Abstract
Objective:
To provide a rigorous and critical review of studies in which formal neuropsychological (NP) testing and measurement of health-related quality of life (HRQL) were conducted pre- and post-parathyroidectomy for primary hyperparathyroidism (PHPT). These data contribute to the discussion on the utility of surgical intervention for nonclassic PHPT.
Summary Background Data:
PHPT is a complex endocrinopathy involving calcium metabolism and a potent hormone made by the parathyroid glands. Approximately 1.5% of Americans age 65 years and older, representing more than 3.9 million people, have PHPT, and the prevalence in postmenopausal women is estimated at 3.4%. Current National Institutes of Health guidelines for curative, surgical intervention of PHPT exclude 80% of patients with hyperparathyroid disease who have subjective neurobehavioral and physical symptoms that affect the quality of their lives.
Methods:
An electronic search was conducted of prospective studies in which cognitive functioning was measured with formal NP tests and HRQL was measured with valid and reliable instruments before and following parathyroidectomy for PHPT.
Results:
In studies conducted pre- and post-parathyroidectomy for PHPT, 6 small studies of cognitive functioning report inconsistent findings; however, 7 well-designed studies of HRQL report improvement across multiple domains following surgery.
Conclusions:
Surgical treatment of PHPT is a viable option for patients with laboratory diagnosed, “nonclassic” PHPT. Formal NP testing and evaluation of HRQL are useful tools that may assist physicians in choosing whom to refer for parathyroidectomy. Further longitudinal study of NP functioning and HRQL in patients with laboratory diagnosed PHPT is warranted.
This review discusses NIH criteria for surgical treatment of primary hyperparathyroidism (PHPT), which excludes 80% of patients with hyperparathyroid disease who experience cognitive and physical symptoms affecting health-related quality of life (HRQL). Six cognitive and 7 HRQL studies, conducted pre- and post-parathyroidectomy, are critically reviewed for their contributions to the debate on surgical management of laboratory diagnosed, “nonclassic” PHPT.
Primary hyperparathyroidism (PHPT) is a complex endocrinopathy involving calcium metabolism and a potent hormone made by the parathyroid glands. The diagnosis is confirmed by inappropriately elevated parathyroid hormone levels accompanied by a high normal or elevated serum calcium. Although the classic presentation of PHPT involves presence of renal stones, bone loss, and gastrointestinal complaints, screening of serum calcium during routine medical examinations has changed the clinical spectrum to include patients with minimal objective symptoms who are referred to as “asymptomatic.”
Current National Institutes of Health (NIH) guidelines1 for curative, surgical intervention are defined by easily measured objective criteria: age younger than 50 years; serum calcium concentration of 1 mg/dL above normal; 24-hour urinary calcium excretion of more than 400 mg; creatinine clearance reduced by more than 30% compared with controls; bone mineral density reduced by more than 2.5 standard deviations below the bone density of age, gender, and race-matched norms; and patients for whom medical surveillance is either not desirable or possible. Only about 20% of patients with hyperparathyroid disease meet these objective indications for surgical intervention.2 Meanwhile, most patients have subjective neurobehavioral symptoms ranging from subtle to severe, but these symptoms and their effects on quality of life have not been used by all as clear criteria for parathyroidectomy.
Subjective neurobehavioral symptoms have been described with PHPT since the 1940s.3,4 Lethargy, drowsiness, depressed mood, neurasthenia, paranoia, hallucinations, disorientation, confusion, and cognitive (mostly memory) complaints have been documented in a number of early case reports.5–10 More recent studies have described biochemically confirmed PHPT to be characterized by a number of symptoms previously thought to be atypical of the condition. These include low energy and/or fatigue,11–13 weakness,11 bone and joint pain,11,12,14 cognitive dysfunction,11,15,16 sleep disorders,13 psychologic and psychiatric symptoms that range from depression and anxiety to psychosis and coma,11,13,14,17–19 decreased ability to complete daily tasks at home12 or work,20 and decreased social interaction.12,14 Some patients with mild hypercalcemia report either no symptoms or only neurobehavioral complaints.21 Presumably, these signs and symptoms of “nonclassic” PHPT have a negative impact on health-related quality of life (HRQL). Despite the number and frequency of these findings, recommendations for their management are not addressed in current NIH guidelines for surgical intervention.
