Abstract
The interferon (IFN) family consisting of alpha IFN (IFN-α), IFN-β, IFN-ω, IFN-δ, IFN-κ, and IFN-τ is a large group of cytokines involved in the innate immune response against various microorganisms. Genes for IFN have been cloned from a variety of mammalian and avian species; however, IFN genes from lower-order vertebrates have not been forthcoming. Here, we report the cloning and characterization of the IFN gene from the zebrafish, Danio rerio. Zebrafish IFN (zfIFN) is 185 amino acids in length, with the first 22 amino acids representing a putative signal peptide. Treatment with the known IFN inducer polyinosinic acid-polycytidylic acid (poly[I]-poly[C]) resulted in an increase in zfIFN mRNA transcripts. zfIFN was also able to activate the IFN-inducible Mx promoter when cotransfected with a plasmid containing the zebrafish Mx promoter upstream of a luciferase reporter gene. To demonstrate antiviral activity, zebrafish cells were transfected with zfIFN and challenged with a fish rhabdovirus. A 36% reduction in plaque number was seen in zfIFN-transfected cells, compared to cells transfected with a control vector. Phylogenetic analysis has shown zfIFN to be approximately equally divergent from avian and mammalian IFN, consistent with its origin from an IFN present in the most recent common ancestor of these divergent lineages. A putative IFN from puffer, Fugu rubripes, was also found when zfIFN was used to search the fugu genome database, demonstrating that zfIFN can be used to find additional fish IFN genes. These results demonstrate that zebrafish can be used as an effective model for studying innate immunity and immune response to infectious disease.
The interferons (IFNs) represent a large family of soluble cytokines with biological and antiviral activity (12). IFN was first discovered by Isaacs and Lindenmann in 1957 as an agent capable of inhibiting the growth of influenza virus in embryonated chicken eggs (15). When induced by viral infection or the synthetic double-stranded RNA molecule poly(I)-poly(C) (9), IFN is synthesized and secreted into the extracellular environment, where it binds to its cognate receptor on neighboring cells (18). This results in a signal transduction cascade, ultimately leading to the stimulation of an array of antiviral proteins, among them 2′-5′-oligoadenylate synthetase, protein kinase p68, and Mx. These proteins are responsible for the degradation, or prevention of synthesis, of viral RNA (32). The Mx gene promoter contains an IFN-stimulated response element (ISRE), which is recognized by an IFN-induced transcription factor composed of signal transducer and activator of transcription (STAT) 1 and 2, bound to IFN regulatory factor 9 (see the review by Taniguchi and Takaoka [36]). By linking the ISRE of the Mx promoter to a reporter gene, the presence of IFN may be detected (3, 10, 30). In addition to having antiviral activity, the IFNs have been implicated in cell proliferation and differentiation, as well as in the suppression of some forms of cancer (2, 16). IFN-β knockout mice have been shown to exhibit diminished antiviral response to vaccinia virus (6), demonstrating the importance of a functional IFN system.
IFNs are divided into two groups, a group consisting of IFN-α, -β, -ω, -δ, -κ, and -τ (IFN-α/β/ω/δ/κ/τ group) and a group consisting of IFN-γ (32). Primary IFN peptides are 185 to 190 amino acids in length, the first 23 to 30 amino acids representing a hydrophobic signal peptide necessary for secretion. The signal peptide is cleaved prior to extracellular translocation of IFN to yield the mature peptide.
Genes for IFN-α/β/ω/δ/κ/τ group IFNs have been cloned from a variety of mammals, including humans (20, 21), mice (5, 33), pigs (22), and several avian species such as ducks (29), chickens (31), and turkeys (34). To date, there have been no reports of an IFN gene being cloned from any fish species, although IFN-like antiviral activity has been demonstrated (25). A sequence for flatfish IFN has been reported (35); however, Magor and Magor (24) found that it had no similarity to other IFNs. Furthermore, analysis with the basic local alignment search tool (BLAST) revealed the >60% amino acid identity of two-thirds of this sequence to sequences from filamentous phage (24). There are several reasons why fish IFN has not previously been cloned, among them the relatively low homology among species of IFN genes, which increases the difficulty of designing effective primers for use in PCR as well as in designing probes for library screening. Also, the lability of IFN transcripts renders isolation of sufficient quantities of IFN mRNA for amplification purposes very difficult. Researchers have thus used downstream effectors of IFN to demonstrate IFN activity indirectly in fish (19, 37, 38).
The zebrafish, Danio rerio, has recently emerged as an ideal model for the study of development and genetics. Genetically, the conservation of synteny among various chromosomal loci of humans and zebrafish has allowed positional cloning of several zebrafish genes (26). Increasingly, these syntenic relationships have been used to find the homologs of genes responsible for human diseases such as Huntington's disease (17) and familial Alzheimer's disease (23). Further, the similarity between humans and zebrafish, in terms of disease, extends beyond gene homology to actual similarities in the proteins involved in pathogenesis (26).
The innate immune response of zebrafish to infectious pathogens is currently not well characterized. However, due to its relative hardiness and resistance to infection and its evolutionary lineage, the zebrafish is likely to provide clues to the early evolution of the innate immune system. We report here the cloning and characterization of an IFN gene from zebrafish. Expression of this gene was inducible by poly(I)-poly(C) and was able to stimulate upregulation of the IFN-inducible Mx promoter. Zebrafish IFN (zfIFN) was also shown to exhibit antiviral activity, as demonstrated by a reduction in plaque formation in zebrafish cell monolayers. Phylogenetic analysis has shown zfIFN to be equally related to mammalian and avian IFN. A putative fugu IFN was also found by using zfIFN to search the fugu genome database (www.jgi.doe.gov/fugu). zfIFN represents the lowest-order vertebrate IFN cloned to date. Future studies of zfIFN will enable a deeper understanding of the IFN system and its importance to innate immunity, both in zebrafish and in humans.
