Abstract
Objective:
To study the predictive value of Nod2/CARD15 gene variants along with disease phenotypic characteristics for requirement of initial surgery and for surgical recurrence in Crohn's disease (CD).
Summary Background Data:
Nod2/CARD15 gene variants play an important role in the susceptibility to CD. Studies of genotype-phenotype relationship suggest that these variants are associated with development of intestinal strictures. Preliminary reports analyzing the association between these variants and need for surgery have produced inconsistent results.
Methods:
A total of 170 CD patients were included prospectively in the study and followed up regularly for a mean of 7.4 ± 6.1 years. Clinical characteristics of CD, time and indication for surgery, and recurrence were registered. Nod2/CARD15 gene variants were determined by DNA sequencing analysis.
Results:
Surgery for stricturing disease was significantly more frequent in patients with Nod2/CARD15 variants in the univariate analysis (odds ratio [OR], 3.63; 95% confidence interval [CI], 1.42–9.27), and it was required at an earlier time (P = 0.004). Only Nod2/CARD15 variants (OR, 3.58; 95% CI, 1.21–10.5) and stricturing phenotype at diagnosis of CD (OR, 9.34; 95% CI, 2.56–33.3) were independent predictive factors of initial surgery for stricturing lesions in the multivariate analysis. Among 70 patients that required surgery, postoperative recurrence was also more frequent in patients with Nod2/CARD15 variants in the univariate and multivariate analysis (OR, 3.29; 95% CI, 1.13–9.56), and reoperation was needed at an earlier time (P = 0.03).
Conclusion:
Nod2/CARD15 variants are associated with early initial surgery due to stenosis and with surgical recurrence in Crohn's disease. Patients with these variants could benefit from preventive and/or early therapeutic strategies.
Nod2/CARD15 gene is a determinant of Crohn's disease susceptibility. This study demonstrates that Crohn's disease patients bearing Nod2/CARD15 gene variants have significantly higher and earlier requirement for initial surgery because of intestinal strictures. Patients with Nod2/CARD15 gene variants also have an increased and earlier need for reoperation.
Despite the advances in our understanding of Crohn's disease (CD), the course of the disease in a particular patient remains unpredictable. Surgery is frequently needed, and a large number of patients require more than 1 operation.1,2 Several articles have attempted to define predictive factors for requirement of initial surgery1,3–5 or reoperation1,5–16 based on the analysis of clinical variables. However, the results of these studies have been inconsistent. This may be attributable to differences in study design concerning patient population, clinical classification used, criteria for variables definition, ethnicity, or duration of CD. Besides, it is important to note that clinical characteristics of CD may change over time;17 therefore, classification of CD patients may significantly benefit from associated stable genetic factors.
Three variants of Nod2/CARD15 gene (R702W, G908R, L1007fsinsC), present in 30% to 50% of CD white patients, are implicated in the susceptibility to CD.18–21 Since a number of genotype-phenotype studies have suggested that Nod2/CARD15 variants are associated with ileal disease and development of intestinal strictures,21–26 it is conceivable that variants of this gene may be also accurate predictors of surgery requirement. A few studies have assessed surgery requirement in relation to Nod2/CARD15 genotype. In 2 recent reports Nod2/CARD15 gene variants were more frequent in patients requiring bowel surgery,25,27 whereas another study found an association only with surgery indicated for the treatment of stricturing lesions.24 However, in none of these studies were the Nod2/CARD15 gene variants independent predictors of surgery requirement. In the only study that evaluated the need of a second surgery, an association with the Nod2/CARD15 gene variants was not found.24
If Nod2/CARD15 genotype were a good predictor of surgery requirement, this genetic factor would allow identification of patients who might benefit from preventive and/or early therapeutic interventions. Therefore, the aim of our study was to ascertain whether the Nod2/CARD15 gene variants are independent predictive factors for both initial surgery and the need of subsequent surgical procedures in patients with CD.
PATIENTS AND METHODS
Patients and Criteria for Diagnosis
Unrelated CD patients attending a single inflammatory bowel disease unit of a tertiary referral center in Spain and regularly followed were included in this study between December 2002 and January 2004. The diagnosis of CD was based on standard clinical, radiologic, endoscopic, and histologic criteria.28 All patients gave their written informed consent to participate in the study after approval of the project by the local ethical committee. The study was performed in accordance to the principles stated in the declaration of Helsinki.
Data Management and Definitions
All clinical data were obtained from a prospective clinical database of our unit. At the initial visit, a complete clinical evaluation was performed. This evaluation included gender, previous appendectomy, smoking habit, family history of inflammatory bowel disease, age at diagnosis of CD, location and behavior of CD, and extraintestinal manifestations of CD.
Smokers were defined as patients who smoked more than 7 cigarettes per week (1 cigarette daily). Ex-smoker was defined as a patient who gave up smoking at least 1 year before diagnosis. Nonsmokers were defined as patients who had never smoked or smoked less than 7 cigarettes per week.15 Location of CD was determined according to x-ray and endoscopic findings; all patients underwent these procedures at least once in our center. Location of CD was registered in not exclusive groups, according to the following criteria: upper gastrointestinal tract, for any disease location proximal to the terminal third of ileum; terminal ileum if evidence of disease existed in the terminal third of ileum with or without spill over into the cecum; colonic disease if evidence of disease existed in the colon; and perianal disease if there was evidence of fistula and/or perianal abscess. Since classification was not exclusive, patients could belong to more than 1 group. For initial behavior of CD, we used a criteria based on the Vienna classification:29 penetrating, for intra-abdominal or perianal fistulas, inflammatory masses, and/or abscess; stricturing, for occurrence of luminal narrowing (radiologic or endoscopic) with prestenotic dilatation or obstructive signs, without concomitant penetrating disease; and inflammatory, for the rest of CD patients.