The prevalence of PHPT varies by age and gender, affecting 1 in 500 females and 1 in 2000 males over the age of 40 years,22 or approximately 1% of the adult population.23 The prevalence of PHPT increases with age in both males and females, but older women have more severe PHPT.24,25 More than 1.5% of Americans age 65 years and older, representing more than 3.9 million people, have PHPT,23 while the prevalence in postmenopausal women is estimated at 3.4%.26
Surgical cure of PHPT has been shown to improve general health,11,12,27 increase bone density,28 and improve psychiatric13,18 and cognitive functioning,15,16 and HRQL.11,12,27,29–31 Nonetheless, the decision to surgically treat PHPT patients who present with mild and vague symptoms is not consistently made because of inadequate data and controversy as to the extent of benefits experienced.2,21,32,33 Consequently, many patients are not referred for surgical evaluation despite the fact that parathyroidectomy is a safe and effective procedure for elderly patients.17,24,34,35
The existing literature in hyperparathyroidism (HPT) is marked by case studies, clinical reports, symptom checklists, small samples, nonrigorous reporting of cognitive dysfunction, and quality of life measured by global reports rather than by standardized measures. Few studies have addressed cognitive functioning with formal neuropsychological (NP) tests, and the literature on HRQL has not benefited from critical discussion. It appears the debate regarding early surgical treatment of PHPT remains unresolved1,21,36 and would be strengthened by rigorous scientific inquiry. This paper will provide a review of studies in which cognitive functioning was measured with formal NP tests, and HRQL was studied with valid and reliable instruments before and following parathyroidectomy. The findings will be summarized and discussed in light of the larger literature on PHPT, including implications for surgical treatment of nonclassic PHPT.
Neuropsychologic Functioning in Patients With Surgically Treated PHPT
A wide variety of psychiatric symptoms ranging from mild personality changes to severe depression and psychosis have been described in patients with PHPT.37–41 However, the NP manifestations of PHPT are incompletely characterized, with some studies showing postsurgical improvement in cognition, while others show no therapeutic effect.
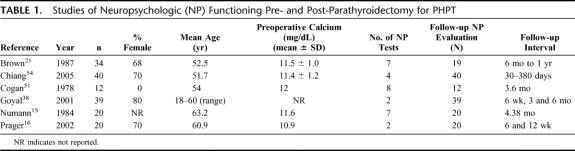
Studies that used NP test batteries pre- and post-surgery to assess multiple cognitive domains include Numann et al15 who compared 10 patients with PHPT who were scheduled for parathyroidectomy to 10 normocalcemic orthopedic patients using 7 cognitive tests: the Wechsler Memory Scale, Form I (WMS)42 the Information, Vocabulary, Similarities, and Block Design subtests of the Wechsler Adult Intelligence Scale (WAIS);43 the Facial Recognition Test;44 the Benton Revised Visual Retention Test;45 Forms A and B of the Trail Making Test;46 and the Finger Tapping Test47 (Table 1). Several tests were clustered to measure specific cognitive domains: the Information and Orientation subtests of the WMS were combined to measure orientation; the Logical Memory and Associate Learning subtests of the WMS were grouped to measure short-term verbal memory; both forms of the Trail Making Test were scored together as measures of visuomotor tracking and planning. Analyses of the data indicated improvement across trials for the hyperparathyroid group on the combined scores of the Logical Memory and Associate Learning subtests of the WMS (measures of short-term verbal memory); improvement in scores of the Digit Span subtest of the WMS (a measure of verbal attention); and improvement on the Similarities subtests of the WAIS (a measure of logical, categorical reasoning). Significant improvements by both groups on the Block Design subtest of the WAIS and the Facial Recognition subtest of the WMS were attributed to practice effects. The results of the Trail Making Test indicated that the hyperparathyroid group had faster times than the orthopedic group both pre- and post-surgery. There were no significant differences between conditions or across trials for the remaining measures. Collectively, these results indicated that PHPT patients demonstrated improved NP functioning across several cognitive skills, including attention, memory, and reasoning following parathyroidectomy.
TABLE 1. Studies of Neuropsychologic (NP) Functioning Pre- and Post-Parathyroidectomy for PHPT
Using some of the same NP tests, Brown et al21 performed NP evaluations prior to treatment in 34 patients with PHPT. Nineteen of these patients, 10 of whom had undergone parathyroidectomy, were retested at 6 months. Measures included the Similarities, Block Design, and Symbol Digit subtests of the WAIS,48 the Trail Making Test, the Short-Term Memory Distractor Test,49 and the Finger Tapping Test.50 The means of the 3 WAIS measures were combined in the data analysis to form a measure of fluid intelligence, ie, the ability to solve novel problems. At the initial evaluation, a significant positive correlation was found between serum calcium levels and cognitive dysfunction, specifically for measures of motor speed, fluid intelligence, and short-term memory. Interestingly, these deficits remained at the 6-month postoperative evaluation, even though serum calcium levels were within normal limits. Collectively, these results suggest that cognitive dysfunction is present in PHPT patients; however, improvement in cognition was not observed within 6 months of surgical intervention.
Likewise, Cogan et al51 used a battery of 8 NP tests to compare 4 subjects with a diagnosis of PHPT, 4 subjects with secondary HPT, and 4 control subjects undergoing similar surgical procedures. All hyperparathyroid subjects underwent parathyroidectomies. Cognitive testing was performed preoperatively and at a mean of 3.6 months postoperatively. The measures included were: the Ravens Progressive Matrices,52 the WMS,42 the Neurologic Index of Mental Impairment,53 Visual Motor Items (VMI),53 the Digit Symbol,48 the Finger Tapping47 and Trail Making Tests,46 and Memory for Designs Test.53 Similar to the findings of Brown et al,21 this study found no significant difference between preoperative and postoperative measures of NP functioning for subjects with PHPT. However, this study did find significant improvements in postoperative performance on measures of general cognitive functioning, nonverbal problem solving, and visual-motor skills for subjects with secondary HPT.
