Abstract
Understanding the function of the distinct amygdaloid nuclei in learning comprises a major challenge. In the two studies described herein, we used c-Fos immunolabeling to compare the engagement of various nuclei of the amygdala in appetitive and aversive instrumental training procedures. In the first experiment, rats that had already acquired a bar-pressing response to a partial food reinforcement were further trained to learn that an acoustic stimulus signaled either continuous food reinforcement (appetitive training) or a footshock (aversive training). The first training session of the presentation of the acoustic stimulus resulted in significant increases of c-Fos immunolabeling throughout the amygdala; however, the pattern of activation of the nuclei of the amygdala differed according to the valence of motivation. The medial part of the central amygdala (CE) responded, surprisingly, to the appetitive conditioning selectively. The second experiment was designed to extend the aversive versus appetitive conditioning to mice, trained either for place preference or place avoidance in an automated learning system (INTELLICAGE). Again, much more intense c-Fos expression was observed in the medial part of the CE after the appetitive training as compared to the aversive training. These data, obtained in two species and by means of novel experimental approaches balancing appetitive versus aversive conditioning, support the hypothesis that the central nucleus of the amygdala is particularly involved in appetitively motivated learning processes.
The amygdala belongs anatomically and functionally to a loosely defined “limbic system,” a group of brain structures involved in the regulation of memory and autonomic and endocrine responses, as well as in mechanisms of attention and cognitive evaluation of the environmental stimuli related to the acquisition of learned emotional responses (for review, see Gallagher and Chiba 1996; Davis and Whalen 2001). The amygdaloid complex consists of several cytoarchitectonically well-defined and internally distinguishable nuclei (Pitkanen et al. 2000; Sah et al. 2003). In concert with the anatomical data, there are also functional differences between various nuclei (Killcross et al. 1997; Savonenko et al. 1999) or even their subdivisions (Repa et al. 2001; Radwanska et al. 2002). However, precise classification of their functions in the context of behavior still remains a major goal of research.
Phylogenetically and morphologically one can discriminate two major subdivisions of the amygdalar complex: the dorsomedial and basolateral groups of nuclei (Johnston 1923; Humphrey 1936; McDonald 1992; Roberts 1992). To explain the functional organization of the amygdala, Wutz and Olds (1963), taking into consideration the results of the self-stimulation studies, proposed that the dorsomedial amygdala acts as a rewarding, and basolateral as a punishing system. Thus, they pointed at the importance of the valence of motivation in the functional descriptions of the amygdala. For the next decades, most of the studies focused on the involvement of the amygdala in negative emotions, but recent evidence supports a role for this structure in processing positive emotions as well (Baxter and Murray 2002; Kelley 2004). Notably, the results obtained predominantly with lesion methods suggested that the subsystems in the amygdala underlying appetitive and aversive learning were functionally similar (see Everitt et al. 2003), although the issue has not been systematically investigated.
To address the question of the importance of the valence of motivation in the functional descriptions of the amygdala, in the present study we compared activity of the various subdivisions of the amygdala during the acquisition of aversively and appetitively motivated behaviors. We used the c-Fos immunolabeling approach, which provides a mapping tool enabling a single-cell resolution and indicates that the structure is involved in processing information related to the task. This method provides the data that are of complementary value to data obtained with other methods, such as the lesion technique.
We have trained the animals in the same environments yet balanced the amount of learning reinforced by either punishment or reward. Moreover, experiments were done both in rats and mice, as well as in nonspatial and spatial paradigms. The results obtained are consistent with the hypothesis that the central nucleus is activated during rewarded instrumental learning.
Results
Experiment 1: Rats
Behavior
The first experiment was designed to provide a systematic comparison between c-Fos activation in various amygdalar nuclei in appetitively versus aversively motivated nonspatial learning in the operant chamber. The acoustic stimuli (CS) with different motivational meanings were used to modulate the on-going bar-pressing instrumental response to noncontinuous food reinforcement. In the case of aversive training, CS signaled a footshock (defensive US), whereas in appetitive procedure, CS signaled more often (contiguous) food reinforcement (alimentary US). The learning was defined as an ability to modify the bar-pressing responses only during exposure to CS. Such data were compared to the pre-CS and post-CS periods. A control group exposed to the noise that did not have any signaling value was also included.
The mean bar-pressing response rates for pre-CS, CS, and post-CS periods (30 sec before, during, and after the acoustic stimulus, respectively) in the Control, FR-1st, FR-10th, Sh-1st, and Sh-10th are depicted in Figure 1. For the Control group, the mean response rate was counted for bar presses within the periods analogous to pre-CS, CS, and post-CS periods in the other groups. No significant differences in the response rates between these periods in the Control group as well as in the NOISE alone group were revealed by one-way ANOVAs (F < 1).
Figure 1.
Mean number of instrumental responses emitted during periods of acquisition (A) and modulation of bar-pressing responses by the acoustic stimulus signaling either food reinforcement (B) or footshock (C). Bars denote response rates that were recorded in consecutive 30-sec periods: immediately before (white bars), during (black bars), and just after the stimulus (hatched bars). For the Control group, the gray bar shows the mean number of responses during three analogous periods of 30 sec. Error bars represent SEM; **P < 0.01; ***P < 0.001. (FR-1st) First day of training with the discriminative stimulus signaling continuous food reinforcement; (FR-10th) last day of training with the discriminative stimulus signaling continuous food reinforcement; (Sh-1st) first day of training with the acoustic stimulus signaling the footshock; (Sh-10th) last day of the training with the acoustic stimulus signaling the footshock.
The introduction of the defensive stimulus (the Sh-1st group) resulted in a significant suppression of the bar-pressing during CS and post-CS periods with respect to pre-CS periods. The analysis with 2 (group) × 3 (periods) ANOVA for repeated measures of the last factor showed significant group (F1,78 = 123.50, P < 0.0001) and period effects (F2,156 = 4.22, P < 0.05), without significant interaction of main factors. Additional two one-way ANOVAs for periods, independently performed for Sh-1st and FR-1st groups, yielded a significant period effect only in the Sh-1st group (F2,78 = 6.58, P < 0.01). Further post hoc Duncan tests revealed significant differences between pre-CS and CS periods (P < 0.01), as well as between pre- and post-CS periods (P < 0.01).
