Abstract
Objective:
To determine whether the outcomes of liver transplantation (LTx) from donation after cardiac death (DCD) donors are equivalent to those from donation after brain death (DBD) donors.
Summary Background Data:
Because of the significant donor organ shortage, more transplant centers are using livers recovered from DCD donors. However, long-term, single-center outcomes of liver transplantation from DCD donors are limited.
Methods:
From January 1, 1993, to July 31, 2002, 553 liver transplants were performed from DBD donors and 36 were performed from DCD donors. Differences in event rates between the groups were compared with Kaplan-Meier estimates and the log-rank test. Differences in proportion and differences of means between the groups were compared with Fisher exact test and the Wilcoxon rank sum test, respectively.
Results:
Mean warm ischemic time at recovery in the DCD group was 17.8 ± 10.6 minutes. The overall rate of biliary strictures was greater in the DCD group at 1 year (33% versus 10%) and 3 years (37% versus 12%; P = 0.0001). The incidence of hepatic artery thrombosis, portal vein stenosis/thrombosis, ischemic-type biliary stricture (ITBS), and primary nonfunction were similar between groups. However, the incidence of both hepatic artery stenosis (16.6% versus 5.4%; P = 0.001) and hepatic abscess and biloma formation (16.7% versus 8.3%; P = 0.04) were greater in the DCD group. Trends toward worse patient and graft survival and increased incidence of ITBS were seen in DCD donors greater than 40 years compared with DCD donors less than 40 years. Overall patient survival at 1 year (DCD, 80%; versus DBD, 91%) and 3 years (DCD, 68%; versus DBD, 84%) was significantly less in the DCD group (P = 0.002). Similarly, graft survival at 1 year (DCD, 67%; versus DBD, 86%) and 3 years (DCD, 56%; versus DBD, 80%) were significantly less in the DCD group (P = 0.0001).
Conclusions:
Despite similar rates of primary nonfunction, LTx after controlled DCD resulted in worse patient and graft survival compared with LTx after DBD and increased incidence of biliary complications and hepatic artery stenosis. However, overall results of LTx after controlled DCD are encouraging; and with careful donor and recipient selection, LTx after DCD may successfully increase the donor liver pool.
Many transplant centers have increased the utilization of livers recovered from donation after cardiac death (DCD) donors for transplantation. Long-term, single-center outcome data exist but are limited by low volume. This analysis compares outcomes of 36 liver transplants (LTx) after DCD to 553 LTx after donation after brain death (DBD).
Liver transplantation continues to be the standard of care for patients with end-stage liver disease, as 1-year graft survival is on average greater than 80% at most centers. Unfortunately, we continue to experience an organ shortage crisis. Based on Organ Procurement and Transplantation Network data as of May 28, 2004, the number of patients on the liver transplant waiting list has increased to 17,478. Although the number of liver transplants increased annually to a high of 5670 in 2003, we are currently only transplanting approximately 32% of the patients on the waiting list. Because of this ongoing problem, the transplant community has attempted to expand the donor organ pool.
Over the last decade, methods to expand the donor organ pool have been developed. These include live donor liver transplantation,1 split-liver transplantation,2 and the use of marginal or expanded donor livers for transplantation.3,4 Included in the marginal group are livers recovered from donation after cardiac death (DCD) donors, formally known as nonheart-beating donors. Based on Organ Procurement and Transplantation Network data, there has been a 4.2% increase in the number of DCD donors in the United States since 1994 to a high of 271 in 2003. Liver transplantation after DCD has increased steadily over the past decade to a high of 158 transplants in 2003.5
DCD can be divided into uncontrolled and controlled donation. In the uncontrolled situation, patients usually sustain cardiopulmonary arrest prior to arrival to the hospital. Upon arrival, they undergo CPR and in some centers are placed on cardiopulmonary bypass to reperfuse the organs for procurement and transplantation.6 Because of prolonged time between cardiac arrest and reperfusion of the organs, the organs usually suffer a severe ischemic insult. As a result, liver transplantation using uncontrolled DCD organs has resulted in inferior 1-year graft survival rates of 17%.7 In the setting of controlled DCD donors, patients undergo withdrawal of care usually in the operating room after adequate consent is obtained from the family. As a result, the time of hypotension and warm ischemia is significantly less than that in the uncontrolled setting. This setting facilitates the removal of the organs after a short period of warm ischemia before preservation with cold solution.
Our center has a long history of using kidneys from controlled DCD donors; and in 1993, we expanded our program to include transplantation of extrarenal organs. We previously have reported our results of 19 liver transplants from DCD donors and compared those results with 364 liver transplants from donation after brain death donors (DBD).8 This report details the short- and long-term results of 36 liver transplants from DCD donors in comparison with 553 liver transplants from DBD donors at our institution.