Chiang et al54 administered 4 NP tests, including the Stroop Color Word Test55 (executive functioning); the Digit Symbol Subtest of the WAIS-R48 (attention, perceptuo-motor coordination); the Royal Melbourne Memory for Prose Test56 (verbal memory); and the Unusual Shapes Test57 (visual recognition) to 20 patients with PHPT and 20 patients scheduled for orthopedic surgery as control subjects matched for age, gender, and intellectual function based on the National Adult Reading Test. A battery of tests was also administered to assess symptoms of anxiety and depression. All tests were administered preoperatively and at a single follow-up interval that varied widely from 30 to 380 days (mean, 125 days) for PHPT patients and 14 to 162 days (mean, 82 days) for the orthopedic patients. No significant changes were found between groups on the NP tests or measures of mood from preoperative to postoperative follow-up periods.
In addition, 2 studies used only 2 NP tests each and reported disparate findings. Goyal et al38 examined the NP manifestations of PHPT and correlations with serum calcium levels in a population of Indian patients. Measures included the Comprehensive Psychopathological Rating Scale, validated in Hindi,58 and the scale for Memory and Intelligence for Hindi-speaking populations.59 Thirty-nine patients were subjects, 14 of whom had PHPT. Thirteen patients with gallbladder disease and 12 patients with thyromegaly, who were scheduled for surgery, comprised the control groups. Patients were assessed at 1 and 6 weeks, 3 months, and 6 months postoperatively. Preoperatively, the PHPT patients revealed pronounced psychiatric symptoms, relative to their companion cohorts, with statistically significant improvement following parathyroidectomy. However, there were no differences from the preoperative to postoperative period on measures of memory or intelligence in the PHPT patients or the control group.
Prager et al16 also conducted formal NP testing using 2 measures of attention and memory in 20 PHPT patients preoperatively and at more than one postoperative interval. The measures used were standard European NP tests, including the d2-Test60 (a measure of attention) and the Numbers-Memorizing subtest of the Wilde Intelligence test61 (a measure of memory). Postoperatively, subjects demonstrated significant improvements at 6 weeks on the d2-Test. Performance on the Numbers-Memorizing subtest also improved; however, this improvement did not reach significance until 12 weeks postoperatively.
Studies of HRQL
HRQL is widely recognized as a multidimensional concept that encompasses physical, psychologic, social, and other domains of functioning specific to a given health condition and the developmental stage of the patient.62 Until recently, the effect of hyperparathyroid disease on quality of life has been infrequently studied, providing scant data for the discussion on when to provide definitive surgical treatment of HPT.
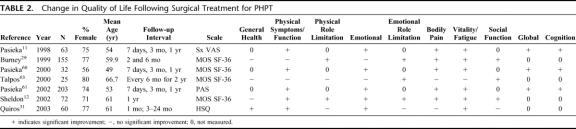
In 1995, Pasieka and Parsons63 provided the initial report of a surgical outcome tool that uses a visual analog scale (VAS) of 0 to 100 (0 = no symptoms; 100 = extreme symptoms) to measure 13 disease-specific items in patients with HPT (examples: weakness, bone pain, forgetfulness), and in 1998 reported further validation of that form.11 (Table 2). The questionnaire was administered preoperatively to 63 patients referred for surgical treatment of PHPT and a comparison group of 54 patients scheduled for surgical treatment of nontoxic thyroid disease. The questionnaire was repeated on postoperative day 7, at 3 months, and 1 year. At 1 year, the patients were also asked to complete global ratings of quality of life and wellness and were queried about general health and satisfaction with surgery. At baseline, the PHPT group was significantly older than the thyroid disease group and reported significantly more symptoms, of which tiring easily and weakness were most common. Other common complaints among PHPT subjects included bone and joint pain, irritability, mood swings, and forgetfulness. The median symptom index score (sum of the median scores of the 13 items on the VAS) was significantly higher in the PHPT group compared with patients with thyroid disease. Following parathyroidectomy, the PHPT patients demonstrated a significant decrease in the median symptom index score at 7 days. Although symptoms continued to decline, there were no further statistically significant declines at 3 and 12 months. The comparison group did not demonstrate a decline in the median symptom index score following thyroidectomy.
