Abstract
Background
Heat and cold shock response are normally considered as independent phenomena. A small amount of evidence suggests instead that interactions may exist between them in two Lactococcus strains.
Results
We show the occurrence of molecular relationships between the mechanisms of cold and heat adaptations in Streptococcus thermophilus, a lactic acid bacterium widely used in dairy fermentation, where it undergoes both types of stress. We observed that cryotolerance is increased when cells are pre-incubated at high temperature. In addition, the production of a protein, identified as ClpL, a member of the heat-shock ATPase family Clp A/B, is induced at both high and low temperature. A knock-out clpL mutant is deficient in both heat and cold tolerance. However lack of production of this protein does not abolish the positive effect of heat pre-treatment towards cryotolerance.
Conclusion
Dual induction of ClpL by cold and heat exposure of cells and reduced tolerance to both temperature shocks in a clpL mutant indicates that the two stress responses are correlated in S. thermophilus. However this protein is not responsible by itself for cryotolerance of cells pre-treated at high temperature, indicating that ClpL is necessary for the two phenomena, but does not account by itself for the relationships between them.
Background
Although cold and heat shock are normally considered as independent processes, recent evidence points to the occurrence of interlock between them is some organisms. Physiological experiments suggest that cold-shock enhances heat tolerance of Lactococcus lactis sp. lactis IL1403 [1]. Possible correlations between the heat and cold shock regulons were suggested by i) the observation of an increased level of Leuconostoc mesenteroides homologues of the heat shock proteins DnaK and GroEL upon cold-shock [2], ii) the cold induction of a group of small heat shock genes in Lactobacillus plantarum [3,4] and iii) a slight protection from freezing of Lactobacillus johnsonii [5] and Lc. lactis sp. lactis [6] cells upon induction of the heat-shock response. Conversely, heat-shock did not improve cryotolerance of Lc lactis sp. cremoris strain MG1363 [7], suggesting a strain specificity of this phenomenon.
An unidentified cold-inducible 45-kDa protein of Lc lactis [8] was proposed to correspond to the heat-inducible ClpX ATPase [9], a member of the large family of closely related ATPases found in both prokaryotic and eukaryotic cells [10], having multiple regulatory functions, included a general chaperone activity and the ability to enable the Clp proteases to recognize their substrates [11,12]. Thus a ClpX function may be to promote the proteolysis of mis-folded proteins after both cold or heat-shock.
In order to gain a deeper insight into the correlation between cold and heat shock, we analyzed the phenomenon in Streptococcus thermophilus, a moderate thermophilic LAB widely used in dairy fermentation, where cold and heat stresses are common. Here we show that the synthesis of a 75 kDa protein is induced in both conditions and this induction is essential for stress tolerance.
Results and discussion
Influence of heat and cold shock on S. thermophilus cryotolerance
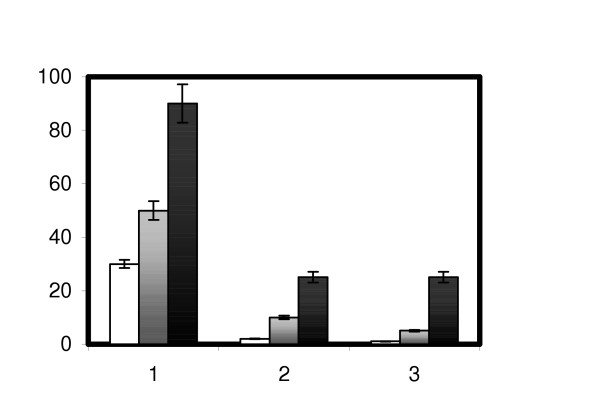
In order to investigate whether cryotolerance of S. thermophilus cells during storage is improved by cold- and/or heat-shock, aliquots of cells grown to middle exponential phase at 42°C were exposed to high and low temperature shocks (30 min at 50°C and 4 h at 20°C, respectively), then fractions of live cells were analyzed after one, two and three days of freeze challenge. Fig. 1 shows that cold shock treatment gives a high protection to freezing, and, in analogy to observations with Lb. johnsonii [5] and Lc. lactis sp. lactis [6], an induction of the heat-shock response gives a slight protection from freezing.
Figure 1.
