Abstract
Objective:
The outcomes of simultaneous pancreas-kidney (SPK) transplantation with donor organs procured from donation after cardiac death (DCD) are compared with transplants performed with donor organs recovered from donation after brain death (DBD).
Summary Background Data:
Concerns exist regarding the utilization of pancreata obtained from DCD donors. While it is known that DCD kidneys will have a higher rate of DGF, long-term functional graft survival data for DCD pancreata have not been reported.
Methods:
A retrospective review of all DCD SPK transplants performed at a single center was undertaken.
Results:
Patient, pancreas, and kidney survival at 5 years were similar between DCD and DBD organs. Pancreas function and outcomes were indistinguishable between the 2 modes of procurement. As expected, the DCD kidneys had an elevated rate of DGF, which had no significant long-term clinical impact.
Conclusion:
SPK transplantation using selected DCD donors is a safe and viable method to expand the organ pool for transplantation.
The long-term outcomes of 37 consecutive simultaneous pancreas-kidney (SPK) transplants performed with organs from donation after cardiac death (DCD) are compared with SPK transplants performed with organs from conventional donation after brain death (DBD). While the DCD kidneys showed an anticipated high rate of delayed graft function (DGF), the pancreatic function was indistinguishable from DBD organs. DCD pancreata can be used to safely expand the donor pool.
The lengthening waiting lists caused by the shortage of available organs and the increasing number of patients with end-stage organ disease have led to the search for new sources of transplantable organs. It has been suggested that, with the current standards of practice, the pancreas is the least likely abdominal organ to be deemed suitable for transplantation.1 Waiting list times for simultaneous pancreas-kidney (SPK) transplantation are increasing. At the end of 1993, there were 855 patients awaiting SPK transplantation, whereas at the end of 2002 there were 2425 (Organ Procurement and Transplantation Network, OPTN). For this reason, attempts to maximize pancreas utilization to satisfy demands is a problem of increasing significance. Alternatives, including the use of less-than-ideal donors,1 living donors,2–4 and pediatric donors,5 have been used in an attempt to expand the donor pool.
Another approach to expanding the donor pool for pancreas transplantation is to use pancreata from donation after cardiac death (DCD). While the use of kidneys and livers from DCD donors is increasing, the use of DCD pancreata is still low (UNOS). DCD is not a novel concept. Prior to the institution of brain death laws in the United States, all donors were DCD donors. Pancreas procurement from DCD donors was described for the first time in 1975.6 However, routine implementation of DCD recovery at many centers has been impeded by ethical concerns, logistical considerations, and fear of poor functional outcomes. Recent studies have shown that DCD renal transplantation can result in outcomes similar to those obtained with kidneys from donation after brain death (DBD) donors.7,8 Experience with DCD liver transplantation is promising but has demonstrated an increased rate of biliary complications as well as a decrease in patient and graft survival.9–11 Limited experience with DCD pancreas transplantation is available, and this is primarily short-term follow-up in a small number of patients.12,13
This report describes the largest single-center experience, with 37 consecutive DCD SPK transplants since the inception of an extrarenal DCD donor program in January 1993, and provides the first data on long-term functional outcomes. In comparison to a contemporaneous cohort of recipients of conventional DBD organs, SPK transplantation from selected DCD donors resulted in similar excellent patient, kidney, and pancreas graft survival.
MATERIALS AND METHODS
Data Collection
The Institutional Review Board at the University of Wisconsin Hospital and Clinics approved this project. Recipient and donor information was retrospectively obtained from the clinical database maintained by the University of Wisconsin Division of Transplantation. A total of 576 SPK transplants were performed between January 1, 1993 and December 31, 2003; 37 were from DCD donors and 539 were from DBD donors.
Definitions
Glomerular filtration rate (GFR) was calculated by the following formula: GFR (ml/min) = 6.7/creatinine (mmol/L) + BW (kg)/4 − urea (mmol/L)/2 − 100/height (m)2 + [35 (male) or 25 (female)].14 Delayed graft function was defined as the need for hemodialysis within the first week posttransplantation.
All kidney transplant rejection episodes were biopsy-proven. Biopsies were performed in the setting of allograft dysfunction (an increase in serum creatinine >0.2 mg/dL in 2 successive measurements). If hyperamylasemia coexisted with documented kidney rejection, the diagnosis of pancreas rejection was also recorded. Pancreas biopsy was considered in those individuals with elevated amylase or lipase, unexplained fever, abdominal discomfort over the graft and glucose intolerance.
For DCD procurements, the warm ischemia time was measured from withdrawal of ventilatory support to the initiation of cold perfusion. Total preservation time for kidneys was measured from the time of aortic cross-clamping and includes both cold storage time and machine pulsatile perfusion time. The pancreas cold storage time was measured from cross-clamping the donor aorta to the time of reperfusion of the pancreas in the recipient. For both the pancreas and kidney, rejection is defined as per treatment, regardless of any existing biopsy results.
Donor Selection
The general selection criteria for pancreas donors are similar to those for other solid organs. The age range for pancreas donation was 6 to 60 years. History of diabetes mellitus, acute necrotizing pancreatitis, or chronic pancreatitis were contraindications to pancreas donation. Further contraindications for pancreas donation included the presence of intra-abdominal sepsis, previous pancreatic surgery, or a history of pancreatic trauma. Hyperglycemia and hyperamylasemia were not considered absolute contraindications.