TABLE 2. Change in Quality of Life Following Surgical Treatment for PHPT
In 2000, Pasieka and Parsons64 further validated the outcome tool in a study of the effects of parathyroidectomy on 32 patients with PHPT, 22 with secondary HPT, 10 with tertiary HPT, and 32 with thyroid disease. Preoperatively, subjects with secondary HPT experienced fatigue, joint and bone pain, weakness, pruritus, and irritability and had the highest median index score based on the VAS among the 4 groups. Subjects with PHPT had slightly lower VAS scores and reported increased fatigue, forgetfulness, mood swings, and irritability. Subjects with tertiary HPT had a preoperative profile similar to those with PHPT with lower VAS scores, and subjects with thyroid disease had fewest symptoms on the scale designed to measure symptoms of HPT. One week postoperatively, subjects with PHPT demonstrated a significant (P < 0.05) decline in the median VAS score and significant declines in fatigue, emotional symptoms including mood swings, irritability, and depression, bone and joint pain, and forgetfulness. Subjects with secondary HPT had significant (P < 0.05) declines in bone pain, irritability, and itching.
The recent work of Pasieka et al65 reports the impact of parathyroidectomy on 203 PHPT patients in a multicenter study. Three centers participated in this study: University of Adelaide, Adelaide, Australia with 27 participants; Medical College of Wisconsin with 54 participants; and University of Calgary with 122 participants with PHPT and 58 comparison participants with nontoxic thyroid disease. Again, the surgical outcome tool developed by Pasieka and colleagues11,63,64 was used resulting in a Parathyroid Assessment of Symptoms (PAS) score based on the median VAS score. There were significant differences between the preoperative PAS scores across the 3 centers; therefore, the data were analyzed separately rather than being pooled. Despite the difference in PAS scores across centers, each center reported “tiring easily” as the most common symptom. There was agreement in rank of other commonly reported symptoms (weakness, depression, mood swings, joint pain, and forgetfulness) across centers despite the difference in total preoperative scores. Also, each center demonstrated a significant decline in the total PAS score from preoperative to the 7-day and/or 3-month postoperative interval in patients with PHPT. Parathyroidectomy significantly reduced the symptoms of PHPT postoperatively and was associated with improved global HRQL. Patients with nontoxic thyroid disease did not demonstrate declines in PAS scores postoperatively.
Owing to previous work by Pasieka and Parsons11,63,64 and Pasieka et al,65 PAS scores are reliable and valid, disease-specific measures of symptoms of HPT. Although the PAS scale was not designed as a quality of life instrument per se, it records a number of HRQL domains specific to HPT. Four items relate to physical symptoms/function (itchy skin, thirst, weakness, difficulty getting out of a chair or car); 3 relate to emotional symptoms (mood swings, depression, and irritability); 4 for pain (bone, joint, abdominal pain and headache); one item relates to fatigue; and one to cognitive functioning (forgetfulness).
Burney et al29 studied 155 men and women referred for parathyroidectomy for PHPT to determine whether patients differed preoperatively and postoperatively on the Medical Outcomes Short Form 36 (MOS SF-36) health survey and on condition-specific indicators by serum calcium levels (<10.9 mg/dL versus ≥10.9 mg/dL). The SF-36 was developed by the Rand Corporation as part of the Medical Outcomes Study.66 It measures 8 domains of health status and function (general health, physical function, physical role limitation, mental health, emotional role limitation, social function, bodily pain, and energy/fatigue). The SF-36 goes beyond reports of symptoms and feelings to measure function. Also, in addition to identifying status by domain, the SF-36 produces a global score and has been widely used in studies of HRQL. At baseline, participants reported lower than expected functioning in all domains compared with a normal reference population. PHPT patients rated themselves particularly less well in domains representing vitality/fatigue, physical and emotional role function, and bodily pain. Six months postoperatively, the patients had improved in all 8 domains and were significantly improved with respect to physical and emotional role functioning, bodily pain, vitality/fatigue, and social functioning. Improvement in health and functional status was independent of serum calcium level.
In 2000, Talpos et al67 conducted a randomized controlled clinical trial on 53 men and women with laboratory confirmed PHPT (serum calcium level of 10.1–11.5 mg/dL) who were otherwise “asymptomatic” of classic disease. Strict eligibility criteria were used to select a group of patients with a similar disease profile, and the “asymptomatic” status was based on history, physical examination, and biochemical parameters. Twenty-five patients were randomized to parathyroidectomy and 28 to observation only; both groups were followed for 2 years. At baseline and 6 months, the treatment and observation groups completed the SF-36 to provide self-reported wellness. A ninth domain (health change) was added because of the prospective design of this study. The SF-36 data showed significant improvement at 6 months that favored the surgical group in 2 domains: emotional role functioning (P < 0.012) and social functioning (P < 0.007), supporting surgical management of mild PHPT.
Sheldon et al12 also used the SF-36 to measure functional status and HRQL in 72 PHPT patients prior to and following parathyroidectomy. Of these, 43 were classified as “asymptomatic” and 29 had classic symptoms of PHPT. The asymptomatic group scored significantly lower preoperatively on 3 of 8 domains (physical functioning, physical role function, and energy/fatigue), and the symptomatic group scored significantly lower on all domains except general health perception, compared with aged-matched national norms. One year following parathyroidectomy, there was overall improvement in 7 of 8 domains in the 72 participants. The asymptomatic group improved significantly in emotional health and energy/fatigue, whereas the group with history of classic symptoms improved in all domains except general health perception.