Survival of S. thermophilus SFi39 after freezing upon direct freezing from 42°C (white bar), heat-shock pre-adaptation (gray bar) and cold-shock pre-adaptation (black bar). Survival (y-axis) is expressed as the percentage of surviving cells compared to the number of cells prior to freezing (100). Cells survival was tested one, two and three days (x-axis) after freezing. Error bars indicate the coefficient of variation in different experiments.
Enhanced cryotolerance was not detected when erythromycin was present at sublethal concentration (2 μg/ml) during heat and cold shock treatments (not shown), which indicated that de novo synthesis of proteins is essential for freezing survival. This is not surprising, since, for example, it is known that GroEL and DnaK are induced by heat and cold stress in other LAB [4] and are involved in heat and cold tolerance of Escherichia coli [13].
Heat and cold induction of a 75 kDa protein
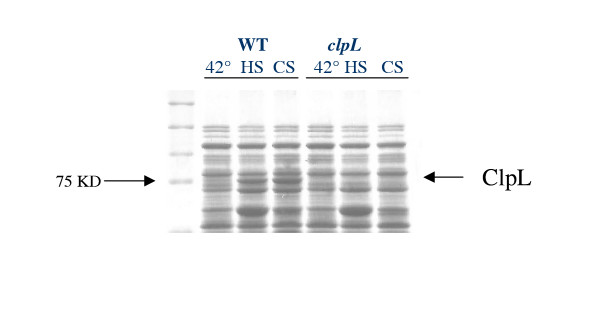
In order to look for proteins induced by heat and cold stresses, we performed a protein extraction followed by SDS PAGE after exposure of S. thermophilus cells to the two temperature shocks. In addition to a few bands most likely corresponding to well known proteins accumulated during the early phase of the heat shock response, a band of apparent molecular mass of 75 kDa was induced by both heat and cold (fig. 2). The two bands were eluted from the HS and CS lanes and N-terminal sequenced. The first 13 amino acids (MNNNFNNMDDLFN) were the same for both and were identical to those of the ClpL protein of S. thermophilus [14] and to a previously reported 75 kDa heat shock induced protein [15]. Based on the N-terminal 10 amino acids of the identified protein, we synthesized a 30 b oligonucleotide that was used to perform Southern hybridization with the KpnI-digested SFi39 chromosome. A 1500 bp fragment was isolated, cloned and sequenced; one 300 aa ORF starting with the expected 13 amino acids was identified (fig. 3) and resulted 99% identical to the class III heat shock protein ClpL of S. thermophilus CNRZ1066, a member of the ClpA/B ATPase family [16].
Figure 2.
SDS-PAGE of cell extracts of S. thermophilus SFi39, wild type and clpLΔ, after growth at 42°C (lanes 1 and 4), after heat shock induction (lanes 2 and 5) and cold shock induction (lanes 3 and 6). Equal amounts of protein were loaded on the gel.
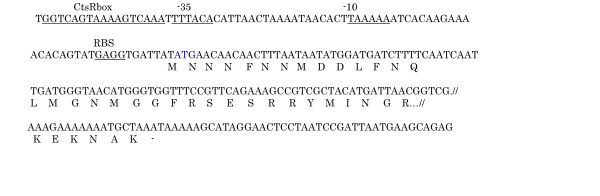
Figure 3.
Promoter region and N-terminal domain of ClpL. The CtsR box, promoter sequences and putative RBS are underlined. The first nucleotide transcribed and the N-terminal amino acids sequenced are in bold.
Most class III heat shock genes are controlled by the class III stress gene repressor CtsR, which binds to a specific direct repeat referred to as CtsR-box [17]. The promoter region of many clp genes contains a sequence homologous to the CtsR box (a directly repeated heptanucleotide, A/GGTCAAANANA/G GTCAAA) [17].
In other LAB, CtsR operators were also found upstream of several clp genes (L. sakei and S. salivarius) [17] and clpP, clpE, clpL, cstR-clpC genes of S. pneumoniae [18] and other Hsp encoding genes, Lo18 of Oenococcus oeni, hsp16 of S. thermophilus [17] and groESL of S. pneumoniae [18]. A putative CtsR consensus sequence is also present in the promoter region of the Sfi39 clpL gene (fig. 3), 44 base pairs upstream of the first transcribed nucleotide (identified by primer extension analysis, data not shown).
clpL disruption reduces tolerance to heat and cold shock
In order to understand the function of ClpL in S. thermophilus, we constructed a SFi39 mutant by cloning an internal fragment of the gene in the pG+host9 vector [19]. SFi39 cells were transformed with the recombinant plasmid and forced for homologous recombination of the plasmid into the chromosome. Chromosomal DNA isolated from both wild-type and erythromycin-resistant cells was analyzed by Southern blotting using a specific clpL sequence as a probe. The absence of gene product in the mutant was confirmed by showing that the ClpL band present in the wild type was not present in the transformed strain (fig. 2). The mutant obtained was named clpLΔ.