The mean bar-pressing response rates in the overtrained rats show that the animals had acquired the conditioned behaviors very effectively, either by increasing the instrumental response (in the FR-10th group) or by decreasing it markedly (in the Sh-10th group) when compared to the 30-sec periods immediately preceding or following the stimulus presentation. It was confirmed by a 2 (group) × 3 (periods) ANOVA for repeated measures of the last factor. This revealed effects of groups (F1,46 = 8.18, P < 0.01), periods (F2,92 = 7.55, P < 0.001), and significant interaction of main factors (F2,92 = 124.22, P < 0.0001). Further one-way ANOVA for periods showed a period effect in the FR-10th group (F2,46 = 124.23, P < 0.0001), and the post hoc Duncan tests showed significant differences between pre-CS and CS periods (P < 0.0001), as well as CS and post-CS periods (P < 0.001). The analogous one-way ANOVA for the Sh-10th group confirmed a period effect (F2,46 = 30.81, P < 0.0001), and further post hoc Duncan tests showed significant differences between pre-CS and CS periods (P < 0.001), as well as between CS and post-CS periods (P < 0.0001).
c-Fos expression in the amygdaloid nuclei: Rats
The level of c-Fos expression in the amygdala was enhanced by both aversively and appetitively motivated behaviors; however, the pattern of activation of the nuclei of the amygdala differed according to the valence of motivation (the results of quantitative analysis of c-Fos expression are given in Table 1 and the representative amygdala sections are shown in Fig. 2). In comparison to the level of c-Fos expression observed in the Control and the NOISE-alone groups, we found that the level of c-Fos expression was increased in the medial part of the central nucleus only by the appetitively motivated behavior. On the other hand, the lateral part of the CE was activated at the same level in the NOISE-alone, FR-1st, and Sh-1st groups (see Fig. 3). In contrast, the dorsal division of the lateral nucleus was activated regardless of the valence of motivation (appetitive or aversive). The level of c-Fos expression in the ventral part of the lateral nucleus was augmented by the aversively motivated behavior. Differences were also seen within the medial (ME) and cortical (CO) nuclei. The level of c-Fos expression following aversively motivated behavior was increased in the ME, whereas appetitively motivated behavior evoked the elevated c-Fos expression in the CO. We did not observe the elevated level of c-Fos expression in the basal nucleus in both experimental groups.
Table 1.
c-Fos expression in the amygdala in Experiment 1: rats
Results of one-way ANOVAs and post hoc Duncan tests. c-Fos levels are shown as numbers of immunopositive cell nuclei per area of each nucleus of the amygdala expressed in mm2 (± standard error of the mean, SEM). Significance levels (Duncan tests) refer to differences between Control and FR-1st or Sh-1st or NOISE-alone (*P < 0.05, **P < 0.01, ***P < 0.001); between NOISE-alone and FR-1st or Sh-1st (^P < 0.05, ^^P < 0.01, ^^^P < 0.001), as well as between FR-1st and FR-10th and between Sh-1st and Sh-10th (#P < 0.05, ##P < 0.01, ###P < 0.001). The level of c-Fos expression was significantly lower in the FD group in comparison to all other groups (significance level at least P < 0.05). (B) Basal nucleus; (CEl) central nucleus, lateral part; (CEm) central nucleus, medial part; (CO) cortical nuclei; (ME) medial nucleus; (Ld) lateral nucleus, dorsal part; (Lv) lateral nucleus, ventral part. FD animals were food-deprived and sacrificed directly from their home cages; Control animals after the last session of instrumental bar-pressing; NOISE-alone animals after the first day of the training with the noise that did not have any signaling value; FR-1st animals after the first day of the training with the discriminative stimulus signaling continuous food reinforcement; Sh-1st animals after the first day of the training with the acoustic stimulus signaling the footshock; FR-10th animals after the last day of the training with the discriminative stimulus signaling continuous food reinforcement; and Sh-10th animals after the last day of the training with the acoustic stimulus signaling the footshock.
Figure 2.
c-Fos expression in the subnuclei of the amygdala after the behavioral training. (A) The Nissl-stained sections show demarcations between the amygdalar nuclei and subnuclei in rat and mouse. Square areas on the sections indicate the part of the amygdala depicted in panel B. (B) c-Fos immunoreactivity in the Ld and CEm of rats and mice under changed motivational conditions. Note the distinct labeling of the medial part of the CE in rats following training of the appetitively motivated behaviors (FR-1st) yet without labeling after training of the aversively motivated behaviors (Sh-1st) and distinct c-Fos expression in the medial part of the CE of mice during early phases of rewarded place preference learning (P-Pref) but not in mice learning a place avoidance task (P-Av). (CEl) Central nucleus, lateral part; (CEm) central nucleus, medial part; (Ld) lateral nucleus, dorsal part; (Lv) lateral nucleus, ventral part; (B) basal nucleus; (ME) medial nucleus; (CO) cortical nuclei of the amygdala.
Figure 3.
Relative increase of c-Fos-labeled cells as compared to respective controls in the medial (CEm) and lateral (CEl) parts of the central amygdala during appetitively and aversively motivated learning in rats (A) and mice (B). Note that the c-Fos expression was clearly enhanced only by the appetitively motivated behavior in the medial part of the CE. Error bars represent SEM; significance level: *P < 0.05; **P < 0.01; ***P < 0.001. For description of the groups, see Figures 5 and 6.
Experiment 2: Mice
Behavior
In the second experiment, mice were exposed to a place preference and place avoidance training in the INTELLICAGE system in order to balance appetitive and aversive conditions. This was achieved by a novel automated test system (INTELLICAGE) permitting us to assess both spatial and operant behavior (see Galsworthy et al. 2005). In the place preference test, the mice were supposed to associate the sweetened water with a specific corner within the large cage, whereas in the aversive training, they were learning to avoid a corner that greeted them with an air-puff.
