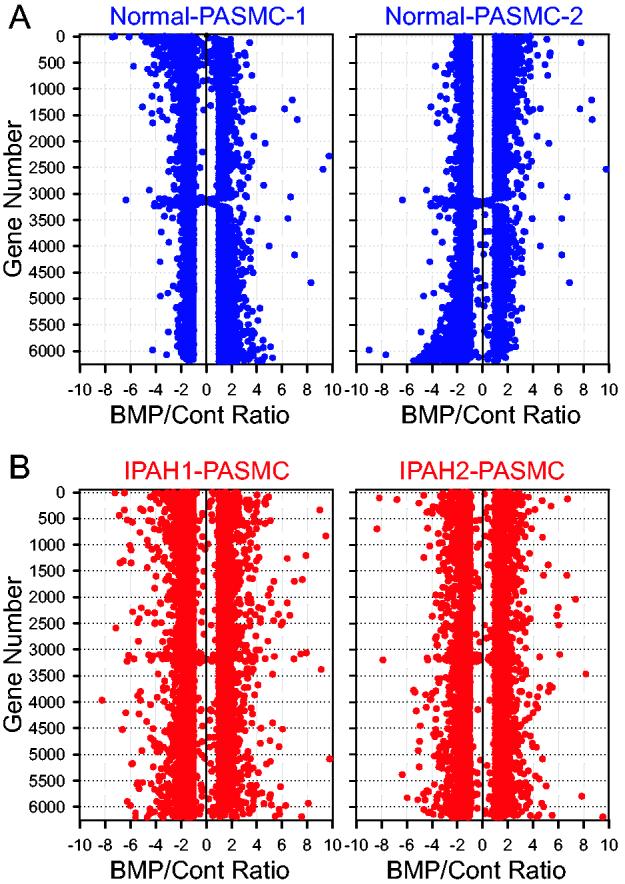
BMP-2-mediated gene expression in PASMC from normal subjects (A) and IPAH patients (B). The number of genes (the ordinate) is plotted with the ratio (the abscissa) of the gene expression level in BMP-2-treated cells to the gene expression level in vehicle-treated (Cont) cells. Each data point represents the fold change of BMP-2-mediated gene expression in two normal PASMC samples (A) and in two IPAH PASMC samples (B). Positive and negative numbers in the abscissa denote upregulation and downregulation, respectively, by BMP-2 treatment.
The BMP-2-mediated changes in gene expression were not always concordant. Therefore, we averaged the values obtained from two normal PASMC samples and from two IPAH PASMC samples treated with BMP-2 (Fig. 3) in order to exclude the genes that were increased in one sample but decreased in the other. After averaging the values in normal (Fig. 3A, left) and IPAH (Fig. 3A, right) PASMC samples, there are 4,466 genes in two normal PASMC samples and 3,623 genes in two IPAH PASMC samples, whose expression levels were changed ≥ 1 fold (either decreased or increased) by BMP-2 treatment (Fig. 3B and C). These genes were then analyzed to define those that are differentially regulated by BMP-2 in normal and IPAH PASMC.
Fig. 3.
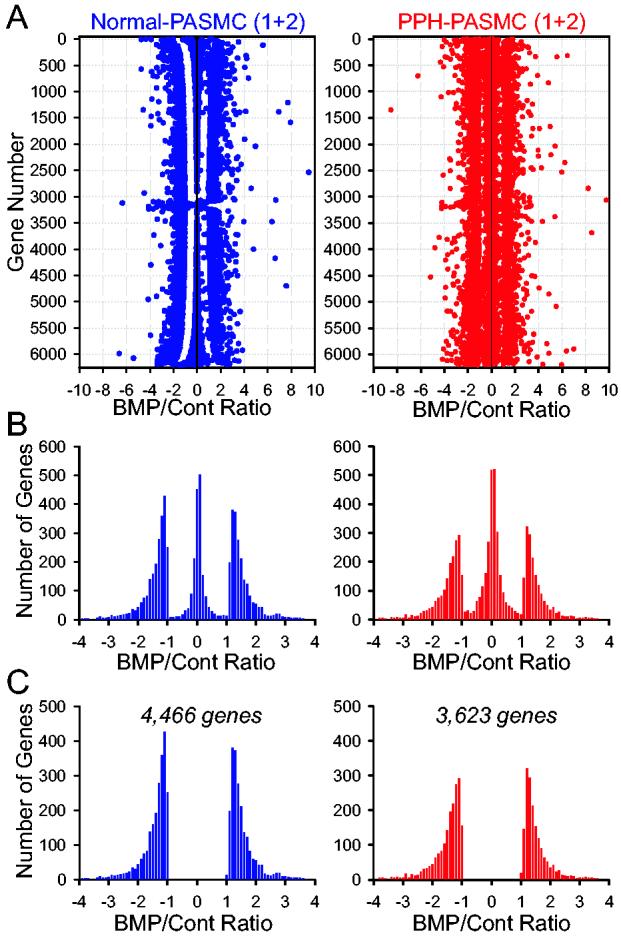
Numbers of genes that have concordant changes in response to BMP-2 in normal and IPAH PASMC. A: Values of the fold changes are averaged in two normal PASMC samples (left) and in two IPAH PASMC samples (right) to indicate the BMP/control ratio (the ratio of the gene expression level in BMP-2-treated cells to the gene expression level in control cells). Positive and negative numbers in the abscissa denote upregulation and downregulation, respectively, by BMP-2 treatment. B: Histogram showing the number of genes with the BMP/control ratio ≤ −1 (downregulated), −1 to +1 (not significantly changed), and ≥ +1 (upregulated) in normal (left) and IPAH (right) PASMC. C: Histogram showing the number of genes with the BMP/control ratio either ≤ −1 or ≥ +1 in normal (left) and IPAH (right) PASMC.