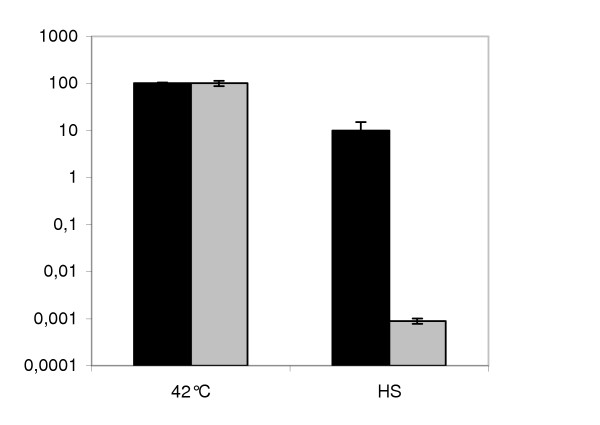
Cells from the two strains were grown to middle exponential phase at 42°C. No difference in growth rate was observed in these conditions. The cells were then heat-shocked at 60°C for 1 hour. As shown in fig. 4, clpLΔ cells were less tolerant to heat-shock: one wild type cell out of ten survived to the treatment, whereas mutant survival decreased dramatically to 1 cell out of 100,000. This result demonstrates that ClpL, in analogy to well known proteins such as GroEL and DnaK, is involved in the protection of cells against heat.
Figure 4.
Streptococcus thermophilus SFi39 (black bars) and clpLΔ (gray bars) survival upon induction of heat shock (HS). Survival is reported as the percentage (y-axis) of colony-forming cells after the treatment compared to that after growth at 42°C (100%). Error bars indicate the coefficient of variation in three different experiments.
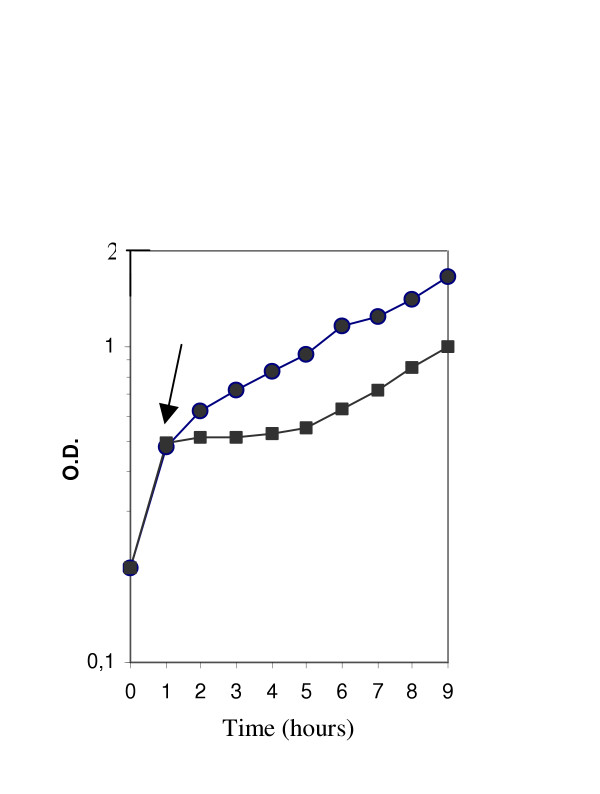
Cold shock tolerance at 20°C of the mutant was tested by measuring its growth, compared to the wild type, after temperature downshift of cells pre-grown exponentially at 42°C. As shown in fig. 5, growth of the wild type continued after temperature downshift at a reduced rate as expected, whereas the clpL mutant had to face a long lag phase before restoration of growth at a rate comparable to that of the wild type, which suggests that ClpL is required for normal response to cold shock.
Figure 5.
Growth curves of SFi39 (circles) and clpLΔ (squares). After the first hour of incubation at 42°C (arrow) cells were incubated at 20°C.