Recipient Selection
No distinctions were made between recipients of DBD and DCD organs. Patients with type 1 diabetes mellitus and uremia, with low C-peptide levels and negative HIV serology, were considered. Recipient age was restricted from 18 to 55 years, as long as there was adequate cardiac reserve with no inducible ischemia. Further restrictions included the absence of significant irreversible liver or lung dysfunction, active smoking or ongoing substance abuse, expected poor compliance, major ongoing psychiatric illness, morbid obesity (body mass index >32 kg/m2), and significant peripheral vascular disease.
Organ Procurement and Transplantation
The technique for organ procurement in DBD procurements has been previously published.15 Briefly, for DBD procurements, the organs are removed after retrograde flushing with 2 L of UW solution (Viaspan, Barr Laboratories, Pomona, NY) through the abdominal aorta and 1 L through the portal vein. The liver and pancreas are procured en bloc and then separated on a back table. The kidneys are isolated in vivo. The technical details of DCD procurements have been previously described.16 Briefly, after consent is obtained from next of kin, the patient is transferred to the operating room where the femoral artery and vein are dissected and exposed under local anesthesia. Heparin and phentolamine are administered prior to the cessation of ventilator support and after declaration of cardiac death, another 5 minutes are allowed to pass,17 during which the femoral vessels are cannulated. Subsequently, the aorta is flushed with 2 L of UW solution in a retrograde fashion through the femoral artery. If consent for precannulation is not given, the femoral vessels are not dissected and the aorta is directly cannulated after the declaration and 5-minute waiting period have elapsed. The viscera extending from the terminal esophagus to the midsigmoid are procured en bloc and then separated on a back table. The en bloc evisceration minimizes in vivo procurement time, allows a more rapid cooling of the organs, and prevents vascular injury.
The technical details of SPK transplantation have been previously published.15 The pancreatic allograft is prepared on the back table as described previously. The stapled ends of the duodenal segment are oversewn with interrupted silk sutures. Bladder drainage is accomplished with a side-to-side anastomosis between the antimesenteric border of the duodenal segment and the bladder. Enteric drainage of pancreata is performed via a side-to-side anastomosis from a point 2 to 3 cm from the end of the duodenal segment to either the distal ileum or the midjejunum. A 2-layer hand-sewn anastomosis is performed in all cases. Drains are not routinely placed. There are no modifications for DCD implants.
Immunosuppression
The initial immunosuppression protocol consisted of quadruple sequential treatment with azathioprine (AZA), prednisone, cyclosporine A, and antibody induction [1993–1996-murine antihuman CD3 monoclonal antibody (OKT3, Muromonab, Ortho Pharmaceuticals, Raritan, NJ); 1996–1997 antithymocyte globulin (ATGAM, Upjohn, Kalamazoo, MI) (basiliximab, Simulect, Novartis Pharmaceuticals, Basel, Switzerland)]. Prednisone 2 mg/kg per day was given on day 0 and then tapered over several months. AZA 2.5 mg/kg per day was given on day 0 if the WBC was >3000/mm3. Cyclosporine A (CSA, Sandimmune, Novartis Pharmaceuticals) 10 mg/kg per day was given when the creatinine fell below 3 and then adjusted to maintain a level of 200 to 300 ng/mL by high-pressure liquid chromatography. In 1995, tacrolimus (Prograf, Fujisawa USA, Deerfield, IL) replaced CSA and was given at 2 mg orally twice per day to maintain levels of 8 to 20 ng/mL. In May 1995, mycophenolate mofetil (CellCept, MMF, Roche Laboratories, Nutley, NJ) replaced AZA and was given at 1.5 to 3 g per day orally. The dose was lowered if recipients experienced significant gastrointestinal symptoms or leukopenia. The current regimen includes induction with anti-IL-2 receptor monoclonal antibody basiliximab 20 mg I.V. day 0 and 4; or daclizumab (Zenapax, Roche Laboratories, Nutley, NJ) 1 mg/kg I.V. × 5 doses or 2 mg/kg I.V. × 2 doses) combined with the previously described doses of MMF, prednisone, and tacrolimus.18,19
Antiviral Therapy
Initial antiviral therapy consisted of high-dose acyclovir (Glaxo Smith Kline Inc., Philadelphia, PA) 800 mg 4 times daily for 12 weeks. As of 1996, ganciclovir (Hoffmann-La Roche Inc., Nutley, NJ) at a dose of 500 to 1000 mg 3 times daily for 12 weeks was given to high-risk patients. Since 2001, valganciclovir (Hoffmann-La Roche Inc.), at a dose of 450 mg twice per day for 12 weeks, has replaced ganciclovir in high-risk patients. High-risk patients include CMV-negative recipients of CMV-positive organs and patients being treated for rejection (additional 12 weeks ganciclovir or valganciclovir according to the era). The drugs were administered intravenously while the patient was not tolerating enteral feedings and then converted to oral once the nasogastric tube was removed. Since 1992, acyclovir has been administered to low-risk patients at a dose of 800 mg 4 times daily.
