Abstract
Objective:
To evaluate the risk factors including tumor histomorphology for survival specific to follicular thyroid carcinoma (FTC) and to apply commonly employed staging systems in predicting survival for patients with FTC.
Summary Background Data:
FTC is usually analyzed collectively with papillary thyroid carcinoma (PTC) in risk group analysis. Risk factors and risk group analysis are important in the management of patients with FTC, although current published therapeutic guidelines call for total thyroidectomy followed by radioactive iodine (I131) ablation for all FTC patients.
Methods:
Over a 40-year period, 156 patients surgically treated for FTC with an average follow-up of 14.4 years were retrospectively studied after histologic reclassification according to the type and degree of invasiveness of the tumor. Potential risk factors for survival were calculated using multivariate analysis, and the prognostic accuracy of AMES risk group stratification, UICC/AJCC pTNM staging, Degroot classification, and MACIS scoring schemes in predicting survival was compared.
Results:
Seventeen (11%) patients had distant metastases at presentation, and bilateral thyroid resection was performed for 131 (84%) patients. Seventeen (11%) patients died of recurrent or metastatic disease. The overall and cancer-specific survival (CSS) rates at 10 years were 79% and 88%, respectively. None of the patients with minimally invasive (n = 49) or angioinvasive (n = 23) carcinomas died compared with 17 of 84 patients with widely invasive carcinomas (P = 0.0007). Using the Cox proportional hazards model, old age, the presence of distant metastases, and incomplete tumor excision were independent prognostic factors for survival. For patients who underwent curative treatment, old age and widely invasive carcinoma were risk factors for poor survival. All staging systems studied accurately predicted CSS, and the pTNM UICC/AJCC staging system yielded the best prognostic information.
Conclusions:
Commonly adopted staging systems can be applied specifically to patients with FTC. The distinction of FTC in minimally invasive and widely invasive carcinoma based on the extent of invasiveness rather than vascular invasion is important in identifying low-risk FTC patients for a more conservative management.
The application of commonly adopted risk group stratification, staging systems, and prognostic scoring schemes for predicting survival specific to follicular thyroid carcinoma remains limited. The extent and type of invasion are commonly used for guiding therapeutic measures. The present study confirmed the applicability of these commonly used prognostic schemes in predicting survival of patients with follicular thyroid carcinoma and the role of histomorphology in guiding the management of patient subgroups.
Prognostic indicators for thyroid cancer have been well documented. Several previously reported schemes have been applied for predicting the survival of thyroid cancer and in the management of patients with well-differentiated thyroid carcinoma (WDTC). However, these prognostic indices have been developed in heterogeneous groups of patients with thyroid cancer,1 collectively in patients with WDTC,2–7 or primarily in patients with papillary thyroid carcinoma (PTC).8–12 Identification of prognostic indicators for survival of follicular thyroid carcinoma (FTC)13–20 and application of these reported schemes specific to FTC patients21–23 have been attempted individually but not concomitantly.
Apart from size and extrathyroidal invasion, histologic features including the type and extent of invasion are not usually incorporated as prognostic indicators in predicting disease recurrence or survival in FTC. However, FTC is classified into minimally invasive or widely invasive carcinoma with distinct clinical course and outcome.24–27 In addition, it has been suggested that minimally invasive FTC with vascular invasion should be distinguished from those with capsular invasion alone because of the greater probability of recurrence and metastases in the presence of angioinvasion.14,28–30 The need of subclassifying minimally invasive carcinoma into tumors with capsular invasion alone (truly minimally invasive) or with angioinvasion (moderately invasive or angioinvasive) remains controversial.17,27–30
The purpose of the present study was to document the prognostic factors for survival specific to FTC with the incorporation of histologic subclassification according to the type and extent of invasion. The role of histologic types in predicting survival as well as in the management of patients with FTC was studied. The applicability of those commonly used prognostic scoring systems or risk group stratification schemes used for PTC or WDTC to predict the disease-specific survival in this group of pure FTC patients treated over the past 4 decades was evaluated and compared.
MATERIALS AND METHODS
Complete data were available for 655 patients with WDTC surgically treated at Queen Mary Hospital from 1961 to 2000. The surgical specimens were reexamined histologically by a single pathologist (K.-Y.L.) and 156 patients with well-differentiated FTC were identified for retrospective analysis. Patients with a diagnosis of occult microcarcinoma during thyroidectomy for benign conditions or with poorly differentiated (insular) thyroid carcinoma were excluded. Patients were classified into histologic subtypes, which consisted of those with minimally invasive (encapsulated) carcinoma or those with widely invasive carcinoma, according to the extent and degree of invasiveness.24,27 Widely invasive carcinoma demonstrates extensive areas of invasion at the gross and microscopic levels, with evidence of tumor extending beyond the tumor capsule and diffusely infiltrating the affected lobe or entire gland.24,28 Minimally invasive FTC was further subdivided into minimally invasive FTC with capsular invasion only or with vascular invasion (angioinvasive) according to the type of invasion. Minimally invasive carcinoma with capsular invasion only (minimally invasive FTC) was diagnosed based on total encapsulation without macroscopic invasion and capsular invasion alone was identified on histologic examination.29 Angioinvasive FTC refers to a grossly encapsulated FTC, not a widely invasive type, with demonstration of vascular invasion.19,28 Extrathyroidal invasion was defined as the extension of tumor beyond the thyroid capsule to the perithyroidal tissue.