The slight protection from freezing of previously heat-shocked wild type cells shown in fig. 1 was observed also with the ClpL mutant (data not shown), which indicates that the molecular relationship between cold and heat stresses is a complex phenomenon in which the ClpL protein plays only part of the role.
Conclusion
It is well established that bacteria display adaptive systems to adjust their metabolism to cold and heat shocks. We show that pre-incubation of S. thermophilus cells at both high and low temperature enhances their resistance to freezing conditions, which supports the idea that cold and heat shocks, normally considered as unrelated phenomena, have in fact some relationships. At least one shock-induced factor, the ClpL protein, is involved in both phenomena, since we show that i) its synthesis is enhanced in both conditions and ii) no correct response to either stress is observed in a clpL knock-out mutant. The finding that this protein is not responsible by itself for the observed phenomenon of cryotolerance of cells pretreated at high temperature indicates that ClpL, although necessary for correct response to both heat and cold stressess, does not account by itself for the moleculr relationships between them, which are most likely based on a more complex regulatory network.
Methods
Bacterial strains and growth conditions
S. thermophilus SFi39 was cultured at 42°C in M17 broth (Oxoid) containing 1% lactose. Growth was monitored by measuring the optical density at 600 nm (OD600) with a JAS.CO spectrophotometer (model 7800). Erythromycin was added at a concentration of 4 μg/ml. E. coli DH5α and EC101 were used as host strains in cloning experiments and were grown in Tryptone Yeast (TY) medium with aeration at 37°C [20]. Ampicillin and erythromycin were used at concentrations of 50 and 100 μg/ml, respectively. To study growth kinetics, 1% inoculated cultures were grown at different temperatures. Growth was monitored by measuring the optical density at 600 nm (OD600).
Freeze-thaw challenge
To study the freeze-thaw survival capacity, with or without preadaptation, S. thermophilus cells were quickly frozen at middle exponential phase (OD600 = 0.5), after heat (30 min at 50°C) and cold shock (4 h at 20°C). Aliquots (1 ml) were spun down (5 min at 6000 rpm), resuspended in 1 ml of fresh LM17 medium, subsequently frozen at -20°C for 24 h, and thawed for 4 min at 30°C in a water bath. The number of CFU was determined just before freezing and after three consecutive freeze-thaw challenges by spread plating decimal dilutions. After 2-day incubations on LM17 plates at 42°C the numbers of CFU were counted. The experiments were performed in duplicate. Coefficient of variation was < 10 %.
Protein extraction and protein analysis by SDS-PAGE gel electrophoresis
Protein extracts were obtained according to [21] and protein concentration was determined by the "BIO-RAD protein assay" method. Protein analysis was performed using SDS PAGE. Equal amounts of protein (15 μg) were applied on the protein gels and the protein content of the extract was determined using Coomassie Brilliant Blue.
N-terminal sequencing
500 μg of protein was loaded on SDS-PAGE gel for detection of the N-terminus of specific bands using conditions identical to the analytical gels. The proteins were blotted on a PVDF membrane using a Trans blot unit according to the instruction of the manufacturer (Biorad, Richmond, USA). The PVDF membrane containing separated proteins was stained 1 min with Coomassie Brilliant Blue R-250 (0.1% in water containing 50% methanol), destained for about 10 min in a solution of 40% methanol plus 10% acidic acid in water, and washed in water. Stained protein band was cut from the membrane and subjected to the Edman procedure. BlastP http://www.ncbi.nlm. nih.gov/BLAST/BLAST.cgi was used to search for similarity in public protein databases.
Southern hybridization with degenerated oligonucleotide
Based on the 10 N-terminal amino acids of the isolated protein (MNNNFNNMDD) a 30 b degenerated oligonucleotide was synthesized (5'-ATGAAYAAYAAYTTYAAYAAYATGGAYGAY-3'; Y=T/C) and used in Southern hybridization with chromosomal DNA. Southern hybridization was performed at 37°C overnight, and the membrane was washed 2 min at 37°C in 3XSSC, 0.1% SDS and 2 min at 37°C in 2XSSC, 0.1% SDS, before exposure to X Ray film [17]. The probe was labeled with polynucleotide kinase (Pharmacia, Biotech) ([γ-32P]-ATP.