Statistics
All statistical analyses were performed using SAS statistical software version 6.12, SAS Institute Inc. (Cary, NC). Continuous variables are summarized by reporting mean and standard deviations (mean ± SD). Percentages are used to summarize categorical variables. Survival estimates are based on the methods of Kaplan-Meier and compared between groups using a log-rank test. To determine whether posttransplant leaks or enteric conversions were associated with worse survival, we used a Cox proportional hazards model with time-varying covariates. P values less than 0.05 were considered significant.
RESULTS
From January 1, 1993 to December 31, 2003, 37 consecutive SPK transplants with DCD organs procured by this institution and 539 SPK transplants with DBD organs were performed at the University of Wisconsin Hospital and Clinics. During this period, 111 DCD procurements were performed, indicating an SPK recovery rate of 31.5%.
Donor Populations
The DBD organs were from a population with a mean age of 30.8 years, 39.5% female, and 95.2% white. The mean serum creatinine was 1.0 ± 0.43 mg/dL and the mean serum glucose was 189.7 ± 92 g/dL (Table 1). The DCD organs were from a population with a mean age of 33.2 years, with 29.7% female and 97.3% white donors. The mean serum creatinine was 0.94 ± 0.51 mg/dL and the mean serum glucose was 169.2 ± 65.3 g/dL.
TABLE 1. Donor Characteristics
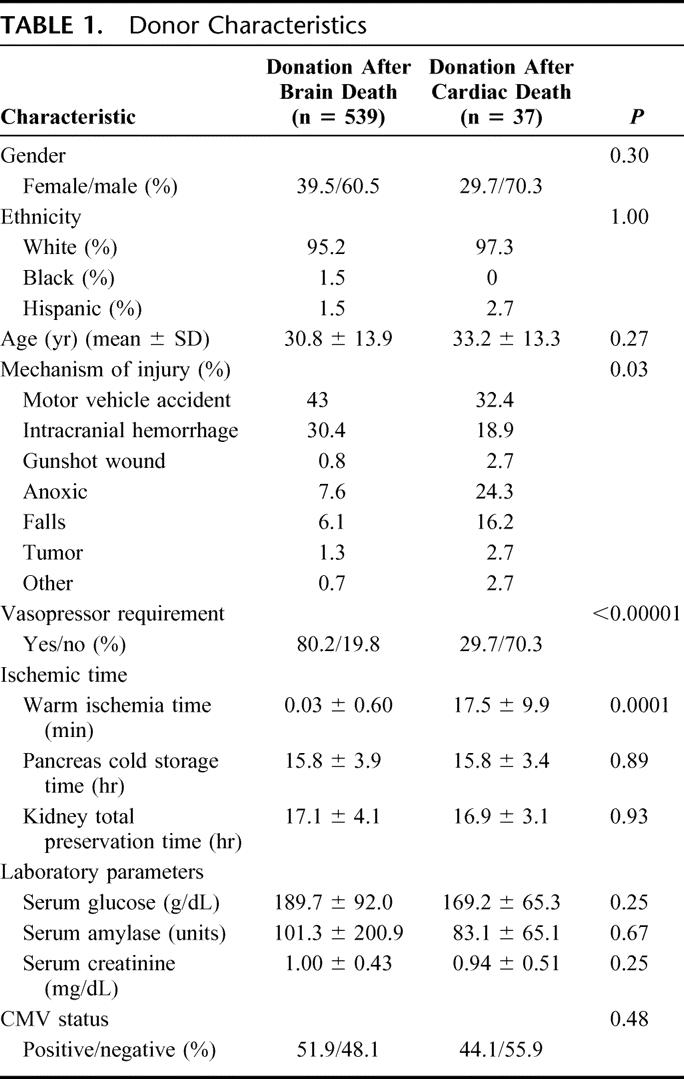
The leading causes of death for the DBD donors were motor vehicle accident (43.0%) and intracranial hemorrhage (30.0%). Other causes accounted for 26.9% (Table 1). For the DCD group, motor vehicle accident (32.4%) and anoxic injury (24.3%) were the most common injuries leading to fatality. However, brain tumors (16.2%) and other various causes (32.5%) were more represented (P = 0.03). While 80.2% of the DBD donors required vasopressors prior to procurement, only 29.7% of the DCD donors received vasopressors (P < 0.0001) (Table 1).
The mean warm ischemia time, as measured from withdrawal of ventilatory support to the initiation of cold perfusion, was 17.5 minutes (range, 6–48 minutes). Twenty of the 37 donors (54%) had a warm ischemia time of 15 minutes or less. Pancreas cold storage time was similar for both populations: 15.7 ± 3.9 hours for DBD and 15.8 ± 3.5 hours for DCD (P = 0.88, Table 1). For DBD kidneys, the mean total preservation time was 17.1 ± 4.1 hours, and for DCD kidneys, 16.9 ± 3.1 hours (P = 0.93, Table 1). Intrinsic to its methodology, the warm ischemia time of organs recovered from DCD donors is significantly longer in comparison to that in DBD organ recoveries (17.5 minutes versus 0 minutes, P = 0.0001, Table 1). The method used to drain pancreatic exocrine secretions was comparable between the 2 populations, with enteric drainage used in 69% of the DBD operations and 73% of the DCD operations. At this center, enteric drainage became the standard technique for pancreatic exocrine drainage for SPK transplantation in 1996.
