Abstract
Objective:
To establish a safer and technically easier retrohepatic dissection for the liver hanging maneuver with the assistance of intraoperative ultrasound (IOUS).
Summary Background Data:
The liver hanging maneuver described by Belghiti et al is an innovative suspending technique of the liver and is useful in difficult major right hepatectomies or in donor operations for living donor liver transplantation. The most important complication of this procedure is injury to the short hepatic veins and subsequent massive bleeding with an incidence of 4% to 6%.
Methods:
After the cranial dissection of the suprahepatic inferior vena cava (IVC) between the middle and left hepatic veins, a long light curved Kelly clamp is inserted from the caudal edge behind the caudate lobe and passed cranially along the anterior midline of the IVC. On the midway of the dissection, the proper hepatic vein draining the caudate lobe (PrCV) is visualized. A safe dissection path is confirmed by IOUS, identifying the position of the clamp tip, PrCV, and the caudal end of the cranial retrohepatic dissection. When IOUS shows that the clamp tip has reached the caudal end of the cranial dissection, the operator can feel the clamp tip with his/her finger and the retrohepatic dissection is completed.
Results:
From September 2003 to July 2004, 50 donor operations were performed for adult living donor liver transplantation. Retrohepatic dissection was feasible in 40 cases (80%). Of these, a US-assisted retrohepatic dissection was performed in 34 donors. PrCVs were visualized by IOUS in 48 donors (96%). The location of these PrCVs varied significantly (60°–175° from the right edge of IVC), and there were no distinct landmarks for identifying the location of PrCVs and safe dissecting course (55°–130°). IOUS found that the dissecting clamp was heading to the PrCV in 3 cases and the direction of dissection was shifted to avoid injury. No substantial bleeding or no other complication related to retrohepatic dissection was encountered in any of the cases.
Conclusions:
With the aid of IOUS, the whole course of the blind dissection between the anterior surface of the IVC and the liver could be clearly visualized. IOUS could also identify the PrCV, the most dangerous point in the retrohepatic dissection.
A safer and technically easier retrohepatic dissection for the liver hanging maneuver was established with the assistance of intraoperative ultrasound. This method visualized the whole course of the blind dissection behind the liver and identified the proper hepatic vein draining the caudate lobe, the most dangerous point in this maneuver.
To control bleeding at the deeper parenchymal plane in right hepatectomy without liver mobilization, Belghiti et al proposed a liver-hanging maneuver using a tape passed between the anterior surface of the inferior vena cava (IVC) and the liver parenchyma.1 This suspending technique is quite useful in difficult major right hepatectomies, requiring so-called anterior approach,2 or in donor operations for living donor liver transplantation, where a hepatic parenchymal transection should be performed before dividing the feeding vessels for the graft.3
The most important step for this maneuver is the dissection of the anterior plane of the IVC, a blind dissection in the retrohepatic space. The safety of the retrohepatic dissection is based on the anatomic assumption that there is a 1-cm-wide avascular space located in the midline of the anterior surface of the retrohepatic IVC.1 This assumption has been challenged by several morphometric studies demonstrating a diffuse distribution of the venous openings of the short hepatic veins.4–7 Bleeding from an injured short hepatic vein is the most important complication of this procedure with a reported incidence of 4% to 6%.1,8 Massive bleeding from the bottom of a deep and narrow surgical field makes the surgeon feel insecure and interrupts the dissection. This may be the main reason why some surgeons are reluctant to try this maneuver. This blind dissection is indeed skipped when the liver hanging maneuver is applied in a recipient operation with IVC preservation: a modified Belghiti hanging maneuver.8,9 A blind retrohepatic dissection is somewhat analogous to a blunt dissection of the esophagus and may face the same criticism as: “No operation is of any value if it cannot be taught to the average resident.”10
To avoid injury to the short hepatic veins during retrohepatic dissection, we applied intraoperative ultrasound (US) to guide dissecting forceps to the safe avascular space. In this report, we propose a US-assisted retrohepatic dissection for a safer and technically easier liver hanging maneuver, which might be acceptable to more liver surgeons.