METHODS
We performed a retrospective review of all liver transplants performed at the University of Wisconsin between January 1993 and July 2002. During that time, 930 organ donors had been referred to the University of Wisconsin Organ Procurement Organization. Eighty-one (8.7%) donors were DCD donors and 849 (91.3%) were DBD donors. Of the 81 DCD donors, 47 were multiorgan, 33 were kidney-only, and one was a pancreas-only recovery. Thirty-six livers were transplanted from 47 extrarenal donors (76.5%) and 11 livers were not used (23.4%). All livers were biopsied and examined by frozen section prior to implantation.
The etiology of donor death was similar between both groups with the exception of anoxic encephalopathy, which was higher in DCD donors (27.8% versus 14.0%; P < 0.01). Likewise, there was no difference in donor characteristics with the exception of more males in the DCD group (3.5:1 versus 1.48:1; P = 0.05) and less need for vasopressors in the DCD group (33.0% versus 76.9%; P = 0.001). Warm ischemic time was defined as the time of extubation of the donor to reperfusion of the organs with cold University of Wisconsin (UW) solution and was 17.8 ± 10.6 minutes for DCD donors. The warm ischemic time during this interval was not complete warm ischemia because there were varying periods of hypotension and hypoxia before cessation of all cardiopulmonary function. Cold ischemic time was defined as the time from infusion of cold UW solution until reperfusion of the liver in the recipient and was not different between groups (8.2 ± 1.9 hours versus 8.3 ± 2.5 hours).
Because DCD donors frequently have preservation of brain stem reflexes, the possibility of continued respiration after extubation must be discussed when obtaining consent from the family. When consent is obtained for DCD, the family is informed that multiorgan donation will not occur beyond 2 hours after which the patient will be returned to the ward, where they will receive end-of-life care, until they die. During the time that consent is obtained, the family is fully informed about the procedure, including any medications that may be given such as phentolamine and heparin. Consent is also obtained for the placement of femoral arterial and venous cannulas that are inserted with local anesthesia before withdrawal of life support. Currently, most extrarenal DCD within our OPO occur in the operating room where a physician, who is not associated with the transplant team, withdraws support and makes the declaration of death. Once death is declared by cardiopulmonary criteria, an additional 5 minutes elapse, as described in the 1997 Institute of Medicine Guidelines,9 before solutions are infused and the incision is made. More recently, to accommodate donor family wishes, support was withdrawn on 2 patients in the intensive care unit. After death was declared, the patients were brought to the operating room for renal and extrarenal organ recovery. These recoveries were performed in 2003 and are not included in this analysis.
Organ Procurement and Preservation
Our techniques of organ procurement and preservation during DCD are previously described.8,10 In summary, all extrarenal DCD donors were brought to the operating room before withdrawal of life support. In most instances, femoral arterial and venous cannulas were placed under local anesthesia. While the patient was fully supported, 10,000 to 20,000 units of heparin and 10 to 20 mg of phentolamine were given intravenously to prevent vasospasm and to facilitate subsequent organ flushing. The patient's physician of record withdrew life support by stopping intravenous medications and extubation. Variable periods of hypotension and hypoxia occurred after the withdrawal of life support and before cessation of all cardiopulmonary function. During this time, the patient was monitored with an arterial line, continuous electrocardiogram, and physical examination. The recovery procedure commenced only after an additional 5 minutes elapsed after the declaration of death. Electrocardiographic silence was not required since the lack of respirations and the lack of a monitored arterial pulse were used as criteria for cessation of cardiopulmonary function.
Five minutes after the declaration of death, cold UW solution was infused into the femoral arterial cannula and the femoral venous cannula was opened to gravity. Median sternotomy and a midline abdominal incision were made and the intra-abdominal organs were removed en bloc. In those instances where femoral cannulas were not placed, the distal aorta was cannulated immediately upon entry into the abdomen. Approximately 1.5 to 3 L of UW solution was infused in situ, and an additional 1 L was used on the back table to flush the portal vein via the superior mesenteric vein as well as the orifices of the celiac, superior mesenteric, and renal arteries. Both the gallbladder and common bile duct were irrigated with UW solution. The entire en bloc preparation was stored in UW solution at 4°C and separated either immediately or upon return to our center. Because minimal dissection was performed in situ, approximately 1 to 1.5 hours of additional back table dissection was required. All livers were transplanted as soon as possible after retrieval. More recently, to decrease cold ischemic time, we have started the recipient operation prior to the return of our procurement team.
Recipient Population
The etiology of liver failure in recipients receiving DCD and DBD livers was similar (data not shown). There were no differences in recipient characteristics, which included age, gender, and mean waiting time. However, the mean patient follow-up was shorter for recipients of DCD livers (3.0 ± 2.6 years versus 4.6 ± 2.9 years; P = 0.002). Standard techniques of liver transplantation were used with livers retrieved from both groups. Livers retrieved from DCD donors, however, were not considered for either reduced or split-liver transplantation. The mean intraoperative blood product usage, operating times, length of stay, and discharge laboratory values are depicted in Tables 1 and 2. Our immunosuppressive protocol was primarily tacrolimus and prednisone with mycophenolate mofetil or basiliximab added if early postoperative renal dysfunction was present. Rejection episodes were treated with high-dose methylprednisolone, increased tacrolimus dosage, and when necessary with either antilymphocyte globulin or OKT3 monoclonal antibody.
