Abstract
Objective:
To compare the clinical outcome of simultaneous bilateral endoscopic totally extraperitoneal inguinal hernioplasty (TEP) using fibrin sealant (FS) and mechanical stapling for prosthetic mesh fixation.
Summary Background Data:
Similar efficacy of FS and mechanical stapling for mesh fixation has been demonstrated in a swine model, but no clinical trial has been conducted to compare the outcomes of TEP using these 2 fixation devices. FS adheres the prosthetic mesh without causing injury to the underlying structures. Whether the application of FS improves early postoperative outcomes, namely, reduction of postoperative pain and seroma formation, has not been examined.
Patients and Methods:
Between July 2002 and February 2004, a total of 93 patients with 186 inguinal hernias who underwent bilateral TEP were randomized to have mesh fixation by either FS (n = 46) or mechanical stapling (n = 47). The primary endpoints were severity of pain, analgesic requirement, and incidence of seroma. Secondary endpoints were length of hospital stay, number of days required to resume normal outdoor activities and work, recurrence rate, and incidence of chronic pain.
Results:
The 2 groups were comparable in age, sex, and types of hernia. TEP were successfully performed in all patients. The FS group consumed significantly less analgesics compared with that of the staple group (P = 0.034). There was no significant difference in the postoperative pain score at rest and on coughing from the day of operation to postoperative day 6 between the groups. The incidence of seroma was significantly higher in the FS group (17.4%) than the staple group (5.3%) (P = 0.009). Length of hospital stay and time taken to resume normal activities and work were comparable between the 2 groups. With a median follow-up of 1.2 years, no recurrent hernia has been detected in either group, but the incidence of chronic pain in the staple group (20.0%) was higher than that of the FS group (13.2%) (P = 0.418).
Conclusions:
This randomized prospective clinical trial demonstrated a significant reduction of analgesic consumption by using FS for mesh fixation during bilateral TEP, but it was associated with an increased incidence of postoperative seroma.
The use of fibrin sealant (FS), instead of mechanical stapling, for mesh fixation during bilateral endoscopic extraperitoneal inguinal hernioplasty conferred a significant reduction of analgesic consumption but resulted in a higher incidence of seroma formation. FS allows biologic fixation of the mesh onto pelvic floor without causing injury to the underlying structures.
Bilateral inguinal hernia is an accepted indication for the performance of endoscopic totally extraperitoneal inguinal hernioplasty (TEP) because of significant reduction of postoperative pain and faster recovery compared with those of open repair.1–3 Inadequate mesh fixation has been reported to be a main cause for hernia recurrence after laparoscopic repair.4–7 Endoscopic stapling has been the most commonly adopted technique for prosthetic mesh fixation. Mechanical anchorage of the mesh not only reduces the risk of mesh migration but also enhances the bursting strength of the repair.8,9 However, neuralgia secondary to nerve entrapment by the staples has been reported.10–12 Application of fibrin sealant (FS) as an adhesive for mesh fixation was first described by Katkhouda et al13 in a swine model in 2001. The tensile strength of repair using FS was found to be comparable to that of stapling. Prosthetic mesh fixation by either stapling or FS significantly reduced the chance of mesh migration compared with those without fixation. FS has been used primarily for hemostasis during surgery.14 The efficacy of FS in the fixation of mesh during TEP has not been examined.
With an increasing emphasis on the evaluation of functional recovery, acute pain has become an important outcome parameter after hernia surgery.15 Biologic fixation of the mesh by FS diminishes the risk of neuromuscular injury and may therefore diminish the severity of postoperative pain. Seroma is the commonest morbidity following TEP, with a reported incidence ranging from 3.4% to 11.7%.16–19 Its occurrence sometimes causes alarm to the patients as it mimics a postoperative recurrence of hernia. Seroma formation is also a prevalent morbidity after mastectomy. Previous studies demonstrated that the application of FS to the dissected area after mastectomy significantly reduced the incidence and volume of postoperative seroma.20,21 The objective of this study was to determine if the replacement of mechanical stapling with FS for the fixation of prosthetic mesh improved early postoperative outcomes, including reduction of postoperative pain and seroma formation. The present randomized prospective trial was designed to test the null hypothesis that the early clinical outcomes of bilateral TEP using FS for the fixation of meshes were equivalent to those using staples.
