Abstract
Recognition memory, involving the ability to discriminate between a novel and familiar object, depends on the integrity of the perirhinal cortex (PRH). Glutamate, the main excitatory neurotransmitter in the cortex, is essential for many types of memory processes. Of the subtypes of glutamate receptor, metabotropic receptors (mGluRs) have received less study than NMDA receptors; thus, the reported experiments examined the role of mGluRs in familiarity discrimination in the rat PRH. Experiments 1 and 2 assessed the effects of systemic administration of MPEP, a group I mGluR (specifically mGluR5) antagonist, and/or LY341495, a group II mGluR antagonist, on a spontaneous object novelty preference task. Simultaneous antagonism of both group I and II mGluRs impaired familiarity discrimination following a 24-h but not a 15-min delay, while antagonism of either mGluR subtype alone had no effect at either delay. The impairment was in acquisition, as in Experiment 3 coadministration of MPEP and LY341495 did not affect recognition memory performance when administered either after the sample phase or prior to test. The impairment in long-term recognition memory was mediated by mGluRs in the PRH, as localized intracortical antagonism of group I and II mGluRs also produced a deficit (Experiment 4). No evidence was found for an involvement of group III mGluRs in the acquisition of long-term familiarity discrimination (Experiment 5). These findings establish that glutamatergic neurotransmission in the PRH via group I and II mGluRs is crucial for the acquisition, but not for the consolidation or retrieval of long-term object recognition memory.
The perirhinal cortex (PRH) in the medial temporal lobe has been shown to be a crucial region for recognition memory performance. Thus, ablation of the PRH produces severe deficits in recognition memory for individual objects in the rat (Mumby and Pinel 1994; Wiig and Bilkey 1995; Ennaceur et al. 1996; Aggleton et al. 1997; Bussey et al. 1999; Mumby et al. 2002; Winters et al. 2004) and monkeys (Gaffan and Murray 1992; Meunier et al. 1993; Suzuki et al. 1993). Furthermore, immunohistochemical studies have shown that neurons within the PRH are activated more by novel than by familiar stimuli (Zhu et al. 1995b, 1996; Wan et al. 1999), a finding consistent with electrophysiological recording studies showing that the PRH contains a high proportion of neurons that respond less to presentations of a familiar stimulus compared to a stimulus never previously encountered (Brown et al. 1987; Zhu et al. 1995a; Brown and Xiang 1998).
Glutamate is the main excitatory neurotransmitter within the cortex (Fonnum 1984), and the PRH has been shown to contain both ionotropic and metabotropic glutamate receptors (mGluRs) (Cho et al. 2000). The mGluR family is divided into eight known subtypes (mGluR1–8), which have been divided into group I (comprising mGluR1 and mGluR5), group II (mGluR2 and mGluR3), and group III (mGluR4, mGluR6, mGluR7, and mGluR8) on the basis of sequence homology, second messenger coupling, and pharmacology. Both group I and group II mGluRs have been shown to be critically involved in perirhinal cortical synaptic plasticity, particularly in the regulation of long-term depression (LTD) (Cho et al. 2000), and it has been hypothesized (Brown and Bashir 2002; Bogacz and Brown 2003) that the mechanisms used in the production of LTD in perirhinal brain slices may be the same mechanisms that give rise to the perirhinal response reductions seen when visual stimuli are repeated.
Previous studies investigating the role of group I and group II mGluRs in learning and memory have shown these receptors to be involved in a variety of behavioral tasks. Thus MCPG, a mixed group I/II mGluR antagonist, impaired acquisition and retention of a spatial alternation task (Riedel et al. 1995b) and a spatial learning task in the water maze (Richter-Levin et al. 1994; Bordi et al. 1996). Attenuation of group I mGluR function either through genetic modification or by administration of MPEP, an mGluR5 antagonist, has been shown to impair spatial learning (Conquet et al. 1994; Lu et al. 1997; Naie and Manahan-Vaughan 2004), while blockade of group II mGluRs by the specific antagonist LY341495 blocked the retention of a passive avoidance task in mice tested 24 h after training (Sato et al. 2004). These results suggest a role for mGluRs in spatial learning paradigms and highlight the potential contribution of mGluRs for the retention of mnemonic information over long delays. The importance of mGluR transmission in visual recognition memory in the rat has yet to be established; thus, the present study examined the effects of manipulations of group I, group II, and group III mGluRs on visual recognition memory in rats and whether the effects were produced by selective antagonism within the PRH. The task used to assess memory performance was the spontaneous object recognition memory test (Ennaceur and Delacour 1988). In this task, the subject is allowed to explore two copies of a familiar object during the “sample” phase. Following a delay period, the subject is simultaneously presented with the familiar object and a novel object during the “test” phase. Recognition memory in the test phase is measured by the preferential exploration of a novel rather than a familiar object. To examine the role of group I mGluRs, the mGluR5 receptor antagonist MPEP was administered both systemically (at two doses) and intracerebrally. Two doses of MPEP were used to exclude the possibility that a lack of effects of MPEP on recognition memory might be due to inadequate blockade of mGluR5 receptors. To examine the role of group II mGluRs, the selective antagonists LY341495 (LY) and EGLU were used. EGLU is a highly selective group II mGluR antagonist, with no antagonist activity at any of the other mGluR subtypes, and does not cross the blood–brain barrier (Jane et al. 1996). It is structurally unrelated to LY (Jane et al. 1996; Kingston et al. 1998) and thus could be used to confirm that results obtained with LY reflected an action at group II mGluR receptors. The effect of combined antagonism of group I and group II mGluRs was also investigated. The effect of antagonism of group III mGluRs was investigated using MSOP, a selective group III antagonist (Thomas et al. 1996), which was administered intracerebrally as at present there are no selective antagonists able to cross the blood–brain barrier.
Here we report that acquisition of long-term recognition memory performance requires either group I or group II mGluRs, but that acquisition of short-term recognition memory does not appear to require mGluR activation. We further report that as blockade of group I and group II mGluRs during both the sample phase and the 24-h test impaired long-term familiarity discrimination, these results do not appear to reflect a state-dependent mechanism.