TABLE 1. Mean Operative Time, Blood Product Usage, and Length of Stay in Recipients of Livers from DCD and DBD Donors
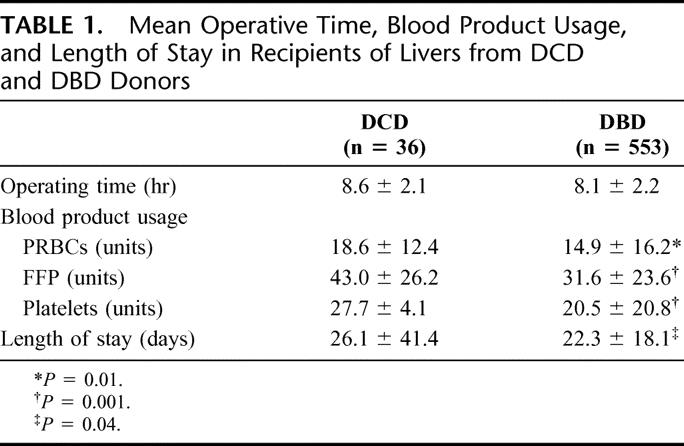
TABLE 2. Recipient Postoperative Laboratory Data
Statistical Analysis
Continuous variables were compared with the rank-sum test. Categorical variables were compared with the Fisher exact test. Patient and graft survival rates, complication rates, and rejection rates were estimated with a Kaplan-Meier product limit estimator and compared with the log-rank test. All tests were 2-sided, and a P value of less than 0.05 was considered to be statistically significant. All analyses were performed with SAS statistical software (SAS Institute Incorporated, Cary, NC).
RESULTS
During the study period, 81 DCD donors were referred to the University of Wisconsin Organ Procurement Organization. Forty-seven were multiorgan donors, 33 were kidney-only, and one was a pancreas-only recovery. Of the 47 multiorgan donors, 36 liver transplants were performed and 11 livers were not used. Two additional DCD donors, one adult donor and one pediatric, were referred and brought to the operating room for recovery. However, both patients continued to have spontaneous respirations beyond 2 hours and were returned to the ward where they later died. These 2 patients were not included in the analysis for the study.
When studying the etiology of donor death, we found that DCD donors had a significantly higher incidence of anoxic encephalopathy compared with DBD donors (27.8% versus 14.0%; P < 0.01). Age of donor was similar between the 2 groups; however, in the DCD group, a higher proportion of donors were males compared with females. DBD donors had significantly higher requirements for vasopressor use compared with DCD donors. This finding had been noted in our earlier series of liver transplantation with DCD donors. There were no significant differences in donor aspartate aminotransferase (AST) levels; however, alkaline phosphatase (ALP) levels were significantly greater in the DCD donors compared with DBD donors (98.2 versus 80.1 U/L; P = 0.04). Seven DCD livers were discarded because of severe steatosis. One liver had centrilobular necrosis that we think was present in the donor before retrieval. One liver was not used because of severe active hepatitis C seen on frozen section biopsy. One liver couldn't be used because it was blood type AB, and we did not have a recipient and were unable to refer the liver to another center. Also, one liver was discarded because of an injured replaced right hepatic artery that occurred when the en bloc technique, which we described, was not strictly adhered to. Since we have accumulated more experience, we would consider using this liver after arterial repair if the injury occurred after the in situ flush.
At the DCD recoveries, mean warm ischemic time was 17.8 minutes, with the longest warm ischemic time being 46 minutes. For this analysis, our protocol was to retrieve extrarenal organs with up to 45 minutes of warm ischemia and retrieve the kidneys with warm ischemic times up to 2 hours. As with all liver transplants at our institution, the livers were transplanted as soon as possible after retrieval. No difference was seen in cold ischemic times between the DCD and DBD groups. This practice has been consistent with the highest rate of initial function and lowest rate of primary nonfunction (PNF) at our center.11
As demonstrated in our previous manuscript, intraoperative blood product usage was significantly higher in DCD donors when compared with DBD donors.8 In this analysis, the number of packed red blood cells, fresh frozen plasma, and platelet transfusions were all significantly higher in the DCD group (Table 1). This likely represents the effects of warm ischemia at the recovery and some initial dysfunction upon reperfusion of the liver because no differences in recipient characteristics were noted. Significant coagulopathy was not a factor postoperatively as no differences in prothrombin time or International Normalized Ratio were noted between groups (Table 2).