METHODS
Surgical Techniques
The liver is exposed through an abdominal or thoracoabdominal approach using either an inverted T-shaped or J-shaped incision. Intraoperative ultrasound (IOUS) is performed using the Aloka SSD-6500 system with a mini-convex 7.5 MHz probe (Aloka Co., Tokyo, Japan). The openings of the major hepatic veins draining the liver to the IVC (left, middle, and right hepatic veins [LHV, MHV, and RHV, respectively]; middle and inferior RHVs [MRHV and IRHV, respectively]11,12) are visualized. The proper hepatic veins for the caudate lobe (PrCV), the thickest short hepatic veins draining the caudate lobe,7,13 are also usually visualized with a magnified view.
The anterior surface of the suprahepatic IVC is exposed, and the pocket-like space between the MHV and RHV is dissected approximately 3 cm in the caudal direction using a long heavy curved Kelly clamp. An IOUS probe is applied on the liver surface, and the tip of the clamp is clearly visualized as a strongly hyperechoic curved structure with a reverberation artifact running between the MHV and RHV (Fig. 1). The caudal end of this cranial retrohepatic dissection is identified as a hyperechoic space after removal of the clamp.
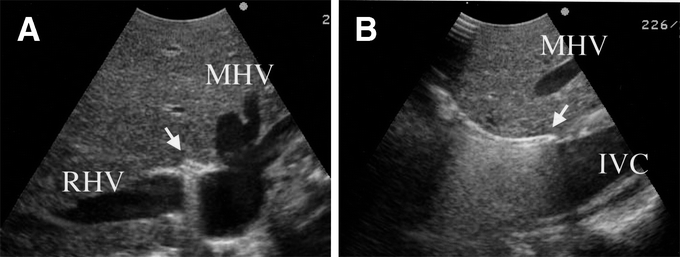
FIGURE 1. IOUS visualization of a cranial retrohepatic dissection (A, transverse view; B, sagittal view). A long heavy curved Kelly clamp is inserted between the pocket-like space between the MHV and RHV. The clamp tip is clearly visualized as a strongly hyperechoic structure with a reverberation artifact (arrow). MHV, middle hepatic vein; RHV, right hepatic vein; IVC, inferior vena cava.
For caudal retrohepatic dissection, the caudal edge of the caudate lobe is lifted from the IVC, and small short hepatic veins are divided. The anterior surface of the lower third of the retrohepatic IVC and IRHV, if present, is exposed. A long light curved Kelly clamp is inserted behind the caudal edge of caudate lobe on the left side of IRHV and passed cranially along the middle plane of the IVC. On the midway of the dissection, or when the operator feels resistance, an IOUS probe is applied on the liver surface and the positions of the clamp tip and the PrCV are checked (Fig. 2). When IOUS shows a dissection line heading to the PrCV or left side of the PrCV, direction of the dissection is shifted to right to avoid the injury. A safe dissection path is also confirmed by IOUS, checking the position of the clamp tip and the caudal end of the cranial retrohepatic dissection mentioned above (Fig. 3).
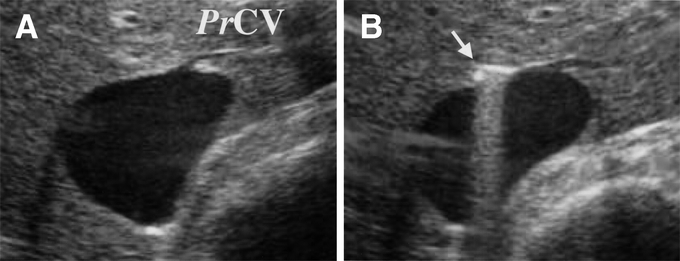
FIGURE 2. IOUS visualization of the clamp tip at the midway point of a retrohepatic dissection (transverse view). The proper hepatic vein for the caudate lobe (PrCV) is visualized (A). A long light curved Kelly clamp is inserted behind the caudal edge of the caudate lobe and passed cranially along the middle plane of the IVC. At the midpoint of the retrohepatic dissection, the relationship between the dissecting course (arrow) and the PrCV is checked (B).