Results
Experiment 1: Role of group I and group II mGluRs in the acquisition of recognition memory
Exploration during the sample phase
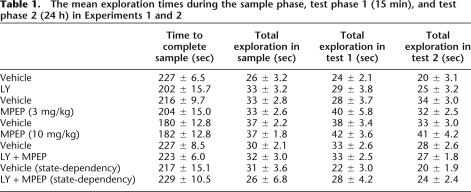
The mean amount of time taken to complete the sample phase and the mean total exploration completed in the sample phase are shown in Table 1. Statistical analysis revealed that there was no significant effect of administration of LY341495 (LY: group II mGluR antagonist) or MPEP (mGluR5 antagonist) on the amount of exploration of objects during the sample phase (LY: F1,11 ≤ 1.0, MPEP 3 mg/kg: F1,11 ≤ 1.0; MPEP 10 mg/kg: F1,11 ≤ 1.0). Analysis of the total time spent in the arena revealed no significant difference between the vehicle and LY-treated groups, F1,11 ≤ 1.0, or between the vehicle and MPEP-treated groups, F1,11 ≤ 1.0.
Table 1.
The mean exploration times during the sample phase, test phase 1 (15 min), and test phase 2 (24 h) in Experiments 1 and 2
Exploration at test
The mean total levels of exploration during test phases 1 and 2 are shown in Table 1. Analysis of the total amount of exploration of the objects during the two test phases revealed no significant effects of administration of LY (drug: F1,11 = 3.58, p > 0.05; delay: F1,11 = 2.69, P > 0.05; drug-by-time interaction: F1,11 ≤ 1.0). Analysis of the amount of exploration at test following administration of MPEP (3 mg/kg) revealed a significant drug-by-time interaction (F1,11 = 5.46, P < 0.05), and analysis of the simple main effects showed a significant main effect of drug administration at test phase 1 (15 min). Inspection of the mean total exploration showed that the MPEP-treated animals completed a greater amount of exploration than the vehicle-treated animals at test phase 1. Administration of MPEP at 10 mg/kg produced no differences in total exploration at either of the test phases (all Fs < 1.0).
Recognition at test—LY (3 mg/kg) or MPEP (3 mg/kg, 10 mg/kg)
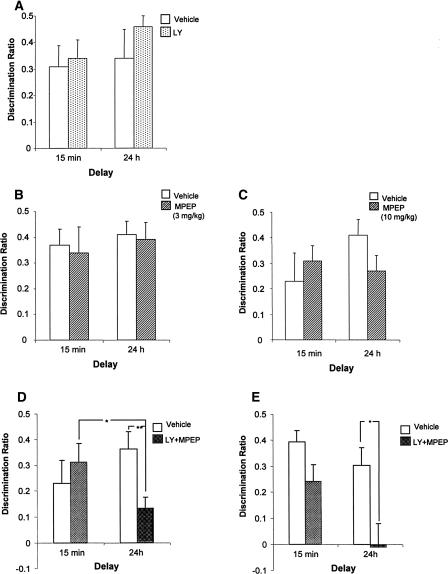
Analysis of the discrimination ratios revealed that systemic administration of LY (3 mg/kg) or MPEP (3 mg/kg or 10 mg/kg) had no significant effect on familiarity discrimination following either the 15-min or the 24-h delay (see Fig. 1A,C), and there was no significant difference between the memory performance at the two test sessions (all Fs < 1.3). In addition, the animals showed significant preference for the novel compared to the familiar object in both the control and drug-treated conditions. As the total amount of exploration completed at the test phase 1 was significantly higher in the MPEP (3 mg/kg) treated animals, the object recognition memory performance of this group was compared to controls using the discrimination index, as it could be argued that the discrimination ratios based on different divisors are not necessarily comparable. Again, statistical analysis revealed no significant difference between the treatment groups using this measure (F < 1.0).
Figure 1.
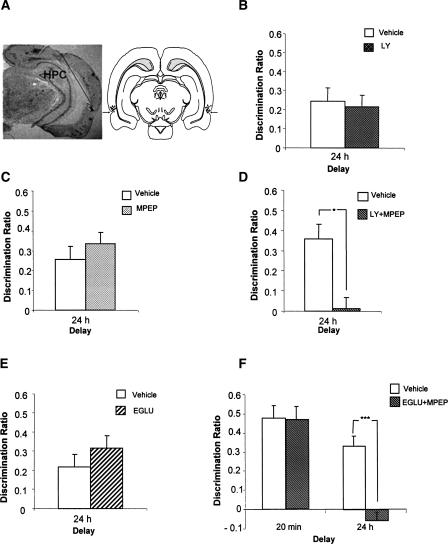
(A) Systemic administration of LY341495 (LY) (3 mg/kg), (B) MPEP (3 mg/kg), (C) MPEP (10 mg/kg), had no effect on familiarity discrimination. Systemic administration of LY341495 and MPEP combined (3 mg/kg each) (LY + MPEP) impaired familiarity discrimination at 24 h, but not at 15 min. (D) Drug administered before acquisition and (E) before acquisition and test. Mean discrimination ratio ±SEM (n = 12). (*) P < 0.05; (**) P < 0.01.
Experiment 2: Effect of combined antagonism of group I and group II mGluRs on the acquisition of recognition memory
Exploration during the sample phase
The mean amount of time taken to complete the sample phase and the mean total exploration completed in the sample phase are shown in Table 1. There were no significant effects of systemic coadministration of LY341495 and MPEP (LY + MPEP) on the amount of exploration of objects during the sample phase (F1,11 ≤ 1.0) or on the amount of time spent in the arena (F1,11 ≤ 1.0).
Exploration during test phase 1 (15 min) and test phase 2 (24 h)
The mean total levels of exploration during test phases 1 and 2 are shown in Table 1. Statistical analysis revealed no significant effect of LY + MPEP drug treatment or delay on the total amount of time spent by the rats exploring the objects in the 15-min or 24-h test phases (F < 1.0), nor was there a significant drug-by-delay interaction (F1,11 ≤ 1.0).
Recognition at test phase 1 (15 min) and test phase 2 (24 h)
Systemic administration of LY + MPEP produced a profound impairment in familiarity discrimination when the animals were tested 24 h, but not 15 min after the sample phase; see Figure 1D (drug-by-time interaction: F1,11 = 4.64, P = 0.05; drug: F1,11 = 2.24, P > 0.1; time: F1,11 ≤ 1.0). Analysis of the simple main effects revealed a significant main effect of drug at 24-h delay (F1,11 = 11.75, P < 0.01); reflecting a decrease in discrimination in the drug group at the longer time delay, however, at 24 h both the control and drug-treated groups continued to demonstrate significant discrimination between the novel and familiar object [vehicle: t(11) = 5.16, P < 0.01; LY + MPEP: t(11) = 2.96, P < 0.01].