MATERIALS AND METHODS
Cell culture and viruses.
Zebrafish liver cells (ZFL), ATCC CRL-2643 (4), were grown in a medium containing a combination of 50% L-15, 35% Dulbecco's minimum essential medium (DMEM), and 15% F-12 with sodium selenite, supplemented with 5% (vol/vol) fetal bovine serum (FBS), 0.5% (vol/vol) trout serum, 0.5% (vol/vol) insulin, and 50 μg each of penicillin, streptomycin, and ampicillin/ml. The embryo fibroblast cell line ZF4, ATCC CRL-2050 (8), was grown in a 1:1 ratio of F-12 and DMEM supplemented with 10% FBS. Spring viremia of carp virus (SVCV) and snakehead rhabdovirus (SHRV) were propagated in ZFL cells. Cells were infected at a multiplicity of infection of 0.1. The supernatant was then collected and filtered to obtain purified virus at a titer of 1.25 × 107 50% tissue culture infectious doses (TCID50)/ml.
Generation of cDNA.
To obtain total RNA, ZFL cells were grown to confluence in 75-cm2 flasks and were treated with either 50 μg of poly(I)-poly(C) (Sigma, St. Louis, Mo.)/ml or 104 TCID50 of SVCV/ml or were not treated. Cells were harvested at 6, 12, 24, 36, and 48 h posttreatment, and total RNA was extracted with the RNeasy minikit (Qiagen, Valencia, Calif.). A reverse transcription reaction was then performed to convert total RNA into cDNA. Briefly, 400 ng of RNA and 0.5 μg of random hexamers were combined and incubated at 70°C for 5 min, followed by a quick chill on ice for 5 min. To this mixture was added ImpromII 5× reaction buffer, 25 mM MgCl2, 10 mM deoxynucleoside triphosphate, 0.5 U of RNase inhibitor, and 1 μl of IMpromII reverse transcriptase (Promega, Madison, Wis.). The reaction mixture was incubated for 1 h at 37°C, followed by a 15-min heat inactivation step at 70°C.
RACE-PCR.
cDNA ready for rapid amplification of cDNA ends (RACE) was prepared with the GeneRacer kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Upstream RACE-PCR was performed by using a gene-specific primer designed from an expressed sequence tag (EST; GenBank accession no. BI708494). This primer, designated 66rev, with the sequence 5′-GCTCTGCGTCTACTTGCGAATGGC-3′, lies at bp 43 to 66 of the EST and primes upstream amplification when used with the downstream GeneRacer primer. This reaction yielded an amplification product containing a region of the 5′ untranslated region (UTR) of zfIFN cDNA, which was subsequently used as template to design primers for downstream RACE.
IFN-specific oligonucleotides were then designed to anneal to the 5′ UTR of zfIFN cDNA and prime downstream amplification. The sequence of the primary IFN primer, which sits at position −73 to −49 relative to the start site and which is designated −73fwd, is 5′-CCAGCACTCTCCATCATGTCTCTG-3′, and the sequence for the nested IFN primer, sitting at position −7 to +18 relative to the start site and which is designated −7fwd, is 5′-CGCAAAGATGAGAACTCAAATGTGG-3′. These primers were used with GeneRacer 3′ primary and nested primers, respectively, in two rounds of PCR. PCR amplifications were carried out according to the manufacturer's instructions (Sigma). Cycling parameters were 94°C for 1 min, 65°C for 1 min, and 72°C for 1 min for a total of 30 cycles. Nested PCRs used the nested primers and first-round product as the template.
The amplified product was subcloned into a pGEM-T Easy vector (Promega) and submitted for sequencing at the University of Maine Sequencing Facility with an ABI 373 DNA sequencer (Applied Biosystems, Foster City, Calif.).
Plasmid construction.
Using the cloned IFN product as the template, PCR was performed with the upstream primer 5′-CAGCCGGGTACCTAAGGAGGCCACCATGAGAACTCAAATGTGGACC-3′, which anneals to bp 1 to 21 and which contains a KpnI site, and the downstream primer 5′-CAGGAATTCTACGAATGCTATTACACTCGAGGATTGAC-3′, which anneals to bp 557 to 540 and which contains an EcoRI restriction site. The modified IFN gene was then subcloned into an FRM expression vector suited for zebrafish, kindly provided by Pat Gibbs, Rosenstiel School of Marine and Atmospheric Sciences, Miami, Fla. (11), and digested with KpnI and EcoRI, thus generating the plasmid pFRMIFN.
Mx promoter transfections.
Transfections were carried out with the cationic liposome reagent TransFast (Promega) according to the manufacturer's instructions. Briefly, 1.0 μg of DNA was added to serum-free ZF4 medium, after which 2.0 μg of TransFast was added, and the resulting mixture was vortexed. The DNA-liposome complex was added to cells, and cells were allowed to incubate at normal growth temperature (28°C) for 1 h, at which time 1.0 ml of ZF4 medium supplemented with 10% FBS was added to each well. The Mx promoter construct contains a region of the zebrafish Mx promoter (GenBank accession no. AF532732) linked to a luciferase reporter gene in a pGL3 series vector (Promega). One treatment group of ZF4 cells was cotransfected with zfIFN DNA and the Mx promoter construct, while another treatment group received the Mx promoter construct with a control pcDNA3 vector in place of zfIFN DNA. A third treatment group was transfected with the Mx promoter construct alone and then induced with 25 μg of poly(I)-poly(C) to serve as the positive control. A fourth treatment group was transfected with the pGL3 basic vector, containing the luciferase reporter gene but lacking the Mx promoter and then induced with 25 μg of poly(I)-poly(C)/ml. Cells were then treated with BrightGlo luciferase assay reagent (Promega) and incubated for 5 min. Luciferase activity was measured in relative light units (RLU) in a Fusion Universal microplate analyzer (Packard, Meriden, Conn.) with a 10-s read time for each well.