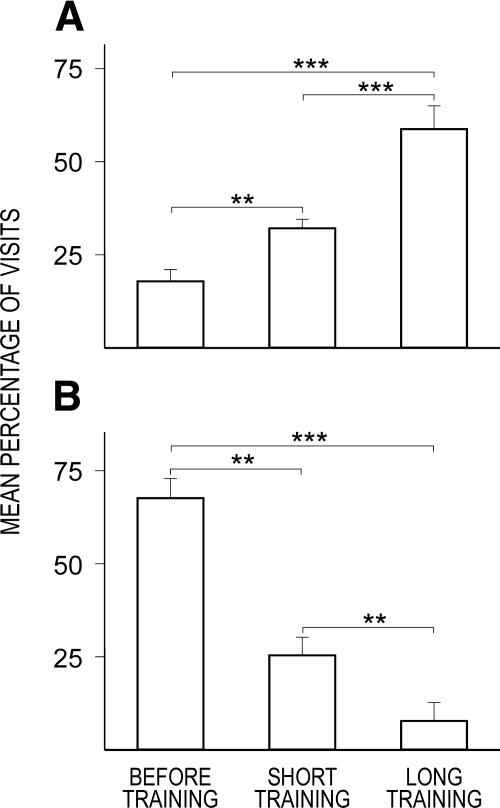
The trained animals rapidly acquired the place preference response (see Fig. 4) as revealed by one-way ANOVA for periods (F2,22 = 26.10, P < 0.0001). Further post hoc Duncan tests revealed that the percentage of corner visits increased after short training (P < 0.01) and after long training (P < 0.0001) with respect to the period before training. Moreover, the percentage of visits after long training was higher than after short training (P < 0.001). Similarly, in the place avoidance group, the fast acquisition of response was also seen. It was confirmed by one-way ANOVA for periods (F2,22 = 25.22, P < 0.0001). Further post hoc Duncan tests revealed that the percentage of corner visits decreased after short training (P < 0.001) and after long training (P < 0.0001) with respect to the period before training. Furthermore, the percentage of visits after long training was lower than after short training (P < 0.01).
Figure 4.
Learning progress in place preference and avoidance of mice within INTELLICAGE. (A) The first bar shows the percentage of visits to the least preferred corner (chance level 25%) during the shaping period (with access to plain water only), followed by a significant increase during the first opportunity of obtaining 10% sucrose in that corner only (the second bar, P-Pref, used for c-Fos immunohistochemistry), and by strong final place preference in those mice left for 3 d in INTELLICAGES (the third bar, P-Pref-long). (B) The first bar shows the spontaneous corner preference as measured during the shaping period (with access to plain water only, chance level 25%), the second bar a significant avoidance of that corner upon receiving air-puffs when entering (P-Av; used for c-Fos immunohistochemistry), and the third bar a strong final place avoidance after 3 d, indicating improved performance (P-Av-long). Error bars represent SEM; significance levels: **P < 0.01; ***P < 0.001.
c-Fos expression in the amygdaloid nuclei: Mice
In the second experiment, the analysis focused on three nuclei, namely, the central nucleus (CE), the dorsal part of the lateral nucleus (Ld), and the basal nucleus (B), in which the differences observed in the first experiment were the most visible. In the CE, we examined the level of c-Fos expression separately in the lateral and medial parts. The results of quantitative analysis of c-Fos expression are given in Table 2, and the representative amygdala sections are shown in Figure 2.
Table 2.
c-Fos expression in the amygdala in Experiment 2: mice
Results of one-way ANOVAs and post hoc Duncan tests. c-Fos levels are shown as numbers of immunopositive cell nuclei per area of each nucleus of the amygdala expressed in mm2 (± standard error of the mean, SEM). Significance levels (Duncan tests) refer to differences between P-Pref and Control-P-Pref groups, P-Av and Control-P-Av groups (*P < 0.05, **P < 0.01), as well as between P-Pref and P-Av groups (#P < 0.001). (B) Basal nucleus; (CEl) central nucleus, lateral part; (CEm) central nucleus, medial part; (Ld) lateral nucleus, dorsal part. (P-Pref) Place preference group comprising mice receiving sweetened water in one corner; (P-Av) place avoidance group comprising mice receiving air-puffs in one corner; (control groups) consisted of mice that were in the same cages as the experimental animals, but obtained sweetened water in all four corners (Control-P-Pref) or received no air-puffs (Control-P-Av), respectively.
In the medial part of the CE, c-Fos expression was clearly enhanced by the appetitively motivated behavior only. In contrast to the medial part, in the lateral part of the CE, we observed the increased c-Fos expression neither in the appetitively nor in the aversively motivated animals (Fig. 3). The level of c-Fos expression in the Ld was elevated in both experimental groups, regardless of the appetitive or aversive motivation. In the B, some increase in c-Fos expression was observed after both kinds of training, albeit reaching statistical significance only after the appetitively motivated one.
Discussion
The major finding of both experiments described herein is that in both, rats and mice, activation of the central amygdala, as measured by increased c-Fos expression, occurs only following appetitive but not aversive instrumental conditioning.
In the first experiment, we describe a novel behavioral training procedure in which the animals that already acquired a bar-pressing response to a discontinuous (partial) food reinforcement are further trained to learn that an acoustic stimulus signals either continuous food reinforcement (appetitive training) or a footshock (aversive training). Our data show that the animals learned the response very effectively, as indicated by a marked increase of the bar-pressing response when an “appetitive” acoustic stimulus was delivered and a dramatic decrease of the response under the influence of the “aversive” acoustic stimulus. Importantly, no overall increase in the responses in the former condition or decrease in the latter was visible during the periods with no acoustic stimulus present, pointing to the specificity of the acquired associations.