To examine whether the impairment in familiarity discrimination observed following the 24-h delay reflected a state-dependent mechanism, the effect of coadministration of LY + MPEP administered before both the sample and the 24-h test phases was investigated. ANOVA revealed a significant effect of drug (F1,8 = 8.75, P < 0.05) and time (F1,8 = 24.77, P < 0.005), but no significant drug × time interaction (F1,8 = 1.44, P > 0.05); see Figure 1E. The impairment at 24 h was confirmed by the finding that the drug-treated group did not show significant discrimination between the novel and familiar objects at this delay [t(8) = 0.097, P > 0.05].
Experiment 3: The role of group I and group II mGlu receptors in consolidation or retrieval of recognition memory
Exploration during the sample phase
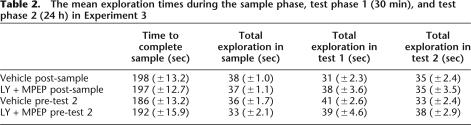
There were no differences in total object exploration or in the duration of the sample phase between the animals, as would be expected because the drugs were not present during the sample phase (the mean values are shown in Table 2).
Table 2.
The mean exploration times during the sample phase, test phase 1 (30 min), and test phase 2 (24 h) in Experiment 3
Exploration during test
The mean total levels of exploration during test phases 1 and 2 are shown in Table 2. There were no differences in total object exploration between the control and drug-treated groups in either the 30-min or 24-h test phase (all Fs < 1.3).
Recognition at test
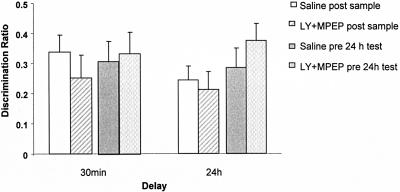
Performance in all drug-treated groups did not differ from performance of the control groups either at the 30-min or the 24-h test; see Figure 2 (drug: F1,13 = 2.30, P > 0.05; time: F1,13 ≤ 1.0; drug × time interaction F1,13 = 1.97, P > 0.05). In addition, both control and drug groups showed significant discrimination between the novel and familiar objects at both delays [vehicle, postsample, test phase 1: t(13) = 5.32, P < 0.01; LY + MPEP, post-sample, test phase 1: t(13) = 2.87, P < 0.05; vehicle, post-sample, test phase 2: t(13) = 4.97, P < 0.01; LY + MPEP, post-sample, test phase 2: t(13) = 3.41, P < 0.01; vehicle, pre-test, test phase 1: t(13) = 3.91, P < 0.01; LY + MPEP, pre-test, test phase 1: t(13) = 4.68, P < 0.01; vehicle, pre-test, test phase 2: t(13) = 4.24, P < 0.01; LY + MPEP, pre-test, test phase 2: t(13) = 6.50, P < 0.01].
Figure 2.
Systemic coadministration of LY341495 (3 mg/kg) and MPEP (3 mg/kg) (LY + MPEP) had no effect on consolidation (injection post-sample) or on retrieval (injection pre-24-h test) of familiarity discrimination. Mean discrimination ratio ±SEM (n = 14).
Experiment 4: The role of perirhinal group I and group II mGlu receptors in acquisition of recognition memory
To confirm that the impairment in familiarity discrimination produced by systemic coadministration of LY and MPEP reflects a specific antagonism of mGlu5 and group II mGlu receptors in the PRH, animals were tested in the spontaneous object recognition task as follows:
The localized intracortical coadministration of LY (5 μM) and MPEP (100 μM) and LY + MPEP. The animals were tested following a 24-h delay.
The intracortical coadministration of EGLU (10 mM), a highly selective group II receptor antagonist (Jane et al. 1996), and EGLU + MPEP. In the EGLU + MPEP study, the animals were tested following a 20-min and a 24-h delay in separate experiments.
Histological examination of the cannulae placements confirmed that infusions were made into perirhinal cortex in all cases (see Fig. 3A).
Figure 3.
Intraperirhinal antagonism of mGluR5 and group II mGluRs impairs familiarity discrimination at 24 h. (A) Histological localization of implanted cannula. (Left) Photomicrograph; cannula tract indicated by a black line; (HPC) hippocampus. (Right) Diagram of the corresponding brain section; arrows indicate location of perirhinal cortex, and the location of each cannula tip is indicated by the dots (Swanson 1998). (B) LY (5 μM) alone (n = 7) animals tested at 24 h. (C) MPEP (100 μM) alone (n = 7) animals tested at 24 h. (D) LY (5 μM) + MPEP (100 μM) animals (n = 6) tested at 24 h, *P < 0.05. (E) EGLU (10 mM) alone (n = 9), animals tested at 24 h. (F) EGLU (10 mM) + MPEP (100 μM) animals (n < 6) tested at 20 min and 24 h, ***P < 0.001. All histograms show the mean discrimination ratio ±SEM.
Exploration during the sample phase
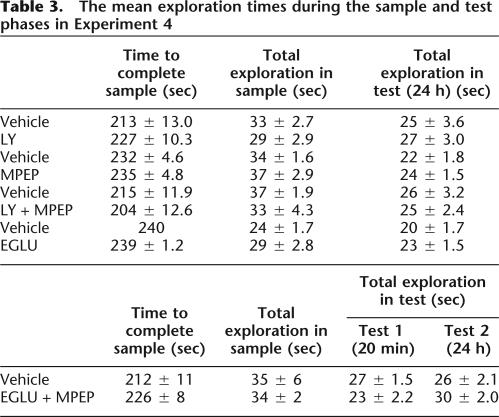
There was no effect of intracerebral administration of LY, MPEP, LY + MPEP, EGLU, or EGLU + MPEP on performance during the sample phase (all Fs < 1.0), nor on the total exploration during the sample phase (all Fs < 1.0). The mean time to complete the sample phase and the mean total exploration completed in the sample phase are shown in Table 3.
Table 3.
The mean exploration times during the sample and test phases in Experiment 4
Exploration at test
Intracerebral administration of LY, MPEP, and LY + MPEP had no effect on total exploration during the test phase following a 24-h delay (all Fs < 1.0; see Table 3 for means).