Bilateral thyroid resection, including total/near-total thyroidectomy or unilateral total and contralateral subtotal lobectomy (Dunhill's procedure), was adopted as the procedure of choice for the majority of patients from 1961 to 1990. Since 1991, it has been reserved for patients with widely invasive FTC after histologic examination. Adjuvant radioiodine (I131) and whole body scintigraphy were used for the majority of patients after bilateral thyroid resection. Postoperative complications with reference to recurrent nerve palsy and hypoparathyroidism were documented by the use of routine postoperative laryngoscopy and serum calcium monitoring as well as during follow-up visit.
Patients were followed by the same surgeon (C.-Y.L.) in conjunction with a clinical oncologist (K.-Y.W.) at a combined thyroid cancer clinic. Thyroxine suppressive therapy has been administered to patients after I131 therapy with thyroglobulin monitoring available since 1990. Follow-up data, including overall mortality, disease-specific mortality, mortality due to other unrelated causes, locoregional recurrence, and distant metastases, were obtained. Information on details of deaths was retrieved from death certificates and autopsy reports. Patient follow-up averaged 14.4 years (range, 0.1–38.6 years). The mean follow-up for patients still alive at last follow-up was 16 years (median, 11.1 years), with 1 year being the minimum. The cancer-specific survival (CSS) rate was calculated at 5 and 10 years after the initial surgical treatment with standard life-table methods by use of the Kaplan-Meier method.31
In the present study, covariates affecting CSS were analyzed in this group of patients with FTC. A univariate analysis was conducted for CSS using the Kaplan-Meier estimation method for the following variables: age at diagnosis (men <40 and women <50 versus men ≥40 and women ≥50 years), sex, tumor size (≤5 cm versus >5 cm), extrathyroidal invasion, lymphovascular invasion, lymph node metastases, initial distant metastases, tumor histologic types, thyroidectomy types (bilateral resection versus unilateral lobectomy), completeness of operation (incomplete versus complete), postoperative I131 ablation therapy, and external beam irradiation. Significance testing for differences in survival was performed with log-rank test for comparison between groups. A multivariate analysis was performed with BMDP statistical package using those variables significantly related to survival derived in the univariate analysis under the assumption of the Cox proportional risk regression hazards model.32 The significant risk factors were given with the hazard ratio (95% confidence interval) of each category. P value <0.05 was regarded as statistically significant. Subgroup analysis was performed to evaluate the role of histologic typing in predicting survival as well as in the management of patients with FTC. In addition, the application of several risk group stratification, staging systems, and prognostic scoring schemes including AMES risk group stratification,2,3 Degroot classification,9 UICC/AJCC pTNM staging system,33 and MACIS scoring scheme11 in predicting CSS was evaluated. Risk group stratification was allocated according to the schemes originally described, and the indices used for different scoring systems were calculated by the formulas obtained from the original studies (Table 1). Comparison between the staging systems was made by calculating the proportion of explained variation (PVE).34 The scoring system associated with a larger PVE would be the better predictor of survival.
TABLE 1. Stratification Schemes and Prognostic Scoring Systems Assessed in the Present Study
RESULTS
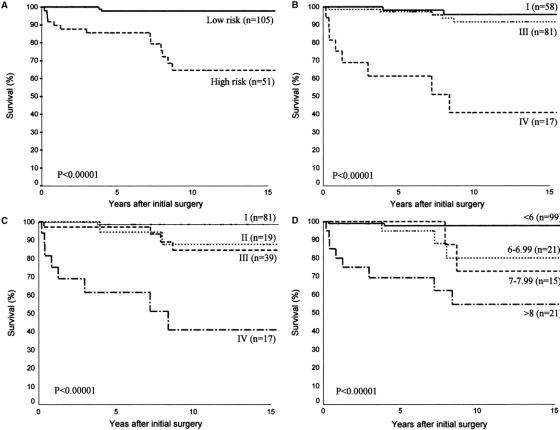
There were 25 men and 131 women with a median age of 44 years (range, 13–84 years). The primary tumor size ranged from 0.3 cm to 14 cm (median, 3 cm). Five (3%) patients had histologically confirmed lymph node metastases, and distant metastases were evident in 17 (11%) patients at presentation either by radiography or whole body scintigraphy. The site of distant metastases included skeletal (n = 7), lung (n = 3), both skeletal and lung (n = 3), brain (n = 2), mediastinum (n = 1), and scalp (n = 1). According to AMES stratification, 105 and 51 patients belonged to the low- and high-risk group, respectively. Fifty-eight, 81, and 17 patients classified as Degroot classes I, III, and IV, respectively. At presentation, after the distribution of patients into stages according to the AJCC/UICC pTNM classification adjusted for age, 81 patients corresponded to stage I, 19 to stage II, 39 to stage III, and 17 to stage IV. There were 99, 21, 15, and 21 patients with MACIS scores of <6, 6 to 6.99, 7 to 7.99, and >8, respectively. The tumors were classified histologically into minimally invasive (n = 49), angioinvasive (n = 23), and widely invasive (n = 84) carcinomas. Extrathyroidal invasion was evident in 16 (10%) patients, whereas lymphovascular invasion was detected in 76 (49%) patients.