PATIENTS AND METHODS
Between July 2002 and February 2004, all patients who presented with bilateral inguinal hernia were assessed for study eligibility. Inclusion criteria were age at least 18 years, medical fitness for general anesthesia, and suitability for TEP. Patients who underwent concomitant operative procedures were excluded. The trial was sponsored by a Research Fund from the Tung Wah Group of Hospitals. The research protocol was approved by the Ethics Committee of Tung Wah Hospital before commencement of the trial. Informed consent was obtained prior to randomization. Eligible patients were randomized to 2 arms of treatment, FS group and staple group, by sealed envelopes containing random number in the operation theater. A prospective collection of data using standardized data entry sheet was performed.
Surgical Technique
Patients were operated in a supine Trendelenburg's position under general anesthesia. Preoperative antibiotic, amoxicillin Na 1 g with clavulanate K 200 mg, was given intravenously on induction of general anesthesia. A 4-port technique was used. Balloon dissection and urinary catheter were not used. A transverse infraumbilical incision was made. After opening the anterior rectus sheath, the rectus muscle was displaced laterally and a 10-mm reusable trocar port was introduced into the preperitoneal space. Carbon dioxide was insufflated to a pressure of 10 mm Hg. Another 10-mm trocar port was then inserted 8 cm proximal to symphysis pubis under endoscopic guidance. The extraperitoneal space was dissected and created using endo-scissors and diathermy. The third and fourth 5-mm trocar ports were placed at about 3 cm proximal to the left and right anterior superior iliac spine, respectively. After reduction of the hernial sac and parietalization of the spermatic cord for a length of approximately 4 cm long, 2 prolene meshes (Prolene Mesh, Ethicon Ltd, Somerville, NJ), each measuring about 10 × 15 cm2, were introduced to cover the posterior wall of the inguinal canal, deep inguinal ring and femoral ring on each side. Hernia types were determined intraoperatively according to the Nyhus classification.22
For the staple group, an endoscopic stapler (EMS Hernia Stapler, Ethicon Ltd) was used to anchor each mesh over the Cooper's ligament, along its medial edge and upper lateral corner. No staples were placed below the iliopubic tract lateral to the Cooper's ligament.
Patients randomized to the FS group had fixation of the mesh using TISSEEL VH 2 mL (Baxter Healthcare Corporation, Glendale, CA). The 2 components of FS, sealer protein solution 2 mL and thrombin solution 2 mL, were reconstituted using the Fibrinotherm heating and stirring device (Baxter Healthcare Corporation) at the commencement of surgery. The 2 solutions were drawn into 2 separate syringes, which were then fitted into the laparoscopic applicator, Duplocath 35 M.I.C. (Baxter AG, Vienna, Austria). Once the 2 meshes were deployed in the desired position, FS 1mL was applied over each Cooper's ligament. The rest of FS (2 mL) was applied to the inferior edge and upper medial corner of the meshes. To ensure the setting FS adhere firmly to the underlying structures, the mesh was steadied in position by graspers for a few minutes until the FS appeared opalescent on the television monitor.
Postoperative Management
For outpatient TEP, after assessment by the operating surgeon and anesthesiologist, patients were discharged in the afternoon on the day of surgery. Postoperative analgesic regimen, including oral diclofenac sodium SR 100 mg daily and compound oral analgesic, proproxyphene 50 mg and paracetamol 325 mg, 4 times daily upon patients’ request, were standardized. All patients had follow-up at the Hernia Clinic 1 week after discharge. During follow-up, all complications and clinical recurrence were recorded. Subsequent follow-ups were scheduled at 3, 6, and 12 months and yearly thereafter.
Outcome Assessment
The primary endpoints were severity of postoperative pain, analgesic requirement, and incidence of seroma. Severity of pain was assessed by a linear analogue pain score on a scale from 0 to 10 daily after operation. All patients were taught to fill in a pain score chart to document their daily pain score at rest and on coughing at home. Total amount of analgesic consumption was based on the total number of analgesic tablets consumed by the patient during the postoperative period. A seroma was defined as the clinical presence of a palpable fluid collection over the groin in the absence of bruising during follow-up.