Follow-up
Patients were visited in the outpatient clinic unit at least twice a year. The following characteristics were registered at each visit: date, smoking habit, use of steroids, use of immunosuppressants, disease activity, change in behavior and extension of disease, and specifically date, indication, and type of surgery and surgical recurrence, and date of medical recurrence.
Each steroid course was registered to obtain a mean number of steroid courses per year of follow-up. For disease activity, each flare of CD was registered. Flare of CD was defined as presence of digestive and/or general symptoms that led to a change in the patient treatment by the physician in charge (J.P., M.S., M.A.L.). A mean number of flares per year of follow-up was also calculated for every patient. Behavior of CD in the follow-up was registered as nonexclusive categories; thus, the presence of intestinal stricturing was also registered if stricturing occurred at a different time than the penetrating lesion, so that patients could belong to both categories, if they occur at different time points of CD evolution. The net effect of this categorization was that patients with stricturing and penetrating behavior, which are only classified as penetrating in the Vienna classification, were categorized as stricturing and penetrating in the nonexclusive categorization.
Surgery was defined as any procedure involving bowel resection or strictureplasty (the latter was performed in only 1 patient). For perianal disease, surgery was defined as requirement of fistulae resection and/or abscess drainage. We considered that surgery for treatment of perianal disease as defined above should be also analyzed since it is an important feature in the treatment of penetrating CD affecting the anorectum.30 Indication for surgery was defined as the primary reason for operation based on clinical presentation, preoperative diagnostic studies, and intraoperative findings. Surgical indications were divided into three groups: stricturing disease, referring to constant intestinal obstruction that does not respond to medical treatment; perforating disease, which included internal or external fistula formation, abscess, or acute free perforation; and other indications, including medical intractability, hemorrhage, toxic dilatation of the colon without perforation, and cancer. Type of surgery refers to the surgical procedure performed. It was divided into 4 types: small bowel resection, colon resection, perianal surgery, and combined procedures for any combination of the previous stated procedures. Those patients who underwent ileostomy, colostomy, planned second-stage surgery, or surgery for a complication of a prior operative procedure were excluded. For the analysis of time for the initial surgery, the start of follow-up (survival time) began at the time of diagnosis and ended at the time of initial surgery or end of follow-up. Surgical recurrence was defined as the need of reoperation for treatment of CD, as has been previously defined.31 For the analysis of time to medical recurrence and surgical recurrence after the first surgery, the start of follow-up (survival time) began at the first surgery and ended when a clinical flare of CD occurred (defined as presence of symptoms that led to a change in treatment) for medical recurrence, at the second surgery for surgical recurrence, or at the end of follow-up if these events had not occurred.
Nod2/CARD15 Genotyping
In all patients, DNA was collected and genotyped for the three main variants of Nod2/CARD15 gene that are associated with CD (MIM 266600). Genomic DNA from whole blood samples was isolated using QIAmp DNA Blood Mini Kit (QIAgen, Hilden, Germany) following the manufacturer's instructions. Specific sequences of exon 4 (missense mutation R702W), exon 8 (missense mutation G908R), and exon 11 (frameshift mutation L1007fsinsC) of Nod2/CARD15 gene were amplified by polymerase chain reaction (PCR), using specific primers and conditions previously published (Primer 4-CF: GAAGTACATCCGCACCGAG and Primer 4-ER: GCTCCCCCATACCTGAAC. Primer 8-F: AAGTCTGTAATGTAAAGCCAC and Primer 8-R: CCCAGCTCCTCCCTCTTC. Primer 11-F: CTCACCATTGTATCTTCTTTTC and Primer 11-R: GAATGTCAGAATCAGAAGGG).21 The PCR products were purified using QIAquick PCR Purification Kit (QIAgen), according to the manufacturer's instructions, sequenced using an ABI BigDye Terminators v1.1 Cycle Sequencing Kit (Foster City, CA) and run on an ABI 3100 automatic sequencer. The investigators who determined the genotypes were blinded to the clinical characteristics of the patients. CD patients categorized as carrying variants were anyone with at least 1 variant of the Nod2/CARD15 gene.
Statistical Analysis
Categorical variables were compared using the χ2 test, applying the Yates correction when necessary. Continuous variables were expressed as the mean ± SD and compared using Student t test. We used univariate analysis to study the association between surgery requirement and clinical characteristics and Nod2/CARD15 genotype. Variables reaching a P value of less or equal than 0.10 in the univariate analysis were entered into the multivariate logistic regression analysis. Probability curves of surgery requirement after diagnosis or after first surgery (surgical recurrence), and probability curves of remaining free of medical recurrence after initial surgery were calculated according to the Kaplan-Meier method and compared using the log-rank test. Patients who did not have medical or surgical recurrence at the end of follow up were censored. All P values were 2-sided, and a value of less than 0.05 was considered to indicate a statistically significant difference. Analysis was carried out using the StatView software package (SAS Institute Inc., Cary, NC).
RESULTS
Patient Population
The study population consisted of 170 patients with CD (91 males, 79 females; mean age, 37.9 ± 14 years). Mean follow-up was 7.4 ± 6.1 years with a median of 5.6 years. Behavior of CD at diagnosis was inflammatory in 123 patients (72.3%), stricturing in 16 patients (9.4%), and penetrating in 31 patients (18.2%). Sixteen cases (9.4%) had lesions in the upper gastrointestinal tract, 128 (75.2%) in the terminal ileum, 107 (63%) in the colon, and 30 (17.6%) had perianal disease.
Behavior of CD changed over time in a significant proportion of patients. Applying the nonhierarchic criteria at the end of follow-up, 73 patients (42.9%) maintained a purely inflammatory phenotype, 54 patients (31.7%) had structuring lesions, and 71 patients (41.7%) had a penetrating phenotype at some time during follow-up.