Quantitative PCR.
Quantitation of IFN cDNA was carried out with the fluorescent SYBR Green nucleic acid stain (7, 28). The primers 66rev and −73fwd were used to amplify a 139-bp fragment of the zfIFN gene. A standard curve was constructed by serially diluting a linearized plasmid containing the open reading frame encoding zfIFN. Zebrafish 18S ribosomal primers (PE Applied Biosystems, Foster City, Calif.) were used to normalize for the starting quantity of RNA. Reactions were performed in an iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, Calif.) according to the manufacturer's instructions. Reactions were carried out in a volume of 25 μl containing 12.5 μl of 2× SYBR Green PCR Master Mix (Qiagen), 1.0 μl of 5.0 μM primers, 10.5 μl of nuclease-free water, and 1.0 μl of cDNA. Cycling parameters were 94°C for 15 min to activate the polymerase followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Fluorescence measurements were taken at each cycle during the 55°C step. RNA levels are expressed as copy number based on the standard curve, after which these values were normalized to the corresponding 18S values to generate the relative copy number.
Antiviral assay.
ZF4 cells were seeded in 12-well plates prior to transfection. One group of cells was transfected with pFRMIFN, and one was transfected with the control vector pcDNA3. A third group of cells, representing the positive control, received 25 μg of poly(I)-poly(C) plus 1 μg of liposome, and a fourth group of cells, representing the negative control, received 1 μg of liposome only. After 16 h, medium was removed and all cells were infected with 400 μl of SHRV at a titer of 1.25 × 103 TCID50/ml. After 24 h, the antiviral assay was terminated by fixing and staining cells overnight in a solution of 1% crystal violet, 0.9% NaCl, and 40% formaldehyde to allow visualization of plaques.
Phylogenetic reconstruction.
Phylogenetic analyses were performed by using algorithms contained in PAUP (version 4.0) (1). Maximum-parsimony and evolutionary-distance methods were used to infer tree topology. In all analyses, branch swapping was by tree-bisection-reconnection with characters weighted equally (weight = 1) and gaps treated as missing data. Bootstrap analyses were performed by using the full-heuristic-search option with 100 replicates for maximum-parsimony and evolutionary-distance analyses. Sequences were aligned with Clustal W, employing the BLOSUM series matrix. The final alignment contained 212 amino acid positions, 185 of which are parsimony informative.
RESULTS
Cloning of zebrafish IFN.
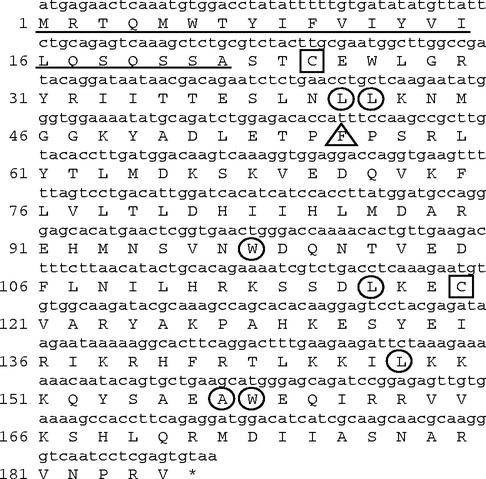
The Zebrafish Information Network EST database was searched by using BLAST with a gene for chicken IFN. Only one EST (accession no. BI708494), containing all but the very 5′ end of the zfIFN gene, was found. Upstream RACE-PCR was performed to obtain the 5′ end of the zfIFN gene by using a primer designed from this EST. PCR amplification yielded a 198-bp product that contained the 5′ end of the zfIFN gene, including the ATG and a portion of the 5′ UTR. To ensure that the amplified product was the IFN gene, 3′ RACE was performed using primers designed from the 5′ UTR, thus spanning the entire open reading frame. The amplified product yielded the full-length zfIFN gene, in addition to approximately 400 bp of 3′ UTR. This gene codes for a protein 185 amino acids in length (Fig. 1), with the first 22 amino acids representing a putative signal peptide that was characterized by analysis with the SignalP program (version 1.1; Center for Biological Sequence Analysis [http://www.cbs.dtu.dk/services/SignalP/]). Amino acid 23 represents the putative first residue of the mature peptide. The N-linked glycosylation motif NXS/T, which is typically seen in other IFNs, was not observed. In addition, zfIFN is alkaline in nature, as opposed to other IFNs, which have an acidic isoelectric point.
FIG. 1.
Nucleotide sequence of the zfIFN gene (lowercase) and deduced amino sequence of zfIFN (uppercase). The two cysteine residues are boxed, and the highly conserved residues are circled. Phe56 is marked by a triangle, and the putative signal peptide is underlined.
Induction of Mx promoter activity by zfIFN.