Laboratory values on postoperative days 1, 3, 7, and at discharge are shown in Table 2. No significant differences in bilirubin were noted between DCD and DBD liver transplants. AST levels were significantly higher in DCD livers on postoperative day 1 when compared with the DBD group. At subsequent time points, AST levels were no different between groups and returned to near-normal levels at the time of discharge. Differences in levels of ALP and gamma-glutamyltransferase (GGT) were seen at various times in the postoperative period. No significant differences in ALP were seen until discharge, whereas levels of GGT were significantly greater in the DCD group at all postoperative time points. While hepatocellular enzymes were elevated initially and decreased to near-normal levels at the time of discharge, bile duct enzymes steadily increased throughout the postoperative course and were highest at the time of discharge.
The overall biliary stricture rate was significantly greater in the DCD group at 1 year (33% versus 10%) and 3 years (37% versus 12%; P = 0.0001) (Fig. 1). There were no significant differences in overall hepatic arterial complications in the 2 groups. However, when the complications were divided into thrombosis and stenosis, the incidence of hepatic artery stenosis (HAS) was significantly higher in the DCD group versus the DBD group (16.6% versus 5.4%; P = 0.001) (Table 3). These stenoses were suspected by abnormal arterial waveforms on Doppler ultrasound examination and confirmed by angiography. One patient developed an anastomotic arterial stricture whereas the other strictures were not at the anastomosis. One patient had unrecognized celiac axis stenosis from medial arcuate ligament compression. The remaining 4 patients developed stenoses of the donor hepatic artery distal to the anastomosis. Eighty-three percent of the DCD recipients with documented HAS developed biliary structures whereas only 37% of DBD recipients with HAS developed biliary strictures. The time to developing strictures after HAS was significantly shorter in the DCD group (P = 0.03). In contrast, the rate of ischemic-type biliary strictures (ITBS), defined as intrahepatic biliary strictures in the presence of a patent, nonstenotic hepatic artery, was similar between the DCD and DBD groups (13.8% versus 8.0%; P=0.07).
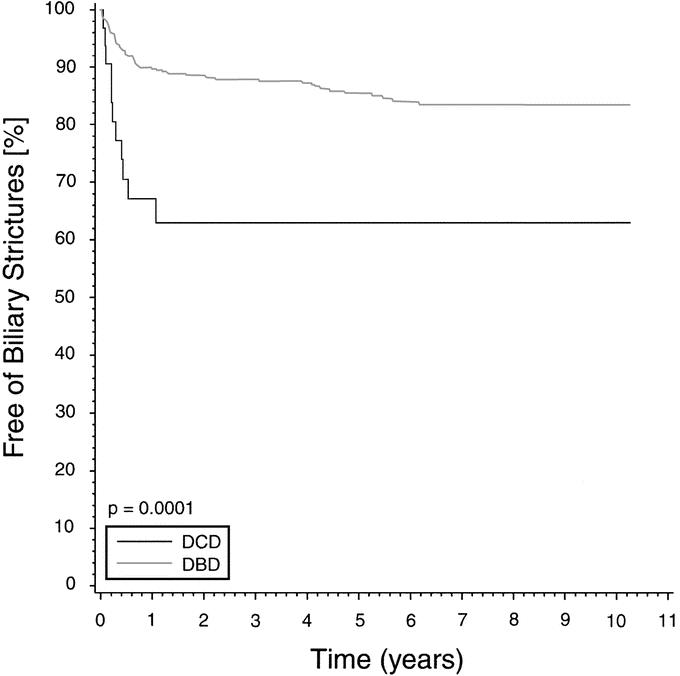
FIGURE 1. Overall biliary stricture rate in liver transplantation after DCD and DBD donation. Kaplan-Meier curves depict the percent free of strictures over time.
TABLE 3. Complications After Liver Transplantation in Recipients of Livers from DCD and DBD Donors
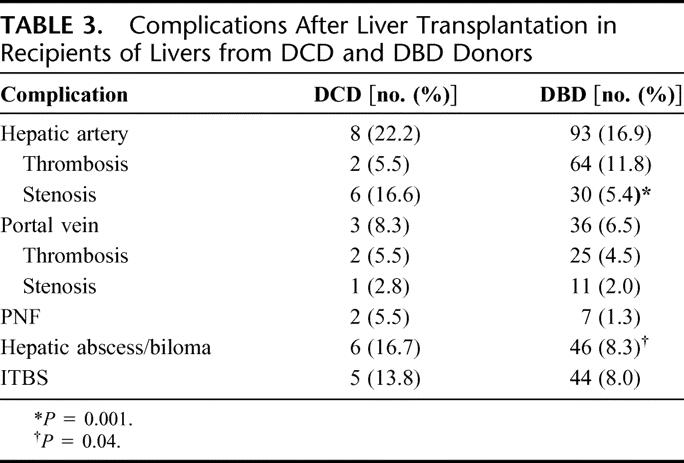
There were no significant differences in portal vein thromboses or portal venous stenoses between the 2 groups. The incidence of hepatic abscess or bilomas was significantly greater in the DCD group versus the DBD group (16.7% versus 8.3%; P = 0.04) (Table 3). Five of these 6 DCD recipients were the same patients who developed either hepatic artery complications or biliary strictures. Likewise, the rate of hepatic abscess/biloma formation in the DCD group at 3 years was approximately 22% versus 8% in the DBD group (P = 0.04). Most of transplants performed were primary transplants, and there was no statistical difference between the DCD group (97.3%) and the DBD group (94.2%).