The aim of the behavioral procedure was to distinguish between appetitive and aversive motivations. However, it should be noted that according to the Dickinson and Balleine (1994) dual-process theory of instrumental motivation, the stimuli used in our study might exert a complex motivational influence, involving both Pavlovian and instrumental elements of conditioning.5 Hence, in order to avoid confusion of these elements and to test the generality of c-Fos expression in appetitive and aversive training, we designed the second experiment. By using the INTELLICAGE system, we were able to balance the conditions of the purely instrumental appetitive and aversive training. In order to make our conclusions more general, we also used a different species of animals, that is, mice. In the place preference training, the mice were supposed to associate the sweetened water with a specific corner within the cage (appetitive motivation), whereas in the aversive training they were learning to avoid a corner where the air-puffs were applied (aversive motivation). As in the first experiment, in the second one, all animals learned the required tasks very effectively.
These behavioral paradigms were used to map the patterns of c-Fos expression in the amygdala. We have found that the presentation of either the appetitively or the aversively paired acoustic stimuli to rats resulted in significant increases in c-Fos immunolabeling following the first training session. However, the pattern of activation of the nuclei of the amygdala differed according to the valence of motivation. The central amygdala (CE) responded selectively to the appetitive conditioning. A very similar pattern of c-Fos expression was observed in the mice. Specifically, in the CE, c-Fos expression was clearly enhanced by the appetitively motivated behavior only.
The CE was previously proposed to mediate stimulus-response representations in Pavlovian appetitive conditioning (Gallagher et al. 1990; Holland and Gallagher 1993a, b; Parkinson et al. 2000; Cardinal et al. 2002) and was suggested to regulate the processing of cues when a predictive relationship between events was first noticed or just altered (Gallagher and Holland 1994). Moreover, it was demonstrated that the CE lesions influenced the Pavlovian-to-instrumental transfer (Hall et al. 2001; Holland and Gallagher 2003). In addition, Killcross et al. (1997) suggested a specific role of the CE in Pavlovian conditioning, but not in the instrumental one (where the basolateral amygdala is, presumably, specifically involved). Those results were consistent with the earlier studies, which showed a significant role of the CE in the classical fear conditioning (Maren and Fanselow 1996; for review, see Rogan and LeDoux 1996; Maren 2003). However, the recent work of Koo et al. (2004), who used fiber-sparing neurotoxic lesions, showed that expression of conditioned fear involves mainly projections that course through the CE en route to downstream fear response structures rather than the CE itself. Furthermore, the lack of involvement of the CE in the instrumental conditioning (aversively motivated) was observed by Duncan et al. (1996) and Savonenko et al. (1999), who saw no c-Fos expression in the CE during the acquisition of an active avoidance response. Similarly, in the place avoidance paradigm, Frenois et al. (2005) have recently shown that re-exposure of rats to a withdrawal-paired environment caused decreased c-Fos responses in the CE but increased responses in the BLA.
Notably, all of the above experiments relied on aversive motivation. Our approach shows for the first time the CE activation in instrumental conditioning with appetitive motivation. Thus, it seems possible that the plastic changes occur in different neuronal circuits, depending on the valence of motivation (appetitive vs. aversive). Incidentally, in the light of our results, one can interpret similarly the results obtained by Hess et al. (1997), who observed an increase in c-Fos expression in the CE during nose-poke for water reward learning and the lack of such an increase in rats acquiring an odor discrimination task, in which responses to odor were punished. Collectively, these and our results challenge the notion about functional parallelism between the subsystems in the amygdala in appetitive and aversive learning (see Everitt et al. 2003).
Interestingly, we observed increased c-Fos expression after appetitively motivated learning only in the medial part of the CE. This is consistent with the results of Lee et al. (2005), who showed the involvement of the medial part of the CE in classical appetitive conditioning. Moreover, we did not observe increased c-Fos expression either in the medial or in the lateral part of the CE after aversively motivated training in both rats and mice. However, the level of c-Fos expression in the lateral part of the CE was enhanced by the acoustic stimulus that did not have any signaling value. This observation seems to be consistent with the postulated role of the CE in attentional processes (Gallagher and Holland 1994).
It should also be noted that the functional significance of c-Fos expression observed in this study is not fully understood. c-Fos immunocytochemistry provides a mapping tool enabling a single-cell resolution, allowing us to recognize the involvement of separate brain regions and even their subdivisions in specific behavioral responses. Since c-Fos is a product of an immediate–early gene and a component of a transcription factor (AP-1), it may orchestrate expression of several other genes, being a marker of neuronal plasticity (for details, see Kaczmarek 1993, 2002).
Our results point to a specific role of c-Fos-related neuronal activity and, presumably, plasticity in the medial part of the central nucleus of the amygdala during acquisition of an instrumental, appetitive response. In particular, comparison of the results obtained in the NOISE-alone and FR-1st groups show that this activity is due to appetitive learning and not merely to sensory stimulation. Since conditioned taste aversion learning was shown to be undisturbed by the CE lesions (Touzani et al. 1997; Morris et al. 1999), the effects that were observed in our study seemed to be motivation-dependent rather than associated with food itself. The increased c-Fos expression seems to be also associated with learning itself because it was much more robust in the place preference group in comparison to its controls, in which the sweetened water appeared as a novel stimulus.
The role of the medial and cortical nuclei in learning, especially in appetitive conditioning, has received limited attention so far. Furthermore, the results of lesion studies were often contradictory (see Oakes and Coover 1997; Holahan and White 2002). In our study, the aversively motivated training evoked an increase in c-Fos expression in the ME. This seems to be consistent with the results of earlier studies (Duncan et al. 1996; Milanovic et al. 1998; Radulovic et al. 1998; Rosen et al. 1998; Savonenko et al. 1999; Schettino and Otto 2001; Silveira et al. 2001). On the other hand, the appetitively motivated training evoked augmented c-Fos expression in the CO. To the best of our knowledge, no data on c-Fos expression evoked by appetitively motivated behaviors in these nuclei have been reported as yet.