Recipient Populations
The 2 recipient populations were similar (Table 2). The mean age of recipients of DBD organs was 38.1 ± 6.4 years and 38.4 ± 6.9 years for recipients of DCD organs (P = 0.83). In the recipients of DBD organs, 39.5% were female and 96.3% were white, while for the recipients of DCD organs, 48.6% were female and 97.3% were white (P = 0.30 and 1.00). The duration of diabetes in the patient populations were similar, with DBD recipients having diabetes for 24.7 ± 6.8 years and DCD recipients for 25.0 ± 7.8 years (P = 0.81). Prior to transplantation, 65.1% of DBD recipients and 64.9% of DCD recipients required dialysis (P = 1.00). The method of dialysis was hemodialysis for 59.2% of DBD patients and 76.0% of DCD patients (P = 0.14).
TABLE 2. Recipient Characteristics
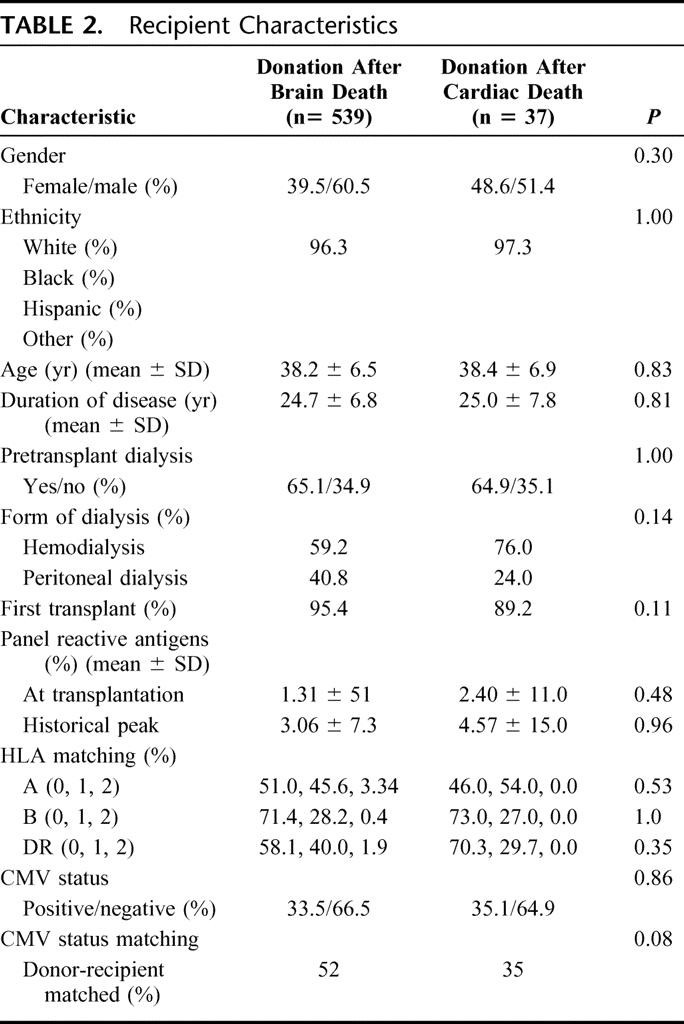
This index SPK transplantation was the first transplantation for 95.4% of DBD recipients and 89.2% of DCD recipients (P = 0.11). At the time of transplantation, the mean PRA was 1.31% ± 5.1% for DBD recipients and 2.40% ± 11.0% for DCD recipients (P = 0.48). The historical peak PRA values were also similar, with DBD 3.06% ± 7.3% and DCD 4.57% ± 15% (P = 0.96). There were no significant differences between the 2 groups with respect to HLA matching for A, B, and DR loci (Table 2). Although not statistically significant, a larger proportion of DBD organ recipients (52%) appeared to have donor- and recipient-matched for CMV status in comparison to the DCD group (35%) (P = 0.07).
Patient Outcomes
The 1- and 5-year patient survival rates were similar between the 2 groups. DBD patient survival was 96.7% at 1 year and 89.1% at 5 years, and DCD patient survival was 91.5% through 5 years (P = 0.85) (Fig. 1). The postoperative lengths of stay were similar at 20.2 ± 16.5 days for DBD recipients and 21.5 ± 12.8 days for DCD recipients (P = 0.55).
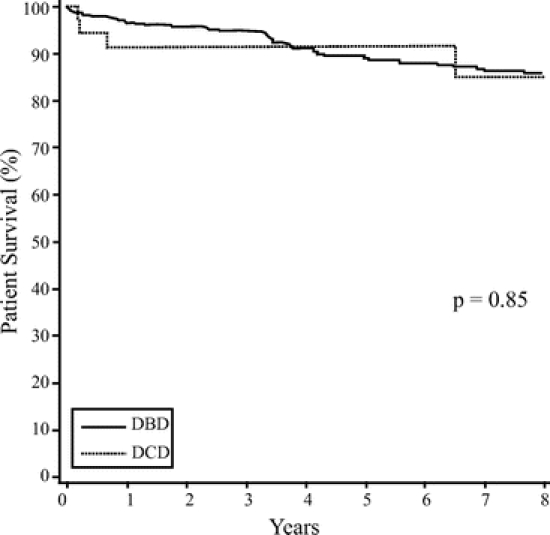
FIGURE 1. Patient survival post simultaneous pancreas-kidney transplantation.
