Abstract
Reference values of blood volume (BV) and plasma volume (PV) of animal species are given as functions of body weight and gender specification generally is not given. Considering the common observation of a decreased hematocrit (Hct) in the females of many species, the BV, the PV, or both must differ between genders. The present study was performed to determine the magnitude of those differences. We measured Hct and PV in 24 female and 23 male Sprague-Dawley rats in their 12th week of life. The rats were surgically prepared with indwelling femoral arterial catheters 4 d prior to the determination of BV. Evan’s Blue dye dilution was used to determine PV in conscious, quietly resting animals. BV was calculated as PV/(1 – Hct). Mean Hct was 2% lower in female rats than males, and PV (mean ± 1 standard deviation) was 4.86 ± 0.54 ml/100 g in females compared with 4.12 ± 0.32 ml/100 g in males. Calculated BV in female rats was 7.84 ± 0.70 ml/100 g compared with 6.86 ± 0.53 ml/100 g in males. When precise estimates of BV or PV are needed for research or dosing purposes, gender differences of 18% for PV and 14% for BV must be considered. In addition, species other than the rat may have similar discrepancies between sexes, and the prudent investigator must determine individual volume assessments of both sexes before assumptions of BV and PV for a species can be made.
Abbreviations: BV, blood volume; Hct, hematocrit; PCV, packed cell volume; PV, plasma volume
Occasionally research studies that use a rat model are conducted under the assumption that blood or plasma volumes of males and females are equivalent. Commonly used laboratory animal references list rat blood volumes (BV) ranging from approximately 5.7 to 7.2 ml/100 g.5,8,9 Similarly, plasma volumes (PV) range from 3.1% to 3.9%.8,9 These are commonly used standard rat values that imply inclusion of both genders and all stocks and strains. Hematocrit (Hct) expressed as percentage of packed cell volume (% PCV) ranges from 34% to 57%.5,8,9 Much of this range is attributable to the increase associated with age.5
It has been generally observed that in the rat, as in many other species, females have a lower Hct than do males. In the rat this feature is generally true for various breeds and all ages.5,8 This general observation requires that, when adjusted for body weight, either the PV, BV, or both differ between females and males. For instance, the BV could be the same with a larger PV in females, or any of several other possibilities, but 1 of the compartments must be different. Limited studies are available that report the effects of gender on BV or PV. Yale and Torhorst11 reported a slight difference in PV in anesthetized Sprague-Dawley rats, with females demonstrating a larger PV, but the BV did not reach statistical significance. Others have reported a larger BV in male rats,10 and others reported no difference in either BV or PV.3 Our study was compiled from several ongoing studies in our institution that were using Sprague-Dawley rats where quantification of these parameters in both sexes was required. The purpose of this report was to collect sufficient animals to determine (with reasonable accuracy) which body fluid volume compartments differ and the magnitude of those differences in the conscious rat.
Materials and Methods
Experimental animals
All experimental animals were acquired and used in compliance with federal, state, and local laws and institutional regulations. The data in this report were combined from 3 separate research protocols that were approved by the Tripler Army Medical Center Institutional Animal Care and Use Committee. The facility and program are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International, and the program is assured by the Office of Laboratory Animal Welfare. Investigators complied with United States Department of Agriculture Animal Welfare Act regulations and the National Research Council’s Guide for the Care and Use of Laboratory Animals.7
The animals used in these studies were F1 offspring from outbred rats (Rattus norvegicus) of Sprague-Dawley stock (NTac:SD) obtained from Taconic Farms (Germantown, NY). The breeders were obtained as murine pathogen-free from the source, and sentinel testing was performed quarterly for serology and histopathology by the University of Missouri Research Animal Diagnostic Laboratory (Columbia, MO). The animals were singly housed in standard shoebox polycarbonate cages (10.5 × 19 × 8 in.) with wire bar lids and Sani-Chips (P.J. Murphy Products, Montville, NJ) hardwood chip bedding, which were changed weekly. Polycarbonate tubes for tunneling behavior and Nylabones (Nylabone Products, Neptune City, NJ) are provided for environmental enrichment. The environmental conditions of the animal housing were maintained 19 to 22 ° C and 45% to 65% relative humidity, with a 12:12-h light:dark cycle (lights on, 0600). The animal housing and experimental conditions were at an altitude of approximately 500 ft. All experiments were performed between 0830 and 1100.