DNA manipulations
Chromosomal S. thermophilus DNA was isolated as described previously [22]. Cells were transformed by electroporation. E. coli cells were transformed by the CaCl2 procedure and plasmid isolation was carried out according to standard procedures [20]. Restriction enzymes, T4 DNA ligase and other DNA-modifying enzymes were purchased from Gibco-BRL Life Technologies, New England Biolabs or Promega, and used as recommended by the manifacturers. Cloning procedures, radiolabelling of DNA fragments, agarose gel electrophoresis and Southern-blot hybridization were performed according to standard procedures [20]. DNA fragments were isolated from agarose gels by using the QIAquick gel extraction Kit (QIAGEN).
Construction of the clpL insertional mutant
To construct an insertional mutant with a disruption in the S. thermophilus clpL gene, a 1500 bp PCR fragment (HCIDWF primer: 5'-CTTTTCAATCAATTG ATGGG-3'; HCIDEL482 primer: CATTTGWGAWACWGGRATWCCWGTCAT-3') was amplifed and cloned in the pG+host9 integrational vector [19] digested with EcoRI-SpeI and the resulting plasmid was designated pGh482. 1 × 108 S. thermophilus competent cells were mixed with 500 ng of plasmid pGh482 and electroporated [23]. Transformed cells were grown in anaerobic conditions in the presence of 4 μg erythromycin ml-1 at permissive temperature (30°C), to allow plasmid replication. Integration of pGh482 into the S. thermophilus chromosome was forced by growing cells at 30°C up to 0.2–0.3 OD600, then shifting to 42°C and allowing cell growth for two generations (up to 1.2–1.5 OD600). Cells were then diluted and plated on LM17 plates supplemented with 4 μg erythromycin ml-1 and incubated anaerobically at 42°C.
Stress tolerance
For heat shock treatment, exponentially growing cells (OD600 = 0.5) of Sfi39 and clpL mutant were transferred to 60°C for 1 h. Cell survival was measured by plating on LM17 at 42°C before and after incubation at 60°C. For cold-shock treatments 50 ml cultures of Sfi39 and clpL mutant were grown in LM17 medium to middle exponential phase (OD600 = 0.5), after which 25 ml of the culture were pelleted (10 min × 4000 g) and re-suspended in the same volume of pre-cooled medium (20°C). Cultures were incubated at 20°C and OD600 values were measured.
Authors' contributions
MV had a predominant role in the design and implementation of the study and in the preparation of the manuscript, SA performed the identification and cloning of the clpL gene and the construction of S. thermophilus mutant, LM carried out the protein preparation for Edman sequencing, DF and GN partecipated in the characterization of clpL mutant phenotype, MDF helped with discussions and suggestions during the work, preparation of the manuscript and funding. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was partially supported by Centro Regionale di Competenza BioTekNet, Naples, Italy.
Contributor Information
Mario Varcamonti, Email: varcamon@unina.it.
Slavica Arsenijevic, Email: arsenijevic@unisi.it.
Luca Martirani, Email: l.martirani@cemon.it.
Daniela Fusco, Email: danielafusco79@libero.it.
Gino Naclerio, Email: gino.naclerio@unimol.it.
Maurilio De Felice, Email: maurilio.defelice@unina.it.