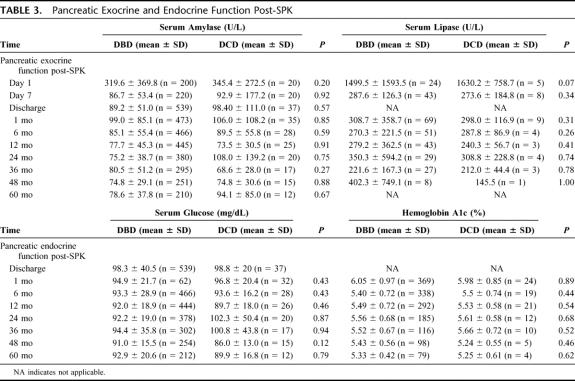
Pancreas Outcomes
One- and 5-year pancreas graft survival rates were similar between the 2 groups (DBD 88.6% and 78.9%, DCD 83.3% and 72.2%) (P = 0.18), respectively (Fig. 2). At discharge, the fasting blood glucose was 98.3 ± 40.5 mg/dL for the DBD group and 98.7 ± 20.0 mg/dL for the DCD group (P = 0.51). The serum amylase at discharge for the DBD group was 89.2 ± 51 IU and for the DCD group it was 98.4 ± 111 IU (P = 0.57). The long-term amylase and lipase levels were similar between the 2 groups (Table 3). Long-term pancreas function was also similar between the 2 groups. Comparing established time points from 1 to 60 months posttransplantation, no significant differences were observed in the serum glucose levels and hemoglobin A1C between the 2 groups (Table 3). Of the 37 SPK transplants performed from DCD donors, 3 patients (8.1%) were receiving oral hypoglycemic agents or insulin to treat mild hyperglycemia a few months after their transplant and remain on long-term supplement therapy. There was no significant difference in comparison to the DBD group, in which 50 patients (9.3%, P = not significant) also required oral hypoglycemic agents or insulin long-term to control mild glucose intolerance.
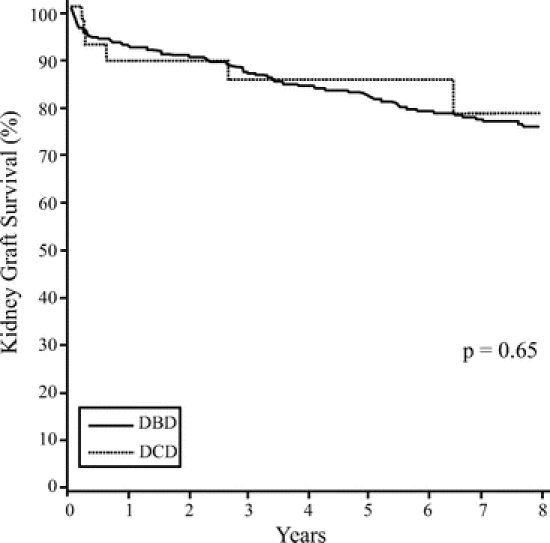
FIGURE 2. Pancreas graft survival post simultaneous pancreas-kidney transplantation.
TABLE 3. Pancreatic Exocrine and Endocrine Function Post-SPK
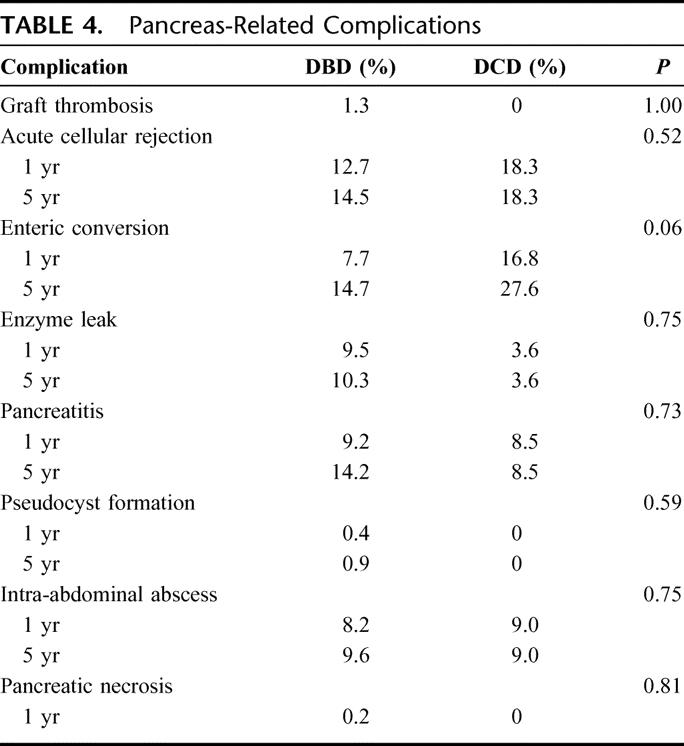
Pancreas-related complication rates were similar between the 2 groups (Table 4). The rejection rate for the DBD pancreata was 12.7% at 1 year and 14.5% at 5 years, and for the DCD pancreata, 13.9% at 1 year and 18.5% at 5 years (P = 0.52). The graft thrombosis rate within the first week posttransplantation was low in both groups. Seven episodes of pancreas graft thrombosis were noted in the immediate postoperative period (less than 1 week) in the DBD group (1.3%) and 0 in the DCD group (P = 1.00). The higher rate of enteric conversion observed in the DCD group approached statistical significance: DBD 7.7% at 1 year and 14.7% at 5 years, DCD 16.8% at 1 year and 27.6% at 5 years (P = 0.06). The enzyme leak rate for the DBD group was 9.1% at 1 year and 9.8% at 5 years, and for the DCD group was 3.6% through 5 years (P = 0.75). The rate of pancreatitis was 9.2% at 1 year and 14.2% at 5 years for the DBD group compared with 8.5% through 5 years for the DCD group (P = 0.73). The rate of pseudocyst formation for the DBD group was 0.4% at 1 year and 0.9% at 5 years, while there were no pseudocysts in the DCD group (P = 0.59). Intra-abdominal abscesses were found in 8.2% at 1 year and 9.6% at 5 years in the DBD group and in 9.0% through 5 years in the DCD group (P = 0.75). The incidence of pancreatic necrosis at 1 year was 0.4% in the DBD group, while none was encountered in the DCD group (P = 0.06).