The offspring used in this study were created as control animals for a series of studies investigating the physiologic and endocrine responses to hemorrhage in young adult rats prenatally exposed to different regimens of orally administered alcohol. For accurate hemorrhage volumes, BVs in these rats were determined. The data reported here were obtained from young adult rats that were F1 offspring from 6 litters of rats from pregnant animals that were fed alcohol-free liquid diets. These maternal rats were used to produce ‘control’ offspring for other protocols and received control liquid diets on days 7 through 21 of pregnancy (producing 20 female and 18 male offspring) or on days 10 through 21 of pregnancy (producing 4 female and 5 male offspring).
The liquid diets (Bio-Serv, Frenchtown, NJ) were control diets and therefore were not supplemented with additives. The diet provided 1000 kcal/l when finally constituted as a liquid upon addition of water, providing 180, 350, and 470 kcal of protein, fat, and carbohydrate, respectively. Fiber constituted 4.6% and ash 3.6% of the weight of the dry diet. The animals were allowed ad libitum access to the liquid diets, consuming approximately 90 kcal/day. The rats had an average weight gain of 126 ± 6 g during pregnancy and produced litters ranging from 11 to 16 pups. Prior to the liquid diets and after the birth of the pups, the pregnant animals were fed pelleted Rodent Diet 5001 (PMI Nutrition, Inc. LLC, Brentwood, MO) and untreated water from bottles ad libitum. The progeny rats were fed by their biological lactating dams and were not exposed to alcohol after birth. Beginning after weaning (when they were 21 days old), they were fed only the pelleted Rodent Diet 5001 and untreated water ad libitum and were studied as young adults.
Surgery
When the offspring were 11 wks old and 4 d prior to the BV determination experiments, the animals had femoral arterial catheters surgically implanted. Anesthesia was induced by placing the rats in a chamber with isoflurane (1% to 5% in O2) and subsequently was maintained with an anesthetic gas delivery system (Surgivet, Waukesha, WI). The left femoral artery was exposed by a midthigh incision on the ventral surface, and the vessel was catheterized with flexible (inner diameter, 0.03 in.) sterile Tygon tubing, which was tunneled under the skin to exit on the dorsal surface between the scapulae. All surgeries were performed under aseptic conditions with sterilized instruments. Oral analgesia was administered 4 h pre- and postsurgically with 7.2 mg ibuprofen in 2-ml flavored gelatin ‘jiggler’ cubes and maintained for 2 d with 60 mg ibuprofen elixir per 100 ml drinking water. After surgery, the catheter was filled with a maintenance solution of 50% heparin (500 U/ml) in 50% dextrose and closed by insertion of a pin into the lumen. The pin was a common sewing pin prepared by blunting the tip and clipping off the head to leave a length of approximately 7 mm. The animals were returned to their cages, with paper towels covering the woodchip bedding, until they were fully mobile, at which time the towels were removed, and the cages and animals were returned to the animal holding facility. Adequacy of the analgesia was assumed because no evidence of restricted activity, restricted eating or drinking behavior, or resistance to handling or cowering was noticed after recovery from the anesthesia.
Experiment
Experiments were performed during the rats’ 12th week of life, 4 d after femoral arterial catheterization. Two prior ‘training’ sessions of approximately 30 and 60 min were performed on separate days, to accustom the animals to mild restraint in plastic experimental chambers (Braintree Scientific, Braintree, MA) used during the experiment.
All rats had ad libitum access to food and water until just prior to the experiment, when they were weighed and placed in the experimental chambers. The arterial catheter then was cleared by withdrawing about 100 μL and flushing with an equal volume of heparized saline. After the animals quietly rested for 45 to 60 min, 50 μl sterile saline containing 250 μg Evans Blue dye (Sigma, St Louis, MO) was injected into the arterial catheter, followed by 200 μl heparized saline to ensure injection of all the dye. After a 5-min equilibration period, a void volume of approximately 150 μl was removed, followed by a 1.5-ml sample. Two microhematocrit tubes were filled from the sample for determination of Hct by centrifugation, and the remaining sample was transferred to a 3-ml sodium heparin phlebotomy tube containing dry heparin. The catheters then were refilled with the maintenance solution and animals returned to their cages.