Bilateral thyroid resection was performed for 131 (84%) patients. Operative procedures included unilateral lobectomy in 25, Dunhill's procedure in 11, and total or near-total thyroidectomy in 120 patients. Total or near-total thyroidectomy was performed either as a primary operation in 59 patients (11 of whom had previous partial thyroidectomy) or a staged completion procedure after unilateral lobectomy in 61 patients. Eight of 111 operations were unilateral lobectomy from 1961 to 1990 compared with 17 of 45 procedures from 1991 to 2000 (P < 0.001; Fisher exact test). The proportion of minimally invasive carcinoma was comparable between these 2 periods (32 of 111, 25% versus 17 of 45, 29%). Ten (3% of nerve at-risk) patients developed transient unilateral recurrent nerve paralysis, whereas 1 (0.4% of nerve at-risk) patient had permanent nerve palsy. Twenty-six (20%) and 8 (6%) patients of 131 patients at-risk had transient and permanent hypocalcaemia.
Adjuvant radioiodine (I131) in a standard dose of 100 mCi was administered to 116 of 131 (89%) patients after bilateral thyroidectomy, while 8 of them, in addition, received external beam irradiation before I131 therapy. All 17 (100%) patients with distant metastases received I131 therapy compared with 99 of 139 (71%) patients without distant metastases (P < 0.007). Three of 6 (50%) patients with residual tumors after an incomplete excision underwent external beam irradiation in comparison with 5 of 150 (3%) patients after macroscopic tumor clearance in the presence of pT4 tumors (P = 0.002).
At the time of the study, 38 patients died over a median duration of 87 months (range, 2.2–317 months). Seventeen (11%) patients died of recurrent and/or metastatic disease over a median duration of 46 months (range, 2.2–104 months), and 21 patients died of unrelated diseases with 8 of which due to other malignancies. The overall 5- and 10-year actuarial survival rates were 89% and 79%, respectively. The 5- and 10-year CSS rates were 94% and 88%, respectively, whereas the disease-free survival rates were 87% and 77%, respectively (Fig. 1). Of the 17 patients presenting with distant metastases, 9 died of thyroid cancer and 3 died of unrelated causes. Three patients were reverted to disease-free 4 to 20 years (median, 12 years) after the initial treatment, whereas 2 patients were alive with metastatic diseases. All except 1 patient with distant metastases had widely invasive carcinoma. That patient had an angioinvasive carcinoma documented to have sternal metastasis with complete response to external irradiation and I131 therapy, but subsequently died of carcinoma of the bladder 2 years after the initial treatment of her thyroid carcinoma. Nine (7%) of 135 patients without distant metastases or residual disease after surgical treatment developed recurrence more than 6 months after the initial surgery, including 5 locoregional, 3 distant, and 1 both. The median time to develop recurrence was 7.3 years (range, 23 months to 13.2 years). One of 71 patients with angioinvasive carcinoma developed locoregional recurrence compared with 8 of 64 patients with widely invasive carcinoma (P = 0.013).