TABLE 4. Pancreas-Related Complications
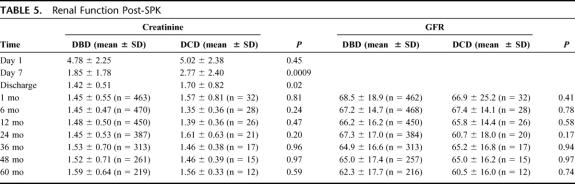
Renal Outcomes
The 1- and 5-year kidney graft survival rates were similar: DBD, 91.6% and 81.6%, respectively; DCD, 88.8% and 84.7, respectively (P = 0.65) (Fig. 3). However, there were differences in renal function between the 2 patient groups. On the first postoperative day, the serum creatinine levels were similar, with DBD 4.8 ± 2.2 mg/dL and DCD 5.0 ± 2.4 mg/dL (P = 0.45). By the seventh postoperative day, the serum creatinine was lower in the DBD group (1.9 ± 1.8 mg/dL) than in the DCD group (2.8 ± 2.4 mg/dL) (P = 0.0009). This difference persisted to the time of discharge, at which time the DBD group had a mean serum creatinine of 1.4 ± 0.5 mg/dL and the DCD group a mean serum creatinine of 1.7 ± 0.82 mg/dL (P = 0.02). The rate of posttransplant DGF was also significantly higher in the DCD group (24.3%) than in the DBD group (5.2%) (P = 0.0002). However, these initial differences disappeared by 1 month posttransplantation and had no apparent direct impact on long-term graft survival. Both the serum creatinine levels and GFR were comparable from 1 to 60 months post-SPK transplantation (Table 5).
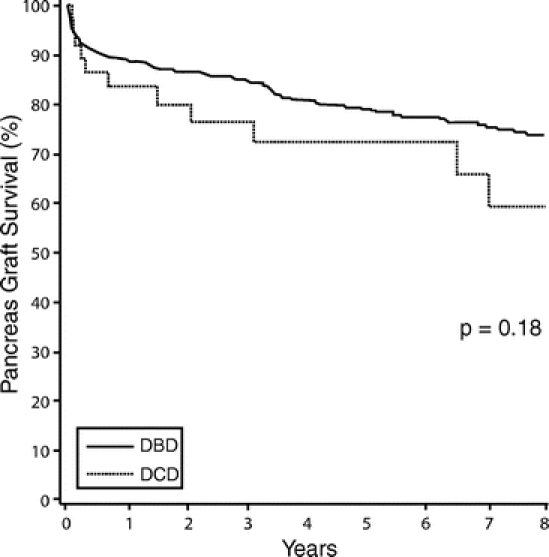
FIGURE 3. Kidney graft survival post simultaneous pancreas-kidney transplantation.
TABLE 5. Renal Function Post-SPK
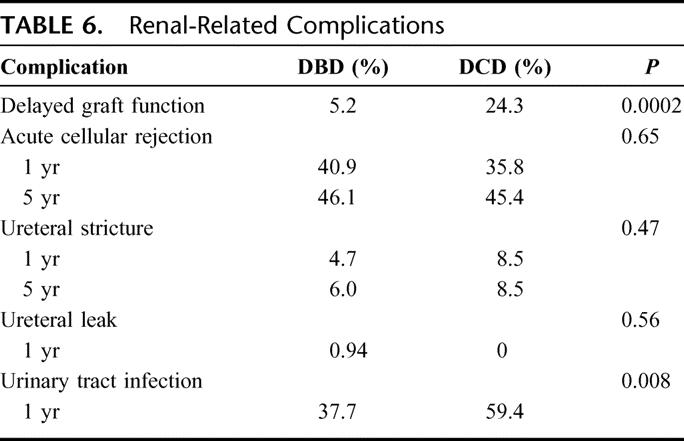
Long-term outcome was comparable with respect to renal allograft survival (Table 6). The 1-year and 5-year rates of acute rejection for DBD recipients (40.9% and 46.1%, respectively) were similar to those of DCD recipients (35.8% and 45.4%) (P = 0.65). The ureteral stricture rate was 4.7% at 1 year and 6% at 5 years for the DBD group and 8.5% through 5 years for the DCD group (P = 0.47). At 1 year posttransplant, the ureteral leak rate was not different (DBD 0.94% and DCD 0%, P = 0.56). However, recipients of DCD organs had a high rate of urinary tract infections (59.4%) compared with recipients of DBD organs (37.7%) (P = 0.002).