In 2003, Quiros et al31 reported longitudinal HRQL data on 60 patients with HPT (56 had PHPT) using the Health Outcomes Institute Health Status Questionnaire (HSQ).68 The HSQ is based on the SF-36 and provides additional information on emotional health. One month following parathyroidectomy, patients reported significant improvement in perception of health status, muscle strength, energy level, and mood. A smaller subset reported persistent improvement at a longer-term follow-up at 3 to 24 months. The improvements in symptoms were positively and significantly correlated with declines in serum calcium and declines in parathyroid hormone levels. The authors concluded that subjective improvement in symptoms represented a valid indication for parathyroidectomy.
DISCUSSION
The NIH has established objective criteria for surgical intervention of PHPT,1,69 that excludes approximately 80% of patients with the disorder.2 Among the excluded are patients with laboratory-documented disease who report vague, subjective symptoms such as fatigue,11–13 weakness,11 bone and joint pain,11,12,14 depressed mood, irritability, anxiety, psychosis,11,13,14,17,18 and forgetfulness as well as other signs of cognitive dysfunction.11,15,16 Although these symptoms may be difficult to interpret, there is increasing longitudinal evidence that patients with these subtle “nonclassic” symptoms of PHPT demonstrate measurable improvement following parathyroidectomy.12,27,29,31,64,65,67 A study by Sywak et al30 found that PHPT patients with only subtle, vague symptoms experienced statistically significant improvement following parathyroidectomy, which was similar to the improvement experienced by surgically treated PHPT patients with classic symptoms. These findings provide support for definitive surgical treatment of patients with mild, otherwise asymptomatic disease, in the absence of classic signs of PHPT. Also, Harrison and Wheeler70 studied 111 patients with laboratory-diagnosed PHPT confirmed by surgery, of whom 83 were “symptomatic” and 28 were “asymptomatic.” There were no differences between the 2 groups with respect to age, parathyroid hormone level (intact, 1–84 PTH), serum calcium level, and excised weight of adenomatous parathyroid tissue. Symptoms of PHPT did not correlate with the degree of biochemical derangement, on which current guidelines for surgery are based. Further, pathologic derangement and clinical assessment alone did not predict severity of disease. Based on the surgical outcomes of these 111 patients, the authors advocated a liberal approach to the selection of asymptomatic patients for operation. It may well be that formal measurement of NP functioning and HRQL associated with mild or “asymptomatic” PHPT could serve as important tools in the decision-making process to select patients who are best served by surgery, much as the current guidelines recommend the use of measurements of age, bone density, and other parameters already used.
Neuropsychologic Findings in Surgically Treated PHPT
Although neurobehavioral symptoms have been described in patients with PHPT for more than 60 years,3 formal NP testing was not applied to the evaluation of these patients until recently. Just as advances in laboratory technology make early identification of PHPT possible,71 advances in the field of neuropsychology provide a means to objectively document the subtle but troublesome manifestations of hyperparathyroidism on thinking, memory, and other cognitive functioning.53 Despite the prominence of neurobehavioral complaints in HPT, formal NP testing has been underused in documenting the effect of PHPT on cognitive functioning.
Of note, the NIH Consensus Panel, which convened to produce the current recommendations for surgical intervention in PHPT, chose not to include “neuropsychological abnormalities” as an indication for parathyroidectomy because “it is not possible at this time to predict which patients will benefit.”1 However, available data suggest that it is possible to identify PHPT patients who exhibit preoperative cognitive decline with formal neurocognitive testing.16,21 Thus, formal NP testing could be used, on a case-by-case basis, as an additional clinical tool to assist the physician in choosing whom to refer for parathyroidectomy. At the same time, current data on cognitive functioning by formal assessment are not adequate to inform general guidelines for surgical treatment of PHPT. Indeed, the lack of such data is striking and suggests that further study is sorely needed. Between 1978 and 2005, only 6 studies15,16,21,38,51,54 reported formal examination of the NP manifestations of PHPT before and following parathyroidectomy (Table 1); and of those studies, only 415,21,51,54 used a battery of NP tests. These studies focused on the cognitive domains of fluid intelligence, attention and concentration, visual and verbal memory, fine motor coordination, and psychomotor speed. The other 2 studies16,38 measured only attention and concentration, and retention. Of these 6 studies, 2 studies15,16 showed improvement in cognition following parathyroidectomy, particularly in verbally coded information, reasoning, and conceptual skills. Another21 documented cognitive deficits associated with elevated calcium levels prior to surgery but showed no improvement in cognition on 6-month follow-up despite normalization of serum calcium. Still another study38 documented psychiatric symptoms preoperatively, which improved following surgery but did not find improvement in the single indicator of cognitive functioning (memory). The remaining 2 studies51,54 showed no difference from the preoperative to postoperative period on cognitive measures.