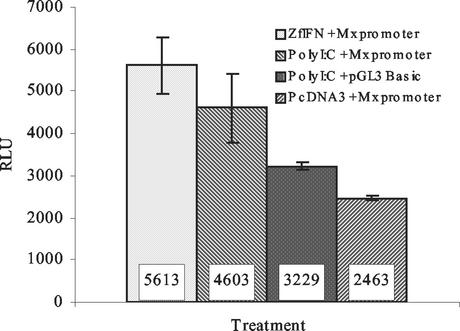
To determine whether zfIFN could induce the Mx gene, cotransfections were performed on ZF4 cells using the Mx promoter-pGL3 luciferase construct and the zfIFN gene (Fig. 2). As a positive control, the Mx promoter construct was transfected alone and then stimulated with the known IFN inducer poly(I)-poly(C). The Mx promoter construct was also cotransfected with the control pcDNA3 vector to demonstrate that random DNA does not significantly induce the Mx gene. In a previous experiment, the FRM and pcDNA3 vector backbones were cotransfected with the Mx promoter to rule out the possibility of differences in CpG content causing increased induction of luciferase (data not shown). As shown in Fig. 2, zfIFN induction of ZF4 cells averaged 5.6 × 103 RLU, while poly(I)-poly(C) induction yielded an average of 4.6 × 103 RLU. A 42% increase in RLU was seen in zfIFN-transfected cells compared to cells transfected with the Mx promoter only, and a 56% increase was seen compared to cells induced with poly(I)-poly(C) but lacking the Mx promoter. Background luminescence, as determined by reading wells containing media only, averaged 2,500 ± 100 RLU. The fact that the construct lacking the Mx promoter showed diminished luciferase activity compared to the Mx promoter-positive construct demonstrates that the Mx promoter is necessary to drive luciferase expression when induced with IFN, making the Mx promoter a useful diagnostic tool for the presence of IFN.
FIG. 2.
Induction of the Mx promoter construct by zfIFN expression. ZF4 cells were either induced with 25 μg of poly(I)-poly(C)/ml, transfected with zfIFN or an empty vector, or untreated. After 24 h, cells were lysed and luciferase activity was measured. The experiment was performed in triplicate, with each bar representing the mean of three samples. Error bars, standard deviations.
Induction of zfIFN by poly(I)-poly(C).
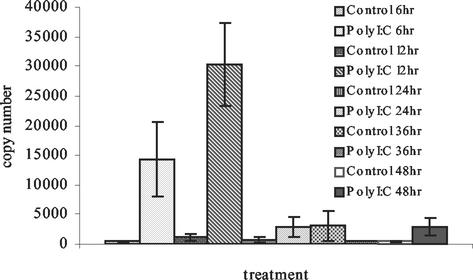
To determine the extent to which zfIFN could be induced by poly(I)-poly(C) in vitro, ZFL cells were treated with 25 μg of poly(I)-poly(C)/ml and RNA was extracted at time points of 6, 12, 24, 36, and 48 h. Quantitative PCR using the dye SYBR Green (Fig. 3) revealed an increase in poly(I)-poly(C)-induced samples of zfIFN mRNA at 6 h to 1.4 × 104 copies, versus 3.6 × 102 copies for the 6-h control group, and a further increase at 12 h to 3.0 × 104 copies, versus 1.1 × 103 copies for the 12-h control group. At 24 h, zfIFN mRNA levels in the induced sample had decreased almost to basal levels (3.0 × 102 to 3.0 × 103 copies), demonstrating the labile nature of IFN transcripts. A low level of constitutive expression was seen, consistent with previous results (13). Control reactions, in which cells were not induced with poly(I)-poly(C), yielded no significant increase in expression levels.
FIG. 3.
Quantitation of zfIFN mRNA upon stimulation with poly(I)-poly(C). ZFL cells were either induced with 25 μg of poly(I)-poly(C)/ml or uninduced, and total cellular RNA was harvested at selected time points. Each bar represents the mean of three replicates. Error bars, standard deviations.
zfIFN protects ZF4 cells from viral infection.
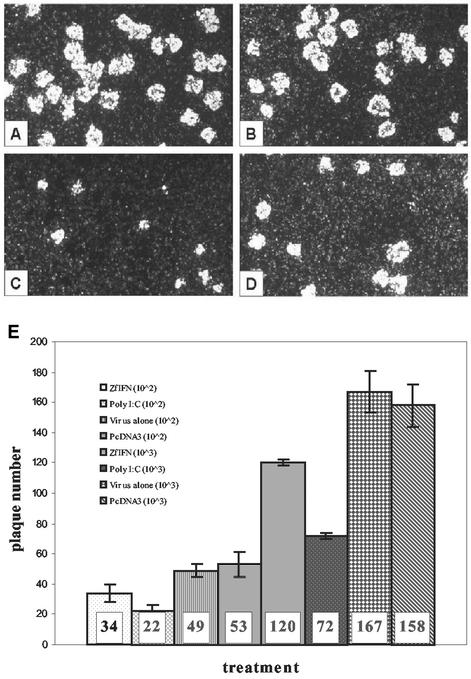
To determine whether zfIFN has antiviral properties, ZF4 cells, chosen for their ability to form plaques when infected with SHRV, were transfected with zfIFN and 16 h later were exposed to SHRV for 1 h. The assay was terminated by addition of a crystal violet fixing and staining solution, allowing visualization of plaques (Fig. 4A to D). At a titer of 102 TCID50/ml, there was a 31% reduction in plaques in zfIFN-transfected cells compared to untransfected cells and a 36% reduction compared to cells transfected with the control pcDNA3 vector. At a virus titer of 103 TCID50/ml, reductions of 28 and 24% from levels in untransfected and pcDNA3-transfected cells, respectively, were seen (Fig. 4E). The size of the plaques in zfIFN-transfected cells was not significantly reduced. Cells treated with poly(I)-poly(C) alone prior to infection with SHRV, however, demonstrated a substantial reduction in both plaque size and number. Relative to levels in untransfected and pcDNA3-transfected cells, the percentages of reduction of plaques at a virus titer of 102 TCID50/ml for poly(I)-poly(C)-treated cells were 55 and 59%, respectively; corresponding percentages of reduction at a virus titer of 103 TCID50/ml were 57 and 55%, respectively (Fig. 4E). These results demonstrate that zfIFN can confer a protective effect on ZF4 cells when administered 16 h prior to viral challenge and that this effect can be seen as a reduction in plaque number.
FIG. 4.