It is known that the performance of well-trained behavior does not evoke c-Fos expression and only a situation that is novel for an animal is accompanied by increased c-Fos expression, suggesting that learning is the critical component in c-Fos induction (see Nikolaev et al. 1992 and Kaczmarek 2002 for extensive discussion on this issue). In this respect, the absence of increase in the c-Fos expression after long training, found in our study, confirms these earlier findings. At the same time, the lack of increased c-Fos expression in the Sh-10th group shows that the shock itself does not evoke c-Fos expression. Note that this conclusion could not have been drawn by studying a control group obtaining shocks unpaired with signaling stimuli, since there would be a strong contextual conditioning.
In conclusion, novel experimental approaches aimed at distinguishing the effects of appetitive versus aversive conditioning on the amygdala allowed us to show a preferential involvement of the central amygdala in appetitive learning as was measured with c-Fos expression. Moreover, it appears that the ME and the CO are involved in the development of the aversively and appetitively motivated conditioned responses, respectively. This result may suggest that the ME and CO provide parallel and, at least partially, independent outputs from the amygdala. This notion is supported by the results of Dayas et al. (1999), who provided evidence that the ME rather than the CE is critical for hypothalamic neuroendocrine cell responses during an emotional response generated by the amygdala, and by the differential organization of neuronal wiring of these nuclei (Pitkanen et al. 2000). The hypothesis that the central nucleus is activated during rewarded but not punished learning certainly requires further studies. However, our results stress that the valence of motivation should be considered as a potentially important factor in the functional descriptions of the amygdala.
Materials and Methods
Experiment 1: Rats
Subjects
Experimental subjects were 26 adult, experimentally naive male Long-Evans rats (250–300 g at the beginning of the experiment), supplied by the Nencki Institute Animal House. Animals were housed individually under a natural light–dark cycle, with water provided ad libitum. Prior to behavioral training, the rats were food-deprived for 10 d and their body weights were gradually reduced to 85% of the free-feeding weight (the low weight levels were maintained in the course of the experiment). Rats were trained once a day at the same time (10 a.m.–2 p.m.) and were fed immediately after the end of the session. Experiments were carried out in accordance with the Polish Act on Animal Welfare, after obtaining specific permission from the First Warsaw Ethical Committee on Animal Research. All efforts were made to minimize the number of animals and their suffering.
Apparatus
Behavioral training was conducted in eight identical operant chambers. Each chamber (23.0 cm width × 29.5 cm depth × 19.5 cm height) was housed in a sound- and light-attenuating box. On the right wall of each chamber was a bar (5.0 cm length × 1.8 cm width × 1.0 cm height) centered 9.5 cm above the grid floor and 12.5 cm from the back of the chamber. A food dispenser delivering 0.045 g of Noyes food pellets was located just below the bar. Footshocks were delivered via the grid floor (0.3-cm-diameter bars at 1.0-cm intervals) from a shock scrambler. A 70-dB wide-band noise (re: 20 μN/m2) was emitted from a speaker mounted below a food supplier under the grid floor. The background noise was 46 ± 2 dB. A light source centered on the top of the back chamber wall provided illumination of 205 ± 5 lux (as measured in the immediate vicinity of the bar). The chambers were equipped with a computer system (located in an adjoining room) monitoring the behavior of the rats.
Procedure and group treatment
The experimental schedule is shown in Figure 5. After 10 d of food deprivation, the brains from three animals were taken for c-Fos immunocytochemistry (FD; n = 3). In the rest of the animals, an alimentary bar-pressing training was conducted over 6 d. A shaping procedure was performed on the initial day (day 0) of the training. During a first 20-min period, rats received 40 “free” food pellets (pressing the bar was not required to obtain a pellet) according to a variable time interval (VI) of 1 min (intervals were randomized from 6 to 176 sec). This procedure attracted the attention of the rats and produced instrumental responses. Then, for ∼20–70 min, each bar-pressing response was reinforced by one food pellet. The session was finished after achieving 80 instrumental responses. On days 1–5, all animals were placed in their respective chambers for 1-h sessions. In order to elicit and maintain stable appetitive instrumental responding over this period, bar presses were reinforced according to a variable time interval of 2.5 min (time intervals were randomized from 13 to 360 sec). After the last day of instrumental bar-pressing training, the brains from three animals were taken for c-Fos immunocytochemistry (Control; n = 3).
Figure 5.
Scheme of the training procedure in rats and time point of c-Fos immunohistochemistry. (Control) Animals after the last session of instrumental bar-pressing; (FR-1st) after the first day of the training with the discriminative stimulus signaling continuous food reinforcement; (Sh-1st) after the first day of the training with the acoustic stimulus signaling the footshock; (NOISE alone) after the first day of the training with the acoustic stimulus that did not have any signaling value; (FR-10th) after the last day of the training with the discriminative stimulus signaling continuous food reinforcement; (Sh-10th) after the last day of the training with the acoustic stimulus signaling the footshock.
The remaining animals continued the 1-h training but with an additional acoustic stimulus for the following 10 d (6th–15th sessions). As in the previous days, bar presses were reinforced according to a VI of 2.5 min. In addition, eight 30-sec acoustic stimuli in the form of 70-dB white noise were now presented. The first stimulus occurred 12.5 min after the beginning of the session, and the following stimuli were given in regular intervals of 5 min.
This acoustic stimulus signaled motivationally different events. In the groups with Food Reinforcement (FR-1st and FR-10th animals), it signaled that every bar-pressing would deliver one pellet during the next 30 sec (fixed ratio FR1), and served as discriminative stimulus. In the groups assigned to shock punishment (Sh-1st and Sh-10th), the acoustic stimulus signaled a footshock of 1.6 mA, lasting for the last 1 sec of the 30-sec noise. In the NOISE-alone group, the acoustic stimulus did not have any signaling value. The number of bar presses emitted in multiple consecutive 30-sec periods in the fifth, sixth, and fifteenth sessions of the training was used as the main measure of behavior. The performance of the animals was characterized by mean rates of instrumental responses in three 30-sec periods: immediately before the acoustic stimulus (pre-CS), during the action of the stimulus (CS), and after the termination of the stimulus (post-CS). The brains for c-Fos immunocytochemistry were taken after the first day (FR-1st; n = 5) and the tenth day (FR-10th; n = 3) of the training with the discriminative stimulus signaling continuous food reinforcement; after the first day (Sh-1st; n = 5) and the tenth day (Sh-10th; n = 3) of the training with the acoustic stimulus signaling the footshock as well as after the first day of the training with the acoustic stimulus that did not have any signaling value (NOISE-alone, n = 4). All animals were sacrificed 90 min after the onset of the training session.