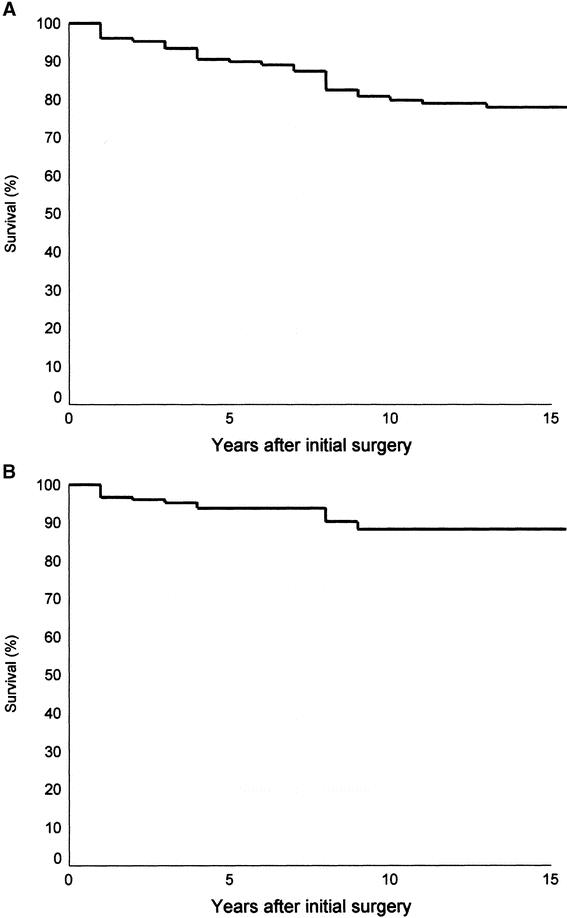
FIGURE 1. Actuarial overall (A) and cancer-specific (B) survival curves for 156 patients with follicular thyroid carcinoma.
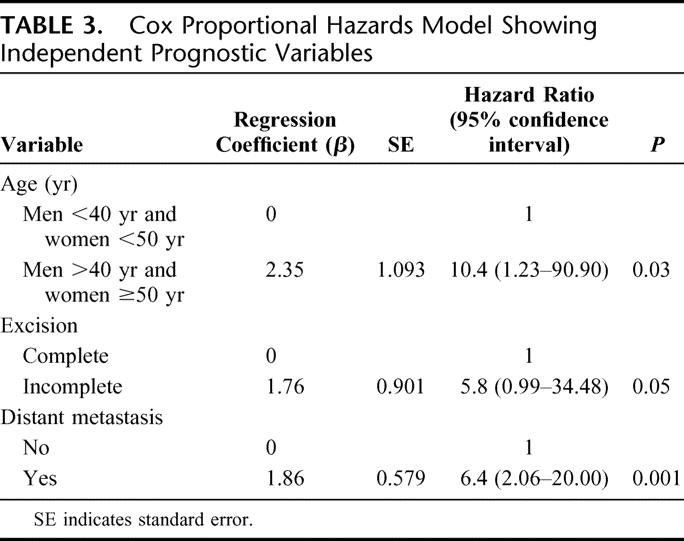
A univariate analysis for CSS showed that old age (men ≥40 years; women ≥50 years), widely invasive follicular carcinoma histology, lymphovascular invasion, presence of distant metastases, incomplete tumor excision, and administration of external beam irradiation are the risk factors for poor survival (Table 2). All young patients except 1 woman <50 years at presentation and all patients with histology type of minimally invasive or angioinvasive carcinoma survived their thyroid cancer. Using Cox proportional hazards model for variables identified to be significant in univariate analysis, only patient age group, initial distant metastases, and completeness of operation were the independent prognostic factors for survival (Fig. 2). Old patients (men ≥40 and women ≥50 years) had a 10.4-fold (1.23–90.9) (95% confidence interval) relative risk of CSS. In addition, patients who underwent an incomplete excision of the tumor with postoperative residual disease as determined by the operating surgeon, and those with distant metastasis at presentation had a 5.8- (0.99–34.48) and 6.4-fold (2.06–20) relative risk of dying from FTC, respectively (Table 3).
TABLE 2. Analysis of Risk Factors for Cause-Specific Mortality (CSM) Using Univariate Analysis
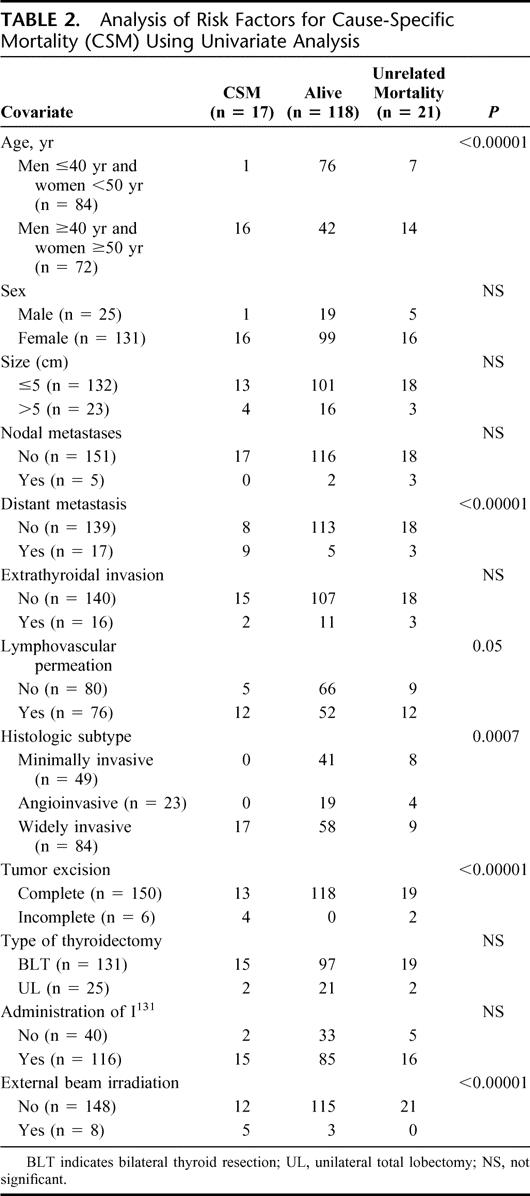
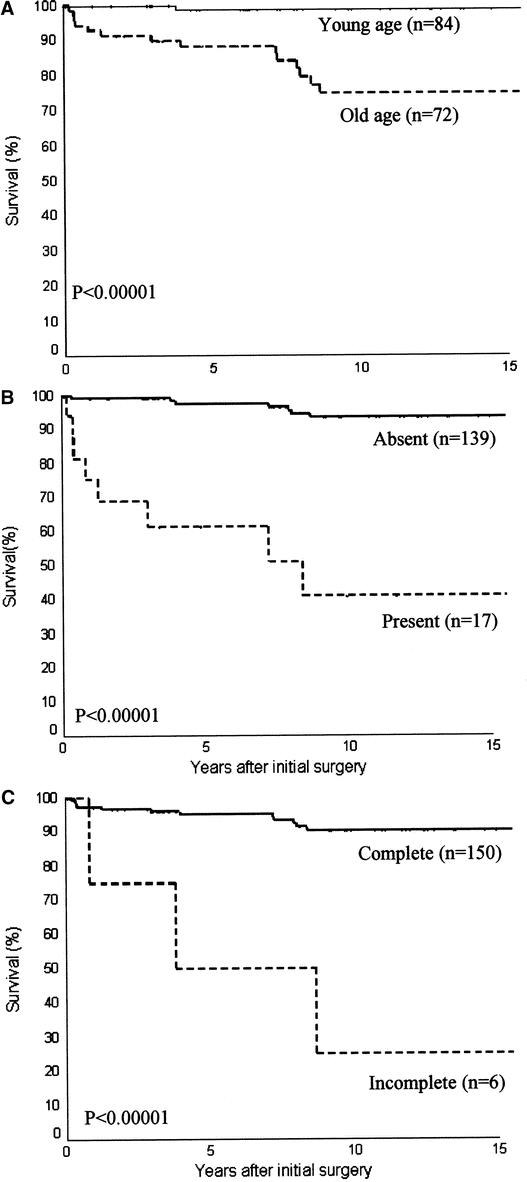
FIGURE 2. Actuarial cancer-specific survival curves of three independent prognostic factors in multivariate analysis under Cox proportional hazards model: age (A), the presence of distant metastasis (B), and completeness of excision (C).
TABLE 3. Cox Proportional Hazards Model Showing Independent Prognostic Variables