Analysis of the total amount of exploration completed in test phase 1 or test phase 2 did not reveal any significant differences between the control and EGLU + MPEP-treated groups [test phase 1 (20 min): F1,7 = 1.48, P > 0.05; test phase 2 (24 h): F1,11 = 1.61, P > 0.05; see Table 3 for means]. In addition, there were no significant differences in the total amount of exploration at test following administration of EGLU alone (F < 1.0; see Table 3 for means).
Recognition at test following blockade of group I and group II mGlu receptors in the perirhinal cortex
Intracerebral administration of LY had no effect on familiarity discrimination when the animals were tested 24 h after the sample phase (F1,7 < 1.0) (see Fig. 3B), and both the control and drug-treated groups showed significant discrimination between the novel and familiar objects [vehicle: t(7) = 2.64, P < 0.05; LY: t(7) = 2.80, P < 0.05]. Intracerebral administration of MPEP alone also had no effect on familiarity discrimination (F1,11 = 1.23, P > 0.05) (see Fig. 3C). Both control and drug-treated groups showed significant discrimination between the novel and familiar objects [vehicle: t(11) = 3.56, P < 0.01; MPEP: t(7) = 5.85, P < 0.001].
Intracerebral administration of LY + MPEP produced a significant impairment in familiarity discrimination when the animals were tested 24 h after the sample phase (F1,5 = 8.170, P < 0.05) (see Fig. 3D). Further analysis showed that the control group was able to discriminate between the novel and familiar objects [t(5) = 4.33, P < 0.01]; however, the LY + MPEP-treated group was not [t(5) = 0.25, P > 0.05].
EGLU administered alone had no effect on familiarity discrimination at 24 h (all Fs < 1.0) (see Fig. 3E), and both vehicle and drug-treatment groups showed significant discrimination between the novel and familiar objects [vehicle: t(8) = 3.10, P < 0.05; EGLU: t(8) = 4.75, P < 0.001].
Coadministration of MPEP and EGLU (MPEP + EGLU) produced a significant impairment of familiarity discrimination following a 24-h delay (drug: F1,11 = 39.1, P < 0.001). Further analysis confirmed that while the control group showed a significant preference for the novel compared to the familiar object [t(11) = 6.04, P < 0.01], the drug-treated group did not [t(11) = 1.19, ns]; see Figure 3F. There was no effect of MPEP + EGLU following a 20-min delay (drug: F1,7 = 1.48, P > 0.05), and at this delay, both groups showed significant discrimination between the novel and familiar objects [vehicle: t(7) = 6.83, P < 0.01; MPEP + EGLU: t(7) = 5.97, P < 0.01].
Experiment 5: The role of perirhinal group III mGlu receptors in the acquisition of recognition memory
Exploration during the sample phase
There were no differences between the total object exploration or the duration of the sample phase between the MSOP-treated and control animals [respectively, F1,11 = 1.67, P > 0.05; F1,11 = 2.67, P > 0.05; means (±SEM), total exploration: MSOP = 39 ± 0.5 sec; Vehicle = 38 ± 1 sec: means (±SEM) duration of sample phase MSOP = 161 ± 13 sec; Vehicle = 191 ± 12 sec].
Exploration during test
There were no differences between the total object exploration during the test phase between the MSOP- and control-treated animals [respectively, F1,11 ≤ 1.0, P > 0.05; means (±SEM) total object exploration: MSOP = 30 ± 2 sec; Vehicle = 31 ± 3 sec].
Recognition at test
No effect of the group III mGluR antagonist MSOP (50 mM) on familiarity discrimination was observed (F1,11 = 1.78, P > 0.05), and both the control and drug groups showed significant preference for the novel compared to the familiar object [vehicle: t(11) = 4.08, P < 0.01; MSOP: t(11) = 3.66, P < 0.01].
Discussion
This study provides the first demonstration that familiarity discrimination of individual objects is dependent on mGluR-mediated transmission. The results obtained using intracerebral administration show that activation of both group I (specifically, mGluR5) and group II mGluRs in the PRH is involved in the acquisition of long-term but not short-term object recognition memory. However, the results also show that activation of these receptors is not necessary for either the consolidation or retrieval of such memories. There was no evidence from these studies for an involvement of group III mGluRs in the acquisition of long-term familiarity discrimination.
To produce impairment in familiarity discrimination, it was necessary to block both group I and group II mGluRs. As LY has weak antagonist activity at group I mGluRs, the effect of administration of MPEP alone was further tested at a higher dose (10 mg/kg compared to 3 mg/kg) to rule out the possibility that the effect of LY + MPEP was due to an effect of blockade of group I mGluRs only. This experiment showed that the higher dose, like the lower dose, had no effect on recognition memory at a long delay; thus, the delay-dependent impairments in familiarity discrimination following combined administration of LY and MPEP were due to the combined effects of blocking both receptor subtypes. In further support of the argument that the lack of effect of systemic MPEP or LY on object recognition memory is not due to an insufficient dose, previous studies have demonstrated significant behavioral effects in animal models of anxiety following the administration of MPEP at doses up to 10 mg/kg (Tatarczynska et al. 2001; Brodkin et al. 2002), and, in addition, LY at a dose of 1 mg/kg was shown to reverse the anxiolytic effects of the group II mGluR antagonist LY354740 (Linden et al. 2005). Thus the doses of MPEP and LY used in the present experiment have been previously shown to produce behavioral effects. Furthermore, an identical pattern of behavioral results (i.e., no effect of single administration of either the group I or group II antagonists, but impairments following combined administration) was obtained following intracerebral administration of the drugs. For MPEP, the IC50 for mGlu5 receptors is 32 nM (Gasparini et al. 1999); for LY, the IC50 for group II mGluRs is 0.21 nM (for mGlu2) and 0.14 nM (for mGlu3) (Kingston et al. 1995; Schoepp et al. 1999); and for EGLU, the IC50 for group II mGluRs is 94 μM. Thus, the doses used in the present experiment would have produced significant antagonism of the mGluR subtypes, and the lack of effect of single administration of these compounds could not be explain by insufficient dose levels used in the study. Finally, the delay-dependent impairment in familiarity discrimination was observed following coadministration of MPEP with both LY or EGLU, and the finding that these two compounds (EGLU and LY) produce the same pattern of results in the object recognition task supports the conclusion that the impairments in familiarity discrimination are the result of selective antagonism of both group II mGluRs and group I mGluRs.