TABLE 6. Renal-Related Complications
DISCUSSION
This series describes an experience with 37 consecutive recipients of SPK transplantation with organs recovered from DCD donors, the largest reported experience from a single center to date. The longest survivor with functioning pancreas and kidney grafts from a DCD donor is now over 11 years posttransplantation. This experience, using selected DCD donors, demonstrates comparable 1-year and 5-year patient and graft survival rates in recipients of DCD and DBD organs.
The pancreas-related outcomes were similar between the 2 recipient populations. From the initial postoperative course to 5 years posttransplant, DCD pancreata functioned as well as DBD organs with respect to glycemic control as measured by fasting serum glucose, HbA1c levels, and assisted glycemic control. By the time of discharge, the pancreata from both donor groups indicated resolution of reperfusion pancreatitis as measured by serum amylase and lipase levels. As predicted from kidney studies,7,8 DCD recipients experienced a significantly higher rate of DGF post-SPK transplantation. The serum creatinine was elevated on the seventh postoperative day and at the time of discharge. However, by the first postoperative month, there was no measurable difference in serum creatinine or GFR between the 2 populations. As noted in previous studies,7,8 a higher rate of DGF did not impact patient or renal allograft survival. Furthermore, despite the higher rate of DGF, there was no increase in hospital length of stay. However, there was a significantly elevated rate of urinary tract infections in DCD recipients. This may be attributable to the higher rate of DGF in DCD kidneys, as has been noted previously.20
The method of procurement limited warm ischemia to a mean of 17.5 minutes. These data suggest that the incurred warm ischemia time does not clinically impact on pancreas function. The true effect of DCD procurement and warm ischemia on pathology at the cellular or molecular level of human pancreata has not been directly studied. However, it has been reported that DCD procurement of pancreata does not impact the number of islets obtained after islet isolations or their insulin secretory capacity.21 Moreover, islets obtained from DCD pancreata have been transplanted and have successfully reversed diabetes.18
Similarly, no experimental studies address the direct impact of brain death on pancreas allograft function. Detrimental effects on the kidney,19,22 liver,23 and heart24 have been demonstrated. Brain death is known to cause hemodynamic instability associated with an increase in serum catecholamines and a cytokine storm. Using a rat model, a recent study compared yield and functionality of β islet cells from pancreata obtained from brain dead animals to those without any brain injury.25 This study demonstrated a significant reduction in islet recovery from the brain dead animals, with an associated decrease in islet viability and increase in β cell apoptosis. Furthermore, a decrease in the insulin secretory response to glucose and arginine was observed in the pancreata from brain dead rats. Hence, there is precedent that brain death, per se, can be damaging to the pancreas.
The hemodynamic insufficiency associated with brain death mandates the usage of vasopressors, which in this experience were seldom required for DCD donors (Table 1). It can be hypothesized that the double-hit phenomenon of vasopressor requirement and elevated inflammatory cytokines associated with DBD procurement may be equivalent to any injury caused by the warm ischemia time in DCD procurements. Further studies are needed to elucidate the underlying mechanisms associated with donor-related organ ischemia/cytokine-mediated injury. Intervention strategies designed to interrupt the pathophysiology in the donor may improve outcomes for organs from both DCD and DBD donors.
The results of this series cannot be generalized to include all DCD donors. The following criteria are proposed to be used as a guide in deciding when to use a DCD donor for SPK transplantation: 1) eligibility is restricted to patients with severe and irreversible brain injury who are not likely to develop criteria of brain death; however, our protocol allows us to consider patients without brain injury in conjunction with an ethics consult; 2) cessation of ventilatory support in the operating room procurement (Type III DCD, Maastricht Protocol);26 3) an observation period of 5 minutes postdeclaration of death, following the guidelines set forth by the Institute of Medicine; and 4) warm ischemia time less than 45 minutes, although kidney donation alone may remain a viable option with longer warm ischemia times.
Currently, DCD pancreata are considered a subset of a larger group of extended criteria or marginal donors. In previous statistical analyses of factors affecting outcome postpancreas transplantation, donor age over 45 years was associated with reduced graft survival rates.1 From this same analysis, pancreas procurement obtained from a DCD donor was not shown to be a risk factor for poor outcomes. These DCD donors were young patients who would otherwise have been considered ideal donors. In this series, DCD organs represented 8.2% of the total SPK transplants performed. Over the last several years, DCD donors have comprised an increasing proportion of the total donor pool. At this institution, DCD now represents up to 25% of all donors while it is 3% to 4% nationally. Therefore, the impact of DCD organs on the donor pool is significant. While not all of these donors will be suitable for pancreas transplantation, DCD may eventually serve to expand the organ pool for both pancreas and islet transplantation.
It is important to emphasize the resilience of the organs recovered from DCD donors using defined protocols and inclusion/exclusion criteria. The organs recovered from DCD donors were not treated differently than those from DBD donors. There were no attempts at decreasing the cold storage time or modifying the posttransplant immunosuppression protocols. In addition, no efforts were made to differentially select recipients of DCD organs. Since the results of SPK using DCD organs are equivalent to the results of DBD organs and represents our standard of care, preoperative consent to use this organs is not obtained. This report proposes that SPK transplantation from donation after cardiac death be considered an alternative that allows the expansion of the ideal donor pool. This proposal is supported by data demonstrating indistinguishable long-term functional outcomes between DCD and DBD SPK transplantation.