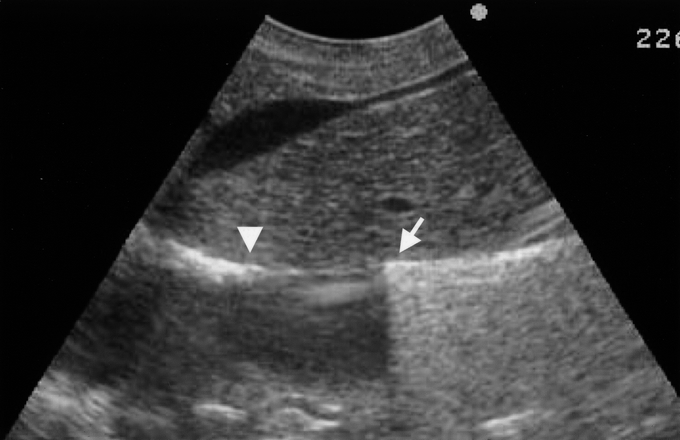
FIGURE 3. A safe dissection course visualized by sagittal IOUS scan. Both the position of clamp tip from the caudal side (arrow) and the extent of cranial dissection (arrowhead) are visualized as a hyperechoic line.
The Kelly clamp is then advanced further toward the tip of the operator's left index finger inserted into the space between the MHV and RHV. When IOUS shows that the clamp tip has reached the caudal end of the cranial dissection, the operator can feel the clamp tip with his/her finger and the retrohepatic dissection is completed. During dissection, the tip of the Kelly clamp is gently swung horizontally to find a space free of short hepatic veins.3 When a resistance-free space cannot be found in the upper third of the retrohepatic IVC with the clamp tip positioned in the right path confirmed by IOUS, the retrohepatic dissection is interrupted because there is a risk of injuring small short hepatic veins, which cannot be visualized by IOUS. In these cases, a tape for liver suspension was passed after the mobilization of right or left hemiliver, a modified retrohepatic dissection.9
IOUS Assessment of the PrCV and the Retrohepatic Dissection Path
The IVC and opening of the PrCV were visualized by IOUS in transverse scan. A straight line was drawn on the long axis of the cross-sectional view of IVC and the right edge of the line was marked 0°. When cross section of IVC was not elliptic but round triangular, a straight line was drawn between the center of the shortest side and the opposite apex. Another line between the opening of PrCV and the center of the long axis was drawn, and the angle between this line and 0° was measured (angle A). IOUS was done after retrohepatic dissection and the dissection line, visualized as a hyperechoic spot, was marked. The angle between the dissection line and 0° was also measured (angle B, Fig. 4).