Nod2/CARD15 Genotype
Fifty-two patients (30.5%) had at least 1 mutation of the Nod2/CARD15 gene. Eight were homozygotes or compound heterozygotes and 44 were simple heterozygotes for these mutations. Distribution of each 1 of the 3 variants was as follows: 30 (17.6%) for R702W, 8 (4.7%) for G908R, and 18 (10.5%) for L1007fsinsC.
At the time of CD diagnosis, only ileal disease (P = 0.008) was associated with Nod2/CARD15 gene variants. A trend toward an earlier age at diagnosis (P = 0.07) was observed in patients carrying these variants. None of other clinical characteristics at the time of CD diagnosis was associated with Nod2/CARD15 variants, including gender, previous appendectomy, smoking habit, family history of inflammatory bowel disease, behavior of CD, and extraintestinal manifestations of CD.
Analysis of Factors Influencing Initial Surgery
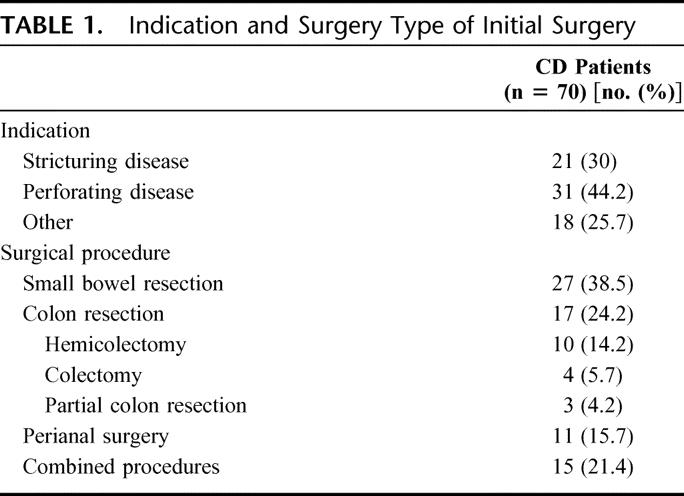
Seventy patients (41.1%) required at least 1 surgical procedure for treatment of CD during the follow-up period. The absolute cumulative frequency of surgery was 22.5%, 35.3%, and 50.4% at 1, 5, and 10 years, respectively, after diagnosis. The mean time of disease duration at the moment of first surgery was 2.8 ± 4.7 years, with a median of 12.0 months. The indication for surgery and type of surgical intervention performed are shown in Table 1.
TABLE 1. Indication and Surgery Type of Initial Surgery
Comparison of clinical characteristics at the time of diagnosis between patients who required surgery and patients who did not are shown in Table 2. Nod2/CARD15 genotype variants, stricturing phenotype, penetrating phenotype, and ileal disease were statistically associated with first surgery requirement, whereas colonic disease and extraintestinal manifestations were associated with a lower proportion of patients requiring surgery. In the group that required surgery, 28 patients (40%) had variants of Nod2/CARD15 compared with 24 patients (24%) in the group that did not require surgery (P = 0.02). On the other hand, 28 of 52 patients (53.8%) with Nod2/CARD15 variants required surgery compared with 42 of 118 patients (35.5%) without Nod2/CARD15 variants (odds ratio [OR], 2.11; 95% confidence interval [CI], 1.08–4.09; P = 0.02). In addition, patients with Nod2/CARD15 variants had an earlier requirement of surgery in the Kaplan-Meier survival analysis (P = 0.0089) (Fig. 1). If we exclude from the analysis the 11 patients who underwent their first surgery for treatment of perianal disease, the significant association between carrying Nod2/CARD15 variants and increased surgery requirements is maintained (OR, 2.32; 95% CI, 1.16–4.64), as well as the significantly reduced period of time between diagnosis of CD and initial surgery according to Kaplan-Meier survival analysis (P = 0.0059).
TABLE 2. Clinical Characteristics at Diagnosis of CD and Nod2/CARD15 Gene Status According to Surgery Requirement
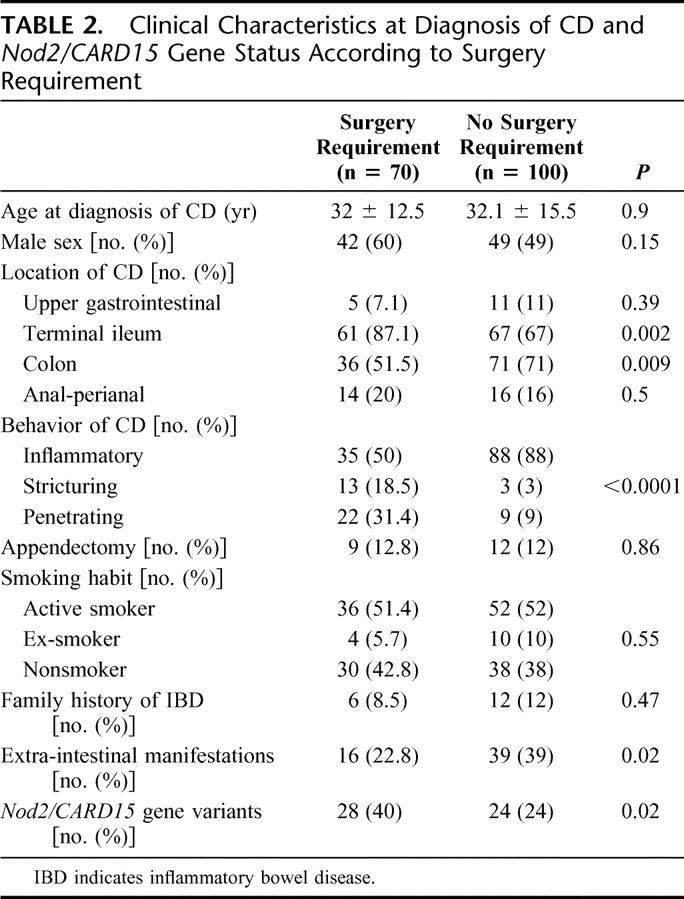
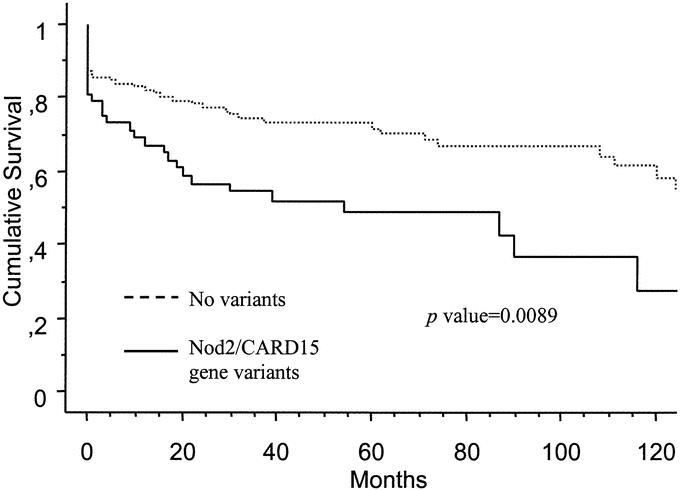
FIGURE 1. Actuarial probability of remaining free of surgery according to Nod2/CARD15 gene status.