What is known about cognitive functioning in PHPT prior to and following parathyroidectomy is severely limited due to the small number and size and disparate designs of these studies. Although some batteries used the same NP tests, findings of improvement in cognitive dysfunction following PHPT remained inconsistent. Also, there were no consistent patterns of improvement by specific cognitive domains following parathyroidectomy. These inconsistencies may also be related to the potential effect of time of testing. Few studies examined the effect of parathyroidectomy on NP functioning beyond the immediate postoperative interval and little data are available up to 6 months postoperatively. Examining patients at multiple postoperative periods enables the potential observation of improvement in NP functioning over time. For example, Prager et al16 reported performance on the number-memorizing subtest improved postoperatively but did not reach significance until 12 weeks postoperatively.
Another potential confounding factor in the results observed may be the presence of depression. HPT has long been associated with subjective neuropsychiatric symptoms such as depression,72 which has been reported to be highly related to cognitive dysfunction.73–77 While studies exploring the organic determinants of depression are ambiguous, cognitive impairment in depressed individuals has been well documented. The nature of the observed deficits has been variable, ranging from impairment of memory77–79 attention,80,81 verbal fluency,76,82 and abstraction83 to deficits in visuomotor and visuospatial abilities.84 These deficits are most frequent and pronounced in the elderly. Unfortunately, only one study of formal NP testing in patients with surgically treated PHPT included standardized measures of depression.54 Collectively, these results suggest that the neurobehavioral syndrome associated with PHPT is ambiguous, and the impact of parathyroidectomy on differential improvement in cognitive functioning has been inadequately examined and may be confounded by the presence of depression. A comprehensive battery of NP tests, in conjunction with effective measures of depression, and long-term follow-up, should provide a more adequate characterization of PHPT and the role parathyroidectomy may play in reversing domain-specific cognitive dysfunction.
HRQL in Surgically Treated PHPT
The findings on HRQL in patients with “asymptomatic” PHPT treated with parathyroidectomy provide a more cohesive picture in support of surgical treatment. Justification of HRQL studies in PHPT is based on the presumption that the vague symptoms, such as fatigue, mood swings, irritability, and physical pain, commonly reported in patients with “nonclassic” PHPT, would affect quality of life and could be self-reported. Seven well-designed studies of HRQL measured with valid and reliable outcome scales have been conducted in patients with PHPT since 1998.11,12,29,31,64,65,67 Three of these12,19,67 used the SF-36, a widely accepted generic instrument originally developed for the Medical Outcome Study.66 Another study31 used the HSQ,68 which is based on the SF-36. These tools function as generic instruments and provide data across several domains of quality of life. Three other studies were based on the work of Pasieka et al11,64,65 as they built a database of patients with PHPT while validating a disease-specific surgical outcome instrument that provides a PAS score.65 These scales differ in scope, in that the SF-36 provides a broad range of items divided into domains with several items for each domain. The SF-36 also allows the patient to report changes in physical and emotional functioning. Meanwhile, the PAS scale focuses on patient-rated symptoms related directly to PHPT, although the scale does contain one item that addresses function (ability to get out of a car or chair). The PAS also includes an item to allow the patient to self-report “forgetfulness,” commonly reported in PHPT, whereas the SF-36 does not measure self-reported changes in cognition.
All 7 studies of HRQL in PHPT reported significant improvement across a number of domains following parathyroidectomy. Improvements in energy level and both physical and emotional well-being were the most commonly reported postoperative findings. There were also significant declines in bodily pain and significant improvements in emotional role functioning and social functioning. These relatively recent HRQL data, which document the salutatory effects of surgical intervention, are especially important when considered against a backdrop of other data that promote surgery as a reasonable treatment of “nonclassic” PHPT.
Progress in Surgical Technique and Operative Management
PHPT is caused by solitary parathyroid adenomas in approximately 80% of patients, and the adenomas are typically located near the thyroid gland (80%–90%).85 Since parathyroid adenomas are typically benign and well encapsulated, the majority of patients with PHPT can be cured with surgery.