The sample was centrifuged (1700 × g for 6 min), and at least 0.5 ml plasma was removed and diluted 1:1 with distilled water. The absorbance of this diluted sample was determined by spectrophotometry at a wavelength of 615 nm (Spectronic 21 D, Milton Roy, Ivyland, PA) and compared with standard Evans Blue solutions in saline, ranging between 0 and 25 μg dye/ml, in order to measure the concentration of dye in the sample. PV then was calculated by dividing the original amount of dye injected (250 μg) by the concentration in the plasma (μg/ml). BV was calculated by dividing PV by (1 - Hct).
Statistical analyses
Data are expressed throughout as mean ± 1 standard deviation. Comparisons between genders were made using unpaired t tests, at the 0.05-level of probability for the alpha error.
Results
The mean body weight of the 23 male rats on the day of the experiment was 389 ± 34 g, approximately 60% greater than that of the 24 females (244 ± 21 g). As would be expected, the absolute PV and BV in the male rats, 16.1 ± 1.8 and 26.7 ± 2.7 ml respectively, was greater than in female rats, 11.9 ± 2.0 and 19.1 ± 2.6 ml respectively. However, the differences were 35% for PV and about 40% for BV, indicating that these volumes, relative to body weight, are greater in female rats.
The data summarized in Figure 1 confirm the preceding deduction and reveal that both PV and BV, expressed as ml/100 g body weight, are greater in female rats than in male rats of a similar age (P < 0.0001). The PV was 18% more and the BV was 14% more in female rats than in males.
Figure 1.
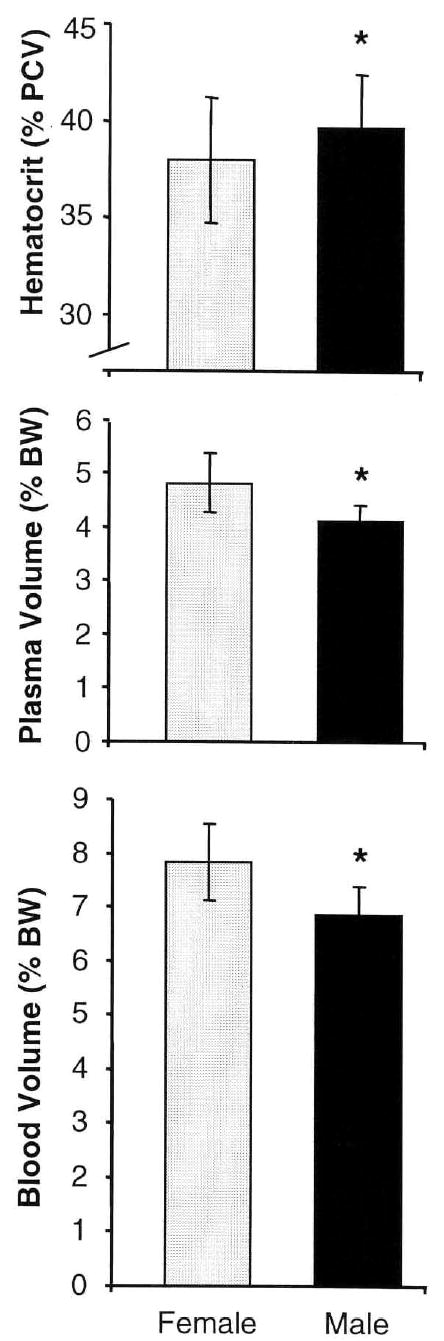
The effects of gender on hematocrit (% packed cell volume [PCV]), plasma volume (% body weight, ml/100 g body weight), and blood volume (% body weight, ml/100 g body weight). Column height corresponds to mean value; error bar, ± 1 standard deviation. *, P < 0.05 compared with the corresponding female value. [1]
The mean body weight of the male rats at surgery (389 ± 35 g) and 4 d later, at the time of BV determination, was unchanged, but the mean pre-surgical female body weight (247 ± 20 g) was reduced by 3 g (P = 0.015). Further analysis was performed to determine whether this weight loss affected the difference in BV distribution between sexes. We analyzed the data from male and female rats that gained weight and from animals that lost weight or remained unchanged. In both the animals that gained weight and those that did not, BV and PV, expressed as a percentage of body weight, did not significantly vary. Furthermore, female rats among the animals that gained weight and those that did not had higher BV (P = 0.004 and P < 0.0001, respectively) and PV (P = 0.002 and P < 0.0001, respectively) than did males. Thus the weight gain or loss between surgery and the day of BV determination did not significantly affect the sex-dependent differences in either BV or PV.