Twenty-one patients had initial distant metastases or residual disease after an incomplete surgical excision and 12 died of thyroid carcinoma during follow-up. Patients without distant metastases or residual disease were regarded as having a curable surgical treatment (n = 135), and only 5 patients died of thyroid carcinoma (P < 0.00001). Those with widely invasive carcinoma had a significantly worse survival compared with those with minimally invasive together with angioinvasive carcinomas. Five of 64 patients with widely invasive carcinoma died compared with none of 71 patients with minimally invasive or angioinvasive carcinomas (P = 0.03). Similarly, survival was significantly worse for older patients (5 of 53 older patients versus 0 of 82 younger patients, P = 0.003). All other factors analyzed were not statistically significant. Subgroup analysis of 64 patients with widely invasive carcinoma who underwent curable surgical treatment showed that either bilateral thyroid resection or the administration of I131 therapy improved CSS, although the difference was only approaching statistical significance. One of 4 (25%) patients who received unilateral lobectomy died compared with 4 of 60 (6.7%) patients who underwent bilateral procedures (P = 0.07). In addition, 3 of 52 (5.8%) patients who received postoperative I131 compared with 2 of 12 (16.7%) who did not receive I131 died of FTC (P = 0.08).
All risk group stratification schemes and prognostic scoring systems evaluated including AMES risk group stratification, Degroot classification, UICC/AJCC pTNM staging, and MACIS scoring systems accurately predicted CSS (Fig. 3). The difference between the respective stages and/or risk groups was highly significant for every stratification scheme and staging system (P < 0.00001) in predicting CSS. The PVE associated with each system was in descending order: 20.12% for UICC/AJCC pTNM staging, 17.51% for MACIS, 16.97% for AMES, and 16.49% for Degroot class.
FIGURE 3. Actuarial cancer-specific survival curves for patients with follicular thyroid carcinoma categorized using AMES risk group stratification (A), DeGroot classification (B), UICC/AJCC pTNM staging (C), and MACIS scoring systems (D).