Secondary outcome measures included operative time, length of hospital stay, number of days required to resume normal outdoor activities and work, recurrence rate, and incidence of chronic groin pain. Operative time was defined as the time from the skin incision to the placement of the last suture. Length of hospital stay was referred to the total number of nights spent in hospital after operation. Chronic groin pain was assessed by a standardized questionnaire by a research assistant at 1 year after operation.23
Statistical Analysis
A sample size analysis was performed using a 2-tailed test with a probability of a type I error (α) of 0.05 and a type II error (β) of 0.2 (power, 80%). Based on our recent review, the mean (SD) pain score on coughing after TEP was 3.4 ± 1.86.15 Assuming that an observed difference of 1.2 exists between the pain scores of the 2 groups, a minimum sample size of 40 patients in each group was required to detect the difference.
Data were analyzed on an intention-to-treat basis. Continuous variables were compared by the Mann-Whitney U test. Categorical variables were compared by the χ2 test or the Fisher exact test when an expected value was less than 5. A P value of <0.05 indicated a statistically significant difference. Statistical analysis was performed using computer software (SPSS/PC+ 9.0, SPSS, Chicago, IL). Values were expressed as medians and interquartile range. Outcome measures were expressed with 95% confidence intervals (CI) when appropriate.
RESULTS
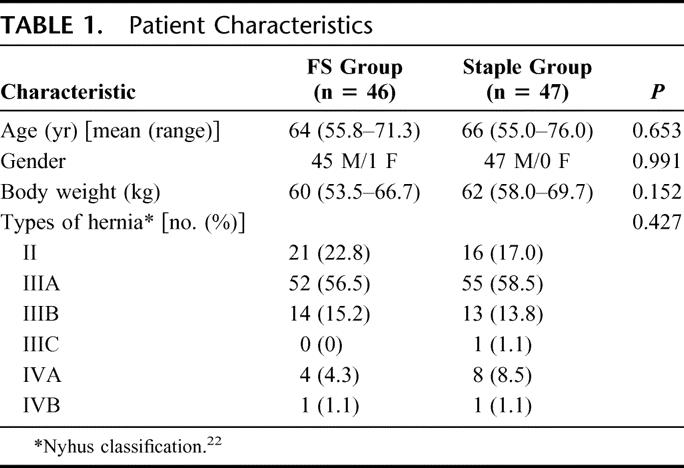
Between July 2002 and March 2004, 108 patients who presented with bilateral inguinal hernia were assessed for study eligibility. Figure 1 shows the profile of the trial according to the CONSORT statement.24 A total of 93 patients with 186 inguinal hernias were randomized to the FS group (n = 46) and the staple group (n = 47). The 2 groups were comparable in sex, age, body weight, and types of hernia (Table 1).
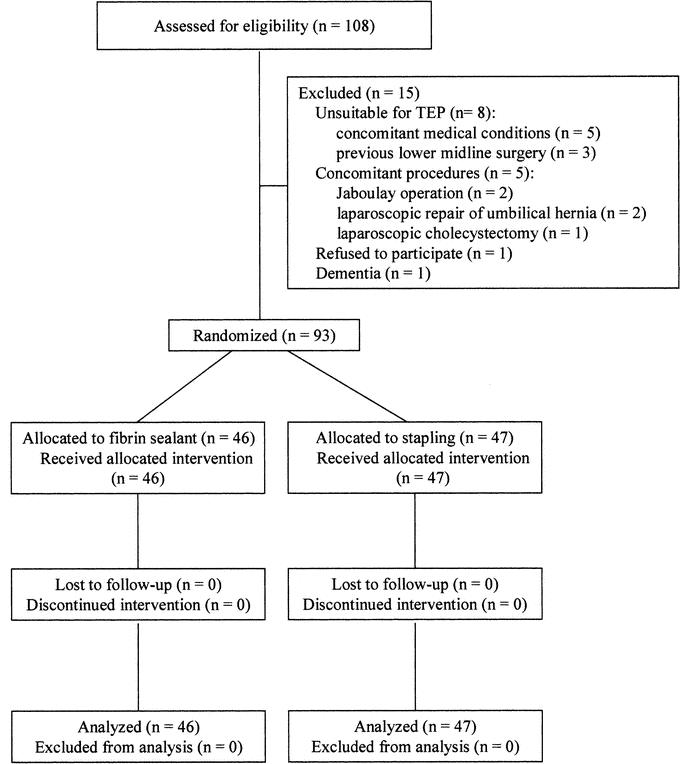
FIGURE 1. Flow diagram of subject progress through the phases of the clinical trial according to the CONSORT statement.