Plaque reduction in cells transfected with zfIFN and infected with SHRV. ZF4 cells were either induced with 25 μg of poly(I)-poly(C)/ml, transfected with zfIFN or the empty pcDNA3 vector, or untreated. All cells received equal amounts of liposome. After 16 h, all cells were challenged with SHRV for 24 h before termination by staining in crystal violet and ethanol. (A) pcDNA3 transfection; (B) liposome only; (C) poly(I)-poly(C) induction plus liposome; (D) zfIFN transfection. (E) Graph of plaque reduction numbers. The first four bars represent experiments done with a virus titer of 102 TCID50/ml; the second four bars represent those done with a virus titer of 103 TCID50/ml. The experiments were performed in triplicate. Bars represent the means of three replicates. Error bars, standard deviations.
Alignment of zfIFN.
A Clustal W alignment was performed on a variety of IFN-α/β/ω/δ/κ/τ group IFN genes from mammals, birds, and zebrafish by using the BLOSUM matrix (Fig. 5). Among the most conserved residues are Leu41, Leu42, Trp98, Leu117, Leu148, Ala157, and Trp158 (positions relative to the zfIFN start codon). Two zfIFN Cys residues, at positions 25 and 120, are conserved among all IFN sequences aligned except that of mammalian IFN-β, while two additional Cys residues are absent in zfIFN but are conserved across all other species aligned. Previous studies have shown that, in human IFN, three residues, Leu30, Arg33, and Phe36 (positions in reference to the mature peptide of human IFN), may be involved in binding to the IFN-α/β/ω/δ/κ/τ group IFN receptor (39). Leu30 and Arg33 do not appear in avian IFN or zfIFN, while Phe36 (corresponding to residue 56 in zfIFN), shown to be important for biological activity (39), is identical in zfIFN but is not conserved in chicken IFN-α or turkey or duck IFN.
FIG. 5.
Alignment of zfIFN with other vertebrate IFNs. Dashes, gaps inserted into the alignment. Shaded residues represent identities among species. Accession numbers are shown in parentheses.
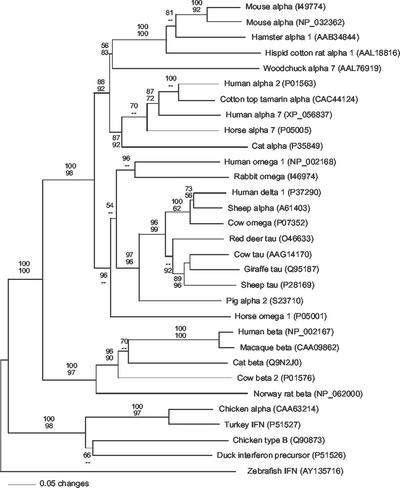
Phylogeny reconstruction.
A single best tree with a minimum evolution score of 5.625 was inferred by evolutionary-distance analysis (Fig. 6). Maximum-parsimony analysis found 18 equally parsimonious best trees of 1,574 steps. All best trees inferred by both methods were identical with respect to all nodes receiving significant bootstrap support (bootstrap proportions, >70%). Results indicate that all mammalian IFNs form a single well-supported clade, as do all avian IFNs, and that these clades exclude zfIFN. Pairwise comparisons of amino acid sequence identity and similarity indicate similar degrees of divergence of zfIFN from mammalian and avian IFN-α and -β subtypes (Table 1). zfIFN has 15 and 14% identity, over the entire sequence, to human IFN-α and -β, respectively, but 25 and 24% identity to human IFN-α and -β, respectively, over the conserved domain. When zfIFN was compared to chicken IFN-α and -β, an increase of only 4 to 5% was seen when aligning the entire sequence versus the truncated sequence, with identities of 18 and 16% to full-length chicken IFN-α and -β, respectively, and 22 and 21% to truncated chicken IFN-α and -β, respectively. A pairwise alignment was also performed on a putative fugu IFN found by searching the fugu genome database with zfIFN. This sequence, containing only 154 amino acids, yielded a 27% identity when aligned with zfIFN. Furthermore, BLAST analysis of the putative fugu IFN revealed a similarity closest to IFN-τ from sheep, with an identity of 27% over the entire fugu sequence. Inclusion of the putative fugu IFN sequence in the phylogenetic analysis showed that the putative fugu IFN forms a branch, independent of the mammalian, avian, and zebrafish branches, that is equidistant from these three lineages. However, these data are not included in Fig. 6 due to the lack of biological support for the putative fugu IFN sequence.
FIG. 6.
Phylogenetic relationships among zfIFN and representative IFN-α, -β, -δ, -ω, and -τ from mammals and birds. Phylogenetic analyses were performed using evolutionary-distance (shown) and maximum-parsimony algorithms. Bootstrap proportions (bp) are presented as the fractional percentage of 100 replicates (top, evolutionary distance; bottom maximum parsimony). Dashes, bp of <50%. Best trees recovered by both algorithms were identical with respect to all well-supported nodes (bp > 70%). The aligned sequence set included 31 sequences of 212 equally weighted positions (185 were parsimony informative). Alignment gaps are treated as missing data. GenBank accession numbers are shown in parentheses.
TABLE 1.
Pairwise alignment of representative sequences of avian and mammalian IFN with zfIFN
| IFN | % Identity/similaritya with:
|
|||||
|---|---|---|---|---|---|---|
| Human IFN-α | Human IFN-β | Mouse IFN-α | Chicken IFN-α | Chicken IFN-β | zfIFN | |
| Human IFN-α | 38/54 | 59/75 | 24/49 | 22/44 | 25/53 | |
| Human IFN-β | 32/52 | 28/49 | 28/51 | 24/47 | 24/44 | |
| Mouse IFN-α | 59/75 | 25/44 | 20/38 | 20/41 | 32/55 | |
| Chicken IFN-α | 20/40 | 21/40 | 19/37 | 55/67 | 22/43 | |
| Chicken IFN-β | 17/41 | 19/38 | 16/36 | 47/61 | 21/41 | |
| zfIFN | 15/34 | 14/36 | 16/34 | 18/38 | 16/39 | |
Numbers below the diagonal represent percentages of identity or similarity over the entire sequence, and numbers above the diagonal represent alignments in which the N-terminal 70 amino acids were removed from each sequence. The region from amino acids 70 to 170 of each group 1 IFN sequence represents a conserved domain.