Experiment 2: Mice
Subjects
Twenty-eight experimentally naive adult (8 wk at the beginning of the experiment) female C57/BL6 mice were tested. A week before the experiment, the animals were housed in groups of four or five with food and water provided ad libitum. They were then anaesthetized by inhalation of isoflurane vapor and subcutaneously injected with glass-covered microtransponders (11.5 mm length, 2.2 mm diameter; Trovan, ID-100) and returned to their cages for 48 h. This passive transponder emits a unique animal identification code when activated by a magnetic field. All animals recovered from the anesthetic within minutes of exposure and were later checked with a handheld scanner for retention of transponders before introduction to the INTELLICAGES. Experiments were approved by the Veterinary office of the Canton of Zurich.
Apparatus
INTELLICAGE is a novel automated learning apparatus assessing spontaneous and learning behavior of group-caged mice (NewBehavior AG; http://www.newbehavior.com). The system fits into a large standard rat cage (Techniplast 2000) measuring 55 × 37.5 cm at the base, 58 × 40 cm at the top, with a height of 20.5 cm (for a detailed description, see Galsworthy et al. 2005). A cover plate holds four operant learning chambers that fit into the corners of the housing cage, covering a triangular 15 cm × 15 cm × 21 cm area of floor space each. Access into the chamber is provided via a tubular antenna reading the transponder codes (50 mm outer and 30 mm inner diameter). This design restricts access to the learning chamber for a single mouse only. The chamber, equipped with a proximity sensor, contains two openings of 13 mm diameter permitting access to the nipples of drinking bottles. These openings are crossed by photobeams recording nose-pokes of the mice. Access to the tubes can be barred by small motorized doors. Aversive stimulation can be delivered in forms of air-puffs directed to the head of the mouse through tubing controlled by electric valves. In addition, each cage contained a sleeping shelter in the center on which the animals could climb to reach the food (ad libitum). The whole set-up of three INTELLICAGES was controlled by a microcomputer recognizing visits, nose-pokes, and tube-lickings of individual mice, and delivering reward (by opening the access to water after a nose-poke) or punishment (by entering the test chamber) according to preprogrammed schedules depending on the assignment of the mice to different test groups within the same cage. All cages were located in a room of the animal facilities. The system ran continually for several days, behavioral activity of the mice being monitored from the experimenter office via Intranet.
Procedure and group treatment
Appetitive and aversive training was performed in three cages each during 8 d. Two 8-d sessions were carried out: (1) the first one with the place preference group and their controls and (2) the second one with the place avoidance group and their controls, with only a 4-d time shift in between (see Fig. 6). Every cage contained four or five mice assigned to the different treatment groups (see below). Sessions took place under a reversed light–dark cycle (dark: 7 a.m. to 7 p.m.; light 7 p.m. to 7 a.m.; a standard schedule in the animal rooms). A session started with a 48-h adaptation period to the cage during which the mice learned to open the gates barring access to plain water from both openings by means of nose-pokes. For the next 3 d, the animals were temporarily deprived of water. They had access to water for 2 h per day, at the same time of the active phase every day. This procedure evoked intense consummatory activity during a limited time span. On the next day at the same time, the mice were assigned to different treatment groups within the cages.
Figure 6.
Training procedure for appetitive and aversive spatial conditioning of the mice whose brains were used for immunocytochemistry. After adaptation and response shaping [(wd) water deprivation], during which all mice received plain water only, the mice of the reward group were allowed, during maximally 2 h, to consume 10% sucrose either in every corner [(Control-P-Pref) with no need to develop a place preference], or only in the corner known to be the least preferred during the shaping period [(P-Pref) place preference]. Individual access to the drinking nipples was controlled by INTELLICAGE computer according to the assignment to a treatment group. In the aversion group, mice were either allowed to drink plain water from every corner [(Control-P-Av) with no need to develop place avoidance or change habits], while the others now received air-puffs upon entering the previously most preferred corner [(P-Av) place avoidance]. As in the reward group, an INTELLICAGE always contained treated and control mice. The brains for immunocytochemistry were taken 85–115 min from the beginning of differentially motivated training.
One group was designed as the “place preference group” (P-Pref). They received sweetened water (10% sucrose) in the corner least preferred during the previous drinking sessions. In the “place avoidance group” (P-Av), the mice now received an air-puff (1 bar) when entering the corner that was the most preferred during the previous drinking session. Control groups consisted of mice that were in the same cages as the experimental mice, but obtained sweetened water in all four corners (Control-P-Pref) or received no air-puffs but only plain water (Control-P-Av), respectively. In the same cages, there were also mice undergoing a prolonged place preference or place avoidance training (P-Pref long and P-Av long, respectively) in order to determine the complete time course of the place conditioning (for 3 d). The P-Pref and P-Av groups consisted of five animals each, the control groups of four animals each, and the P-Pref long and P-Av long groups of five animals each. The whole experimental schedule in the second and third cage for each type of training was shifted by 1 and 2 h, respectively, with respect to the schedule in the first cages in order to allow timely removal of animals for immunohistochemistry.
The brains for immunocytochemistry were taken from P-Pref, P-Av, Control-P-Pref, and Control-P-Av groups on the first day of place conditioning, from three mice from each cage. The mice were removed 85–115 min after the start of the training. In this period, c-Fos expression is stable. Care was taken to remove the mice in an order balanced for treatment groups in order to minimize possible stress effects of the mice remaining in the cage induced by picking up their cage mates.