Identification of genes that are oppositely regulated by BMP-2 in PASMC from normal subjects and IPAH patients. Among the BMP-2-regulated 4,466 genes in normal PASMC samples and 3,623 in IPAH PASMC, 1,123 genes were regulated oppositely by BMP-2 in normal and IPAH PASMC (Fig. 4A). There were 523 genes that were downregulated (by ≥1-fold) by BMP-2 in normal PASMC but upregulated (by ≥1-fold) in IPAH PASMC (Fig. 4A, left panel), whereas 540 genes were upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC (Fig. 4A, right panel). Among the 1,123 genes whose expression levels were oppositely regulated by BMP-2, the fold change of BMP-2-mediated inhibitory or augmenting effect was negatively correlated (r = −0.1233 to −0.2821; P < 0.01) between normal and IPAH PASMC (Fig. 4C), i.e., the extent of BMP-2-induced gene down- or up-regulation in normal PASMC is inversely correlated to that of the same gene in IPAH PASMC.
Fig. 4.
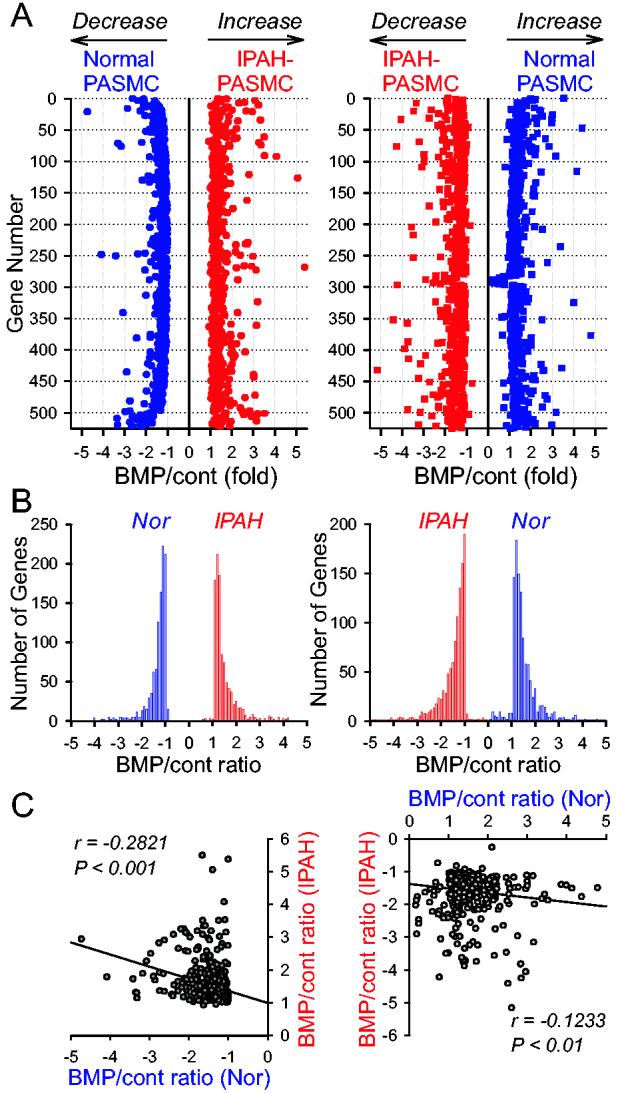
Numbers of genes that are regulated oppositely by BMP-2 in normal and IPAH PASMC. A: The number of genes that are downregulated by BMP-2 in normal PASMC but upregulated in IPAH PASMC (left panel) and that are upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC (right panel). B: Histogram showing the number of genes with the BMP/control ratio ≤ −1 or ≥ +1 in normal and IPAH PASMC. C: Correlation of BMP-2 mediated opposite effects on gene expression in normal and IPAH PASMC. Left panel shows the genes that were downregulated by BMP-2 in normal PASMC but upregulated in IPAH PASMC. Right panel indicates the genes that were upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC.
It may be argued that a 1-fold change in gene expression may represent a low threshold for analysis and that this may contribute to the large number (e.g., 523 and 540, as shown in Fig. 4) of genes that were differentially regulated by BMP-2. By using a 1.5-fold cut-off for the data set, we observed that 155 (out of 523 genes) were downregulated in normal PASMC but upregulated in IPAH-PASMC by BMP-2, whereas 204 genes (out of 540 genes) were upregulated in normal PASMC but downregulated in IPAH PASMC. These results indicate that more than 30% of the genes showed altered expression after BMP-2 treatment if we used a 1.5-fold cut-off for data analysis.
Among others, BMP-2 downregulated a series of genes in normal PASMC that relate to cell growth and contraction; these genes were upregulated in IPAH PASMC (Table 2). In contrast, BMP-2 upregulated several apoptosis-related genes in normal PASMC, but downregulated these genes in IPAH PASMC (Table 3). The BMP-2-mediated upregulation of the growth-related genes and downregulation of the proapoptotic genes in IPAH PASMC may explain why BMP-2 enhances PASMC proliferation in IPAH patients [9,10].
Table 2.