None of the drugs tested either individually or in combination had any effect on behavior during the sample phase, or on recognition memory following a short delay. These results exclude the possibility that the performance deficits observed at test can be explained either by nonspecific drug effects on alertness, attentional processes, or as a simple perceptual deficit. In addition, there were no differences in exploration levels between test phases 1 and 2, which could have accounted for the differences in recognition memory performance seen following the two delay periods. There was a significant effect of MPEP at 3 mg/kg on exploration in test phases 1; however, statistical analysis using the discrimination index ([time spent with novel] − [time spent with familiar]) confirmed that there was no effect of MPEP on recognition memory performance.
The lack of nonspecific drug effects on general levels of exploration during the sample or test phases is consistent with previous reports of a lack of effect of MPEP or LY341495 on locomotor activity or on general exploratory behavior (Henry et al. 2002; Smolders et al. 2004). Furthermore, the demonstration that the blockade of mGluR5 and group II mGluRs during both the acquisition and the 24-h test impaired familiarity discrimination at 24 h rules out the possibility that the selective long-term object recognition impairment was due to a state-dependent mechanism and supports the proposition that the impairment in familiarity discrimination produced by blockade of group I and group II mGluRs reflects a purely mnemonic deficit. Impairments in long-term spatial memory have been reported following administration of either MPEP or the group I/group II antagonist MCPG (Riedel et al. 1995b; Balschun and Wetzel 2002).
Coantagonism of mGluR5 and group II mGluRs immediately post-sample phase had no effect on short-term or long-term object memory performance, indicating that the consolidation of object recognition memory is independent of group I and II mGluR activation. These results are consistent with previous studies showing that blockade of mGlu5 receptors or co-blockade of group I and group II mGlu receptors had no effect on spatial learning when administered post-sample (Riedel et al. 1995a; Balschun and Wetzel 2002). Recent results from our laboratory have shown that post-sample infusions of the AMPA/kainate antagonist CNQX disrupted familiarity discrimination following short delays, indicating that the retrieval of recognition memory is dependent on neuronal activation by AMPA/kainate receptors in the PRH (Barker et al. 2003). However, the present study demonstrated that blockade of mGluR5 and group II mGluRs had no effect on retrieval.
Selective blockade of mGluR5 and group II mGluRs by localized intracerebral infusions into the PRH produced a significant mnemonic impairment. Unpublished observations from our laboratory, using Indian ink to visualize the extent of the drug spread following the intracerebral injections, have indicated that the area of the perirhinal cortex infused is ∼1 mm3, and this estimation of the drug spread is supported by previous studies investigating the spatial localization of drug effects using autoradiography (Martin and Ghez 1999). While the present study cannot rule out the possibility that the drug infusions spread to some extent outside the perirhinal cortex, recent studies by Winters and Bussey demonstrated that infusions of lidocaine into the perirhinal cortex spread preferentially anterior-dorsally along the extent of the PRH without encroaching into the area TE or the entorhinal cortex (Winters and Bussey 2005).
Hence, glutamatergic neurotransmission via mGluRs in the PRH is crucial for long-term recognition memory. As the impairment was produced only following the blockade of both mGluR5 and group II mGluRs, acquisition of information required for long-term familiarity discrimination is supported by neurotransmission via both or either type of receptor. The lower discrimination ratios obtained in the experiments in which either EGLU or MPEP was administered intracerebrally, compared to the discrimination ratios obtained when the drugs were administered together, probably reflect the low discriminability of the particular objects used in these experiments (not intentionally), as the effect was found in both the control and drug-treated groups and the level of discrimination in this type of recognition task, which relies on the animal’s spontaneous behavior, is highly dependent on the nature of the objects used.
Neither the group I, group II, or group III MGlu receptor was found to be involved in shorter-term (15–20 min) recognition memory. That shorter-term familiarity discrimination may be supported by a region other than the PRH is unlikely, as lesions of the PRH or intraperirhinal administration of scopolamine or lorazepam disrupt recognition memory following a 15-min delay (Bussey et al. 1999; Warburton et al. 2003; Wan et al. 2004; Winters et al. 2004). Moreover, systemic administration of group I with group II mGluR antagonists had no effect on such shorter-term recognition memory. The results therefore suggest that any neuronal processes that support retention of the mnemonic information over a short delay are independent of group I and II mGluR activation, while the processes underlying the long-term storage of information do require these mGluRs.
Neuronal response reductions within the PRH have been proposed to signal relative familiarity of visual stimuli (Brown and Xiang 1998; Warburton et al. 2003), and LTD has been proposed as a candidate model for such decreases in neuronal activation (Brown and Bashir 2002). Since group I and II mGlu receptors are involved in the induction of LTD (Cho et al. 20002002) but not long-term potentiation (Ziakopoulos et al. 1999) in the PRH, the present results support the hypothesis that common mechanisms may underpin LTD and long-term familiarity discrimination. Group I and group II mGluRs are linked to separate intracellular signaling cascades, but both cascades have been linked to putative memory consolidation mechanisms. Thus, group I receptors are coupled to phospholipase C and activate protein kinase C (PKC). The activation of PKC has been shown to be a key stage in the intracellular signaling pathways that are hypothesized to be necessary for the consolidation of mnemonic information (Bernabeu et al. 1997; Vianna et al. 2000). Group II mGluRs, however, are negatively coupled to adenylate cyclase, and activation of group II mGluRs has been shown to reduce cAMP levels (Kingston et al. 1998) and inhibit protein kinase A (PKA). Previous research has suggested that inhibition of the cAMP/PKA pathway impairs learning and memory (Baldwin et al. 2002); another study has shown that increases in cAMP (by the administration of LY341495) also impaired memory, leading to the suggestion that the relationship between cAMP and memory performance may be represented by an inverted U-shape (Sato et al. 2004). One explanation for the present results might be that in the PRH it may be necessary to both block the PKC pathway and stimulate the PKA pathway to prevent plasticity related to long-term recognition memory. Clearly, further research is required to clarify the contribution of the PKC and PKA pathways in long-term recognition memory.