TABLE 1. Patient Characteristics
All TEP were successfully performed, and there were no conversions to open repair or transabdominal preperitoneal inguinal hernioplasty. The median operative time was 76 minutes (range, 63.0–86.5 minutes) and 75 minutes (range, 65.0–90.0 minutes) in the FS and the staple groups, respectively (P = 0.348). There were no intraoperative complications or hospital mortality.
The total number of analgesic tablets consumed by the FS group (4.5 tablets; range, 2–10 tablets) was significantly less than that of the staple group (7 tablets; range, 4–10 tablets) (P = 0.034). A total of 17 patients (18.3%), 9 in the FS group and 8 in the staple group, did not require any analgesia after returning home (P = 0.551). Comparison of daily pain scores at rest and on coughing between the 2 groups showed no significant difference from the day of operation to postoperative day 6 (Figs. 2 and 3).
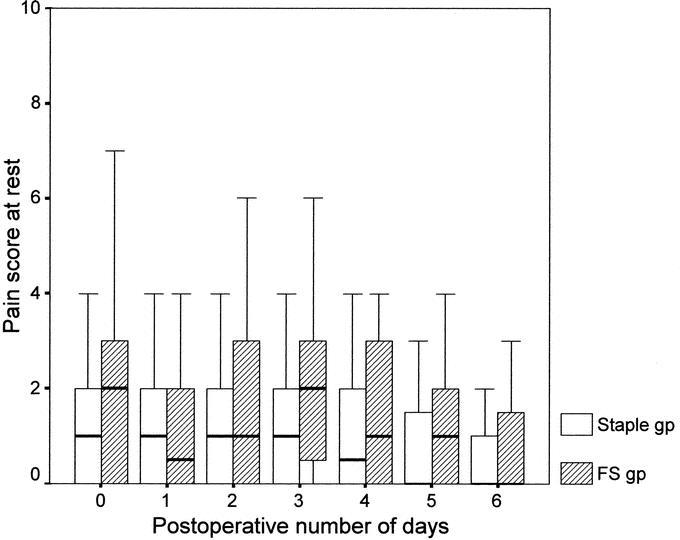
FIGURE 2. Box-plot of median value and interquartile range of daily postoperative pain score at rest in staple and FS group (P > 0.05, Mann-Whitney U test).
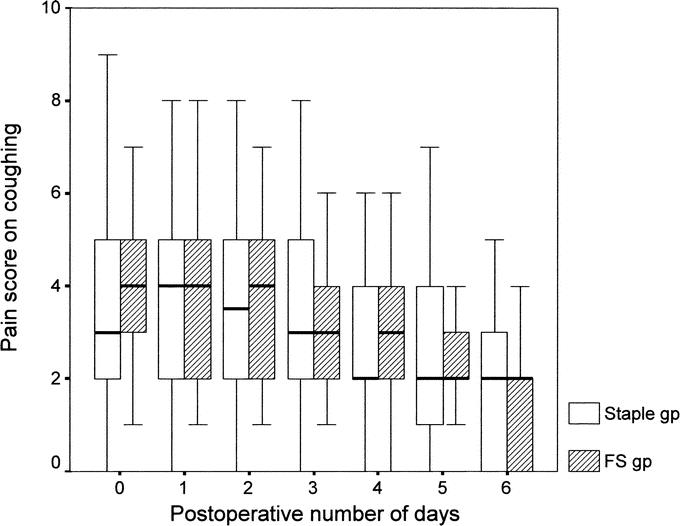
FIGURE 3. Box-plot of median value and interquartile range of daily postoperative pain score on coughing in staple and FS group (P > 0.05, Mann-Whitney U test).