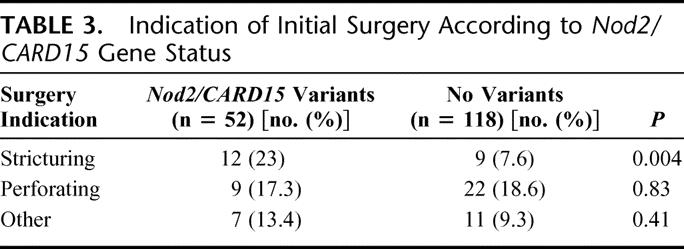
We assessed the relationship between gene status and surgery indications to determine whether the association was with all surgery indications or only with some specific indication (Table 3). Among indications for initial surgery, only the proportion of patients that underwent surgery for stricturing disease was significantly higher in patients with Nod2/Card15 variants 12 of 52 (23%), compared with 9 of 118 patients (7.6%) without variants (OR, 3.63; 95% CI, 1.42–9.27; P = 0.004). There was no difference in indications for perforating disease or other indications between patients with Nod2/Card15 variants and those without.
TABLE 3. Indication of Initial Surgery According to Nod2/CARD15 Gene Status
In the univariate analysis of factors associated with initial surgery for stricturing disease, a significant association was found with behavior at diagnosis (stricturing disease) (P < 0.0001), absence of colonic disease (P = 0.002) and Nod2/CARD15 gene variants (P = 0.004). In addition to these, ileal disease (P = 0.08) and extraintestinal manifestation (P = 0.09) had also a P < 0.1 in the univariate analysis. The multivariate analysis looking for independent predictive factors for initial bowel surgery for stricturing disease at the time of diagnosis, including all the aforementioned variables, demonstrated that only Nod2/CARD15 gene variants (OR, 3.58; 95% CI, 1.21–10.5) and stricturing behavior at the time of diagnosis (OR, 9.34; 95% CI, 2.56–33.3) were independent predictive factors for initial bowel surgery for stricturing disease. Ileal location of CD (OR, 1.27; 95% CI, 0.22–7.3), colonic location (OR, 032; 95% CI, 0.10–1.01) or extraintestinal manifestations (OR, 0.7; 95% CI, 0.10–3.31) were not significant independent predictive factors. In addition, Nod2/CARD15 gene variants were associated with an earlier requirement of initial surgery due to stricturing disease in the Kaplan-Meier analysis (P = 0.004) (Fig. 2).
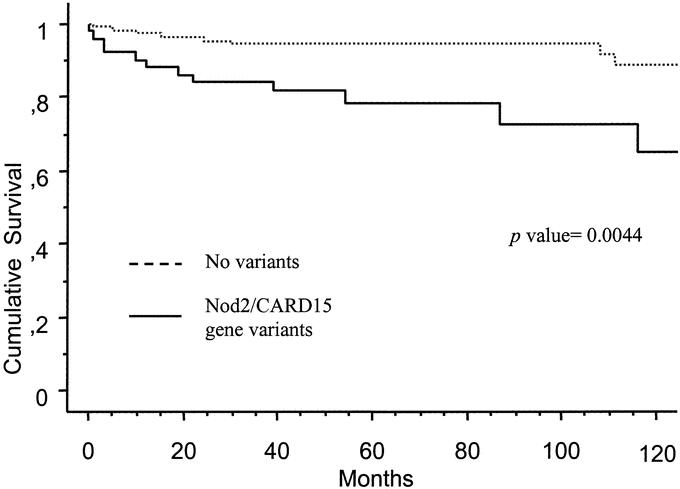
FIGURE 2. Actuarial probability of remaining free of initial surgery for treatment of stricturing lesions according to Nod2/CARD15 gene status.
Analysis of Factors Influencing Surgical Recurrence
Of 70 CD patients requiring an initial operation, 23 patients (33%) required a second surgery. The cumulative relapse rates were 15.4% and 28.0% at 1 and 5 years, respectively, after the first surgery. The time of follow-up was similar between patients that required reoperation (6.2 ± 6.3 years) and those who did not (7.8 ± 6.7 years; P = 0.3).