Two episodes of PNF that occurred in the DCD group were seen in the first 6 liver transplant patients. One PNF was technical in origin; and after this initial experience, our recipients were treated with intravenous prostaglandin E1 (0.2–0.8 mg/kg per hour), vitamin E (1000 u), and N-acetylcysteine (6 g) via the nasogastric tube intraoperatively before reperfusion of the liver and postoperatively. There have not been any episodes of PNF in the last 30 livers transplanted from DCD donors since this protocol was instituted.
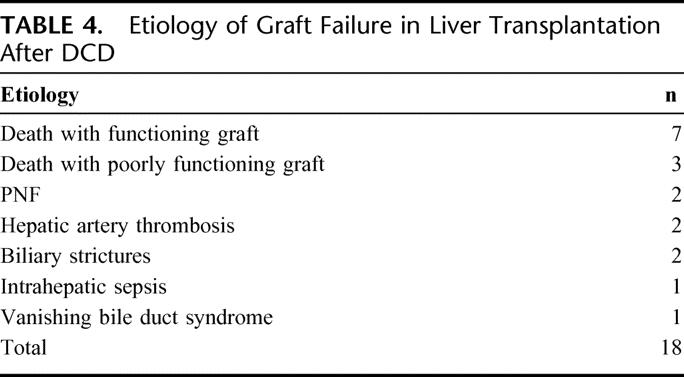
When 1-year rates of rejection were examined, no difference between DCD donors and DBD donors was seen (61% versus 56%). Although patient survival at 3 years was not significantly different in our previous report of 19 patients after DCD, this study revealed a lower patient survival in the DCD group versus the DBD group at 1 year (80% versus 91%) and 3 years (68% versus 84%; P = 0.002) (Fig. 2). As depicted in Figure 3, both 1-year (67% versus 86%) and 3-year (56% versus 80%) graft survival was significantly worse in the DCD group versus the DBD group (P = 0.0001). Eighteen grafts failed and the causes of graft loss in the DCD group are listed in Table 4. The most common causes of graft failure were death with functioning graft (7) followed by death with a poorly functioning graft (3). The latter group clearly had hepatic dysfunction but that was not the ultimate cause of death. The retransplant rate was higher in the DCD group (19.4% versus 7.0%; P < 0.01). Seven patients in the DCD group required retransplantation. The indications for retransplantation were PNF (2), ITBS (1), hepatic artery thrombosis (1), celiac artery stenosis/bilomas (1), intrahepatic sepsis (1), and vanishing bile duct syndrome (1).

FIGURE 2. Patient survival with liver transplantation after DCD and DBD donation.
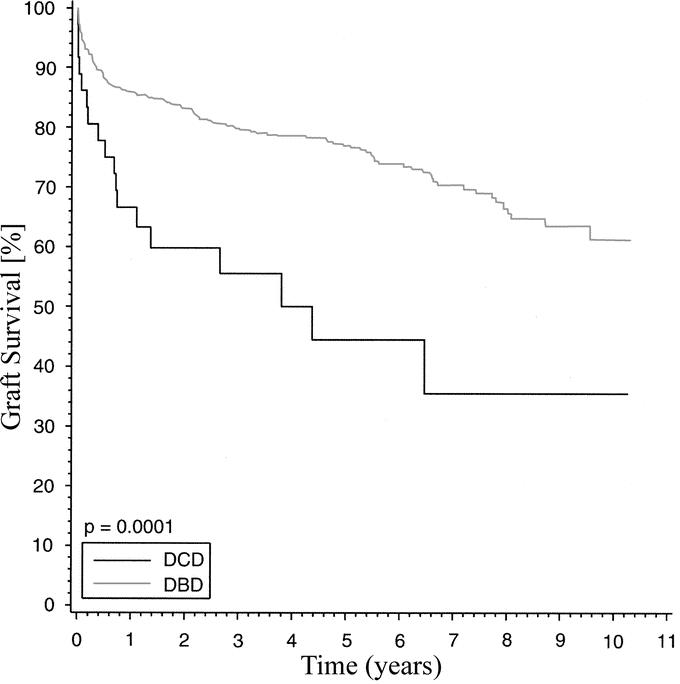
FIGURE 3. Graft survival with liver transplantation after DCD and DBD donation.