Immunocytochemistry
Rats were anesthetized with an overdose of chloral hydrate and mice with Nembutal (Abbott Lab). Then, the animals were perfused intracardially with ice-cold saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and stored in the same fixative for 24 h at 4°C, and subsequently immersed in 30% sucrose with 0.01% sodium azide at 4°C. Then the brains were slowly and gradually frozen and sectioned at 40 μm on a cryostat. The coronal brain sections containing amygdaloid nuclei (1.0–3.3 mm posterior to bregma in rats and 1.46–1.70 mm posterior to bregma in mice) were collected (Paxinos and Watson 1982; Paxinos and Franklin 2004). The immunocytochemical staining was performed on free-floating sections. The sections were washed three times in phosphate-buffered saline (PBS at pH 7.4; Sigma), incubated for 10 min in 0.003% H2O2 in PBS, washed twice in PBS, and incubated with a polyclonal antibody (anti-c-Fos, 1:1000; Santa Cruz Biotechnology no. sc-52) in PBS and normal goat serum (3%; Vector) for 48 h at 4°C. The sections were then washed three times in PBS with 0.3% Triton X-100 (Sigma), incubated with goat anti-rabbit biotinylated secondary antibody (1:1000; Vector) in PBS/Triton and normal goat serum (3%) for 2 h at room temperature, washed three times in PBS/Triton, incubated with avidin–biotin complex (1:1000 in PBS/Triton; Vector ABC kit) for 1 h at room temperature, and washed three times in PBS. The immunostaining reaction was developed using the oxidase-diaminobenzidine-nickel method. The sections were incubated in distilled water with diaminobenzidine (DAB; Sigma), 0.5 M nickel chloride, and peroxidase (Sigma) for 5 min. The staining reaction was stopped by three washes with PBS. The reaction resulted in a dark brown stain within the nuclei of c-Fos immunoreactive neurons. The sections were mounted on slides, air dried, dehydrated in ethanol solutions and xylene, and coverslipped with Entellan (Merck).
The measure of c-Fos immunopositivity was expressed as density, determined in the following manner. For each brain section, the number of c-Fos immunopositive nuclei in a given amygdalar structure was counted and divided by the area occupied by this structure (in mm2). The borders of the subnuclei were determined with the use of the Nissl-stained adjacent section (see Fig. 2). Image analysis was done with the aid of an image analysis computer program (Image J) on four sections per animal from rat brains or two sections per animal from mouse brains.
Data analysis
Behavioral data
To evaluate the within- and between-group differences in bar-pressing response rate of rats, a one-way ANOVA for repeated measures (pre-CS, CS, and post-CS periods) and a two-way mixed design ANOVA for one independent factor (group) and one dependent factor (period) were performed. To assess differences between the stages of the place preference and place avoidance trainings in mice (before training, after short training, and after long training), two one-way ANOVAs were applied, independently for P-Pref and P-Av groups. The measure of learning was the number of visits in the preferred or avoided corner divided by the number of all visits in all corners during the given time (expressed as percentage values). For statistical analysis the arcsin correction for percentage values was used (arcsin √n, n = percent).
Morphometry
The number of c-Fos immunopositive cell nuclei was analyzed by a one-way ANOVA, independently for each amygdalar nucleus. Further post hoc Duncan tests were conducted for more detailed comparisons of behavioral and c-Fos expression results.
Acknowledgments
This work was supported by the State Committee for Scientific Research (KBN, Poland) grant no. 6P05A01420 (to T.W.), the Swiss National Foundation for Scientific Research, and the NCCR “Neural Plasticity and Repair.”
Footnotes
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.54706
Instrumental conditioning, in which an organism learns a new motor response in order to obtain a positive outcome or avoid a negative one, allows modifying the current environment through new behaviors to produce more favorable conditions. In contrast, Pavlovian (classical) conditioning results in making associations (or pairings) between previously neutral stimulus (e.g., tone) and an unconditioned stimulus, like a footshock. It allows an animal to respond in a specific way but not to control the environment.
References
- Baxter M.G., Murray E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Lachenal G., Halkerston K.M., Rudarakanchana N., Hall J., Morrison C.H., Howes S.R., Robbins T.W., Everitt B.J. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav. Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dayas C.V., Buller K.M., Day T.A. Neuroendocrine responses to an emotional stressor: Evidence for involvement of the medial but not the central amygdala. Eur. J. Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dickinson A., Balleine B. Motivational control of goal-directed action. Anim. Learn. Behav. 1994;22:1–18. [Google Scholar]
- Duncan G.E., Knapp D.J., Breese G.R. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Cardinal R.N., Parkinson J.A., Robbins T.W. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann. NY Acad. Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Frenois F., Stinus L., Di Blasi F., Cador M., Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J. Neurosci. 2005;25:1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Chiba A.A. The amygdala and emotion. Curr. Opin. Neurobiol. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Holland P.C. The amygdala complex: Multiple roles in associative learning and attention. Proc. Natl. Acad. Sci. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Graham P.W., Holland P.C. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. J. Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsworthy M.J., Amrein I., Kuptsov P.A., Poletaeva I.I., Zinn P., Rau A., Vyssotski A., Lipp H.P. A comparison of wild-caught wood mice and bank voles in the INTELLICAGE: Assessing exploration, daily activity patterns and place learning paradigms. Behav. Brain Res. 2005;157:211–217. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Hall J., Parkinson J.A., Connor T.M., Dickinson A., Everitt B.J. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hess U.S., Gall C.M., Granger R., Lynch G. Differential patterns of c-fos mRNA expression in amygdala during successive stages of odor discrimination learning. Learn. Mem. 1997;4:262–283. doi: 10.1101/lm.4.3.262. [DOI] [PubMed] [Google Scholar]