In the univariate analysis of variables measured at the time of diagnosis, including gender, previous appendectomy, smoking habit, family history of inflammatory bowel disease, age at diagnosis of CD, location and behavior of CD, and extraintestinal manifestations of CD, only Nod2/CARD15 gene variants were associated with surgical recurrence; in patients carrying variants, 14 of 28 (50%) presented surgical recurrence compared with 9 of 42 (21%) of those without variants (P = 0.01). None of other clinical variables at the time of CD diagnosis reached a P value <0.10. To exclude other confounding factors, Nod2/CARD15 gene variants, along with location, behavior of CD, and clinical factors associated with requirement of initial surgery, were entered in a multivariate analysis for surgical recurrence. Nod2/CARD15 gene variants were the only independent predictive factor for surgical recurrence (OR, 3.66; 95% CI, 1.28–10.42; P = 0.01). When only patients with an intestinal resection in the initial surgery were analyzed, excluding patients operated exclusively for perianal disease, Nod2/CARd15 gene variants remained significantly associated with surgical recurrence (OR, 4.17; 95% CI,1.33–13.1; P = 0.01).
In the univariate analysis including clinical characteristics of CD after first surgery listed in Table 4, and Nod2/CARD15 gene variants, only the latter was associated with requirement of a second surgery. A trend toward a higher rate of smoking (P = 0.07) and lower use of immunosuppressants (P = 0.10) was observed in patients requiring reoperation. In the multivariate analysis including these 3 variables, only Nod2/CARD15 gene variants (OR, 3.18; 95% CI, 1.07–9.47) were an independent predictive factor for surgical recurrence. If we exclude from this analysis patients that were operated for treatment of perianal disease in the initial surgery, the Nod2/CARD15 gene status (OR, 3.79; 95% CI, 1.17–12.21) remains as an independent predictive factor for surgical recurrence. In the survival analysis of patients remaining free of surgical recurrence, Nod2/CARD15 genotype was significantly associated with an early requirement of surgery in the Kaplan-Meier analysis (P = 0.03) (Fig. 3). Nod2/CARD15 genotype was not a predictive factor for medical recurrence after surgery; medical recurrence leading to changes in medical treatment was observed in 21 of the 28 patients carrying variants of Nod2/CARD15 (75%), and in 28 of the 42 patients without these variants (66.6%) (OR, 1.58; 95% CI, 0.50–4.92; P = 0.4). Survival time free of medical recurrence was not different between patients carrying Nod2/CARD15 genotype variants and those without these variants (P = 0.54).
TABLE 4. CD Characteristics During Follow-up and Nod2/CARD15 Genotype According to Surgical Recurrence
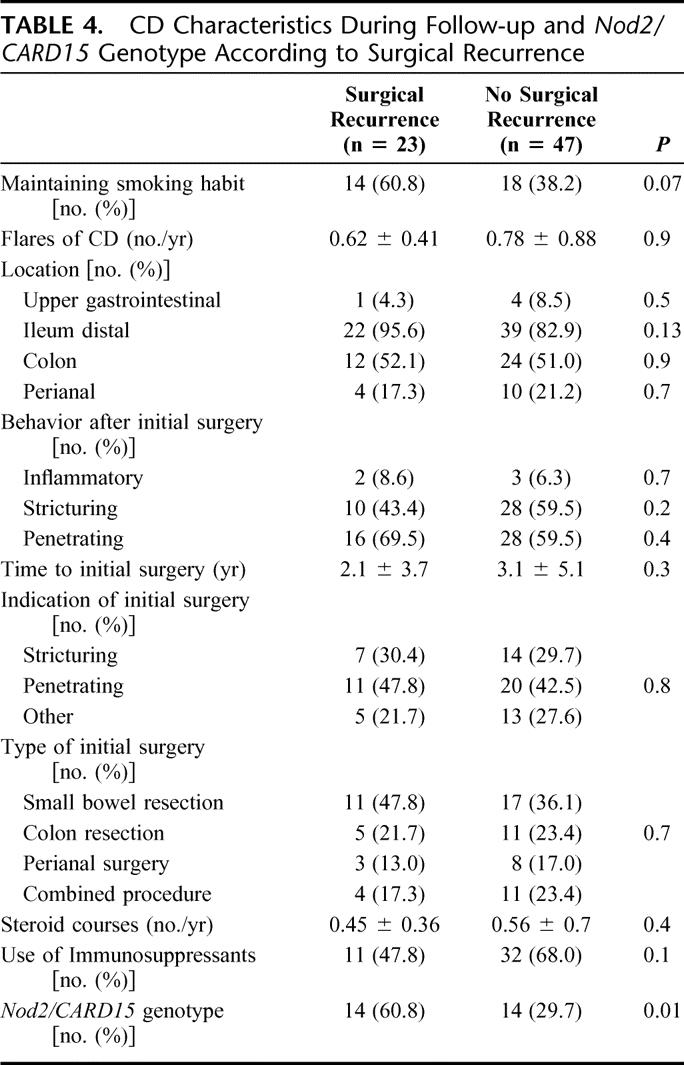
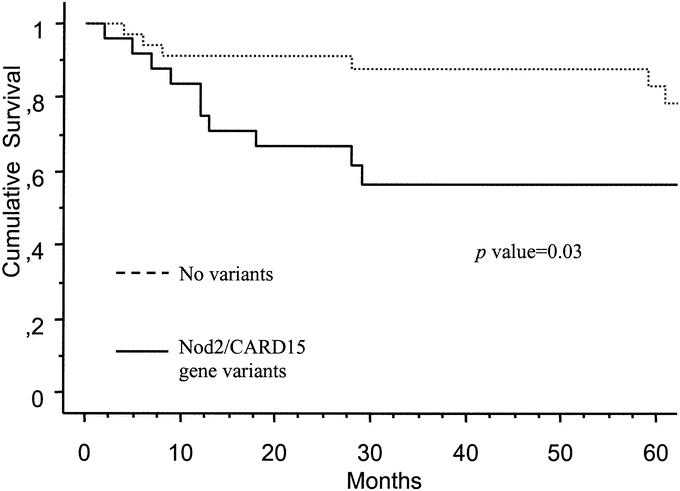
FIGURE 3. Actuarial probability of remaining free of surgical recurrence according to Nod2/CARD15 gene status.