Group III mGluRs have been shown to produce anxiolytic- as well as antidepressant-like effects in behavioral tests after central administration in rats (Palucha et al. 2004). In addition, it has been demonstrated that group III mGluRs are involved in the process of visual response habituation in the superior colliculus (Cirone and Salt 2000, 2001), yet their role in mnemonic processing has not been extensively explored. The results from the present experiment found no evidence for any involvement of perirhinal group III mGlu receptors alone. However, the role of these receptors in combination with either group I or group II mGluRs in long-term object recognition memory has yet to be investigated.
Thus, these findings indicate that the activation of group I and group II mGluRs in the PRH is necessary for the acquisition of long-term, but not short-term familiarity discrimination. However, these receptors appear not to be involved in either the consolidation or in the retrieval of short-term or long-term object recognition memory.
Materials and Methods
All experiments were conducted in male pigmented rats (DA strain, Bantin and Kingman, Hull UK, weighing 200–250 g at the start of the experiments). The animals were housed under a 12-h light/12-h dark cycle (light phase 18:00–6:00 h). Behavioral training and testing were conducted during the dark phase of the cycle. All animal procedures were performed in accordance with the United Kingdom Animals Scientific Procedures Act (1986) and associated guidelines. All efforts were made to minimize any suffering and the number of animals used.
All statistical analyses used a significance level of 0.05.
Object preference test
Apparatus
Exploration occurred in an open-topped arena (50 × 90 × 100 cm) made of wood. The walls inside the arena were surrounded with a black cloth to a height of 1.5 m so that no external stimuli could be seen during the experiment, and the floor of the arena was covered with sawdust. An overhead camera and a videorecorder were used to monitor and record the animal’s behavior for subsequent analysis. The stimuli presented were triplicate copies of objects composed of “Duplo” (Lego UK Ltd.) that varied in shape, color, and size (9 × 8 × 5 cm to 25 × 10 × 5 cm), and were too heavy for the animal to displace.
Training
After being handled for a week, the animals were habituated to the arena without stimuli for 10–15 min daily for 2 d prior to the commencement of the spontaneous recognition procedure. The procedure itself comprised an acquisition or sample phase followed by either one or two preference tests after a delay (test 1, test 2), depending on the experiment. In the sample phase, duplicate copies (A1 and A2) of an object were placed near the two corners at either end of one side of the arena (10 cm from each adjacent wall). The animal was placed into the arena facing the center of the opposite wall and then allowed a total of either 40 sec of exploration of A1 and A2, or 4 min in the arena. Exploratory behavior was defined as the animal directing its nose toward the object at a distance of <2 cm. Any other behavior such as looking around while sitting on or resting against the object was not considered as exploration. The delay between the sample phase and test 1 was either 15, 20, or 30 min or 24 h (depending on the nature of the experiment). If a second test phase was run, this always occurred 24 h after the sample phase. At test (3 min duration), the animal was replaced in the arena, presented with two objects in the same positions: One object (A3 for the first test phase or A4 if a second test phase was run) was an additional copy of the triplicate set of the objects used in the sample phase, and the other was a novel object (B3 for the first test phase or C3; i.e., a different novel object, if a second test phase was run). The positions of the objects in the test and the objects used as novel or familiar were counterbalanced between the animals in a group and between the drug-treated or control groups.
Systemic administration
Systemic drug administration
The systemically active group II antagonist LY341495 (2S,1′S,2′S)-2(9-xanthylmethyl)-2-(2′-carboxycyclopropyl)glycine (LY, 3 mg/kg; Tocris Cookson) was dissolved in 0.1 M NaOH and made up to volume with sterile 0.9% saline solution. The group I antagonist MPEP [6-methyl-2-(phenylethynyl)pyridine; 3 mg/kg, 10 mg/kg; Tocris Cookson] was dissolved in sterile 0.9% saline solution. Drug solutions were adjusted to a pH of 7.4–7.8 with 1 M HCL before administration. Control vehicle injections were an equivalent volume of normal saline. All drugs were administered by intraperitoneal injection 30 min prior to the sample phase, with the experimenter being blind to whether the injection was of drug or of vehicle.
Subjects and Experimental Design
Experiments 1 and 2: The role of group I and group II mGluRs in acquisition of recognition memory, and effect of combined antagonism of group I and group II mGluRs
The subjects were 12 naive rats. A within-subjects cross-over experimental design was used in all experiments, and the rats were tested both 15 min and 24 h after acquisition. Vehicle, LY341495 (LY), MPEP, or both drugs (LY + MPEP) were administered prior to the sample phase, over a 10-wk period. Thus, in week 1, half the rats received LY and half received vehicle; 6 d later (week 2), the rats that had received LY received vehicle, and the vehicle-treated rats received LY341495. In weeks 3 and 4, the rats received either MPEP (3 mg/kg) or vehicle. In weeks 5 and 6, the rats received either MPEP (10 mg/kg) or vehicle. In weeks 7 and 8, the rats received either LY + MPEP or vehicle.
A state-dependence experiment was run in weeks 9 and 10 using nine subjects from the original cohort of 12. Thus, the effect of systemic coadministration of LY + MPEP before both the sample and the 24-h test phases was investigated, using a cross-over design as for the other experiments.
Experiment 3: Role of group I and group II mGluRs in consolidation or retrieval of recognition memory
The subjects were 14 naive rats and were tested 30 min and 24 h after acquisition. Vehicle or LY + MPEP was administered either immediately after the sample phase (to test for an involvement in consolidation processes) or just prior to the 24-h test phase (to test for an involvement in retrieval processes) in a Latin square design. Test phase 1 was conducted after a delay of 30 min, to ensure that the drug given systemically immediately after the sample phase would be centrally active at the first test phase.
Localized infusion into the PRH
Cannulation surgery
Twelve rats were implanted bilaterally with guide cannulae directed at the perirhinal cortex. Each rat was anaesthetized with isoflurane (induction 4%, maintenance 2%–3%). The rat was secured in a stereotaxic frame with the incisor bar set at 3.3 mm below the interaural line. Two stainless steel guide cannulae (26 gauge; Plastics One) were implanted through burr holes in the skull at an angle of 20° to the vertical, using the coordinates AP, −5.6 mm; L, ±4.47 mm (relative to bregma), V, −6.7 mm (relative to surface of the skull). The guide cannulae were anchored to the skull by two stainless steel skull screws (Plastics One) and dental cement. Between infusions the cannulae were closed by dummy cannulae. Following surgery, each animal was given fluid replacement therapy (5 mL of saline, s.c.) and analgesia (0.05 mL of Temgesic, i.m.), and was housed individually. It was allowed to recover for at least 10 d before habituation to the testing arena began.