DISCUSSION
FTC is a relatively rare form of thyroid cancer accounting for 4% to 39% of all thyroid malignancies,27 and they usually constitute only a small proportion of the study cohorts for thyroid cancer.2–8 Despite classified collectively as WDTC, PTC, and FTC have distinct clinicopathologic features, biologic behavior, and clinical outcome.4–7 FTC is generally considered to be a more aggressive tumor than PTC and is associated with a worse prognosis. Patients with FTC often present with more advanced stage diseases and a higher incidence of distant metastases because of the propensity of vascular invasion. In contrast, lymph node metastasis is quite uncommon in FTC with an average incidence of <10%.27 However, patients with FTC and PTC have similar prognosis when they are matched for age and stage.18 Various variables including older age,13,18 distant metastases at presentation,13,14,17,35 extrathyroidal invasion,27 nodal metastases,37 larger tumor size,20 marked vascular invasion,13,19 widely invasive histologic subtype,24,26 and postoperative locoregional disease35 have been identified as the independent prognostic factors for survival in multivariate analysis. In our previous study on 64 patients with FTC having a minimum follow-up of 7 years, the presence of distant metastases was the only independent factor for poor survival in the multivariate analysis.17 In the present study, by cumulating a larger number of patients with a longer follow-up period, old age and incomplete tumor excision were identified to be the additional independent risk factors for survival.
Risk group stratification has been applied in the management of patients with WDTC.2,3 Some of the risk group stratification schemes were applied for both histologic types of WDTC,2,6,7 whereas others were evaluated for PTC only.9,10 In the past, the scores for metastases, age, completeness of resection, invasiveness, and size (MACIS, Mayo Clinic),10 and intrathyroidal tumor, nodal metastases, extrathyroidal tumor, and distant metastases classification (Degroot classification, University of Chicago)9 were derived from patients with PTC. In addition, the scores for the age, metastases, extrathyroidal invasion, and size (AMES, Lahey Clinic)2,3 and UICC/AJCC pTNM staging33 had been applied collectively for patients with WDTC. In the present study, it was shown that these risk group stratification, classification, staging system, and prognostic scoring schemes could be applied with prognostic accuracy specifically for patients with FTC. The applicability of these stratification schemes and scoring systems to patients with FTC was consistent with the identification of the 3 independent prognostic factors in the multivariate analysis for survival. These factors, including old age at presentation, the presence of distant metastases, and incomplete excision, are commonly incorporated as prognostic factors in these staging systems and for determining the allocation of scores in these scoring systems.
The theoretical application of these risk-related groups is to define a set of patients in which a limited operation and possibly no I131 treatment would be appropriate to avoid morbidity of a more aggressive treatment. However, despite the identification of these prognostic factors, current published therapeutic guidelines call for total thyroidectomy followed by I131 ablation for all patients with FTC7,36 because of the poorer survival of FTC compared with PTC patients. Although there have been no ultrastructural, morphometric, flow cytometry, immunohistochemical, or oncogene expression studies proven to be reliably predictable in separating benign from malignant neoplasms or between grades of carcinomas,27 histomorphologic criteria have been frequently adopted and applied to separate these categories in FTC. Nevertheless, it remains as a source of confusion because of the different classification and histologic criteria used by different pathologists.27,30 Apart from classifying the tumors into intrathyroidal or extrathyroidal based on thyroid capsular extension,35,37 FTC was more commonly classified as either “minimally invasive encapsulated” or “widely invasive” by the Armed Forces Institute of Pathology classification,28 which followed the WHO guidelines.24 A diagnosis of minimally invasive carcinoma can be made either on the basis of capsular invasion alone, vascular invasion alone, or the presence of both types of invasions. A pathologic distinction between low-grade minimally invasive (encapsulated) and widely invasive follicular thyroid carcinoma as suggested by WHO24 is valid for prognosis and has been adopted in guiding treatment of FTC.26,27 Minimally invasive carcinoma as defined as such is associated with excellent prognosis and long-term recurrence rate of <5%.27
There is no doubt that FTC with capsular invasion alone behaves like a benign tumor, with a zero disease-specific mortality as well as distant metastases at 10 years’ follow-up, regardless of primary treatment.14 FTC with capsular invasion alone should be managed with a more conservative surgical approach in the form of hemithyroidectomy without additional therapy in view of its excellent survival rate.14,30 On the other hand, the presence of vascular invasion alone increased the disease-specific mortality to 28% at 10 years,14 although in multivariate analysis, the presence of distant metastases was the only independent prognostic factor for survival. Vascular invasion alone may be a better indication of malignancy than capsular penetration4,25 because of the greater probability of recurrence and metastases caused by angioinvasion. It was suggested that minimally invasive encapsulated FTC with vascular invasion should be distinguished from those with capsular invasion only.29 Encapsulated FTC with capsular invasion alone should be termed minimally invasive carcinoma, whereas those with vascular invasion should be coined angioinvasive29 or moderately invasive carcinoma.19 However, the association of vascular invasion in minimally invasive carcinoma with poorer prognosis was demonstrated in some14,19 but not all studies.27,38
Interestingly, the most frequently used prognostic systems did not include the extent and degree of invasion (minimally invasive versus widely invasive FTC) as well as vascular invasion in their criteria. In our present study, we incorporated the histologic subtypes of minimally invasive (encapsulated), angioinvasive, and widely invasive carcinomas into the multivariate analysis to define its role in predicting survival. However, similar to others,14,27,38 we were unable to document the significance of either vascular invasion or histologic group as an independent prognostic factor in the multivariate analysis. None of our patients with minimally invasive FTC with or without vascular invasion died of thyroid cancer. Patients with widely invasive carcinoma were presumed to have a number of other confounding factors, such as older age, incomplete excision due to widespread invasive growth, and the presence of distant metastases at presentation.