We also analyzed if Nod2/CARD15 gene status was associated with surgery requirement for stricturing lesions at any time during the course of CD, irrespective of whether surgery for stricturing disease was required in the initial surgery or in the subsequent surgeries. In the multivariate analysis including the variables at the time of diagnosis listed in Table 2 with a P value ≤0.10 in the univariate analysis, variants of Nod2/CARD15 gene (OR, 3.61; 95% CI, 1.38–9.45), stricturing behavior at diagnosis (OR, 7.09; 95% CI, 2.06–24.3), and perforating behavior at diagnosis (OR, 3.77; 95% CI, 1.14–12.5) were independent predictive factors for subsequent surgery requirement due to stricturing lesions. Ileal disease (OR, 1.55; 95% CI, 0.28–8.43), colonic disease (OR, 0.44; 95% CI, 0.16–1.2) or perianal disease (OR, 0.13; 95% CI, 0.015–1.24) were not significant independent predictive factors for surgery requirement for stricturing lesions. The Kaplan-Meier survival analysis showed again a significant association between Nod2/Card15 genotype and early surgery requirement for treatment of stricturing lesions at any time during the course of CD (P = 0.003) (Fig. 4).
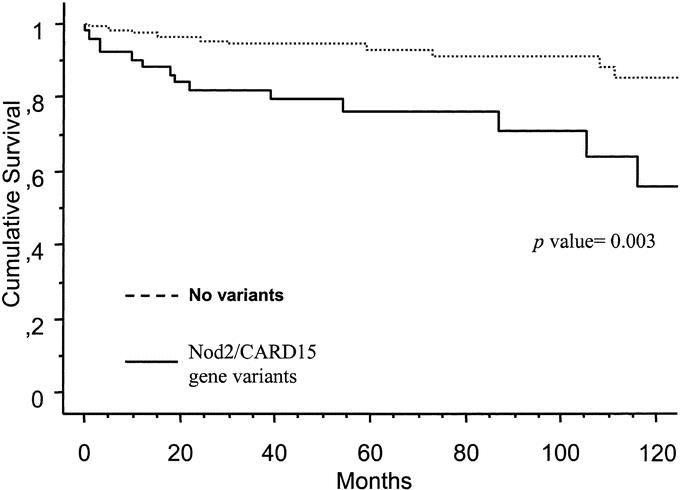
FIGURE 4. Actuarial probability of remaining free of surgery for treatment of stricturing lesions during the course of CD according to Nod2/CARD15 gene status.
None of 3 variants of Nod2/CARD15 gene (R702W, G908R, L1007fsinsC) alone was significantly associated with any surgical requirement, although this is probably the result of the low number of patients remaining in each group.
DISCUSSION
Identification of genes that cause polygenic disorders promises new approaches to dissect complex diseases such as CD. The finding of an association between Nod2/CARD15 gene and CD has raised great expectations on the clinical implications that could directly result from this discovery. This study is the first to evaluate in depth the association of Nod2/CARD15 gene with an important clinical event of CD course such as surgery requirement. There are probably several factors that influence surgical requirements in the course of CD, and these factors could be different according to the particular indication of surgery. The present study provides a step forward toward identification of stable factors, such as gene status of patients, that would allow prediction of the future course of the disease and, optimally, development of preventive clinical strategies.
Initial Surgery
We found specifically that Nod2/CARD15 genotype is an independent predictive factor for early surgery requirement due to stricturing lesions. Despite the more conservative surgical approach that has been adopted during the past few decades in CD, there is yet 1 indication for surgery, particularly independent of personal inclinations of patients, physicians, or medical institutions, as is a complete and/or irreversible intestinal stricturing.
Previous reports have found that small bowel disease and absence of colonic involvement were associated with requirement of surgery,1,4 both clinical conditions associated with Nod2/CARD15 variants.32 Our findings are in keeping with reports that have found an association between this gene and a stricturing phenotype,21,23,26 especially with the most severe forms of stricturing disease as indicated by the need for surgery. A previous report suggested that patients with fibrosis and stenosis that require surgery were a specific subgroup of CD patients, rather than being inevitable phases of disease evolution,33 and our results indicate that carrying the Nod2/CARD15 variant is a major genetic factor determining this CD subgroup. Therefore, classifying indications for surgery in 2 groups (perforating and nonperforating), as previous reports have done,10,16,34,35 may be too simplistic; and in our opinion, there is a genetic basis to classify indications for surgery in at least 3 groups: perforating, stricturing, and others. The reason for the association between Nod2/CARD15 genotype and need for early surgery for structuring lesions is not readily apparent, but some studies have hypothesized that intestinal immune response in patients with these variants shifts T cells toward transforming growth factor-β cytokine production and increased collagen deposition by smooth muscle cells and fibroblasts in the intestine.26
As expected, stricturing behavior at the time of diagnosis was another clinical variable independently associated with surgery requirement due to stricturing lesions. This phenotype has been previously associated with the development of complications and need for surgery.33 However, since characteristics of disease phenotype are not stable over time17 and misclassifications of clinical variables may be frequent,36 genotyping of patients may offer additional information, which, according to our findings, may be more reliable that clinical phenotypic characteristics of the disease in stratifying patients and establishing a prognosis. In that regard, 1 of the key points in the studies of risk factors for surgery is the time of clinical evaluation and the duration of CD. Since the majority of patients with CD are operated within the first years after diagnosis and the rates of surgery increase according to disease duration,1–3 it may be of great importance to identify at the time of diagnosis those factors that certainly predict the need of initial surgery in the first years of the disease course.