TABLE 4. Etiology of Graft Failure in Liver Transplantation After DCD
We then divided the DCD donors into those over and under 40 years of age. Based on Kaplan-Meier estimates, there was a trend toward worse patient survival when the older DCD livers were used. Three-year patient survival in the under 40 group was 80% versus 55% in the over 40 group (P = 0.06). When graft survival of DCD donors over 40 was compared with DCD donors under 40, there was also a trend toward lower graft survival in the older group. Three-year graft survival in the over 40 group was worse when compared with the under 40 group (45% versus 65%; P = 0.1). However, these differences were not statistically significant. There was also a trend toward increased ITBS in the over 40 group, but statistical significance was not reached. At 3 years, there was a 32% incidence of ITBS in the over 40 group and a 7% incidence of ITBS in the under 40 group (P = 0.12). No differences in rejection rates, vascular complications, or primary nonfunction were seen when the over 40 and under 40 year groups were compared. Statistical analyses revealed a large amount of variability, and no significant differences were seen. This was likely secondary to limited events in each group.
DISCUSSION
Because of the increasing number of patients on the liver transplant waiting list and the continued organ shortage crisis, more centers and organ procurement organizations are developing protocols for DCD. Because kidneys tolerate periods of warm ischemia more than extrarenal organs, the experience of DCD with kidney transplantation has been much greater compared with other organs. Both short-term and long-term results of kidney transplantation after DCD have been described.12–14 In our recent analysis, no differences in patient and graft survival are seen at 5, 10, and 15 years after renal transplantation.15 Our experiences with simultaneous pancreas and kidney transplantation from DCD donors have also been described, and results are similar to that from brain-dead donors.16 Our experiences continue to show that an increase in the number of organ donors, approximately 8% to 10%, can be achieved through a DCD program. However, our more recent experience indicates that a 15% to 25% increase can be expected.
Initial results with liver transplantation from non-heartbeating donors were suboptimal because of poor preservation methods, immunosuppression, and transplant surgical techniques. After brain death criteria were established in 1968, with the Harvard Neurologic Definition and Criteria for Death,17 non-heartbeating donation programs went out of favor. However, because of the organ shortage crisis, there has been a resurgence of DCD programs across the United States.
We previously described our single-center results of 19 patients undergoing liver transplantation from DCD donors and compared those outcomes with 364 liver transplants from DBD donors.8 In that analysis, we demonstrated a higher rate of PNF in the DCD liver transplants and worse allograft survival at 1 and 3 years. However, no differences in vascular complications, infectious complications, or patient survival were seen. We have now nearly doubled the number of liver transplants after DCD, and we demonstrate some similar and some different outcomes. Donor characteristics were similar in our 2 series. Male-to-female ratio, alkaline phosphatase, and warm ischemic time were statistically greater in the DCD group, whereas vasopressor use was significantly greater in the DBD group. The latter is likely due to the retention of brain stem function in the DCD donors and thus more hemodynamic stability in that group of donors. No differences were seen in donor age or AST levels between DCD and DBD donors.
In a recent analysis of the UNOS database, Abt et al reviewed 144 liver transplants after non-heartbeating donation (NHBD).18 A greater incidence of PNF in the NHBD group was seen when compared with recipients after heartbeating donation (HBD). Also, multivariate analysis revealed that cold ischemic time was the only significant factor influencing NHBD graft failure within the first 60 days. In grafts that failed, mean cold ischemic time was 9.4 ± 3.3 hours versus 7.6 ± 2.69 hours in those grafts that did not fail.18 In our study, we found no significant difference in cold ischemic time between the 2 groups with mean values of 8.1 and 8.6 hours. Rates of PNF and retransplantation decreased significantly at our center when attempts at extended preservation were limited as a result of a study by Ploeg et al.11 The importance of transplanting these organs from DCDs as soon as possible needs to be emphasized, but it does not explain the differences and outcomes in our series. However, current practice is to begin the recipient operation prior to our procurement team returning and to avoid using DCD livers in technically challenging situations (ie, retransplantation) in attempts to further reduce cold ischemic time.
Recipient characteristics were not different between the 2 groups except for mean follow-up time being longer in the DBD group. However, mean follow-up time of 2.8 ± 2.6 years in the DCD group was adequate to show differences in outcomes. Although PNF was similar between the 2 groups, initial liver dysfunction was demonstrated by blood product usage. Our greater usage of PRBCs, FFP, and platelets in the DCD group suggests initial intraoperative liver dysfunction. Despite this, our operating time was not prolonged in the DCD group. Higher transfusion rates in the DCD group could also be due to the anesthesiologists’ anticipation of a dysfunctional graft and not necessarily due to increased blood loss. Nonetheless, postoperative coagulation parameters, prothrombin time, and International Normalized Ratio were similar throughout the entire postoperative course.