List of selected genes that are downregulated by BMP-2 in normal PASMC but upregulated in IPAH PASMC
| Growth factors and ligands | Signal transduction proteins and cytoplasmic proteins |
| Y15915: Platelet-derived growth factor β | AF035752: Caveolin-2 |
| U12471: Thrombospondin-1 | U12022: Calmodulin |
| M96956: Teratocarcinoma-derived growth factor 3 | AF035606: Ca2+ binding protein ALG-2 |
| D16431: Hepatoma-derived growth factor | M72393: Ca2+-dependent phospholipid-binding protein |
| M60828: Keratinocyte growth factor | J03191: Profilin |
| M74587: Insulin-like growth factor binding protein | U49436: Translation initiation factor 5 (eIF5) |
| M96995: EGF receptor-binding protein GRB2 | M84711: v-fos transformation effector protein (Fte-1) |
| J02783: Thyroid hormone binding protein (p55) | U49957: LIM protein (LPP) |
| U66469: Cell growth regulator CGR19 | X15875: cAMP response element binding protein-1 |
| U47741: CREB-binding protein (CBP) | |
| Membrane receptors, channels, and transporters | |
| M91467: Serotonin receptor (5HT1E) | Transcription factors |
| U48861: Nicotinic ACh receptor subunit β4 | M92287: Cyclin D3 (CCND3) |
| U43672: Transmembrane receptor IL-1Rrp | M58603: NF-κB |
| L27080: Melanocortin 5 receptor (MC5R) | D89667: c-Myc binding protein |
| U38480: Retinoid X receptor-γ | M13929: c-Myc-P64 |
| AF091890: G-protein coupled receptor RE2 | K00650: c-Fos |
| M62762: Vacuolar H+ ATPase proton channel subunit | X52541: Early growth response protein 1 (hEGR1) |
| Protein kinases | Enzymes and mitochondrial proteins |
| U33284: Protein tyrosine kinase PYK2 | U50136: Leukotriene C4 synthase (LTC4S) |
| M64174: Protein tyrosine kinase JAK1 | X96924: Mitochondrial citrate transport protein |
| D10495: Protein kinase Cδ | X69907: Mitochondrial ATP synthase c subunit |
| L32976: Protein kinase MLK-3 | X83218: ATP synthase |
| Y12735: Protein kinase Dyrk3 | M32304: Metalloproteinase inhibitor |
| Z11695: Protein kinase related to rat ERK2 | J02947: Extracellular-superoxide dismutase (SOD3) |
| L20321: Serine/threonine kinase stk2 | AF067139: NADH-ubiquinone oxidoreductase |
| X07767: cAMP-dependent protein kinase | NDUFS3 subunit |
| U08316: Insulin-stimulated protein kinase 1 (ISPK-1) |
Table 3.
List of selected genes that are upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC
| Apoptotic inducers or proapoptotic factors | X59892: IFN-inducible γ2 protein |
| X83492: Fas/Apo-1 | L77864: Stat-like protein (Fe65) |
| AF006041: Fas-binding protein (DAXX) | AL049176: Chordin-like protein/vWF type C domain |
| AF031167: Interleukin 15 precursor (IL-15) | AF022385: Apoptosis-related protein TFAR15 |
| X82540: Activin β-C chain | U88666: Serine kinase SRPK2 |
| M34064: N-cadherin | U89896: Casein kinase I γ2 |
| D21255: OB-cadherin-2 | U17714: Tumor suppressor ST13 (ST13) |
| M33308: Vinculin | AF061836: Tumor suppressor protein (RDA32) |
| M63838: Interferon-γ induced protein (IFI 16) | |
| Transcription factors | |
| Membrane receptors, ion channels, transporters | X15218: Ski |
| U20860: Angiotensin II type 2 receptor gene | U47677: Transcription factor E2F1 |
| D13168: Endothelin-B receptor (hET-BR) | X96717: Transcription factor TFE3 |
| U01062: Inositol 1,4,5-trisphosphate receptor-3 | J04111: c-Jun protooncogene |
| M58286: Tumor necrosis factor receptor | Z30093: Basic transcription factor 2t |
| U22662: Nuclear orphan receptor LXR-α | AF041259: Breast cancer transcription factor ZABC1 |
| L00352: Low density lipoprotein receptor | |
| AB026833: Chloride channel protein | Enzymes |
| U46569: Aquaporin-5 (AQP5) gene | D13146: 2 ,3 -cyclic-nucleotide 3 -phosphodiesterase |
| AJ010953: Ca2+-transporting ATPase | AL035079: Catalase |
| M95549: Na+/glucose cotransporter-like protein | U34683: Glutathione synthetase |
| AB016243: Regulatory factor 2 of Na+/H+ exchanger | Z82244: Heme Oxygenase 1 (HO-1) |
| U45285: Vacuolar H+ pump subunit (OC-116kDa) | M19961: Cytochrome c oxidase subunit Vb (coxVb) |
| U46116: Receptor tyrosine phosphatase γ (PTPRG) | |
| Signal transduction proteins and protein kinases | M64929: Protein phosphatase 2Aα subunit |
| U05770: Annexin V (ANX5) | X05409: Mitochondrial aldehyde dehydrogenase I |
| J04444: Cytochrome c-1 | Y00264: Amyloid A4 precursor of Alzheimers disease |
| M68891: GATA-binding protein (GATA2) | M83772: Flavin-containing monooxygenase form II |
To confirm the BMP-2-mediated upregulation and downregulation of mRNA expression of genes shown in Tables 2 and 3, we performed real time RT-PCR experiments on three genes that were downregulated by BMP-2 in normal PASMC but upregulated in IPAH PASMC (Table 2), and three genes that were upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC (Table 3), as indicated by the microarray experiments. Treatment with BMP-2 (200 nM for 24 hrs) had no effect on mRNA expression of GAPDH (Fig. 5A). However, it significantly decreased mRNA levels of caveolin-2, c-Myc binding protein, and c-Fos in normal PASMC, but markedly increased mRNA expression levels of these genes in PASMC from IPAH patients (Fig. 5B). In these experiments, we used a maximum of 45 cycles to detect mRNA levels, where a higher required cycle number indicates a low mRNA level. In contrast, BMP-2 treatment significantly upregulated mRNA expression of annexin V, cytochrome c-1, and GATA-binding protein 2 in normal PASMC, but downregulated these genes in IPAH PASMC (Fig. 5C). These results, which are in agreement with the results shown in Tables 2 and 3, suggest that the qualitative data obtained by microarray experiments using the Affymetrix platform are reliable and demonstrate convincing differences between normal and IPAH PASMC in response to BMP-2.
Fig. 5.