Bilateral TEP was performed as an outpatient procedure in 15 (32.6%) patients of the FS group and 10 (21.3%) patients of the staple group. All these patients were discharged uneventfully on the day of operation. For inpatient TEP, the median length of hospital stay was 1 day (range, 1–1 day) in the FS group and 1 day (range, 1–2 days) in the staple group (P = 0.428).
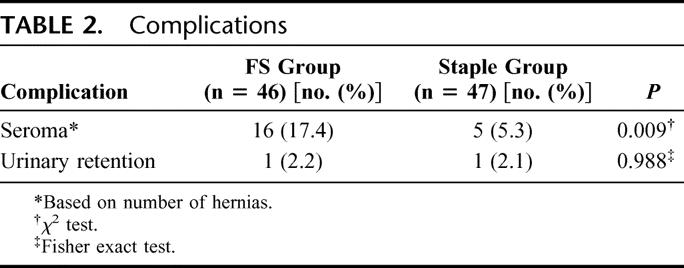
Table 2 summarizes the postoperative complications in detail. There were no major complications, and none of the patients had wound infection. The FS group had a significantly higher incidence of postoperative seroma (17.4%; 95% confidence interval [CI], 6.4%–28.4%) than the staple group (5.3%; 95% CI, 0%–11.7%) (P = 0.009). All these morbidities resolved spontaneously without the need for surgical intervention.
TABLE 2. Complications
The time taken to resume normal outdoor activities was comparable between the FS group (3 days; range, 2–5 days) and the staple group (3 days, range, 2–4 days) (P = 0.681). At the time of operation, 58 patients were either retired (n = 50) or unemployed (n = 8). Thirty-five patients had assessment of their time taken to return to work, which was similar between the FS group (8 days; range, 4–10 days) and the staple group (6 days; range, 5–10 days) (P = 0.915).
The total cost for the use of FS was U.S. $270, inclusive of TISSEEL VH 2 mL (Baxter Healthcare Corporation) (U.S. $230) and Duplocath 35 M.I.C. (Baxter AG, Vienna, Austria) (U.S. $40), which was greater than that for an endoscopic stapler (EMS Hernia Stapler, Ethicon Ltd) (U.S. $130). There were no differences in other costs between the groups.
Follow-up ranged from 8 to 27 months. With a median follow-up of 1.2 years, none of the patients was found to have recurrence. A total of 78 patients had follow-up exceeding 1 year. Of these, the incidence of chronic pain was 20% (95% CI, 7.6%–32.3%) (n = 8) in the staple group, which was higher than that of the FS group (13.2%; 95% CI, 2.5%–23.9%) (n = 5) (P = 0.418).
DISCUSSION
Efficacy of TEP for the repair of inguinal hernia has been well proven by a number of randomized prospective trials,1–3 but TEP remains an evolving technique. Some concern has arisen regarding the potential complications of prosthetic stapling, such as sensory nerve entrapment. To address this issue, in addition to that of cost containment, performance of TEP without fixation of the mesh has been advocated. Recent reports25–27 demonstrated comparable early recurrence rates after TEP with and without prosthetic mesh stapling, but long-term results remain to be proven. Besides, recurrence of hernia after laparoscopic repair has been attributed to an inadequate mesh fixation.28–30 Phillips et al7 suggested secure stapling of the mesh to reduce recurrence rate following laparoscopic hernioplasty. At the time of this study, endostapler was the most commonly adopted modality for prosthetic mesh fixation. Biologic fixation of the mesh with FS has only been reported in a swine model.13 No prospective clinical trials have been conducted to compare the outcomes of biologic versus mechanical fixation of the mesh during TEP.
In the present trial, the use of FS conferred a significant reduction of analgesic requirement compared with that of the staple group. This finding was consistent with our earlier report15 for reduced acute pain by avoiding prosthetic stapling during unilateral TEP. Biologic fixation with FS avoids somatic and neurologic injury associated with the use of staples.
The lower incidence of chronic pain in the FS group also favored biologic prosthetic mesh fixation. Chronic pain after inguinal hernia repair has been classified broadly as either somatic or neuropathic in origin. Somatic pain may arise from tissue injury, tissue ischemia, or the placement of staples or nonabsorbable sutures on the pubic bone.7 Meralgia paresthetica is a rare but serious potential complication of prosthetic mesh stapling.10,31,32 Tetik et al5 conducted a multicenter study recruiting 1514 laparoscopic repairs of inguinal hernia and reported 2 neurologic complications that required repeat laparoscopy and staple removal.