AST levels were significantly greater on postoperative day 1 in the DCD group compared with the DBD group. This suggests greater initial hepatocellular injury with the recovery/preservation process in the DCD group. However, subsequent normalization of AST levels suggests the resolution of hepatocellular integrity in both groups. Alkaline phosphatase and GGT were elevated at various time points in the postoperative period. GGT was significantly greater in the DCD group at all postoperative time points, whereas alkaline phosphatase was significantly greater in the DCD group only at discharge. These levels demonstrate greater degrees of bile duct injury in the DCD group.
In our previous analysis, we did see significantly higher alkaline phosphatase levels at the time of discharge in the DCD group, but no differences in hepatic arterial complications or ITBS were noted. Because no differences in ITBS were seen, we did not further evaluate the overall incidence of biliary strictures in that analysis. However, in this analysis, there were statistically higher rates of hepatic artery stenosis, hepatic abscess/biloma formation, and total biliary strictures in the DCD group with similar rates of hepatic artery thrombosis.
The cause of bile duct injury in our series is likely multifactorial. Because the incidence of ITBS was not significantly different between the 2 groups, ischemic injury from the procurement is not the only cause of biliary complications. The new finding in this analysis is the significantly higher incidence of HAS in the DCD group and the overall increase in biliary strictures. Although 1 patient had a hemodynamically significant stenosis of the celiac axis and another had an arterial anastomotic stricture, 4 of the 6 patients in the DCD group had donor proper hepatic arterial stenoses proximal to the bifurcation. It is unclear why the majority of the stenoses occurred in the donor hepatic artery distal to the anastomosis. Our techniques of procurement have not changed since our first series, and we routinely do not dissect the hepatic artery to the level of the bifurcation. It is possible that the extrahepatic arteries are susceptible to warm ischemia during the DCD recovery, resulting in subsequent scar and stenoses. Because 83% of the DCD recipients with HAS developed biliary strictures and only 37% of the DBD recipients with HAS developed strictures, we think that DCD livers are more susceptible to postoperative arterial ischemia than are DBD livers. We have been aggressive in diagnosing and managing HAS in all of our liver transplants where there is evidence of bile duct injury.
Other groups have documented higher rates of bile duct injury in NHBDs compared with HBDs. Abt et al compared outcomes of liver transplantation with 15 NHBDs to those after 221 HBDs at the University of Pennsylvania. Although patient and graft survival were similar at 1 and 3 years, the incidence of major biliary complications was significantly greater in the DCD group (33.3% versus 9.5%; P < 0.01).19 Warm ischemic time was the only factor that differed between the NHBD and HBD groups.19
In another single-center experience of 8 liver transplants from NHBDs, Reich et al reported significant and progressive hyperbilirubinemia with cholestasis in their NHBD transplant recipients.20 The cholestasis ultimately resolved after 3 weeks. Although there was a 50% rejection rate in those NHBD livers, the cholestasis preceded a rejection and continued after the rejection was successfully treated. Even though no biliary complications were seen, ischemic bile duct injury was suggested in their results.20 We saw similar intrahepatic biliary strictures and beading in the DCD donor livers, and we suspect that these were caused by warm ischemia at procurement and postoperative arterial ischemia secondary to hepatic arterial stenoses. However, the mechanisms of bile duct injury remain unclear.
It is well recognized that liver transplantation from older DBD donors can achieve satisfactory results.21,22 Some have suggested that short-term results of liver transplantation from older DCD donors are similar to those from younger DCD donors.23 To address this issue in our analysis, we further divided our DCD group into donor age greater than 40 and donor age less than 40 and studied the same outcomes. Our analysis suggested that recipients receiving DCD livers from donors over age 40 have worse patient and graft survival. Similarly, the incidence of ITBS in the over 40 group was also higher than that in the under 40 group. However, statistical significance was not attained. We suspect that the lack of statistical significance in these outcomes is likely due to insufficient power as each group only had 18 patients. Our data with increased numbers and longer follow-up suggest a trend toward worse patient and graft survival and increased incidence of ITBS in the older donors. We are not sure what the age cutoff should be in DCD donors. We chose 40 in our analysis so that we would have equal numbers in each group. Based on our observations and outcomes, it is currently our practice to not use donors greater than 50 years of age and limit warm ischemic time to 30 minutes.
Other single-center series with smaller numbers of transplants and shorter follow-up have demonstrated no difference in graft survival at 1 year.19,20,23 In both our previous and current analyses, overall graft survival was worse in the DCD group when compared with the DBD group. Similar statistical differences in graft survival at 1 and 3 years were seen in the recent UNOS database analysis with larger numbers of patients.18 However, in the latter study, differences in patient survival were not significant. In this analysis, patient survival at both 1 and 3 years were significantly worse in the DCD group. Our survival at 3 years in the DCD group (68%) is similar to that recorded in the UNOS database (67.8%). However, our 3-year patient survival in the DBD group is higher (84%) than that seen in the UNOS database (72.1%). Therefore, significant differences seen in our patient survival data may be related to better outcomes in the DBD group.