For those patients who underwent a complete excision in the absence of distant metastases, both older age and widely invasive carcinoma were the risk factors for survival. Histologic classification according to the extent of invasion into minimally invasive or widely invasive carcinoma remains useful and reliable for guiding the management of this subgroup of patients after potential curative surgical treatment. Either bilateral thyroid resection or I131 therapy was associated with an improved survival in this selected group of patients with widely invasive carcinoma, although the number of patients was probably too small to reach statistical significance. On the other hand, total thyroidectomy with I131 treatment should also be adopted for patients with distant metastases or residual disease because patients can be reverted back to disease-free with long-term survival after therapy during follow-up.
CONCLUSION
Commonly adopted staging systems for WDTC or PTC can be applied specifically to patients with FTC. The UICC/AJCC pTNM staging system remains the most accurate prognostic system for FTC and is the staging system of choice for comparison purposes as well as guiding management. Despite the lack of significance as an independent prognostic indicator, the distinction of FTC between minimally invasive and widely invasive carcinomas based on histomorphology criteria using the degree and extent of invasion is important and practical in the identification of low-risk patients for a more conservative management. Vascular invasion in minimally invasive FTC can be associated with distant metastases and locoregional recurrence, but this does not seem to adversely affect the long-term prognosis. Hemithyroidectomy can be considered as adequate treatment of patients with minimally invasive carcinoma with or without vascular invasion after histologic confirmation, whereas total thyroidectomy followed by adjuvant I131 therapy should be reserved for patients with widely invasive carcinoma based on histomorphologic criteria or for those high-risk patients using the commonly adopted staging systems.
ACKNOWLEDGMENTS
The authors thank Mr. Ping-Yin Lee, BSocSc (Statistics) for providing advice on statistical analysis.
Footnotes
Reprints: Chung-Yau Lo, MS(HK), Division of Endocrine Surgery, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Pokfulam, Hong Kong, China. E-mail: cylo@hkucc.hku.hk.
REFERENCES
- 1.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma: a study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–1041. [DOI] [PubMed] [Google Scholar]
- 2.Cady B, Rossi R, Silverman M, et al. Further evidence of the validity of risk group definition in differentiated thyroid carcinoma. Surgery. 1985;98:1171–1178. [PubMed] [Google Scholar]
- 3.Cady, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 4.Simpson WJ, McKinney SE, Carruthers JS, et al. Papillary and follicular thyroid cancer: prognostic factors in 1578 patients. Am J Med. 1987;83:479–488. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MP, Duda RB, Recant W, et al. Survival discriminants for differentiated thyroid cancer. Am J Surg. 1990;160:344–347. [DOI] [PubMed] [Google Scholar]
- 6.Lerch H, Schober O, Kuwert T, et al. Survival of differentiated thyroid carcinoma studied in 500 patients. J Clin Oncol. 1997;15:2067–2075. [DOI] [PubMed] [Google Scholar]
- 7.Loh KC, Greenspan FS, Gee L, et al. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–3562. [DOI] [PubMed] [Google Scholar]
- 8.Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–388. [PubMed] [Google Scholar]
- 9.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 11.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058. [PubMed] [Google Scholar]
- 12.Sebastian SO, Rodriguez Gonzalez JM, Paricio PP, et al. Papillary thyroid carcinoma; prognostic index for survival including the histological variety. Arch Surg. 2000;135:272–277. [DOI] [PubMed] [Google Scholar]
- 13.Brennan MD, Bergstrahl EJ, van Heerden JA, et al. Follicular thyroid cancer treated at Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11–22. [DOI] [PubMed] [Google Scholar]
- 14.van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a non-threatening malignancy. Surgery. 1992;112:1130–1136. [PubMed] [Google Scholar]