References
- Panoff JM, Thammavongs B, Laplace A, Hartke A, Boutibonnes P, Auffray Y. Cryotolerance and cold adaptation in Lactococcus lactis subsp. lactis IL1403. Cryobiol. 1995;32:516–520. doi: 10.1006/cryo.1995.1053. [DOI] [Google Scholar]
- Salotra P, Singh DK, Seal KP, Krishna N, Jaffe H, Bhatnagar R. Expression of DnaK and GroEL homologs in Leuconostoc mesenteroides in response to heat shock, cold shock or chemical stress. FEMS Microbiol lett. 1995;131:57–62. doi: 10.1111/j.1574-6968.1995.tb07754.x. [DOI] [PubMed] [Google Scholar]
- Spano G, Capozzi V, Vernile A, Massa S. Cloning, molecular characterization and expression analysis of two small heat shock genes isolated from wine Lactobacillus plantarum. Journal of Applied Microbiol. 2004;97:774–782. doi: 10.1111/j.1365-2672.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- Spano G, Beneduce L, Perrotta C, Massa S. Cloning and characterization of the hsp 18.55 gene, a new member of the small heat shock genes family isolated from wine Lactobacillus plantarum. Research in Microbiol. 2005;200:19–224. doi: 10.1016/j.resmic.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Walker DC, Girgis HS, Klaenhammer TR. The groESL chaperone operon of Lactobacillus johnsonii. Appl Environ Microbiol. 1999;65:3033–41. doi: 10.1128/aem.65.7.3033-3041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent JR, Lin C. Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiol. 1999;39:88–102. doi: 10.1006/cryo.1999.2190. [DOI] [PubMed] [Google Scholar]
- Wouters JA, Jeynov B, Rombouts FM, de Vos WM, Kuipers OP, Abee T. Analysis of the role of 7 kDa cold- shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology. 1999;145:3185–94. doi: 10.1099/00221287-145-11-3185. [DOI] [PubMed] [Google Scholar]
- Wouters JA, Rombouts FM, de Vos WM, Kuipers OP, Abee T. Cold shock proteins and low-temperature response of Streptococcus thermophilus CNRZ302. Appl Environ Microbiol. 1999;65:4436–42. doi: 10.1128/aem.65.10.4436-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MM, Trempy JE. Expression of clpX, an ATPase subunit of the Clp protease, is heat and cold shock inducible in Lactococcus lactis. J Dairy Sci. 2001;84:1783–5. doi: 10.3168/jds.S0022-0302(01)74615-2. [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Glover JR, Singer MA, Lindquist S. HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci. 1996;21:289–96. doi: 10.1016/0968-0004(96)10038-4. [DOI] [PubMed] [Google Scholar]
- Wawrzynow A, Wojtkowiak D, Marszalek J, Banecki B, Jonsen M, Graves B, Georgopoulos C, Zylicz M. The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J. 1995;14:1867–77. doi: 10.1002/j.1460-2075.1995.tb07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S, Gottesman S, Skowyra D, Hoskins J, McKenney K, Maurizi MR. A molecular chaperone, ClpA, functions like DnaK and DnaJ. Proc Natl Acad Sci U S A. 1994;91:12218–22. doi: 10.1073/pnas.91.25.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KC, Tung WL. Overexpression of dnaK/dnaJ and groEL confers freeze tolerance to Escherichia coli. Biochem Biophys Res Commun. 1998;253:502–5. doi: 10.1006/bbrc.1998.9766. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol. 2004;22:1554–8. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giliberti G, Naclerio G, Martirani L, Ricca E, De Felice M. Alteration of cell morphology and viability in a recA mutant of Streptococcus thermophilus upon induction of heat shock and nutrient starvation. Gene. 2002;295:1–6. doi: 10.1016/S0378-1119(02)00830-2. [DOI] [PubMed] [Google Scholar]
- Huang DC, Huang XF, Novel G, Novel M. Two genes present on a transposon-like structure in Lactococcus lactis are involved in a Clp-family proteolytic activity. Mol Microbiol. 1993;7:957–65. doi: 10.1111/j.1365-2958.1993.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Derre I, Rapoport G, Devine K, Rose M, Msadek T. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol Microbiol. 1999;32:581–93. doi: 10.1046/j.1365-2958.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- Chastanet A, Prudhomme M, Claverys JP, Msadek T. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J Bacteriol. 2001;183:7295–307. doi: 10.1128/JB.183.24.7295-7307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguin E, Prèvost H, Ehrlich SD, Gruss A. Efficient insertional mutagenesis in Lactococci and other Gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. second. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 1989. [Google Scholar]
- Gonzalez-Marquez H, Perrin C, Bracquart P, Guimont C, Linden G. A 16 kDa protein family overexpressed by Streptococcus thermophilus PB18 in acid environments. Microbiology. 1997;143:1587–94. doi: 10.1099/00221287-143-5-1587. [DOI] [PubMed] [Google Scholar]
- Limauro D, Falciatore A, Basso AL, Forlani G, De Felice M. Proline biosynthesis in Streptococcus thermophilus: characterization of the proBA operon and its products. Microbiology. 1996;142:3275–82. doi: 10.1099/13500872-142-11-3275. [DOI] [PubMed] [Google Scholar]
- Baccigalupi L, Naclerio G, De Felice M, Ricca E. Efficient insertional mutagenesis in Streptococcus thermophilus. Gene. 2000;258:9–14. doi: 10.1016/S0378-1119(00)00449-2. [DOI] [PubMed] [Google Scholar]