Surgical Recurrence
In the current study, we observed that one third of patients need reoperation after initial surgery. This proportion is in keeping with previous reports.1,2,31 We ascertained whether the Nod2/CARD15 gene variants contribute to the need of reoperation in patients with CD. For this purpose, the predictive value for surgical recurrence of these genetic variants along with other clinical characteristics was studied. We found that Nod2/CARD15 variants were an independent predictive factor for surgical recurrence in the multivariate analysis that included other clinical variables obtained at the time of diagnosis or during follow-up. Moreover, the variants of Nod2/CARD15 gene were associated with earlier recurrence in the Kaplan-Meier analysis. Although these 2 statistical analysis concur in indicating that Nod2/CARD15 gene variants are associated with the need for reoperation, the relatively small number of patients that are included in this analysis (n = 70) implies that the possibility of a type 1 error may exist.
Despite a study indicating that the Nod2/CARD15 genotype was not associated with requirement of second surgery,24 other studies have found predictive clinical factors for surgical recurrence linked to Nod2/CARD15 gene variants, such as a younger age at onset or small bowel disease.1,37 Nod2/CARD15 gene variants may be 1 of the factors that may contribute to explain the initial observation that patients with a shorter duration of CD previous to first surgery had also a shorter time to recurrence6,38 and supports the notion that patients bearing this variants have a complicated evolution of the disease.27 The lack of association between Nod2/CARD15 gene variants and medical recurrence after surgery may be related to the fact that in many subjects the initial recurrence after surgery had an inflammatory phenotype, and this phenotype is not associated with Nod2/CARD15 gene variants.
A possible reason to explain the association of Nod2/CARD15 gene variants with a higher rate of surgical recurrence could be related to an altered regulation of the inflammatory response and/or repairing in patients carrying these variants. A marker of this pathologic condition would be the predisposition to form fibrotic and stricturing lesions at site of intestinal inflammation in patients with these variants.26 Thus, this alteration would contribute to abnormal repairing at the site of surgical intervention and consequently to an earlier surgical recurrence, irrespective of the cause that led to initial surgery.
It is important to note that CD is a clinically heterogeneous disorder, and there is no universally applicable classification of CD phenotypes.10,29,35,39 The widely used Vienna classification29 has been recently criticized in different reports because it does not account for all the observed variations of CD phenotype.17,32,40 This classification is based only on 3 clinical parameters and applies a hierarchical criteria for disease behavior. Thus, patients with both stricturing and penetrating disease are only classified as penetrating not allowing a reliable genotype-phenotype analysis for stricturing phenotype. In the majority of large published studies demonstrating that carriage of Nod2/CARD15 variants may predispose to stricturing disease, the Vienna classification was not used to classify disease behavior.18,21,26 The categories that we have used in relation to CD location and behavior in the follow-up are not hierarchical or exclusive as in the Vienna classification, with the aim to evaluate each category separately and avoid losing information.
Smoking habit has been 1 of the few clinical factors more constantly associated with surgical recurrence.15 Moreover, 2 recent reports have suggested that the use of immunosuppressants may decrease the need for reoperation.41,42 A trend toward a higher risk of reoperation in those patients maintaining an active smoking habit (P = 0.07) and without use of immunosuppressants (P = 0.1) was observed in our study. However, the limited number of patients with surgical recurrence in the present study probably limits the statistical power for exploring these associations.
One important limitation of the available studies in genetic area of CD is that most participating patients came from tertiary referral centers. Our hospital serves as a referral center for patients with inflammatory bowel disease; thus, some patients may have a disease of greater complexity with greater need for surgery, and it has been reported that the prognosis of the disease is more benign in population-based studies43,44 than in centers with a special interest in CD surgical centers where possibly more severe cases are referred.45
CONCLUSION
Our results indicate that Nod2/CARD15 genotyping may have a useful clinical application as a major marker of evolution of CD, particularly an early need of initial surgery due to stricturing disease and need of reoperation. Although determination of the Nod2/CARD15 genotype cannot be used currently to guide indications for surgery, it might define a subgroup of patients with a severe course of the disease, who may require a more aggressive therapeutic strategy to prevent the appearance of complications. Confirmation of these results in future studies is necessary before interventional studies based on NOD2/CARD15 genotyping are designed.
Footnotes
Supported by Ministerio de Ciencia y Tecnología Grant No. SAF2002-02211, Instituto de Salud Carlos III Grant No. C03/02, MARATO TV3 Grant No. 003110, and Asociación Española de Gastroenterología Grant No. 2004.
Reprints: Julián Panés, MD, Gastroenterology Department, Hospital Clinic, Villarroel 170, 08036 Barcelona, Spain. E-mail: panes@ub.edu.