Discussion
The Hct values reported in the present study fall within the broad ranges of referenced values.5,8,9 The BV, and more clearly the PV, values reported in the present study for both sexes is larger than the referenced values.8,9 Unfortunately, not all of the sources are cited, but that of Yale and Torhorst,11 reported female and male PV values of 3.30 and 3.08 ml/100 g, respectively (P < 0.05), and BV values to be 5.8 ml/100 g for both sexes. Although the values in the present report support the gender difference for PV values, the values for both sexes are about 30% higher in the current work. The studies of Yale and Torhorst11 were done with rats of similar age and breed and using similar methodology as for the present studies. However, important differences are evident. The rats in the previous studies had their food removed (but with ad libitum water continued) 18 h prior to the study, and they were anesthetized and their jugular veins catheterized for their Evans Blue dye dilution studies, which were conducted within minutes of these procedures.
There are 2 factors that may account for our findings of increased PVs. First, with removal of food, one can expect that ad libitum water consumption will be reduced, because the osmotic load of the food ultimately contributes to the thirst stimulus. Therefore, the animals may have been slightly more dehydrated in the studies by Yale and Torhorst11 than in our experiments. This situation is suggested by the Hct values of their rats, which were approximately 5% PCV higher for both sexes than we noted as well as being higher than commonly referenced values.5 However, like we did, Yale and Torhorst11 found that Hct in female rats was lower than in males. As a 2nd consideration, Barbee and colleagues2 have observed that PV determinations conducted in conscious mice were 21% higher than those obtained from anesthetized mice. Therefore the anesthetized state and possible slight dehydration may have contributed to the lower PV values of Yale and Torhorst.11 In support of this possibility are the studies of Crofton and Share,3 who determined Hct and PV values in the same range as those in this present report. Their studies were conducted on conscious rats in a euhydrated condition. It is well established that the state of anesthesia and the effects of specific anesthetics and surgery have profound influences on vasopressin release,4 and the period of recovery where the animals experience temporary reduced drinking and eating behavior clearly predict water balance disturbances. The current experiments were done 4 d after surgery when the animals had restored mobility with 66% of the animals exhibited a weight gain with the remainder showing either no gain or a loss in weight demonstrating restored eating and drinking behavior. Most importantly, whether the animals gained weight or lost weight, the effects of gender on blood and plasma volumes were not different.
Most reports indicate that male rats have higher Hct than female rats by 1% to 3% PCV at ages similar to those used in these studies, and at older ages, the difference becomes exaggerated.3,5,8,11 Yet there are unexplained reports in which female rats in proestrus have been found to have higher Hct values than males,10 whereas other investigators3 report that female rats during proestrus, estrus, and metestrus demonstrated reduced Hct compared with male rats and that Hct values in female rats during diestrus approached male values. Clearly the estrus cycle has a potential influence on these parameters. However, the pattern is not clear, and unfortunately estrus cycle information was not obtained in the present studies. When we compared the female values, reflecting random stages of the estrus cycle, with the male values, the female Hct was 1.8% PCV lower than the male value, in general agreement with previous work.3,5,8,11
The PV values reported in the current study were approximately 4.9 ml/100 g in female rats compared with 4.1 ml/100 g in males. A similar gender-based difference has been reported previously,11 with the reported difference being approximately 9% greater in female rats, although the absolute values were lower than those we report here. Others have reported PV in female rats during proestrus, estrus, and metestrus to be approximately 10% greater than in male rats, but this difference did not reach statistical significance, probably because of sample size.3 Although one might suspect an association between animal size and the observed gender difference, we could detect no significant correlation within sexes between body weight and PV. Furthermore, in a weight matched study, where PV was determined in both sexes, where female rats were approximately 25 wks old, and the male rats were 9 wks old, female rats also had higher plasma volumes.6
The BV calculated in the present experiments, although higher than referenced values5,8,9 are in agreement with recent work in which PV is measured by an indicator dilution technique1,3 and BV calculated by PV/(1 – Hct). Argent and colleagues1 found the methods of plasma dilution of 125I-labeled albumin and 113m indium to yield similar results in BV determination (7.2 ml/100 g by either method) in male Wistar rats. However, when 51Cr-labeled red blood cells are used alone10 or with 125I-labeled albumin for independent measures of red blood cell volume and PV,1 the reported values are about 10% less.