Progress in the preoperative radiographic localization of parathyroid adenomas and in operative management provides surgeons treatment options in addition to conventional neck exploration. Preoperative localization of parathyroid adenomas by scintigraphy, with sensitivity ranging from 73% to 89%86 provides the surgeon the option of planning a minimally invasive parathyroidectomy. The many benefits of minimally invasive directed parathyroidectomy include less dissection of the cervical region, possible elimination of general anesthesia and fewer subsequent respiratory complications, decreased hospital stay, and improved cosmetic result. When necessary, single photon emission computed tomography (SPECT) imaging may be used to provide definitive localization of the lesion.87 Specifically, SPECT imaging can help differentiate superior from inferior parathyroid adenomas, which alters the site of incision in an effort to minimize dissection and maximize efficiency of dissection. In addition to better imaging, progress in operative guidance has been seen with the use of a rapid intraoperative parathyroid hormone assay, which facilitates complete excision of all hyperfunctioning parathyroid tissue or alerts the surgeon to further explore to achieve a successful parathyroidectomy.88 With this technology and skilled endocrine surgeons, minimally invasive parathyroidectomy can be performed under attended local anesthesia with same-day discharge, even in elderly patients.89
Gains Afforded by Parathyroidectomy
The gains afforded by parathyroidectomy described in the recent HRQL data focus attention on data that suggest that PHPT increases risk for premature death, mainly from cardiovascular disease and malignancy, and this increased risk can be reversed by parathyroidectomy.22,90–92 Stefenelli et al93 reported reversible left ventricular hypertrophy and decreased progression of calcification after successful parathyroidectomy. Palmer et al94 found increased mortality in patients with untreated hypercalcemia, probably of PHPT origin. Increased survival following parathyroidectomy has been reported in all age groups, including patients 75 years of age and older.91
Further Study Is Needed, Particularly Among the Elderly
The prevalence of PHPT increases with age; and while the benefits of parathyroidectomy for PHPT with advanced symptomatology are widely known, less is known about the degree to which older adults with nonclassic symptoms benefit from parathyroidectomy. Chigot et al95 conducted a retrospective study of 78 patients over age 75 years (mean, 79.1 years) who had undergone neck exploration for PHPT over a 15-year period and reported overall postoperative mortality of 3.8%. Significant complications occurred in 3.8%, and the average length of postoperative stay was 4 days. Of 65 cases with follow-up data (mean, 3 years), 94% reported an improvement in symptoms. The HRQL studies discussed above were conducted in women and men in their middle 50s to early 60s. Only Talpos et al67 reported HRQL data on participants with mean age over 65 years. What is known is that the older population is not readily referred for surgical evaluation,24,96 even though “subclinical” or unrecognized symptoms may be major contributors to disability and functional decline in older adults with this disease. A New Zealand study found that the delay between diagnosis and referral for surgery for PHPT ranged from 8 days to 10 years, exceeding 2 years in 24% of patients.96 This study also showed that those referred for surgical intervention tended to have more severe disease. In a study conducted at Johns Hopkins hospital, Chen et al24 found that PHPT patients older than 70 years present with the most advanced disease, supporting the notion that elderly adults are referred for surgery later in the disease process. The elderly also have a higher prevalence of hypercalcemic crisis as a presenting diagnosis.24 Delay in referral for PHPT in the elderly means more severe parathyroid disease and higher risk of functional impairments. Bony fracture risks in patients with PHPT are significantly associated with increasing age and female gender and are threats to functional status and HRQL. Bone density tends to improve during the 1 to 2 years after successful parathyroidectomy,28 and it is logical to propose that functional risk would also be reduced, especially if muscle strength and balance improve.97 Functional consequences of disease are one of the most important issues faced by older people, researchers in aging, and our society in general.98,99 Recent evidence has shown that progression of functional impairment, particularly in lower extremity function beyond certain threshold limits indicates high risk for future loss of ability to live independently in the community.100 New and more rigorous studies must be developed to determine whether early treatment of PHPT, including adults in their 60s and 70s, will reduce the progression of disability in this growing population.
Footnotes
Reprints: Laura H. Coker, PhD, Wake Forest University School of Medicine, 1 Medical Center Blvd., Winston-Salem, NC, 27157. E-mail: lcoker@wfubmc.edu.
REFERENCES
- 1.Bilezikian JP, Potts JT, Fuleihan GE-H, et al. Summary statement from a workshop on asymptomatic primary hyperthyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–5361. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg SJ, Bilezikian JP, Bone HG, et al. Therapeutic controversies in primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84:2275–2285. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen HE, Steffensen K. Geographic distribution and surgical therapy of hyperparathyroidism in connection with 2 cases. Nord Med. 1941;9:115–121. [Google Scholar]