REFERENCES
- 1.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg. 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmer A. Natural history and prognosis: an evidence-based approach. In: Satsangi J, Sutherland LR, eds. Inflammatory Bowel Disease. London: Churchill Livingstone, 2003:301–317. [Google Scholar]
- 3.Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn's disease: relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818–1825. [DOI] [PubMed] [Google Scholar]
- 4.Basilisco G, Campanini M, Cesana B, et al. Risk factors for first operation in Crohn's disease. Am J Gastroenterol. 1989;84:749–752. [PubMed] [Google Scholar]
- 5.Michelassi F, Balestracci T, Chappell R, et al. Primary and recurrent Crohn's disease: experience with 1379 patients. Ann Surg. 1991;214:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Dombal FT, Burton I, Goligher JC. Recurrence of Crohn's disease after primary excisional surgery. Gut. 1971;12:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lock MR, Farmer RG, Fazio VW, et al. Recurrence and reoperation for Crohn's disease: the role of disease location in prognosis. N Engl J Med. 1981;304:1586–1588. [DOI] [PubMed] [Google Scholar]
- 8.Frikker MJ, Segall MM. The resectional reoperation rate for Crohn's disease in a general community hospital. Dis Colon Rectum. 1983;26:305–309. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg DM, Allan RN, Thompson H, et al. Excisional surgery with ileostomy for Crohn's colitis with particular reference to factors affecting recurrence. Gut. 1974;15:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenstein AJ, Lachman P, Sachar DB, et al. Perforating and non-perforating indications for repeated operations in Crohn's disease: evidence for two clinical forms. Gut. 1988;29:588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimann TM, Greenstein AJ, Lewis B, et al. Prediction of early symptomatic recurrence after intestinal resection in Crohn's disease. Ann Surg. 1993;218:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borley NR, Mortensen NJ, Jewell DP. Preventing postoperative recurrence of Crohn's disease. Br J Surg. 1997;84:1493–1502. [PubMed] [Google Scholar]
- 13.Strong SA. Prognostic parameters of Crohn's disease recurrence. Baillieres Clin Gastroenterol. 1998;12:167–177. [DOI] [PubMed] [Google Scholar]
- 14.Caprilli R, Corrao G, Taddei G, et al. Prognostic factors for postoperative recurrence of Crohn's disease: Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Dis Colon Rectum. 1996;39:335–341. [DOI] [PubMed] [Google Scholar]
- 15.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn's disease. Gastroenterology. 1994;106:643–648. [DOI] [PubMed] [Google Scholar]
- 16.Lautenbach E, Berlin JA, Lichtenstein GR. Risk factors for early postoperative recurrence of Crohn's disease. Gastroenterology. 1998;115:259–267. [DOI] [PubMed] [Google Scholar]
- 17.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampe J, Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925–1928. [DOI] [PubMed] [Google Scholar]
- 19.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 20.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. [DOI] [PubMed] [Google Scholar]
- 21.Lesage S, Zouali H, Cezard JP, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuthbert AP, Fisher SA, Mirza MM, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. [DOI] [PubMed] [Google Scholar]
- 23.Radlmayr M, Torok HP, Martin K, et al. The c-insertion mutation of the NOD2 gene is associated with fistulizing and fibrostenotic phenotypes in Crohn's disease. Gastroenterology. 2002;122:2091–2092. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad T, Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn's disease. Gastroenterology. 2002;122:854–866. [DOI] [PubMed] [Google Scholar]
- 25.Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet. 2002;359:1661–1665. [DOI] [PubMed] [Google Scholar]
- 26.Abreu MT, Taylor KD, Lin YC, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679–688. [DOI] [PubMed] [Google Scholar]
- 27.Helio T, Halme L, Lappalainen M, et al. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut. 2003;52:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6. [DOI] [PubMed] [Google Scholar]
- 29.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn's disease. Gastroenterology. 2003;125:1508–1530. [DOI] [PubMed] [Google Scholar]
- 31.Sachar DB. Patterns of postoperative recurrence in Crohn's disease. Scand J Gastroenterol Suppl. 1990;172:35–38. [DOI] [PubMed] [Google Scholar]
- 32.Colombel JF. The CARD15 (also known as NOD2) gene in Crohn's disease: are there implications for current clinical practice? Clin Gastroenterol Hepatol. 2003;1:5–9. [DOI] [PubMed] [Google Scholar]
- 33.Prantera C, Levenstein S, Capocaccia R, et al. Prediction of surgery for obstruction in Crohn's ileitis: a study of 64 patients. Dig Dis Sci. 1987;32:1363–1369. [DOI] [PubMed] [Google Scholar]
- 34.Aeberhard P, Berchtold W, Riedtmann HJ, et al. Surgical recurrence of perforating and nonperforating Crohn's disease: a study of 101 surgically treated patients. Dis Colon Rectum. 1996;39:80–87. [DOI] [PubMed] [Google Scholar]
- 35.Greenway SE, Buckmire MA, Marroquin C, et al. Clinical subtypes of Crohn's disease according to surgical outcome. J Gastrointest Surg. 1999;3:145–151. [DOI] [PubMed] [Google Scholar]
- 36.Silverberg MS, Daly MJ, Moskovitz DN, et al. Diagnostic misclassification reduces the ability to detect linkage in inflammatory bowel disease genetic studies. Gut. 2001;49:773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Post S, Herfarth C, Bohm E, et al. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn's disease. Ann Surg. 1996;223:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachar DB, Wolfson DM, Greenstein AJ, et al. Risk factors for postoperative recurrence of Crohn's disease. Gastroenterology. 1983;85:917–921. [PubMed] [Google Scholar]
- 39.Steinhart AH, Girgrah N, McLeod RS. Reliability of a Crohn's disease clinical classification scheme based on disease behavior. Inflamm Bowel Dis. 1998;4:228–234. [DOI] [PubMed] [Google Scholar]
- 40.Arnott ID, Satsangi J. Crohn's disease or Crohn's diseases? Gut. 2003;52:460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanauer SB, Korelitz BI, Rutgeerts P, et al. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723–729. [DOI] [PubMed] [Google Scholar]
- 42.Ardizzone S, Maconi G, Sampietro GM, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology. 2004;127:730–740. [DOI] [PubMed] [Google Scholar]
- 43.Gollop JH, Phillips SF, Melton LJ III, et al. Epidemiologic aspects of Crohn's disease: a population based study in Olmsted County, Minnesota, 1943–1982. Gut. 1988;29:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellers G. Crohn's disease in Stockholm county 1955–1974: a study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl. 1979;490:1–84. [PubMed] [Google Scholar]
- 45.Lindhagen T, Ekelund G, Leandoer L, et al. Pre- and post-operative complications in Crohn's disease with special reference to duration of preoperative disease history. Scand J Gastroenterol. 1984;19:194–203. [PubMed] [Google Scholar]