Histology
At the end of the experiment, each rat was anaesthetized with Euthetal and perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde. The brain was post-fixed in paraformaldehyde for a minimum of 2 h before being transferred to 30% sucrose in 0.2 M phosphate buffer and left overnight. Coronal sections were cut at 50 μm on a cryostat and stained with cresyl violet. Cannula locations were checked against standardized sections of the rat brain (see Fig. 3A).
Intraperirhinal drug administration
MPEP and the group III antagonist MSOP (RS)-a-Methylserine-O-phosphate were dissolved in sterile 0.9% saline solution to the appropriate concentration (100 μM MPEP, 50 mM MSOP; both drugs from Tocris Cookson). The group II antagonist EGLU (S)-α-ethylglutamic acid (a group II antagonist that is structurally unrelated to LY but does not cross the blood–brain barrier) or LY was dissolved in 0.1 M NaOH and made up to the appropriate concentration using vehicle (10 mM EGLU; Tocris Cookson; 5 μM LY). The drug concentrations for MPEP, EGLU, and MSOP used were calculated using either available IC50 values or the equivalent. The drug concentration used for the infusions of LY was chosen from pilot dose-response experiments conducted in the laboratory (data not shown). Control animals received 0.9% saline or saline with an equivalent concentration of NaOH. Injections were made into the PRH through a 33-gauge infusion cannula (Plastics One) attached to a 5-μL Hamilton syringe via a length of polyethylene tubing. A volume of 1.0 μL was injected into each hemisphere over a 2-min period, using an infusion pump (Harvard). The infusion cannula was then left in place for a further 5 min. The sample phase of the object recognition test began 15 min after the start of drug infusion and followed the procedures detailed above.
Subjects and experimental design
Experiment 4: Role of perirhinal group I and group II mGluRs in acquisition of recognition memory
A within-subjects cross-over experimental design was used with a week between each stage, and the rats were tested either 20 min or 24 h after acquisition in separate experiments. The effects of intracerebral infusions of LY (n = 7), MPEP (n = 12), EGLU (n = 9), and LY + MPEP (n = 6) were examined following a delay of 24 h only. The effects of EGLU + MPEP (n = 12) were examined following delays of both 20 min and 24 h.
Experiment 5: Role of perirhinal group III mGlu receptors acquisition of recognition memory
A within-subjects cross-over experimental design was used with a week between each stage. The effect of infusion of MSOP (n = 12) was tested 24 h after acquisition.
Statistical analysis
All measures of exploration were made with the experimenter blind as to the treatment. The discrimination ratio was calculated as the difference in time spent by each animal exploring the novel compared to the familiar object divided by the total time spent exploring both objects in the 3-min test period. In cases in which the total amount of exploration at test varied significantly, a discrimination index, that is, difference in time spent exploring the novel compared to the familiar object, was used. Comparisons between the vehicle and drug-treated conditions used a within-subject analysis of variance (ANOVA) or one sample paired t-tests against no preference. Any subjects that failed to complete a minimum of 20 sec of exploration in a test phase were excluded from the analysis.
Acknowledgments
This work was supported by the MRC. The authors are grateful to B. Fry, Jane Robbins, and K. Narduzzo for their expert technical assistance.
Footnotes
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/NA
References
- Aggleton J.P., Keen S., Warburton E.C., Bussey T.J. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res. Bull. 1997;43:279–287. doi: 10.1016/s0361-9230(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Baldwin A.E., Sadeghian K., Holahan M.R., Kelly A.E. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Balschun D., Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharm. Biochem. Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Barker G.R., Brown M.W., Warburton E.C.2003The role of glutamate receptors in recognition memory. Soc. Neurosci. Abs. 721.9. [Google Scholar]
- Bogacz R., Brown M.W. Comparison of computational models of familiarity discrimination in the perirhinal cortex. Hippocampus. 2003;13:494–524. doi: 10.1002/hipo.10093. [DOI] [PubMed] [Google Scholar]
- Bordi F., Marcon C., Chiamulera C., Reggiani A. Effects of metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology. 1996;35:1557–1565. doi: 10.1016/s0028-3908(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Brodkin J., Busse C., Sukoff S.J., Varney M.A. Anxiolytic-like activity of the mGluR5 antagonist MPEP—A comparison with diazepam and buspirone. Pharmacol. Biochem. Behav. 2002;73:359–366. doi: 10.1016/s0091-3057(02)00828-6. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Bashir Z.I. Evidence concerning how neurons of the perirhinal cortex may effect familiarity discrimination. Philos. Trans. R Soc. Lond. B Biol. Sci. 2002;357:1083–1095. doi: 10.1098/rstb.2002.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.W., Xiang J.Z. Recognition memory: Neuronal substrates for the judgment of prior occurrence. Prog. Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Wilson F.A.W., Riches I.P. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Bussey T.J., Muir J.L., Aggleton J.P. Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J. Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K., Kemp N., Aggleton J.P., Brown M.W., Bashir Z.I. A new form of long term depression in the perirhinal cortex. Nat. Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Cho K., Brown M.W., Bashir Z.I. Mechanisms and physiological role of enhancement of mGlu5 receptor function by group II mGlu receptor activation in rat perirhinal cortex. J. Physiol. 2002;540:895–906. doi: 10.1113/jphysiol.2001.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone J., Salt T.E. Physiological role of group III metabotropic glutamate receptors in visually responsive neurones of the rat superficial superior colliculus. Eur. J. Neurosci. 2000;12:847–855. doi: 10.1046/j.1460-9568.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Cirone J., Salt T.E. Group II and III metabotropic glutamate receptors contribute to different aspects of visual response processing in the rat superior colliculus. J. Physiol. 2001;534:169–178. doi: 10.1111/j.1469-7793.2001.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F., Bashir Z.I., Davies C.H., Daniel H., Ferraguti F., Bordi F., Franzbacon K., Reggiani A., Matarese V., Conde F., et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A., Neave N., Aggleton J.P. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of formix transaction in the rat. Behav. Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J. Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Gaffan D., Murray E.A. Monkeys (macaca-fascicularis) with rhinal cortex ablations succeed in object discrimination-learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav. Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gasparini F., Lingenhöhl K., Flor P.J., Heinrich M., Vranesic I., Biollaz M., Allgeier H., Heckendorn R., Urwyler S., Varney M.A., et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent and selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Henry S.A., Lehmann-Masten V., Gasparini F., Geyer M.A., Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Jane D.E., Thomas N.K., Tse H.-W., Watkins J.C. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Kingston A.E., Burnett J.P., Mayne N.G., Lodge D. Pharmacological analysis of 4-carboxyphenylglycine derivatives—comparison of effects on mglur1-α and mglur5a subtypes. Neuropharmacology. 1995;34:887–894. doi: 10.1016/0028-3908(95)00069-i. [DOI] [PubMed] [Google Scholar]