- Holahan M.R., White N.M. Conditioned memory modulation, freezing, and avoidance as measures of amygdala-mediated conditioned fear. Neurobiol. Learn. Mem. 2002;77:250–275. doi: 10.1006/nlme.2001.4012. [DOI] [PubMed] [Google Scholar]
- Holland P.C., Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav. Neurosci. 1993a;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland P.C., Gallagher M. Effects of amygdala central nucleus lesions on blocking and unblocking. Behav. Neurosci. 1993b;107:235–245. doi: 10.1037//0735-7044.107.2.235. [DOI] [PubMed] [Google Scholar]
- Holland P.C., Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The telencephalon of the bat. The non-cortical nuclear masses and certain pertinent fiber connections. J. Comp. Neurol. 1936;65:603–711. [Google Scholar]
- Johnston J.B. Further contributions to the study of evolution of the forebrain. J. Comp. Neurol. 1923;36:143–192. [Google Scholar]
- Kaczmarek L. Molecular biology of vertebrate learning: Is c-Fos a new beginning? J. Neurosci. Res. 1993;34:377–381. doi: 10.1002/jnr.490340402. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L.2002. c-Fos in learning: Beyond the mapping of neuronal activity. In Handbook of chemical neuroanatomy. Immediate early genes and inducible transcription factors in mapping of the central nervous system function and dysfunction (eds. L. Kaczmarek and H.A. Robertson) pp. 189–216. Elsevier; Amsterdam [Google Scholar]
- Kelley A.E. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Killcross S., Robbins T.W., Everitt B.J. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Koo J.W., Han J.S., Kim J.J. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J. Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Groshek F., Petrovich G.D., Cantalini J.P., Gallagher M., Holland P.C. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J. Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann. NY Acad. Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Maren S., Fanselow M.S. The amygdala and fear conditioning: Has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- McDonald A.J.1992. Cell types and intrinsic connections of the amygdala. In The amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction (ed. J.P. Aggleton) pp. 67–96. Wiley-Liss; New York [Google Scholar]
- Milanovic S., Radulovic J., Laban O., Stiedl O., Henn F., Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Morris R., Frey S., Kasambira T., Petrides M. Ibotenic acid lesions of the basolateral, but not the central, amygdala interfere with conditioned taste aversion: Evidence from a combined behavioral and anatomical tract-tracing investigation. Behav. Neurosci. 1999;113:291–302. doi: 10.1037//0735-7044.113.2.291. [DOI] [PubMed] [Google Scholar]
- Nikolaev E., Werka T., Kaczmarek L. c-Fos protooncogene expression in rat brain after long-term training of two-way active avoidance reaction. Behav. Brain Res. 1992;48:91–94. doi: 10.1016/s0166-4328(05)80143-3. [DOI] [PubMed] [Google Scholar]
- Oakes M.E., Coover G.D. Effects of small amygdala lesions on fear, but not aggression, in the rat. Physiol. Behav. 1997;61:45–55. doi: 10.1016/s0031-9384(96)00348-4. [DOI] [PubMed] [Google Scholar]
- Parkinson J.A., Robbins T.W., Everitt B.J. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur. J. Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J.2004. The mouse brain in stereotaxic coordinates. 2nd ed. Elsevier Academic Press; San Diego. [Google Scholar]
- Paxinos G., Watson C.1982. The rat brain in stereotaxic coordinates. Elsevier Academic Press; New York. [Google Scholar]
- Pitkanen A., Jolkkonen E., Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol. (Warsz) 2000;59:1–23. [PubMed] [Google Scholar]
- Radulovic J., Kammermeier J., Spiess J. Relationship between fos production and classical fear conditioning: Effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J. Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K., Nikolaev E., Knapska E., Kaczmarek L. Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. Neuroreport. 2002;13:2241–2246. doi: 10.1097/00001756-200212030-00015. [DOI] [PubMed] [Google Scholar]
- Repa J.C., Muller J., Apergis J., Desrochers T.M., Zhou Y., LeDoux J.E. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat. Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Roberts G.W.1992. Neuropeptides: Cellular morphology, major pathways, and functional considerations. In The amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction (ed. J.P. Aggleton) pp. 115–142. Wiley-Liss; New York. [Google Scholar]
- Rogan M.T., LeDoux J.E. Emotion: Systems, cells, synaptic plasticity. Cell. 1996;85:469–475. doi: 10.1016/s0092-8674(00)81247-7. [DOI] [PubMed] [Google Scholar]
- Rosen J.B., Fanselow M.S., Young S.L., Sitcoske M., Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S., De Lopez Armentia M., Power J. The amygdaloid complex: Anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Savonenko A., Filipkowski R.K., Werka T., Zielinski K., Kaczmarek L. Defensive conditioning-related functional heterogeneity among nuclei of the rat amygdala revealed by c-Fos mapping. Neuroscience. 1999;94:723–733. doi: 10.1016/s0306-4522(99)00331-0. [DOI] [PubMed] [Google Scholar]
- Schettino L.F., Otto T. Patterns of Fos expression in the amygdala and ventral perirhinal cortex induced by training in an olfactory fear conditioning paradigm. Behav. Neurosci. 2001;115:1257–1272. doi: 10.1037//0735-7044.115.6.1257. [DOI] [PubMed] [Google Scholar]
- Silveira M.C., Zangrossi H., de Barros Viana M., Silveira R., Graeff F.G. Differential expression of Fos protein in the rat brain induced by performance of avoidance or escape in the elevated T-maze. Behav. Brain Res. 2001;126:13–21. doi: 10.1016/s0166-4328(01)00233-9. [DOI] [PubMed] [Google Scholar]
- Touzani K., Taghzouti K., Velley L. Increase of the aversive value of taste stimuli following ibotenic acid lesion of the central amygdaloid nucleus in the rat. Behav. Brain Res. 1997;88:133–142. doi: 10.1016/s0166-4328(96)02273-5. [DOI] [PubMed] [Google Scholar]
- Wutz R.H., Olds J. Amygdaloid stimulation and operant reinforcement in the rat. J. Comp. Physiol. Psychol. 1963;56:941–949. doi: 10.1037/h0042033. [DOI] [PubMed] [Google Scholar]