- 15.Emerick GT, Duh QY, Siperstein AE, et al. Diagnosis, treatment, and outcome of follicular thyroid carcinoma. Cancer. 1993;72:3287–3295. [DOI] [PubMed] [Google Scholar]
- 16.Shaha AR, Loree RT, Shah JP. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery. 1995;118:1131–1138. [DOI] [PubMed] [Google Scholar]
- 17.Lo CY, Lorentz TG, Wan KY. Follicular carcinoma of the thyroid gland in Hong Kong Chinese. Br J Surg. 1995;82:1095–1097. [DOI] [PubMed] [Google Scholar]
- 18.DeGroot LJ, Kaplan EL, Shuka MS, et al. Morbidity and mortality in follicular thyroid cancer. J Clin Endocrinol Metab. 1995;80:2946–2953. [DOI] [PubMed] [Google Scholar]
- 19.D'Avanzo A, Treseler P, Ituarte PH, et al. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;15:1123–1129. [DOI] [PubMed] [Google Scholar]
- 20.Zidan J, Kassem S, Kuten A. Follicular carcinoma of the thyroid gland: prognostic factors, treatment, and survival. Am J Clin Oncol. 2000;23:1–5. [DOI] [PubMed] [Google Scholar]
- 21.Davis NL, Bugis SP, McGregor GI, et al. An evaluation of prognostic scoring systems in patients with follicular thyroid cancer. Am J Surg. 1995;170:476–480. [DOI] [PubMed] [Google Scholar]
- 22.D'Avanzo A, Ituarte P, Treseler P, et al. Prognostic scoring systems in patients with follicular thyroid cancer: a comparison of different staging systems in predicting the patient outcome. Thyroid. 2004;14:453–458. [DOI] [PubMed] [Google Scholar]
- 23.Passler C, Prager G, Scheuba C, et al. Application of staging system for differentiated thyroid carcinoma in an endemic region with iodine substitution. Ann Surg. 2003;237:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedinger CE, Williams ED, Sobin LH. Histological typing of thyroid tumours. In: Hedinger CE, ed. International Histological Classification of Tumors, vol. 11. Berlin: Springer-Verlag, 1988:7–68. [Google Scholar]
- 25.Lang W, Choritz H, Hundehagen H. Risk factors in follicular thyroid carcinomas: a retrospective follow-up covering a 14 year period with emphasis on morphological findings. Am J Surg Pathol. 1986;10:246–255. [PubMed] [Google Scholar]
- 26.Gemsenjager E, Heitz PU, Martina B. Selective treatment of differentiated thyroid carcinoma. World J Surg. 1997;21:546–552. [DOI] [PubMed] [Google Scholar]
- 27.Thompson LDR, Wieneke JA, Paal E, et al. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer. 2001;91:505–524. [DOI] [PubMed] [Google Scholar]
- 28.Rosai J, Carcangiu ML, DeLellis RA. Tumours of the thyroid gland. In: Rosai J, Sobin LH, eds. Atlas of Tumour Pathology, 3rd series, fascicle 5. Washington, DC: Armed Forces Institute of Pathology, 1992:49–63. [Google Scholar]
- 29.Baloch ZW, Livolsi VA. Pathology of the thyroid gland. In: Livolsi VA, Asa SL, eds. Endocrine Pathology. New York: Churchill Livingstone, 2002:61–88. [Google Scholar]
- 30.Delbridge L, Parkyn R, Philips J, et al. Minimally invasive follicular thyroid carcinoma: completion thyroidectomy or not. ANZ J Surg. 2002;72:844–845. [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparameteric estimation from incomplete observation. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 32.Cox DR. Regression models and life tables. J R Stat Soc (B). 1972;34:187–220. [Google Scholar]
- 33.International Union Against Cancer. In: Sobin LH, Wittlekind C, eds. TNM Classification of Malignant Tumours, 5th ed. New York: Wiley, 1997. [Google Scholar]
- 34.Schemper M, Stare J. Explained variation in survival analysis. Stat Med. 1996;15:1999–2012. [DOI] [PubMed] [Google Scholar]
- 35.Simpson WJ, Panzarella T, Carruthers JS, et al. Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988;14:1063–1075. [DOI] [PubMed] [Google Scholar]
- 36.Hay ID, Feld S, Garcia M, et al. AACE clinical practice guidelines for the management of thyroid carcinoma. Endocr Pract. 1997;3:60–71. [Google Scholar]
- 37.Saadi H, Kleidermacher P, Esselstyn C Jr. Conservative management of patients with intrathyroidal well-differentiated follicular thyroid carcinoma. Surgery. 2001;130:30–35. [DOI] [PubMed] [Google Scholar]
- 38.Furlan JC, Bedard YC, Rosen IB. Clinicopathologic significance of histologic vascular invasion in papillary and follicular thyroid carcinomas. J Am Coll Surg. 2004;198:341–348. [DOI] [PubMed] [Google Scholar]