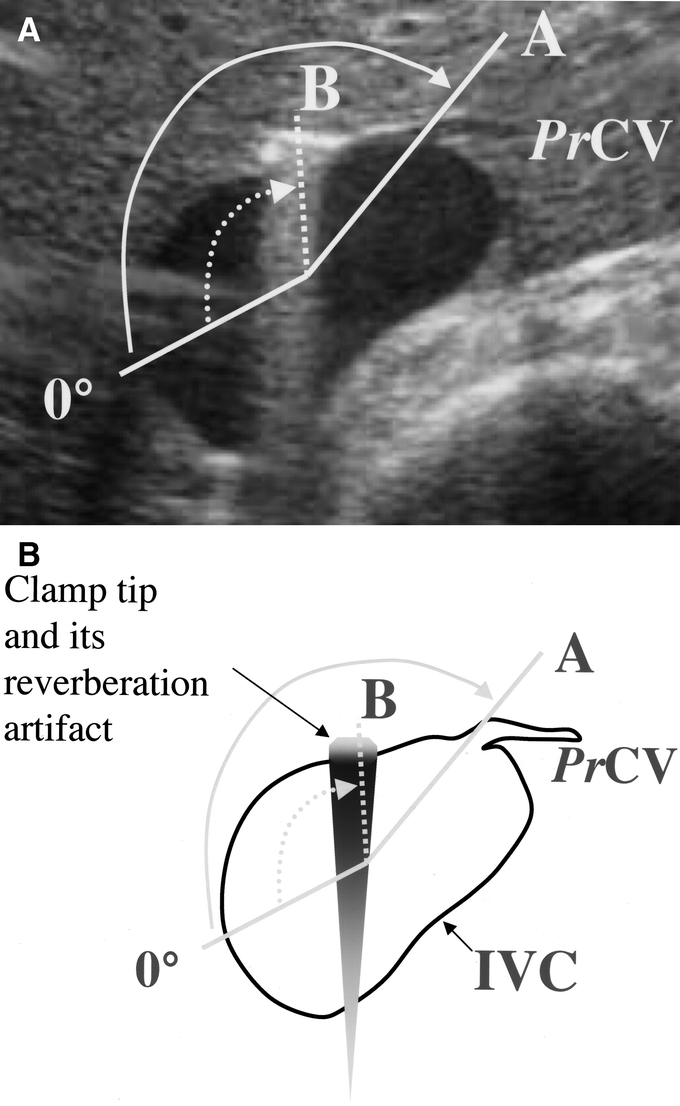
FIGURE 4. IOUS assessment of the PrCV and the retrohepatic dissection path (A, IOUS picture; B, a schematic drawing). A straight line was drawn on the long axis of cross-sectional view of IVC, and right edge of the line was marked 0°. Another line between the opening of the PrCV and the center of the long axis was drawn, and the angle between this line and 0° was measured (angle A). IOUS was done after retrohepatic dissection, and the dissection path (clamp tip) visualized as a hyperechoic spot with reverberation artifact was marked (dashed line). The angle between the dissection path (dashed line) and 0° was also measured (angle B).
RESULTS
From September 2003 to July 2004, 52 consecutive donors underwent liver grafting for adult living donor transplantation. Graft types consisted of the following: left liver with caudate lobe, 20 cases; right liver, 17 cases; right liver with MHV, 12 cases; left liver, 2 cases; and right lateral sector; 1 case. Excluding 2 cases of left liver graft, which does not require transection of the caudate lobe, 50 donors underwent the liver hanging maneuver. Of these, retrohepatic dissection was successful in 40 donors (80%), and a modified retrohepatic dissection was applied in the remaining 10 donors. A US-assisted dissection was performed in 34 of the 40 donors. In the remaining 6 donors, Kelly's clamp was easily passed behind the liver without US assistance. No substantial bleeding or any other complication related to retrohepatic dissection was encountered in any of the cases.
The proper caudate veins (PrCV) were visualized by IOUS in 48 donors (96%). Diameter of visualized PrCV in transverse scan ranged from 1.3 mm to 5.3 mm (3.0 ± 1.1 mm, mean ± SD). Of these, 42 had a so-called typical PrCV located around the midportion of the retrohepatic IVC.7 The remaining 6 had nontypical PrCVs draining to the LHV (3 cases) or to the IVC wall very close to the opening of LHV + MHV (3 cases). Angle A for the typical PrCV was 152° ± 24° (range, 60°–175°, n = 25), and angle B for retrohepatic dissection line was 102° ± 21° (range, 55°–130°, n = 26). Angle A-B was 49° ± 21° (range, −22 to 95°, n = 20). IOUS demonstrated that the dissecting clamp was heading to the PrCV in 3 cases, and the direction of dissection was shifted to avoid the injury. The initial direction of dissection was too much to the left in 1 case and it was adjusted under IOUS guidance.