ACKNOWLEDGMENTS
The authors thank the multidisciplinary team who provided excellent care to the patients as well as the thorough and dedicated efforts of the database team and Janet Fox and Karen Heim for their editorial support.
Footnotes
Reprints: Anthony M. D'Alessandro, MD, University of Wisconsin, Division of Organ Transplantation, H4/787 Clinical Science Center, 600 Highland Avenue, Madison, WI 53792-7375. E-mail: am.dalessandro@hosp.wisc.edu.
REFERENCES
- 1.Krieger NR, Odorico JS, Heisey DM, et al. Underutilization of pancreas donors. Transplantation. 2003;75:1271–1276. [DOI] [PubMed] [Google Scholar]
- 2.Gruessner RW, Sutherland DE, Drangstveit MB, et al. Pancreas transplants from living donors: short- and long-term outcome. Transplant Proc. 2001;33:819–820. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Najarian JS, Gruessner R. Living versus cadaver donor pancreas transplants. Transplant Proc. 1998;30:2264–2266. [DOI] [PubMed] [Google Scholar]
- 4.Zielinski A, Nazarewski S, Bogetti D, et al. Simultaneous pancreas-kidney transplant from living related donor: a single-center experience. Transplantation. 2003;76:547–552. [DOI] [PubMed] [Google Scholar]
- 5.Van der Werf WJ, Odorico J, D'Alessandro AM, et al. Utilization of pediatric donors for pancreas transplantation. Transplant Proc. 1999;31:610–611. [DOI] [PubMed] [Google Scholar]
- 6.Calne RY, Williams R. Liver transplantation in man: I. Observations on technique and organization in five cases. Br Med J. 1968;4:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudich SM, Kaplan B, Magee JC, et al. Renal transplantations performed using non-heart-beating organ donors: going back to the future? Transplantation. 2002;74:1715–1720. [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Dindo D, Demartines N, et al. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–255. [DOI] [PubMed] [Google Scholar]
- 9.Abt P, Crawford M, Desai N, et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. [DOI] [PubMed] [Google Scholar]
- 10.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Liver transplantation from controlled non-heart-beating donors. Surgery. 2000;128:579–588. [DOI] [PubMed] [Google Scholar]
- 12.D'Alessandro AM, Odorico JS, Knechtle SJ, et al. Simultaneous pancreas-kidney (SPK) transplantation from controlled non-heart-beating donors (NHBDs). Cell Transplant. 2000;9:889–893. [DOI] [PubMed] [Google Scholar]
- 13.Tojimbara T, Teraoka S, Babazono T, et al. Long-term outcome after combined pancreas and kidney transplantation from non-heart-beating cadaver donors. Transplant Proc. 1998;30:3793–3794. [DOI] [PubMed] [Google Scholar]
- 14.Nankivell BJ, Gruenwald SM, Allen RDM, et al. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995;59:1683–1689. [DOI] [PubMed] [Google Scholar]
- 15.Sollinger HW, Ploeg RJ, Eckhoff DE, et al. Two hundred consecutive simultaneous pancreas-kidney transplants with bladder drainage. Surgery. 1993;114:736–743. [PubMed] [Google Scholar]
- 16.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation. 1995;59:977–982. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine, Division of Health Care Services. Non-heart-beating organ transplantation: practice and protocols. Washington DC: National Academy Press; 2000. [Google Scholar]
- 18.Markmann JF, Deng S, Desai NM, et al. The use of non-heart-beating donors for isolated pancreatic islet transplantation. Transplantation. 2003;75:1423–1429. [DOI] [PubMed] [Google Scholar]
- 19.Koo DD, Welsh KI, McLaren AJ, et al. Cadaver versus living donor kidneys: impact of donor factors on antigen induction before transplantation. Kidney Int. 1999;56:1551–1559. [DOI] [PubMed] [Google Scholar]
- 20.Muller V, Becker G, Delfs M, et al. Do urinary tract infections trigger chronic kidney transplant rejection in man? J Urol. 1998;159:1826–1829. [DOI] [PubMed] [Google Scholar]
- 21.Clayton HA, Swift SM, Turner JM, et al. Non-heart-beating organ donors: a potential source of islets for transplantation? Transplantation. 2000;69:2094–2098. [DOI] [PubMed] [Google Scholar]
- 22.Pratschke J, Wilhelm MJ, Kusaka M, et al. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343–348. [DOI] [PubMed] [Google Scholar]
- 23.van Der Hoeven JA, Ter Horst GJ, Molema G, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000;232:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novitzky D, Wicomb W, Cooper D, et al. Prevention of myocardial injury during brain death by total cardiac sympathectomy in the chacma baboon. Ann Thorac Surg. 1986;41:520–524. [DOI] [PubMed] [Google Scholar]
- 25.Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52:2935–2942. [DOI] [PubMed] [Google Scholar]
- 26.Kootstra G. Statement on non-heart-beating donor programs. Transplant Proc. 1995;27:2965. [PubMed] [Google Scholar]