In contrast to our hypothesis that seroma formation would be reduced by the use of FS, the prevalence of postoperative seroma was significantly higher in the FS group than the staple group. FS has been shown to reduce seroma formation after mastectomy.20,21 Formation of a physiologic clot by the application of FS decreases the drainage from transected small blood vessels and lymphatics. Besides, FS adheres the skin flap and chest wall, thereby diminishing potential space for fluid collection. Our findings could be explained by the fact that FS was used to affix the mesh onto the Cooper's ligament and pelvic floor, instead of applying it for sealing the potential space of the hernial cavity. Besides, FS may have stimulated a more intense inflammatory reaction in the tissues that increase exudation and hence seroma formation. Katkhouda et al13 demonstrated that histologic inflammatory response was significantly stronger in the FS fixed graft than in grafts fixed by stapling in the swine model. Another important consideration in the use of FS for its adhesive properties is the timing of action.20 To maximize the sealing effect and adhesive strength of FS, deflation and closure of the potential space should be undertaken immediately after its application. Once polymerization of the sealant has occurred, tissue adherence can no longer be achieved.
Our findings showed no difference in early recurrence rates between the 2 groups. FS, distinguished from mechanical stapling, allows fixation of the mesh onto the pelvic floor, where major neurovascular structures traverse underneath. Provided adequate parietalization of spermatic cord has been achieved, affixing the inferior edge of the mesh onto pelvic floor prevents mesh lifting or folding by the returned peritoneum, seroma, or hematoma. Lowham et al4 conducted a multicenter study to evaluate the mechanisms leading to hernia recurrence after laparoscopic and traditional preperitoneal herniorrhaphy. Mesh lifting by hematoma and inadequate inferior mesh fixation represented the most common causes of recurrence for surgeons experienced in traditional or laparoscopic preperitoneal hernia repair. In another retrospective review of 7661 patients with 10,053 laparoscopic hernia repairs by Felix et al,6 inadequate lateral and medial fixation of the mesh were the chief mechanisms causing recurrence. As stapling of the mesh is contraindicated below the iliopubic tract, FS serves as a complementary tool for prosthetic mesh fixation.
Fixation of the mesh during TEP can be achieved by mechanical or biologic methods. Endostapler has been the conventional mechanical technique for prosthetic fixation during TEP. Alternative mechanical methods include suturing, helical fastener, anchors, etc. Inherent problems of these techniques include pain, bleeding, nerve irritation, and neuralgia. Reports on biologic fixation of the mesh have been limited.13 In contrast to mechanical staples, FS is a biodegradable and biocompatible agent as it will be completely absorbed during wound healing. Other benefits of FS include its well-proven safety profile, added hemostatic effect, and its ability to affix the mesh without injuring the underlying structure. This may help to reduce the incidence of chronic pain in the long run. FS possessed both adhesive and hemostatic properties, the ultimate effect of which was dependent on the relative concentrations of fibrinogen and thrombin.33 The fibrinogen component is responsible for the tensile strength and adhesive properties, while the thrombin component accounts for the clotting time and promotes fibroblast proliferation. The concentrations of fibrinogen and thrombin should therefore be modified in accordance with the particular clinical indications, depending on the requirements for hemostasis or adhesiveness. Further studies should be conducted to identify the best formulation of FS for prosthetic mesh fixation. The main drawback of using FS in this study was its cost, which was double that of an endoscopic stapler. Whether this could be offset by the reduction of analgesic medications and avoidance of potential complications like neuralgia remain to be determined. Other disadvantages of FS include long preparation time, the requirement of cold storage for the agents, and the use of multiple devices. The best fixation method, which should be effective, safe, ready to use, affordable, and biocompatible, has yet to be developed.
CONCLUSION
This randomized prospective trial demonstrated a significant reduction of analgesic requirement by using FS for mesh fixation during bilateral TEP, but it was associated with an increased incidence of postoperative seroma. FS is a safe and efficacious alternative to mechanical stapling for the anchorage of mesh during TEP. It allows biologic fixation of the mesh onto the pelvic floor without the risk of causing neurovascular injury and serves as an additional tool for prosthetic mesh fixation during TEP.