DISCUSSION
Although the cranial landmarks of the MHV and RHV for retrohepatic dissection are distinct, caudal landmarks are unclear. The safe dissecting path should lie along the middle plane of the IVC1 or the midline of the IVC, but it is difficult to consistently determine the direction from the caudal side. Further, the long axis or the centerline of the IVC is not always a straight perpendicular line but may be slightly curved to the left.4,5,7 As shown in the present study, the location of the dissecting path on the circumference of the IVC varied significantly. Using US assistance, a safe dissection path, which was neither necessarily straight nor on the centerline of the VC, could be determined by checking the opening of the PrCVs and the caudal end of the cranial dissection.
IOUS could visualize the PrCV in 96% of donors with varying location on the circumference of the IVC. Fourteen percent of the visualized PrCV (6 of 42) were the so-called nontypical PrCV draining to the LHV or to the IVC wall very close to the opening of LHV + MHV.7 The IVC opening of nontypical PrCV is another dangerous point in retrohepatic dissection.
According to a meticulous anatomic study by Kogure et al,13 the hepatic venous system of the caudate lobe consisted of 1 or 2 PrCVs and the pleural small accessory hepatic veins (AcHV). The number and size of the AcHVs varied in each case, and they were distributed in all areas of the caudate lobe. AcHVs are too small to be visualized by IOUS. Using a term “minimum hepatic vein openings,” a diffuse distribution of AcHVs has also been demonstrated in previous anatomic studies.4,5
Because of the diffuse distribution of short hepatic veins (AcHV), retrohepatic dissection may not be possible in 100% of the patients. The success rate of 80% in the present series was lower than those in our initial report (98.6%)3 and the report by Ettorre et al with 92% feasibility.8 After our initial report, we experienced bleeding from an injured AcHV. We then decided not to push harder in the present series when the operator felt resistance in the cranial third of retrohepatic IVC with the clamp tip positioned in the right path as confirmed by IOUS. When the retrohepatic dissection was interrupted, a routine hemiliver mobilization was applied from the right or left side. In most of such cases, there were several thread-sized AcHVs and/or firm fibrous tissue in the resistant region, which were divided by a mobilization procedure (our unpublished observation). According to morphometric studies by Hirai et al7 and Sato et al,6 openings of medium-sized AcHVs may present in some cases near the midline in the cranial third of the retrohepatic IVC. The frequency of cranially located AcHVs is estimated to be 2.6% to 18.8% according to Sleiman Raad Camargo et al.5 Because these AcHVs are too small to be visualized by IOUS, a resistance in the right upper course of direction confirmed by IOUS suggests a high risk of AcHV injury.
Safe and dangerous dissecting courses behind the liver are schematically shown in Figure 5. In the lower two thirds of dissection, a typical PrCV can be visualized and be avoided by IOUS. The IRHV can also be visualized by IOUS, but it can be easily avoided by direct visual inspection from the caudal side behind the liver. The MRHV is generally located more dorsolaterally,7 so that there is no risk of injury. There is a risk of injuring a nontypical PrCV in 14% of donors if the dissecting course deviates to the left. This can be avoided using IOUS to check the position of the tip of the forceps and the caudal end of the cranial dissection.
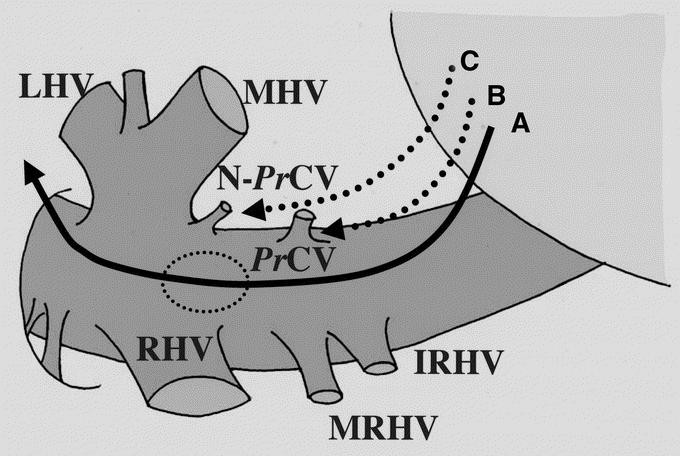
FIGURE 5. Schematic drawing of retrohepatic dissection. Line A indicates a recommended dissection course. When a resistance-free space cannot be found in the upper third of this course (dashed ellipse), there is a risk of injuring accessory hepatic veins. B, A dissecting course heading for a typical PrCV. C, A dissecting course heading for a nontypical PrCV. LHV, MHV, and RHV, left, middle, and right hepatic veins, respectively; IRHV and MRHV, inferior and middle right hepatic veins, respectively; PrCV, proper hepatic vein for the caudate lobe; N-PrCV, nontypical PrCV.