DISCUSSION
In this paper we report the cloning of an IFN gene from zebrafish coding for a protein 185 amino acids in length. Several lines of evidence support the characterization of this protein as an IFN, including sequence similarity to known IFNs, ability to induce the Mx promoter, upregulation in response to poly(I)-poly(C), and the ability to reduce viral plaque formation in a zebrafish cell line. The first 22 amino acids of zfIFN, which are highly hydrophobic, represent a putative signal peptide. Although zfIFN contains eight Asn residues, none are part of the NXS/T motif needed for N-linked glycosylation, suggesting that zfIFN is not glycosylated. Since the antiviral activity of zfIFN has been demonstrated, glycosylation does not appear to be necessary for zfIFN to exert its antiviral effect. The low degree of similarity between zfIFN and mammalian and avian IFNs is also evident in zfIFN being highly basic whereas mammalian and avian IFNs are typically more acidic. The pH stability exhibited by most IFNs has made purification of these proteins more efficient than that of pH-labile proteins and has also allowed the inactivation of complement by treatment with low pH. If the basic nature of zfIFN is a characteristic shared by other fish IFNs, it may help to explain why the purification of IFN in this lineage has proven so difficult.
Real-time PCR analysis showed that, when ZFL cells were induced with poly(I)-poly(C), there was a significant increase in IFN transcripts at 6 and 12 h, followed by a rapid decrease to basal levels by 24 h, consistent with previous results (34). The copy number of IFN transcripts in both the control and poly(I)-poly(C)-treated groups ranged from several hundred to several thousand per sample due to low levels of constitutive IFN expression (13). The labile nature of IFN transcripts is evidenced by the rapid decline in copy number between 12 and 24 h in the poly(I)-poly(C)-treated group. These results also demonstrate that ZFL cells are capable of producing IFN.
To demonstrate the antiviral nature of zfIFN, ZF4 cells were transfected with a zfIFN expression plasmid and were later challenged with virus. In these cells, compared with cells transfected with control vector, a 36% reduction in plaque number was seen. Cells induced with poly(I)-poly(C) and then challenged saw the greatest reduction in plaque number, as well as a significant reduction in plaque size. This result confirms that ZF4 cells do indeed produce and respond to biologically active IFN. These results also demonstrate that zfIFN, when administered prior to viral infection, confers an antiviral effect on ZF4 cells.
One important limitation of fish cells is the relatively low transfection efficiencies afforded by these cell lines, compared to those afforded by mammalian cell lines (C. Kim, personal communication). The ZF4 cells used in these experiments have a low transfection efficiency, ranging from 1 to 5%, as determined by transfection with a plasmid encoding a constitutively expressed green fluorescent protein (data not shown). Because of this limitation, the difference between the zfIFN treatment and the pcDNA3 treatment in Fig. 2 was necessarily reduced by the inability of the ZF4 cells to take up the plasmids effectively. The reason for this is that the negative control remains constant regardless of the transfection efficiency, whereas the zfIFN-transfected cells produce more product in proportion to the number of cells transfected. Thus, the approximately twofold difference in cells cotransfected with zfIFN and the Mx promoter construct, as opposed to the control pcDNA3 vector and Mx promoter construct, would be greater if the general efficiency of transfection could be increased.
The poor transfectability of zebrafish cells can also help to explain the higher luciferase induction by pIFNFRM but the greater protection of ZF4 cells by poly(I)-poly(C). In the plaque assay, methylcellulose was added 16 h after induction with either IFNFRM or poly(I)-poly(C). Due to the low transfection efficiency of plasmid DNA, the number of cells overexpressing IFNFRM after 16 h would most likely be less than the number of cells induced by poly(I)-poly(C). The addition of methylcellulose would then effectively inhibit further dissemination of extracellular IFN to neighboring cells. Thus, a comparable group of cells induced with poly(I)-poly(C) would show greater coverage of cells with IFN after 16 h. The Mx promoter assay mixture was allowed to incubate for 24 h because the expressed protein was not IFN but rather the more stable intracellular protein luciferase, allowing for maximal accumulation of protein with minimal degradation.
Phylogenetic analyses indicate that mammalian and avian IFNs form distinct clades to the exclusion of zfIFN. Furthermore, pairwise comparisons of amino acid sequence identity and similarity indicate similar degrees of divergence between zfIFN and all mammalian and avian IFN subtypes. These results indicate that the divergence of zfIFN from an ancestral IFN occurred prior to the divergence of mammalian and avian IFN and that these divergences, in turn, preceded the divergence of extant IFN subtypes in birds and mammals. These results are consistent with the origin of all IFN in a single common ancestral IFN present in the most recent common ancestor of fish, birds, and mammals. This extends the minimum estimated time of origin of IFN from the divergence of the ancestors of birds and mammals in the Carboniferous Period, approximately 310 million years ago (mya), to the fish-tetrapod transition, estimated to have occurred at least 360 to 370 mya.