ACKNOWLEDGMENTS
The author thanks Tze-Ching Tan, MD, for editing the manuscript.
Footnotes
Supported in part by the Tung Wah Group of Hospitals Research Fund. The work will be submitted as a chapter of the thesis to the University of Hong Kong for the degree of Doctor of Medicine.
Presented as a poster at the 90th Annual Clinical Congress of American College of Surgeons, October 10–14, 2004, New Orleans, LA.
Reprints: Hung Lau, MD, Department of Surgery, University of Hong Kong Medical Center, Tung Wah Hospital, 12 Po Yan Street, Sheung Wan, Hong Kong SAR, China. E-mail: lauh@hkucc.hku.hk.
REFERENCES
- 1.Liem MS, van der Graaf Y, van Steensel CJ, et al. Comparison of conventional anterior surgery and laparoscopic surgery for inguinal hernia repair. N Engl J Med. 1997;336:1541–1547. [DOI] [PubMed] [Google Scholar]
- 2.MRC Laparoscopic Groin Hernia Trial Group. Laparoscopic versus open repair of groin hernia: a randomized comparison. Lancet. 1999;354:185–190. [PubMed] [Google Scholar]
- 3.Bringman S, Ramel S, Heikkinen TJ, et al. Tension-free inguinal hernia repair: TEP versus mesh-plug versus Lichtenstein. A prospective randomized controlled trial. Ann Surg. 2003;237:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowham AS, Filipi CJ, Fitzgibbons RJ Jr, et al. Mechanism of hernia recurrence after preperitoneal mesh repair: traditional and laparoscopic. Ann Surg. 1997;225:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetik C, Arregui ME, Dulucq JL, et al. Complications and recurrences associated with laparoscopic repair of groin hernias: a multi-institutional retrospective analysis. Ann Surg. 1994;8:1316–1323. [DOI] [PubMed] [Google Scholar]
- 6.Felix E, Scott S, Crafton B, et al. Causes of recurrence after laparoscopic hernioplasty: a multicenter study. Surg Endosc. 1998;12:226–231. [DOI] [PubMed] [Google Scholar]
- 7.Phillips EH, Rosenthal R, Fallas M, et al. Reasons for recurrence following laparoscopic hernioplasty. Surg Endosc. 1995;9:140–145. [DOI] [PubMed] [Google Scholar]
- 8.Dion YM, Laplante R, Charara J, et al. The influence of the number of endoclips and of mesh incorporation on the strength of an experimental hernia patch repair. Surg Endosc. 1994;8:1324–1328. [DOI] [PubMed] [Google Scholar]
- 9.Hollinsky C, Gobl S. Bursting strength evaluation after different types of mesh fixation in laparoscopic herniorrhaphy. Surg Endosc. 1999;13:958–961. [DOI] [PubMed] [Google Scholar]
- 10.Eubanks S, Newman L 3rd, Goehring L, et al. Meralgia paresthetica: a complication of laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3:381–385. [PubMed] [Google Scholar]
- 11.Stark E, Oestreich K, Wendl K, et al. Nerve irritation after laparoscopic hernia repair. Surg Endosc. 1999;13:878–881. [DOI] [PubMed] [Google Scholar]
- 12.Tucker JG, Wilson RA, Rarnshaw BJ, et al. Laparoscopic herniorrhaphy: technical concerns in prevention of complications and early recurrence. Am Surg. 1995;61:36–39. [PubMed] [Google Scholar]
- 13.Katkhouda N, Mavor E, Friedlander MH, et al. Use of fibrin sealant for prosthetic mesh fixation in laparoscopic extraperitoneal inguinal hernia repair. Ann Surg. 2001;233:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenk WG III, Burks SG, Gagne PJ, et al. Fibrin sealant improves hemostasis in peripheral vascular surgery: a randomized prospective trial. Ann Surg. 2003;237:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau H, Patil NG. Acute pain following endoscopic totally extraperitoneal (TEP) inguinal hernioplasty: multivariate analysis of predictive factors. Surg Endosc. 2004;18:92–96. [DOI] [PubMed] [Google Scholar]