This study demonstrates that liver transplantation after DCD results in inferior patient and graft survival when compared with that after DBD. The overall incidence of biliary strictures, hepatic abscess/biloma formation, and hepatic arterial stenosis are increased in the DCD group, whereas no differences in PNF and portal vein complications are noted. Despite these differences, the results of liver transplantation after DCD should not preclude the use of these livers. Attention should be directed at further reducing both warm and cold ischemic time as well as possibly reducing donor age, all of which might yield improved results. Likewise, additional investigations into protecting biliary epithelium, reducing hepatic arterial ischemia and the formation of subsequent biliary strictures may further improve results with DCD livers. With more experience and careful selection of recipients and donors, liver transplantation after DCD should continue to be explored as a method to expand the organ donor pool.
ACKNOWLEDGMENTS
The authors thank Barbara Voss and Delores Robillard for their technical assistance with data collection.
Footnotes
Reprints: Anthony M. D'Alessandro, MD, Department of Surgery, University of Wisconsin Hospital, 600 Highland Avenue, Madison, WI 53792-7375. E-mail: am.dalessandro@hosp.wisc.edu.
REFERENCES
- 1.Malago M, Testa G, Frilling A, et al. Right living donor liver transplantation: an option for adult patients. Single institution experience with 74 patients. Ann Surg. 2003;238:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renz JF, Emond JC, Yersiz H, et al. Split-liver transplantation in the United States: outcomes of a national survey. Ann Surg. 2004;239:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 4.Tisone G, Manzia TM, Zazza S, et al. Marginal donors in liver transplantation. Transplant Proc. 2004;36:525–526. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond VA; University Renal Research and Education Association, Ann Arbor, MI. 2004 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. www.optn.org/AR2004.
- 6.Alvarez J, del Barrio R, Arias J, et al. Non-heart-beating donors from the streets: an increasing donor pool source. Transplantation. 2000;70:314–317. [DOI] [PubMed] [Google Scholar]
- 7.Casavilla A, Ramirez C, Shapiro R, et al. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Liver transplantation from controlled non-heart-beating donors. Surgery. 2000;128:579–588. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine DoHCS. Non-heart-beating organ transplantation: In: Medical and Ethical Issues in Procurement. Washington, DC: National Academy Press, 1997. [PubMed] [Google Scholar]
- 10.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation. 1995;59:977–982. [DOI] [PubMed] [Google Scholar]
- 11.Ploeg RJ, D'Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation: a multivariate analysis. Transplantation. 1993;55:807–813. [DOI] [PubMed] [Google Scholar]
- 12.Koffman G, Gambaro G. Renal transplantation from non-heart-beating donors: a review of the European experience. J Nephrol. 2003;16:334–341. [PubMed] [Google Scholar]
- 13.Sanchez-Fructuoso AI, Prats D, Torrente J, et al. Renal transplantation from non-heart beating donors: a promising alternative to enlarge the donor pool. J Am Soc Nephrol. 2000;11:350–358. [DOI] [PubMed] [Google Scholar]
- 14.Gerstenkorn C. Non-heart-beating donors: renewed source of organs for renal transplantation during the twenty-first century. World J Surg. 2003;27:489–493. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JT, Chin LT, Krieger NR, et al. Donation after cardiac death: the University of Wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490–1494. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessandro AM, Odorico JS, Knechtle SJ, et al. Simultaneous pancreas-kidney (SPK) transplantation from controlled non-heart-beating donors (NHBDs). Cell Transplant. 2000;9:889–893. [DOI] [PubMed] [Google Scholar]
- 17.Ad Hoc Committee of the Harvard Medical School. A definition of irreversible coma: report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of bran death. JAMA. 1968;205:337–340. [PubMed] [Google Scholar]
- 18.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abt P, Crawford M, Desai N, et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. [DOI] [PubMed] [Google Scholar]
- 20.Reich DJ, Munoz SJ, Rothstein KD, et al. Controlled non-heart-beating donor liver transplantation: a successful single center experience, with topic update. Transplantation. 2000;70:1159–1166. [DOI] [PubMed] [Google Scholar]
- 21.Emre S, Schwartz ME, Altaca G, et al. Safe use of hepatic allografts from donors older than 70 years. Transplantation. 1996;62:62–65. [DOI] [PubMed] [Google Scholar]
- 22.Washburn WK, Johnson LB, Lewis WD, et al. Graft function and outcome of older (≥60 years) donor livers. Transplantation. 1996;61:1062–1066. [DOI] [PubMed] [Google Scholar]
- 23.Fukumori T, Kato T, Levi D, et al. Use of older controlled non-heart-beating donors for liver transplantation. Transplantation. 2003;75:1171–1174. [DOI] [PubMed] [Google Scholar]