Assuming a single common ancestor of IFNs, it is possible to infer potential structural and functional features of ancestral and modern IFNs based on conserved features of the extant IFN types. For example, four Cys residues are widely conserved among IFNs. One pair (in positions 51 and 151 of zfIFN) is conserved throughout all lineages but is absent in zebrafish. A second pair (Cys 25 and Cys 120 in zfIFN) is conserved in all lineages except mammalian IFN-βs. This pattern can be explained most parsimoniously in one of two ways given the known phylogeny of the host organisms. Both pairs may have been present in the ancestral IFN, with subsequent loss of Cys51 and Cys151 in the branch leading to zebrafish and loss of Cys25 and Cys120 in the branch leading to mammalian IFN-β. Alternatively, Cys25 and Cys120 may have been present in the ancestral IFN and subsequently lost in the branch leading to mammalian IFN-β, while Cys51 and Cys151 were acquired in the branch leading to mammalian and avian IFNs. In either case, these Cys residues have been gained or lost in pairs and have been broadly conserved in IFN evolution, indicating that they are likely involved in disulfide bond formation and may be critical to correct conformation and function of IFN. Similarly, other highly or universally conserved residues and motifs are likely to be critical to IFN function and should become targets of more intense scrutiny.
It is also clear from these data that proliferation of this gene family is relatively recent and has occurred independently in birds and mammals (14). Although the known diversity of tissue-specific IFN types is greatest in mammals, followed by birds and fish, it is not yet clear whether this is a sampling artifact or an actual evolutionary trend. Experiments to determine if the family including the zebrafish IFN gene is a multigene family with distinct subtypes in bony fish are under way. In addition, the discovery of a single putative fugu IFN sequence, by searching the completed fugu genome database with zfIFN, suggests that teleost fish, such as fugu and zebrafish, contain only one form of IFN. Interestingly, the putative fugu IFN has only a 27% identity with zfIFN and does not cluster with zfIFN when included in the phylogenetic analysis.
All animals, vertebrate and invertebrate, possess an innate immune response. Adaptive immunity, complete with antigen-specific memory cells, is believed to have developed around the time when jawed and jawless vertebrates diverged, some 450 mya (27). The zebrafish, representing one of the lowest orders of jawed vertebrates, is well suited for studying the evolution of the innate immune response and the link to adaptive immunity. As more IFN sequences from fish and other lower-order vertebrates are identified and characterized, we can begin to construct a clearer picture of the evolution of this diverse gene family.
Acknowledgments
We thank Marta Gomez-Chiarri, Marc Johnson, Keith Hutchison, Paul Millard, Stephen Pelsue, and Con Sullivan for helpful discussion and comments on the manuscript. We also thank Meagan Pressley and Katie Churchill for their technical assistance.
This study was supported by National Institutes of Health grant R15 AI49237-01 and funds administered through the Maine Agricultural and Forest Experiment Station.
Footnotes
Maine Agricultural and Forest Experiment Station publication 2568.
REFERENCES
- 1.Berlocher, S. H., and D. L. Swofford. 1997. Searching for phylogenetic trees under the frequency parsimony criterion: an approximation using generalized parsimony. Syst. Biol. 46:211-215. [DOI] [PubMed] [Google Scholar]
- 2.Chawla-Sarkar, M., D. W. Leaman, and E. C. Borden. 2001. Preferential induction of apoptosis by interferon (IFN)-β compared with IFN-α2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin. Cancer Res 7:1821-1831. [PubMed] [Google Scholar]
- 3.Collet, B., and C. J. Secombes. 2001. The rainbow trout (Oncorhynchus mykiss) Mx1 promoter. Structural and functional characterization. Eur. J. Biochem. 268:1577-1584. [PubMed] [Google Scholar]
- 4.Collodi, P., C. L. Miranda, X. Zhao, D. R. Buhler, and D. W. Barnes. 1994. Induction of zebrafish (Brachydanio rerio) P450 in vivo and in cell culture. Xenobiotica 24:487-493. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty, B., D. Martin-Zanca, B. Kelder, K. Collier, T. C. Seamans, K. Hotta, and S. Pestka. 1984. Isolation and bacterial expression of a murine alpha leukocyte interferon gene. J. Interferon Res. 4:635-643. [DOI] [PubMed] [Google Scholar]
- 6.Deonarain, R., A. Alcami, M. Alexiou, M. J. Dallman, D. R. Gewert, and A. C. Porter. 2000. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J. Virol. 74:3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhar, A. K., M. M. Roux, and K. R. Klimpel. 2002. Quantitative assay for measuring the Taura syndrome virus and yellow head virus load in shrimp by real-time RT-PCR using SYBR Green chemistry. J. Virol. Methods 104:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driever, W., and Z. Rangini. 1993. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell Dev. Biol. Anim. 29A:749-754. [DOI] [PubMed] [Google Scholar]
- 9.Eaton, W. D. 1990. Antiviral activity in four species of salmonids following exposure to poly I:C. Dis. Aquat. Org. 9:193-198. [Google Scholar]
- 10.Fray, M. D., G. E. Mann, and B. Charleston. 2001. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J. Immunol. Methods 249:235-244. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs, P. D., and M. C. Schmale. 2000. GFP as a genetic marker scorable throughout the life cycle of transgenic zebra fish. Mar. Biotechnol. 2:107-125. [DOI] [PubMed] [Google Scholar]
- 12.Gresser, I. 1997. Wherefore interferon? J. Leukoc. Biol. 61:567-574. [DOI] [PubMed] [Google Scholar]