We conclude that the majority of studies indicate that for normally hydrated, conscious rats, female rats have lower Hct than do males. This fact mandates that the blood volume compartments of red cell mass and plasma differ between sexes. The present work emphasizes, clarifies, and adds precision to the magnitude of the differences. PV was 18% greater and BV was 14% greater in female rats at random stages of the estrus cycle than in male rats. For experiments and dosing regimens using rats of both sexes and requiring an accuracy greater than 18% variation, individual assessment of plasma and blood volumes seems advisable. When not possible, PV of female rats should be assumed to be at least 10% greater than that of males.
Acknowledgments
The authors gratefully acknowledge the conscientious animal care provided by Robin Grain and the veterinary technical expertise of SSG David Watters and SPC Kristopher Craighead. We are also grateful for the grant support provided from the Hawaii Community Foundation (no. 20030617) and from a Research Centers in Minority Institutions Award from the National Center for Research Resources, National Institutes of Health (no. P20 RR11091). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources, National Institutes of Health. The views expressed are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or US Government.
References
- 1.Argent NB, Liles J, Rodham D, Clayton CB, Wilkinson R, Baylis PH. A new method for measuring the blood volume of the rat using 113-indium tracer. Lab Anim. 1994;28:172–175. doi: 10.1258/002367794780745218. [DOI] [PubMed] [Google Scholar]
- 2.Barbee WR, Perry BD, Ré RN, Murgo JP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol (Regul Integr Comp Physiol) 1992;263:R728–R733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- 3.Crofton JT, Share L. Sexual dimorphism in vasopressin and cardiovascular response to hemorrhage in the rat. Circ Res. 1990;66:1345–1353. doi: 10.1161/01.res.66.5.1345. [DOI] [PubMed] [Google Scholar]
- 4.Forsling ML, Ullmann EA. 1977. Non-osmotic stimulation of vasopressin release. In: Moses AM, Share L, editors. Neurohypophysis. Basel: S Karger. p 128–135.
- 5.Kohn DF, Clifford CB. 2002. Biology and diseases of rats. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine. New York: Academic Press. p 121–165.
- 6.Kuebler JF, Toth B, Rue LW, Wang P, Bland K, Chaudry IH. Differential fluid regulation during and after soft tissue trauma and hemorrhagic shock in males and proestrus females. Shock. 2003;20:144–148. doi: 10.1097/01.shk.0000072127.33223.f1. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council. 1996. Guide for the care and use of laboratory animals. Washington (DC):National Academy Press.
- 8.Ringler DH, Dabich L. 1979. Hematology and clinical biochemistry In: Baker HJ, Lindsey JR, Weisbroth SH, editors. The laboratory rat. Orlando (FL): Academic Press. p 105–121.
- 9.Sharp PE, LaRegina MC. 1998. Important biological features. In: Suckow MA, editor. The laboratory rat, vol 1: biology and disease. Boca Raton (FL): CRC Press. p 1–20.
- 10.Slimmer LM, Blair ML. Female reproductive cycle influences plasma volume and protein restitution after hemorrhage in conscious rat. Am J Physiol (Regul Integr Comp Physiol) 1996;271:R626–R633. doi: 10.1152/ajpregu.1996.271.3.R626. [DOI] [PubMed] [Google Scholar]
- 11.Yale CE, Torhorst JB. Critical bleeding and plasma volumes of the adult germfree rat. Lab Anim Sci. 1972;22:497–502. [PubMed] [Google Scholar]