CONCLUSION
A new approach to a safer and easier retrohepatic dissection for a liver hanging maneuver is established using IOUS. IOUS could visualize most of the typical and nontypical PrCVs, the most dangerous points in retrohepatic dissection. The location of these PrCVs varied significantly, and there were no distinct landmarks for identifying their location. With the aid of IOUS, the entire course of the blind dissection between the anterior surface of IVC and the liver can be actually visualized in real time.
Footnotes
Reprints: Norihiro Kokudo, MD, Hepato-Biliary-Pancreatic Surgery Division, Department of Surgery, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan. E-mail: KOKUDO-2SU@h.u-tokyo.ac.jp.
REFERENCES
- 1.Belghiti J, Guevara OA, Noun R, et al. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC, Fan ST, Lo CM, et al. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314–317. [DOI] [PubMed] [Google Scholar]
- 3.Kokudo N, Sugawara Y, Imamura H, et al. Sling suspension of the liver in donor operation: a gradual tape-repositioning technique. Transplantation. 2003;76:803–807. [DOI] [PubMed] [Google Scholar]
- 4.Chang RWH, Shan-Quan S, Yen WWC. An applied anatomical study of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava. J Anat. 1989;164:41–47. [PMC free article] [PubMed] [Google Scholar]
- 5.Sleiman Raad Camargo AM, Gracioli Teixeira G, Ortale JR. Anatomy of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava. J Anat. 1996;188:59–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Sato TJ, Hirai I, Murakami G, et al. An anatomical study of short hepatic veins, with special reference to delineation of the caudate lobe for hanging maneuver of the liver without the usual mobilization. J Hepatobiliary Pancreat Surg. 2002;9:55–60. [DOI] [PubMed] [Google Scholar]
- 7.Hirai I, Murakami G, Kimura W, et al. How should we treat short hepatic veins and paracaval branches in anterior hepatectomy using the hanging maneuver without mobilization of the liver? Clin Anat. 2003;16:224–232. [DOI] [PubMed] [Google Scholar]
- 8.Ettorre GM, Vennarecci G, Boschetto A, et al. Feasibility of hanging maneuvers in orthotopic liver transplantation with inferior vena cava preservation and in liver surgery. J Hepatobiliary Pancreat Surg. 2004;11:155–158. [DOI] [PubMed] [Google Scholar]
- 9.Ettorre GM, Vennarecci G, Santoro R, et al. Modified liver hanging maneuver during orthotopic liver transplantation with inferior vena cava preservation. Transplantation. 2003;75:247–249. [DOI] [PubMed] [Google Scholar]
- 10.Orringer MB, Sloan H. Esophagectomy without thoracotomy. (comment by Belsey R). J Thorac Cardiovasc Surg. 1978;76:643–654. [PubMed] [Google Scholar]
- 11.Makuuchi M, Hasegawa H, Yamazaki S, et al. The inferior right hepatic vein: ultrasonic demonstration. Radiology. 1983;148:213. [DOI] [PubMed] [Google Scholar]
- 12.Makuuchi M, Hasegawa H, Yamazaki S, et al. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet. 1987;164:69. [PubMed] [Google Scholar]
- 13.Kogure K, Kuwano H, Fujimaki N, et al. Relation among portal segmentation, proper hepatic vein, and external notch of the caudate lobe in the human liver. Ann Surg. 2000;231:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]