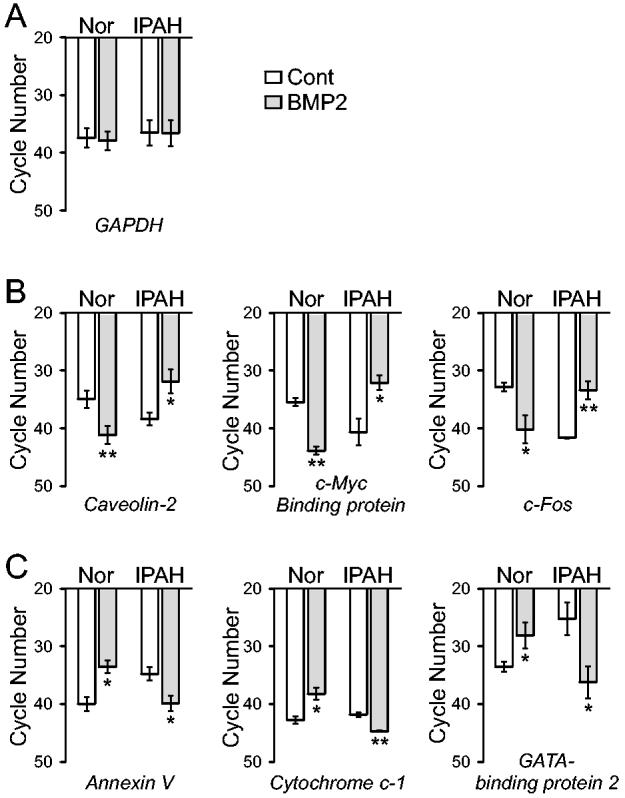
Comparison of mRNA expression levels of genes in normal and IPAH PASMC treated with or without BMP-2. Expression of mRNA for GAPDH, caveolin-2, c-Myc binding protein, c-Fos, annexin V, cytochrome c-1, and GATA-binding protein 2 was assessed by real-time PCR. A maximum of 45 cycles were used to detect mRNA levels, where a higher required cycle number indicates a lower mRNA level. Open bars show mRNA levels of various genes in control PASMC (not treated with BMP-2) while shaded bars represent data from PASMC treated with 200 nM BMP-2 for 24 hours. The mRNA expression level is reported as a function of cycle number for GAPDH controls (A), selected genes (caveolin-2, c-Myc binding protein, c-Fos) that are downregulated in normal PASMC but upregulated in IPAH PASMC (B), and selected genes (annexin V, cytochrome c-1, and GATA-binding protein 2) that are upregulated in normal PASMC but downregulated in IPAH PASMC (C) following BMP-2 exposure. ** P<0.01, * P<0.05 vs. untreated control cells (open bars).
Comparison of expression levels of the BMP-2-sensitive genes between normal and IPAH PASMC. Previous investigation has demonstrated that the mRNA expression levels of many genes are well correlated with PAP in rats with hypoxia-induced pulmonary hypertension [24,25]. Whether expression levels of the BMP-2-sensitive genes correlate with PAP was therefore examined in a normal subject (the PAP is assumed to be ∼16 mmHg) and two IPAH patients. Among the 6,206 BMP-2-sensitive genes, the expression level of 876 genes was much higher (i.e., the difference between gene expression levels is > +100 arbitrary units), whereas the level of 858 genes was much lower (i.e., the difference between gene expression levels in IPAH and normal PASMC is < −100), in IPAH PASMC than in normal PASMC. As shown in Figure 6, each solid circle represents a difference of expression level of a gene between normal and IPAH PASMC (i.e., the averaged expression level of a gene in IPAH-PASMC minus the expression level of the gene in normal PASMC). For most of the BMP-2-sensitive genes, the expression level differences between normal and IPAH PASMC were small, with the difference between the gene expression levels between normal and IPAH PASMC ranging from −100 to +100 arbitrary units (Fig. 6A, lower panel). However, the expression level differences of some genes in PASMC between normal subjects and IPAH patients were larger than 500 arbitrary units; the difference was ≥ +500 in 161 genes, and ≤ −500 in 287 genes.
Fig. 6.
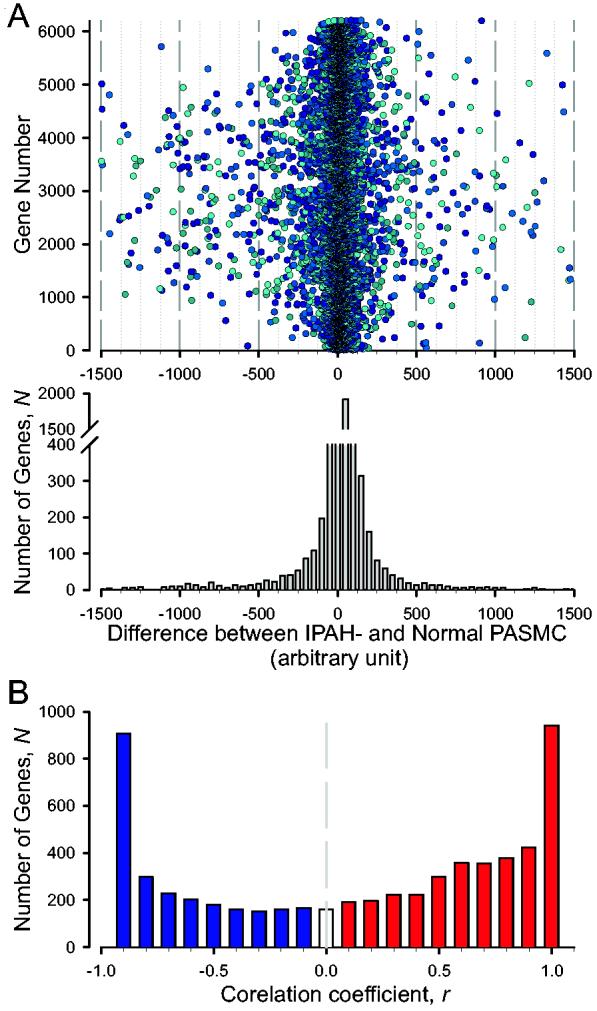
Distribution of genes, whose expression levels are correlated to the mean PAP, as a function of the correlation coefficient r. A: The number of genes (the ordinate), whose expression levels are different between normal and IPAH PASMC. The abscissa denotes the difference of gene expression levels (arbitrary units) between IPAH PASMC and normal PASMC. The negative and positive values indicate that the gene expression level in IPAH PASMC is less than (negative) or greater than (positive) in normal PASMC, respectively. Lower panel: Histogram showing the number of genes with various differences of gene expression levels. B: The ordinate N is the number of genes in the interval (r, 1) for positive r or in (−1, r) for negative r.