- 13.Gresser, I., C. Maury, T. Kaido, M. T. Bandu, M. G. Tovey, M. T. Maunoury, L. Fantuzzi, S. Gessani, G. Greco, and F. Belardelli. 1995. The essential role of endogenous IFN alpha/beta in the anti-metastatic action of sensitized T lymphocytes in mice injected with Friend erythroleukemia cells. Int. J. Cancer 63:726-731. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, A. L., and R. M. Roberts. 2000. Independent origin of IFN-alpha and IFN-beta in birds and mammals. J. Interferon Cytokine Res. 20:737-739. [DOI] [PubMed] [Google Scholar]
- 15.Isaacs, A., and J. Lindenmann. 1987. Virus interference. I. The interferon. J. Interferon Res. 7:429-438. [DOI] [PubMed] [Google Scholar]
- 16.Kang, D. C., R. V. Gopalkrishnan, Q. Wu, E. Jankowsky, A. M. Pyle, and P. B. Fisher. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 99:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlovich, C. A., R. M. John, L. Ramirez, D. Y. Stainier, and R. M. Myers. 1998. Characterization of the Huntington's disease (HD) gene homologue in the zebrafish Danio rerio. Gene 217:117-125. [DOI] [PubMed] [Google Scholar]
- 18.Karpusas, M., M. Nolte, C. B. Benton, W. Meier, W. N. Lipscomb, and S. Goelz. 1997. The crystal structure of human interferon beta at 2.2-Å resolution. Proc. Natl. Acad. Sci. USA 94:11813-11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, C. H., M. C. Johnson, J. D. Drennan, B. E. Simon, E. Thomann, and J. A. Leong. 2000. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol 74:7048-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn, R. M., J. Adelman, T. J. Dull, M. Gross, D. Goeddel, and A. Ullrich. 1981. DNA sequence of two closely linked human leukocyte interferon genes. Science 212:1159-1162. [DOI] [PubMed] [Google Scholar]
- 21.Lawn, R. M., M. Gross, C. M. Houck, A. E. Franke, P. V. Gray, and D. V. Goeddel. 1981. DNA sequence of a major human leukocyte interferon gene. Proc. Natl. Acad. Sci. USA 78:5435-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefevre, F., and C. La Bonnardiere. 1986. Molecular cloning and sequencing of a gene encoding biologically active porcine alpha-interferon. J. Interferon Res. 6:349-360. [DOI] [PubMed] [Google Scholar]
- 23.Leimer, U., K. Lun, H. Romig, J. Walter, J. Grunberg, M. Brand, and C. Haass. 1999. Zebrafish (Danio rerio) presenilin promotes aberrant amyloid beta-peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry 38:13602-13609. [DOI] [PubMed] [Google Scholar]
- 24.Magor, B. G., and K. E. Magor. 2001. Evolution of effectors and receptors of innate immunity. Dev. Comp. Immunol. 25:651-682. [DOI] [PubMed] [Google Scholar]
- 25.Nygaard, R., S. Husgard, A. I. Sommer, J. A. Leong, and B. Robertsen. 2000. Induction of Mx protein by interferon and double-stranded RNA in salmonid cells. Fish Shellfish Immunol. 10:435-450. [DOI] [PubMed] [Google Scholar]
- 26.Postlethwait, J. H., I. G. Woods, P. Ngo-Hazelett, Y. L. Yan, P. D. Kelly, F. Chu, H. Huang, A. Hill-Force, and W. S. Talbot. 2000. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 10:1890-1902. [DOI] [PubMed] [Google Scholar]
- 27.Rast, J. P., M. K. Anderson, S. J. Strong, C. Luer, R. T. Litman, and G. W. Litman. 1997. Alpha, beta, gamma, and delta T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity 6:1-11. [DOI] [PubMed] [Google Scholar]
- 28.Schmittgen, T. D., B. A. Zakrajsek, A. G. Mills, V. Gorn, M. J. Singer, and M. W. Reed. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285:194-204. [DOI] [PubMed] [Google Scholar]
- 29.Schultz, U., J. Kock, H. J. Schlicht, and P. Staeheli. 1995. Recombinant duck interferon: a new reagent for studying the mode of interferon action against hepatitis B virus. Virology 212:641-649. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher, B., D. Bernasconi, U. Schultz, and P. Staeheli. 1994. The chicken Mx promoter contains an ISRE motif and confers interferon inducibility to a reporter gene in chick and monkey cells. Virology 203:144-148. [DOI] [PubMed] [Google Scholar]
- 31.Sekellick, M. J., A. F. Ferrandino, D. A. Hopkins, and P. I. Marcus. 1994. Chicken interferon gene: cloning, expression, and analysis. J. Interferon Res. 14:71-79. [DOI] [PubMed] [Google Scholar]
- 32.Sen, G. C., and P. Lengyel. 1992. The interferon system. A bird's eye view of its biochemistry. J. Biol. Chem. 267:5017-5020. [PubMed] [Google Scholar]
- 33.Shaw, G. D., W. Boll, H. Taira, N. Mantei, P. Lengyel, and C. Weissmann. 1983. Structure and expression of cloned murine IFN-alpha genes. Nucleic Acids Res. 11:555-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suresh, M., K. Karaca, D. Foster, and J. M. Sharma. 1995. Molecular and functional characterization of turkey interferon. J. Virol. 69:8159-8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamai, T., S. Shirahata, T. Noguchi, N. Sato, S. Kimura, and H. Murakami. 1993. Cloning and expression of flatfish (Paralichthys olivaceus) interferon cDNA. Biochim. Biophys. Acta 1174:182-186. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi, T., and A. Takaoka. 2001. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell. Biol. 2:378-386. [DOI] [PubMed] [Google Scholar]
- 37.Trobridge, G. D., P. P. Chiou, C. H. Kim, and J. C. Leong. 1997. Induction of the Mx protein of rainbow trout Oncorhynchus mykiss in vitro and in vivo with poly I:C and infectious hematopoietic necrosis virus. Dis. Aquat. Org. 30:91-98. [Google Scholar]
- 38.Trobridge, G. D., and J. A. Leong. 1995. Characterization of a rainbow trout Mx gene. J. Interferon Cytokine Res. 15:691-702. [DOI] [PubMed] [Google Scholar]
- 39.Waine, G. J., M. J. Tymms, E. R. Brandt, B. F. Cheetham, and A. W. Linnane. 1992. Structure-function study of the region encompassing residues 26-40 of human interferon-α4: identification of residues important for antiviral and antiproliferative activities. J. Interferon Res. 12:43-48. [DOI] [PubMed] [Google Scholar]