- 16.Aeberhard P, Klaiber C, Meyenberg A, et al. Prospective audit of laparoscopic totally extraperitoneal inguinal hernia repair: a multicenter study of the Swiss Association for Laparoscopic and Thoracoscopic Surgery (SALTC). Surg Endosc. 1999;13:1115–1120. [DOI] [PubMed] [Google Scholar]
- 17.Ferzli GS, Kiel T. The role of endoscopic extraperitoneal approach in large inguinal scrotal hernias. Surg Endosc. 1997;11:299–302. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgibbons RJ Jr, Camps J, Cornet DA, et al. Laparoscopic inguinal herniorrhaphy: results of a multicenter trial. Ann Surg. 1995;221:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau H, Lee F. Seroma following endoscopic extraperitoneal inguinal hernioplasty: incidence and risk factors. Surg Endosc. 2003;17:1773–1777. [DOI] [PubMed] [Google Scholar]
- 20.Moore M, Burak WE Jr, Nelson E, et al. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: a randomized prospective clinical trial. J Am Coll Surg. 2001;192:591–599. [DOI] [PubMed] [Google Scholar]
- 21.Sanders RP, Goodman NC, Amiss LR Jr, et al. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. J Surg Res. 1996;61:65–70. [DOI] [PubMed] [Google Scholar]
- 22.Nyhus LM. Individualization of hernia repair: a new era. Surgery. 1993;114:1–2. [PubMed] [Google Scholar]
- 23.Lau H, Patil NG, Yuen WK, Lee F. Prevalence and severity of chronic groin pain after endoscopic totally extraperitoneal inguinal hernioplasty. Surg Endosc. 2003;17:1620–1623. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Schulz KF, Altman DG, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-randomized trials. JAMA. 2001;285:1987–1991. [DOI] [PubMed] [Google Scholar]
- 25.Spitz JD, Arregui ME. Sutureless laparoscopic extraperitoneal inguinal herniorrhaphy using reusable instruments: two hundred three repairs without recurrence. Surg Laparosc Endosc Percutan Tech. 2000;10:24–29. [PubMed] [Google Scholar]
- 26.Beattie GC, Kumar S, Nixon SJ. Laparoscopic total extraperitoneal hernia repair: mesh fixation is unnecessary. J Laparoendosc Adv Surg Tech A. 2000;10:71–73. [DOI] [PubMed] [Google Scholar]
- 27.Smith AI, Royston CM, Sedman PC. Stapled and nonstapled laparoscopic transabdominal preperitoneal (TAPP) inguinal hernia repair: a prospective randomized trial. Surg Endosc. 1999;13:804–806. [DOI] [PubMed] [Google Scholar]
- 28.Ferzli GS, Frezza EE, Pecoraro AM Jr, et al. Prospective randomized study of stapled versus unstapled mesh in a laparoscopic preperitoneal inguinal hernia repair. J Am Coll Surg. 1999;188:461–465. [DOI] [PubMed] [Google Scholar]
- 29.Khajanchee YS, Urbach DR, Swanstrom LL, et al. Outcomes of laparoscopic herniorrhaphy without fixation of mesh to the abdominal wall. Surg Endosc. 2001;15:1102–1107. [DOI] [PubMed] [Google Scholar]
- 30.Lau H, Patil NG. Selective non-stapling of mesh during unilateral endoscopic total extraperitoneal inguinal hernioplasty: a case-control study. Arch Surg. 2003;138:1352–1355. [DOI] [PubMed] [Google Scholar]
- 31.Broin EO, Horner C, Mealy K, et al. Meralgia paresthetica following laparoscopic inguinal hernia repair: an anatomical analysis. Surg Endosc. 1995;9:76–78. [DOI] [PubMed] [Google Scholar]
- 32.Felix EL, Harbertson N, Vartanian S. Laparoscopic hernioplasty: significant complications. Surg Endosc. 1999;13:328–331. [DOI] [PubMed] [Google Scholar]
- 33.Spotnitz WD, Falstrom JK, Rodeheaver GT. The role of sutures and fibrin sealant in wound healing. Surg Clin North Am. 1997;77:651–669. [DOI] [PubMed] [Google Scholar]