Correlation coefficient between the mean PAP and gene expression level. To investigate the relationship between the PAP and expression level of BMP-2-sensitive genes, the correlation coefficient, r, was computed by the formula:
| [1] |
where x is the mean PAP (which were 16, 51, and 53 mmHg for the subjects), y is the gene expression level, the gene number i is from 1 to 6,206 (i.e., i = 1, 2, 3, … 6,206) in our case, and the sample number n is 3 in this study. Values of r near +1 imply a strong positive association between the mean PAP and the gene expression level, while values near −1 imply a strong negative association (values near 0 imply little or no association). Figure 6B shows the distribution of the number of genes, N (the abscissa), as function of the correlation coefficient r (the ordinate) between the mean PAP and gene expression level. There are 939 genes having values of r between +0.9 and +1, and 907 genes having values of r between −1 and −0.9.
After computing the correlation coefficients, the genes were then aligned according to their values of r. The correlation coefficients r(x, y) can be positive or negative, i.e., PAP may be caused by an increase or decrease in gene expression. Therefore, we tabulated the genes with positive r separately from those with negative r. The genes with r = −1 → −0.9 and with r = 0.9 → 1 are listed in Tables 4 and 5, respectively. It is noticed that the large number of genes with r of either less than −0.9 or greater than +0.9 is probably because of the small number of patients.
Table 4.
List of selected genes whose expression levels are correlated negatively (r = −0.9 to −1) to the pulmonary artery pressure (PAP)
| Ligands and growth factors | AF089814: Growth suppressor related (DOC-1R) |
| X70340: Transforming growth factor α (TGF-α) | AF005775: Caspase-like apoptosis regulatory protein 2 |
| M60315: Transforming growth factor-β (TGF-β) | S78085: Programmed cell death-2 (PDCD2) |
| M22488: Bone morphogenetic protein 1 (BMP-1) | U78733: Smad-2 |
| X02910: Tumor necrosis factor (TNF-α) | L13720: Growth arrest-specific protein (gas) |
| X83490: Fas/Apo-1 | S46622: Calcineurin A catalytic subunit |
| AF024710: Vascular endothelial growth factor (VEGF) | AF043250: Mitochondrial outer membrane protein |
| J03764: Plasminogen activator inhibitor-1 | X96924: Mitochondrial citrate transport protein |
| Membrane receptors, ion channels, transporters | Transcription factors |
| L17075: TGF-β receptor type I | U58334: Bcl2-p53 binding protein Bbp |
| D50683: TGF-β receptor type IIα | AF002697: Bcl-2-binding protein Nip3 |
| J04027: Plasma membrane Ca2+ pumping ATPase | U71267: Transcriptional repressor NOT4Hp |
| AF016266: TRAIL receptor 2 | L19067: NF-κB transcription factor p65 subunit |
| X99101: Estrogen receptor | M58603: NF-κB DNA binding subunit (NF-κB) |
| L10338: Na+ channel β1 subunit (SCN1B) | S79639: Tumour suppressor/hereditary multiple |
| M60459: Erythropoietin receptor | exostoses candidate gene |
| AL050404: Voltage-gated K+ channel (KCNG1) | AF041381: Transcriptional repressor E2F-6 |
| Signal transduction, cytoplasmic and mitochondrial proteins | Enzymes |
| D83402: Prostacyclin synthase | |
| AF006041: Fas-binding protein (DAXX) | S72370: Pyruvate carboxylase |
| M55654: TATA-binding protein | X07834: Manganese superoxide dismutase |
| U19599: BAX-δ | X69819: ICAM-3 |
| U83981: Apoptosis associated protein (GADD34) | U04636: Cyclooxygenase-2 (hCox-2) |
Table 5.
List of selected genes whose expression levels are correlated positively (r = +0.9 to +1) to the pulmonary artery pressure (PAP)
| Ligands and growth factors | M19311: Calmodulin |
| M62302: Growth/differentiation factor 1 (GDF-1) | X62535: Diacylglycerol kinase |
| J05081: Endothelin 3 (EDN3) | AF074382: IkB kinase γ subunit (IKK- γ) |
| D00017: Lipocortin II | AF031416: IkB kinase β subunit (IKK-β) |
| M57399: Nerve growth factor (HBNF-1) | U68111: Protein phosphatase inhibitor 2 (PPP1R2) |
| X16323: Hepatocyte growth factor (HGF) | U12779: MAP kinase-activated protein kinase 2 |
| Membrane (receptors, ion channels, transporters) and cytoplasmic proteins | Transcription factors |
| AF017307: Ets-related transcription factor (ERT) | |
| M81590: Serotonin receptor 1D (5-HT1D) | U48730: Transcription factor Stat5b |