- Kingston A.E., Ornstein P.L., Wright R.A., Johnson B.G., Mayne N.G., Burnett J.P., Belagaje R., Wu S., Schoepp D.D. LY341495 is a nanomolar potent and selective antagonist at group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Linden A.-M., Shannon H., Baez M., Yu J.L., Koester A., Schoepp D.D. Anxiolytic-like activity of the mGlu2/3 receptor agonist LY354740 in the elevated plus maze is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl.) 2005;179:284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- Lu Y.-M., Jia Z., Janus C., Henderson J., Gerlai R., Wojtowicz J.M., Roder J.J. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. Neuroscience. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.H., Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J. Neurosci. Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M., Murray E.A. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J. Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby D.G., Pinel J.P.J. Rhinal cortex lesions and object recognition in rats. Behav. Neurosci. 1994;108:11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Mumby D.G., Glenn M.J., Nesbitt C., Kyriazis D.A. Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behav. Brain Res. 2002;132:215–226. doi: 10.1016/s0166-4328(01)00444-2. [DOI] [PubMed] [Google Scholar]
- Naie K., Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: Relevance for learning and memory formation. Cereb. Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Palucha A., Tatarczynska E., Branski P., Szewczyk B., Wieronska J.M., Klak K., Chojnacka-Wojcik E., Nowak G., Pilc A. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology. 2004;46:151–159. doi: 10.1016/j.neuropharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G., Errington M.L., Maegawa H., Bliss T.V.P. Activation of metabotropic glutamate receptors is necessary for long-term potentiation in the dentate gyrus and for spatial learning. Neuropharmacology. 1994;33:853–857. doi: 10.1016/0028-3908(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Riedel G., Casabona G., Reyman K.G. Inhibition of long-term potentiation in the dentate gyrus of freely moving rats, by the metabotropic glutamate receptor antagonist MCPG. J. Neurosci. 1995a;15:87–98. doi: 10.1523/JNEUROSCI.15-01-00087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G., Wetzel W., Reyman K.G. Metabotropic glutamate receptors in spatial and nonspatial learning in rats studied by means of agonist and antagonist application. Learn. Mem. 1995b;2:243–265. doi: 10.1101/lm.2.5.243. [DOI] [PubMed] [Google Scholar]
- Sato T., Tanaka K., Ohnishi Y., Teramoto T., Irifune M., Nishikawa T. Inhibitory effects of group II mGluR-related drugs on memory performance in mice. Physiol. Behav. 2004;80:747–758. doi: 10.1016/j.physbeh.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Schoepp D.D., Jane D.E., Monn J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Smolders I., Lindekens H., Clinckers R., Meurs A., O’Neill M.J., Lodge D., Ebinger G., Michotte Y. In vivo modulation of extracellular hippocampal glutamate and GABA levels and limbic seizures by group I and II metabotropic glutamate receptor ligands. J. Neurochem. 2004;88:1068–1077. doi: 10.1046/j.1471-4159.2003.02251.x. [DOI] [PubMed] [Google Scholar]
- Suzuki W.A., Zola-Morgan S., Squire L.R., Amaral D.G. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J. Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L.W.1998. Brain maps: Structure of the rat brain. Elsevier; Amsterdam. [Google Scholar]
- Tatarczynska E., Klodzinska A., Chojnacka-Wojcik E., Palucha A., Gasparini F., Kuhn R., Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br. J. Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N., Jane D., Tse H., Watkins J. α-Methyl derivatives of serine-O-phosphate as novel, selective competitive metabotropic glutamate receptor antagonists. Neuropharmacology. 1996;35:637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- Vianna M.R.M., Barros D.M., Silva T., Choi H., Madche C., Rodrigues C., Medina J.H., Izquierdo I. Pharmacological demonstration of the differential involvement of protein kinase C isoforms in short- and long-term memory formation and retrieval of one-trial avoidance in rats. Psychopharmacology (Berl.) 2000;150:77–84. doi: 10.1007/s002130000396. [DOI] [PubMed] [Google Scholar]
- Wan H., Aggleton J.P., Brown M.W. Different activation of the hippocampus and perirhinal cortex to recognition memory. J. Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Warburton E.C., Zhu X.O., Koder T.J., Park Y., Aggleton J.P., Cho K., Bashir Z.I., Brown M.W. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur. J. Neurosci. 2004;20:2214–2224. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- Warburton E.C., Koder T., Cho K., Massey P.V., Duguid G., Barker G.R.I., Aggleton J.P., Bashir Z.I., Brown M.W. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Wiig K.A., Bilkey D.K. Lesions of rat perirhinal cortex exacerbate the memory deficit observed following damage to the fimbria-fornix. Behav. Neurosci. 1995;109:620–630. doi: 10.1037//0735-7044.109.4.620. [DOI] [PubMed] [Google Scholar]
- Winters B.D., Bussey T.J. Transient inactivation of perirhinal cortex disrupts encoding, retrieval and consolidation of object recognition memory. J. Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B.D., Forwood S.E., Cowell R.A., Saksida L.M., Bussey T.J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.-O., Brown M.W., Aggleton J.P. Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices. Eur. J. Neurosci. 1995a;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Zhu X.-O., Brown M.W., McCabe B.J., Aggleton J.P. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995b;69:821–829. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
- Zhu X.-O., McCabe B.J., Aggleton J.P., Brown M.W. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7:1871–1875. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]
- Ziakopoulos Z., Tillett C.W., Brown M.W., Bashir Z.I. Input- and layer-dependent synaptic plasticity in the rat perirhinal cortex in vitro. Neuroscience. 1999;92:459–472. doi: 10.1016/s0306-4522(98)00764-7. [DOI] [PubMed] [Google Scholar]