| X77307: Serotonin receptor 2B (5-HT2B) | X96717: Transcription factor TFE3 |
| Y12505: Serotonin receptor 4B (5-HT4B) | AF040253: Transcription factor Tat-CT1 |
| M23263: Androgen receptor | D26155: Transcriptional activator hSNF2a |
| U25441: Dopamine D3 receptor (DRD3) | X51688: Cyclin A |
| M62424: Thrombin receptor | M73812: Cyclin E |
| X76079: Platelet derived growth factor α receptor | AF075587: Myc protein |
| M34641: Fibroblast growth factor receptor-1 | L13689: Protooncogene BMI-1 |
| D26070: Inositol 1,4,5-trisphosphate receptor type 1 | L20861: Protooncogene Wnt-5a |
| AF038962: Voltage-dependent anion channel protein | M23379: GTPase-activating protein ras p21 (RASA) |
| AB026833: Chloride channel protein | X77956: Id1 |
| Signal transduction, cytoplasmic and mitochondrial proteins | Enzymes |
| U34683: Glutathione synthetase | |
| U49020: Myocyte-specific enhancer factor 2A | M28713: NADH-cytochrome b5 reductase (b5R) |
| (MEF2A) | Z35307: Endothelin-converting-enzyme 1 |
| L11285: ERK activator kinase (MEK2) | M98539: Prostaglandin D2 synthase |
| AF035752: Caveolin-2 | M64231: Spermidine synthase |
To confirm the qualitative difference of gene expression between normal PASMC and PASMC from IPAH patients, we conducted real time RT-PCR experiments on six selected genes whose mRNA expression was correlated negatively or positively to the PAP. In PASMC isolated from normal subjects (PAP ≈ 16 mmHg). The mRNA level of GAPDH was comparable between normal and IPAH PASMC (Fig. 7A). The mRNA levels of transforming growth factor β receptor I (TGF- β-RI), TGF-β receptor IIα (TGF-β-RIIα), and apoptosis associated protein GADD34 were significantly lower in PASMC from IPAH patients (PAP ≈ 51-53 mmHg) (Fig. 7 B). Furthermore, in normal PASMC, the mRNA levels of serotonin receptor 2B (5-HT receptor 2B), thrombin receptor, and inositol 1,4,5-trisphosphate (IP3) receptor type I were markedly higher in IPAH PASMC (Fig. 7C). These results from real-time RT-PCR experiments are consistent with the microarray experiments, at least for the 6 genes examined. These findings support the microarray data presented in Tables 4 and 5, showing that the mRNA expression levels of TGF-β-RI, TGF-β-RIIα, and apoptosis associated protein GADD34 were negatively correlated to the PAP, whereas the mRNA expression levels of 5-HT receptor 2B, thrombin receptor, and IP3 receptor 1 were positively correlated to the PAP.
Fig. 7.
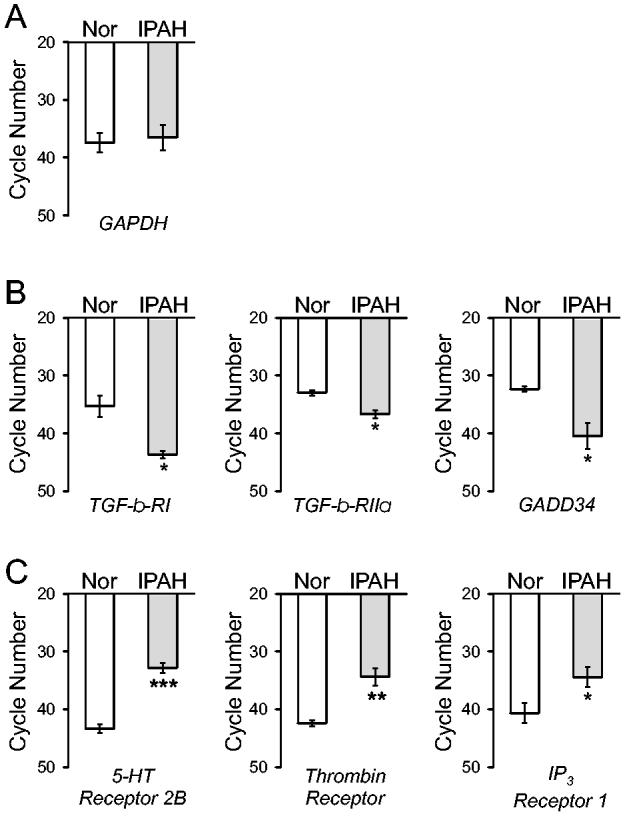
Comparison of mRNA expression levels of BMP-2-sensitive genes in normal PASMC and IPAH PASMC. Expression of mRNA for GAPDH, TGF-β receptor I (TGF-β-RI), TGF-β receptor RIIα (TGF-β-RIIα), apoptosis associated protein GADD34, 5-HT receptor 2B, thrombin receptor, and IP3 receptor 1 was assessed by real-time PCR. A maximum of 45 cycles were used to detect mRNA levels, where a higher required cycle number indicates a lower mRNA level. The mRNA expression is reported as a function of cycle number for GAPDH controls (A), selected genes (TGF-β-RI, TGF-β-RIIα, and apoptosis associated protein GADD34) that are negatively correlated with PAP (B), and selected genes (5-HT receptor 2B, thrombin receptor, and IP3 receptor 1) that are positively correlated with PAP (C), in PASMC from normal subjects (open bars) and PASMC from IPAH patients (shaded bars). *** P<0.001, ** P<0.01, * P<0.05 vs. PASMC from normal subjects (open bars).
It has to be emphasized that the comparison of gene expression between normal subjects and IPAH patients has some limitations in this study because of small sample numbers, genomic variability of the human population, and gender and age differences in the subjects from whom the cells were isolated.
Discussion
PASMC from IPAH patients have undergone many phenotypical changes in comparison to PASMC from normal subjects, such as elevated intracellular Ca2+ which triggers PASMC contraction and stimulates PASMC growth [20], and enhanced proliferation and inhibited apoptosis that lead to vascular wall thickening as a result of medial hypertrophy [1,11]. The phenotypical changes of PASMC in IPAH patients may lead to different responses to vasoconstrictive and mitogenic agonists as well as antiproliferative and proapoptotic factors.
BMPs, similar to other members of the TGF-β superfamily, play a critical role in the regulation of cell proliferation, differentiation, and apoptosis [8,9]. In human lung tissues, BMPs can be synthesized and secreted in different types of cells (e.g., PASMC, vascular endothelial cells, and fibroblasts). The secreted BMPs in the intercellular milieu then exert angiogenic, antiproliferative and proapoptotic effects via an autocrine or paracrine mechanism that involves activation of sarcolemmal BMP receptors. Binding of BMP ligands to BMP receptors (either BMP-RI or -RII) leads to the hetero-dimerization of BMP-RI and BMP-RII and the formation of ligand-receptor complex, which in turn activates the downstream signaling elements such as the receptor-activated Smads (R-Smads) [8,26,27]. Dimerization of the R-Smads (e.g., Smad-1, -5, and -8) with the co-Smad (e.g., Smad-4) forms a signaling complex that can translocate into the nucleus to regulate transcription of the Smad-responsive genes by directly binding to the specific Smad binding sequence (5′-CAGAC-3′ or 5′-GTCTG-3′) in their promoter. In addition to Smad proteins, signal transduction downstream of BMP receptors also involves MAP kinase pathway [28].
Since numerous genes contain the Smad-binding sequence in the promoter, BMPs can exert their functional (antiproliferative, proapoptotic, and angiogenic) effects on PASMC through transcriptional regulation of multiple genes. The end outcome of BMP-mediated effects on PASMC is thus determined by the integrative coordination among many gene products.
In this study, we observed that BMP-2 (for 24 hrs) oppositely regulated more than 1,000 genes in normal and IPAH PASMC (selected examples shown in Tables 2 and 3). In addition to these genes regulated by relatively long-time treatment of BMP-2, there are many genes in PASMC that would be activated or downregulated at earlier time points than the 24 hr interval. The reason we chose this time point was based on the experimental data showing that exposure to BMP-2 (100-200 nM) for 24-48 hrs results in increased PASMC apoptosis [10] and decreased PASMC proliferation [9,14]. Therefore, the genes listed in Tables 2 and 3 do not include the genes that might be transiently regulated by BMP-2.
The genes that were decreased by 24 hr treatment of BMP-2 in normal PASMC but increased in IPAH-PASMC include: a) growth factors and ligands (e.g., teratocarcinoma-derived growth factor, keratinocyte growth factor, PDGF-β), b) membrane receptors (e.g., nicotinic ACh receptor, 5-HT receptor 1E), c) signal transduction proteins and kinases (e.g., CREB-binding protein, PYK2, protein kinase Dyrk3, calmodulin, profilin), d) transcription factors (e.g., cyclin D3, c-Fos, c-Myc binding protein, and NF-κB), and e) enzymes (e.g., leukotriene C4 synthetase, ATP synthase) (selected genes in Table 2). In contrast, the genes that were upregulated by BMP-2 in normal PASMC but downregulated in IPAH PASMC include: a) apoptotic inducers or proapoptotic factors (e.g., Fas/Apo-1, cytochrome c, annexin V, GATA-binding protein), b) membrane receptors, and ion channels or transporters (e.g., endothelin receptor B, TNF receptor, angiotensin-II type 2 receptor, IP3 receptor, aquaporin 5, Cl- channel, and Na+-glucose cotransporter), c) transcription factors (e.g., Ski, transcription factors E2F1 and TFE3, breast cancer transcription factor ZABC1), and d) cytoplasmic enzymes and kinases (e.g., catalase, heme oxygenase-1, serine kinase SRPK2, apoptosis-related protein TFAR15, tumor suppressor ST13) (selected genes in Table 3). The genes listed in Tables 2 and 3 were selected mainly based on their function. We grouped these representative genes based on their potential relationship to signaling pathways that contribute to the increased proliferation and/or decreased apoptosis, ion channel dysfunction, and enzymatic deregulation that have been previously characterized as potential etiological mechanisms underlying the development or onset of IPAH.
The divergent effects of BMP-2 on the gene expression profile indicate that PASMC may undergo significant phenotypic changes in IPAH patients during development of the disease. These results are also consistent with in vitro experiments indicating that a) BMP-2 inhibits normal PASMC proliferation, whereas the antiproliferative effect is converted to be proliferative in IPAH PASMC [9]; and b) BMP-2 induces apoptotic effect on normal PASMC, and the proapoptotic effect is significantly inhibited in IPAH PASMC [10]. Based on the data from this study, we speculate that the transition of BMP-2 from antiproliferative to proliferative may be due to the differential effects of BMP-2 on gene expression of various growth factors, membrane receptors, signal transduction proteins, and transcription factors in IPAH PASMC.
Taken together, these results indicate that BMP signaling pathway is important for a) limiting excessive cell growth, b) inducing “normal” apoptosis to remove “misguided” or hypertrophied PASMC, and c) controlling expression levels of genes that promote PASMC proliferation in normal subjects. However, dysfunctional BMP signaling (due to mutations in BMPR2 and/or acquired downregulation of BMP receptors) leads to i) imbalanced proliferation and apoptosis in PASMC causing pulmonary vascular medial hypertrophy, and ii) upregulation of mitogen receptors and growth-promoting transcription factors and downregulaton of proapoptotic factors causing excessive cell proliferation in PASMC from IPAH patients. It is unknown whether the IPAH patients from whom we obtained PASMC have germline mutations in the BMPR2 gene, or whether PASMC from these IPAH patients contain somatic mutations in the BMPR2 gene. Therefore, we cannot exclude the possibility that the divergent effect of BMP-2 on gene expression pattern in normal and IPAH PASMC is due solely to mutations of the BMPR2 gene. We are currently conducting experiments to define whether transfection of different mutant BMPR2 (based on the mutations identified from IPAH patients) into normal PASMC affects BMP-2-mediated gene expression and which mutations identified from IPAH patients in the BMPR2 gene affect BMP-2-mediated transcriptional regulation on the genes examined in this study.
By comparing gene expression levels in PASMC between normal subjects and IPAH patients, we observed that there are many genes whose expression levels correlate negatively or positively with the PAP. The data indicate that the bulk of the genes have markedly different expression levels between normal and IPAH PASMC. Among the genes whose expression levels are strongly correlated negatively (r < −0.9) or positively (r > +0.9) to the PAP, several are consistent with the report by Geraci et al. [29]. For example, the expression levels of IP3 receptors, Cl- channels, caveolin and Myc are much greater, whereas expression levels of BMPs, voltage-gated Na+ and K+ channels, mitochondrial outer membrane protein, mitochondrial citrate transport protein, and calcineurin are much less in IPAH PASMC than in normal PASMC.
The genes that were found to have a correlation to PAP were the same as those that were differentially regulated between normal and IPAH PASMC. In other words, we only analyzed the genes whose expression levels were changed by BMP-2 treatment (or BMP-2-sensitive genes) in normal and IPAH APSMC. However, in this analysis, we did not take into account whether the genes had been up- or down-regulated by BMP-2 treatment or the BMP-2-mediated effect on mRNA expression was opposite or parallel in normal and IPAH PASMC. The correlation was based solely on the potential link between gene expression and the phenotypic differences in PAP. That the findings closely mirror those for the normal vs. IPAH analysis is inevitable and may simply represent a repetition of the differences already described between normal and IPAH cells. Nevertheless, in establishing the correlation coefficient between PAP and expression levels, we more clearly identify those genes that may be involved in the phenotypic changes observed in IPAH tissues.
We realize that our patient sample size was small, with two normotensive and 2 IPAH patient samples tested, and that this may present some limitations in terms of the validity of our findings. However, it is a problem that could not be surmounted due to the difficulty in obtaining tissues from normal and IPAH patients. Therefore, since tissue availability was an issue, we substantiated our microarray findings by performing real-time RT-PCR experiments on mRNA isolated from PASMC from the same patients. As shown in Figs. 5 and 7, these experiments confirmed our microarray findings, at least vis-à-vis the genes we tested (i.e., caveolin-2, c-Myc binding protein, c-Fos, annexin V, cytochrome c1, GATA binding protein 2, TGF-β-RI, TGF-βb-RII, GADD34, 5-HT2B, thrombin receptor, IP3R1), leading us to believe that our microarray findings are credible and may be representative of a larger population.
Although we should be cautious in interpreting the data because of limited patient samples and the potential pitfalls as mentioned above, the list of genes shown in Tables 4 and 5 provides useful information for further investigation on different gene expression patterns in normal and IPAH PASMC. Understanding the difference of gene expression patterns in PASMC between normal subjects and IPAH patients will also provide important insights to develop therapeutic approaches for the disease.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL064945, HL054043 and HL66012), and from the NASA Goddard Space Flight Center and the National Health Research Institutes in Taiwan (to W. Huang). We thank B. Lapp, N. Elliot, and A. Nicholson for their technical assistance.
References
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 3.Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JAI, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliott G. Genetic basis of pulmonary arterial hypertension: Current understanding and future directions. J Am Coll Cardiol. 2004;43:S33–S39. doi: 10.1016/j.jacc.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Nakaoka T, Gonda K, Ogita T, Otawara-Hamamoto Y, Okabe F, Kira Y, Harii K, Miyazono K, Takuwa Y, Fujita T. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest. 1997;100:2824–2832. doi: 10.1172/JCI119830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamura Y, Hua X, Bergelson S, Lodish HF. Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- 6.Yanagisawa K, Osada H, Masuda A, Kondo M, Saito T, Yatabe Y, Takagi K, Takahashi T, Takahashi T. Induction of apoptosis by Smad3 and down-regulation of Smad3 expression in response to TGF-β in human normal lung epithelial cells. Oncogene. 1998;17:1743–1747. doi: 10.1038/sj.onc.1202052. [DOI] [PubMed] [Google Scholar]
- 7.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BLM. Bmp signaling regulates proximal-distal diferentiation of endoderm in mouse lung development. Development. 1999;126:4005–4016. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- 8.Massague J, Chen Y-G. Controlling TGF-β signaling. Genes & Development. 2000;14:627–644. [PubMed] [Google Scholar]
- 9.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β1 and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX-J. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L740–L754. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 11.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JAI, Newman J, Williams D, Galiè N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Dionnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited mechanism for primary pulmonary hypertension. Am J Hum Gen. 2001;68:92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX-J, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 15.Wotton D, Lo RS, Lee S, Massaguè J. A Smad transcriptional corepressor. Cell. 1999;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 16.Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with smads. J Biol Chem. 1999;274:35269–35277. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- 17.Luo K, Stroschein SL, Wang W, Chen D, Martens E, Zhou S, Zhou Q. The Ski oncoprotein interacts with the Smad proteins to repress TGFβ. Genes Dev. 1999;13:2196–2206. doi: 10.1101/gad.13.17.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Liu X, Ng-Eaton E, Lodish HF, Weinberg RA. SnoN and Ski protooncoproteins are rapidly degraded in response to transforming growth factor β signaling. Proc Natl Acad Sci U S A. 1999;96:12442–12447. doi: 10.1073/pnas.96.22.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y. Structural insights on Smad function in TGFβ signaling. BioEssays. 2001;23:223–232. doi: 10.1002/1521-1878(200103)23:3<223::AID-BIES1032>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Yuan JX-J, Aldinger AM, Juhaszova M, Wang J, Conte JVJ, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 21.Fu M, Zhu X, Zhang J, Lian J, Lin Y, L. Z, Ehrengruber MU, Chen YE. Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene. 2003;315:33–41. doi: 10.1016/s0378-1119(03)00730-3. [DOI] [PubMed] [Google Scholar]
- 22.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 23.Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Sher Y-P, Delgado-West D, Wu JT, Peck K, Fung YC. Tissue remodeling of rat pulmonary artery in hypoxic breathing. I. Changes of morphology, zero-stress state, and gene expression. Ann Biomed Eng. 2001;29:535–551. doi: 10.1114/1.1380416. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Sher Y-P, Peck K, Fung YC. Correlation of gene expression with physiological functions: Examples of pulmonary blood vessel rheology, hypoxic hypertension, and tissue remodeling. Biorheology. 2001;38:75–87. [PubMed] [Google Scholar]
- 26.Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-β/BMP signaling. J Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 28.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Geraci M, Moore M, Gesell T, Yeager M, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]