- 4.Nielson H. Familial occurrence, gastrointestinal symptoms and mental disturbances in hyperparathyroidism. Acta Med Scand. 1955;151:359–366. [DOI] [PubMed] [Google Scholar]
- 5.Fitz TE, Hallman BL. Mental changes associated with hyperparathyroidism. Arch Intern Med. 1952;89:547–551. [DOI] [PubMed] [Google Scholar]
- 6.Reinfrank RF. Primary hyperparathyroidism with depression. Arch Intern Med. 1961;108:606–610. [DOI] [PubMed] [Google Scholar]
- 7.Agras S, Oliveau DC. Primary hyperparathyroidism and psychosis. Can Med Assoc J. 1964;91:1366–1367. [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan TA, Goodwin DW, Alderson P. Psychiatric illness in a large family with familial hyperparathyroidism. Br J Psychiatry. 1970;117:693–698. [DOI] [PubMed] [Google Scholar]
- 9.Gatewood JW, Organ CH Jr, Mead BT. Mental changes associated with hyperparathyroidism. Am J Psychiatry. 1975;132:129–132. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt S, Faillace LA. Psychiatric manifestations of hyperparathyroidism. Tex Med. 1977;73:59–60. [PubMed] [Google Scholar]
- 11.Pasieka JL, Parsons LL. Prospective surgical outcome study of relief of symptoms following surgery in patients with primary hyperparathyroidism. World J Surg. 1998;22:513–519. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon DG, Lee FT, Neil NJ, et al. Surgical treatment of hyperparathyroidism improves health-related quality of life. Arch Surg. 2002;137:1022–1028. [DOI] [PubMed] [Google Scholar]
- 13.Joborn C, Hetta J, Johansson H, et al. Psychiatric morbidity in primary hyperparathyroidism. World J Surg. 1988;12:476–481. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto T, Kamo T, Obara T. Outcome study of psychological distress and nonspecific symptoms in patients with mild primary hyperparathyroidism. Arch Surg. 2002;131:779–784. [DOI] [PubMed] [Google Scholar]
- 15.Numann PJ, Torppa AJ, Blumetti AE. Neuropsychologic deficits associated with primary hyperparathyroidism. Surgery. 1984;96:1119–1123. [PubMed] [Google Scholar]
- 16.Prager G, Kalaschek A, Kaczirek K, et al. Parathyroidectomy improves concentration and retentiveness in patients with primary hyperparathyroidism. Surgery. 2002;132:930–6. [DOI] [PubMed] [Google Scholar]
- 17.Rastad J, Joborn C, Akerstrom G, et al. Incidence, type and severity of psychic symptoms in patients with sporadic primary hyperparathyroidism. J Endocrinol Invest. 1992;15(suppl):149–156. [PubMed] [Google Scholar]
- 18.Joborn C, Hetta J, Lind L, et al. Self-rated psychiatric symptoms in patients operated on because of primary hyperparathyroidism and in patients with long-standing mild hypercalcemia. Surgery. 1989;105:72–78. [PubMed] [Google Scholar]
- 19.Doherty GM. Parathyroid glands. In: Greenfield LJ, Mulholland MW, Oldham KT, et al, eds. Surgery: Scientific Principles and Practice. Philadelphia: Lippincott, Williams & Wilkins, 2001:1284–1306. [Google Scholar]
- 20.Lundgren E, Szabo E, Ljunghall S, et al. Population based case-control study of sick leave in postmenopausal women before diagnosis of hyperparathyroidism. BMJ. 1998;317:848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown GG, Preisman RC, Kleerekoper M. Neurobehavioral symptoms in mild primary hyperparathyroidism: related to hypercalcemia but not improved by parathyroidectomy. Henry Ford Hospital Med J. 1987;35:211–215. [PubMed] [Google Scholar]
- 22.Clark OH. Diagnosis of primary hyperparathyroidism. In: Clark OH, Duh G-Y, eds. Textbook of Endocrine Surgery. Philadelphia: Saunders, 1997:297–301. [Google Scholar]
- 23.Heath H III, Hodgson SF, Kennedy MA. Primary hyperparathyroidism: incidence, morbidity, and potential economic impact in a community. N Engl J Med. 1980;302:189. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Parkerson S, Udelsman R. Parathyroidectomy in the elderly: do the benefits outweigh the risks? World J Surg. 1998;22:531–536. [DOI] [PubMed] [Google Scholar]
- 25.Khosla S, Melton LJ III, Wermers RA, et al. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700–1707. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren E, Hagstrom EG, Lundin J, et al. Primary hyperparathyroidism revisited in menopausal women with serum calcium in the upper normal range at population-based screening 8 years ago. World J Surg. 2002;26:931–936. [DOI] [PubMed] [Google Scholar]
- 27.Burney RE, Jones KR, Wilson Coon J, et al. Assessment of patient outcomes after operation for primary hyperparathyroidism. Surgery. 1996;120:1013–1019. [DOI] [PubMed] [Google Scholar]
- 28.Minisola S, Rosso R, Romagnoli E, et al. Trabecular bone mineral density in primary hyperparathyroidism: relationship to clinical presentation and biomarkers of skeletal turnover. Bone Miner. 1993;20:113–123. [DOI] [PubMed] [Google Scholar]
- 29.Burney RE, Jones KR, Christy B, et al. Health status improvement after surgical correction of primary hyperparathyroidism in patients with high and low preoperative calcium levels. Surgery. 1999;125:608–614. [PubMed] [Google Scholar]
- 30.Sywak MS, Knowlton ST, Pasieka JL, et al. Do the National Institutes of Health consensus guidelines for parathyroidectomy predict symptom severity and surgical outcome in patients with primary hyperparathyroidism? Surgery. 2002;132:1013–1020. [DOI] [PubMed] [Google Scholar]
- 31.Quiros RM, Alef MJ, Wilhelm SM, et al. Health-related quality of life in hyperparathyroidism measurably improves after parathyroidectomy. Surgery. 2003;134:675–681. [DOI] [PubMed] [Google Scholar]
- 32.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg SJ. Non-classical target organs for primary hyperparathyroidism. J Bone Miner Res. 2002;17:N117–N125. [PubMed] [Google Scholar]
- 34.Chigot J-P, Menegauz F, Achrafi H. Should primary hyperparathyroidism be treated surgically in elderly patients older than 75 years? Surgery. 1994;117:397–401. [DOI] [PubMed] [Google Scholar]
- 35.Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–1875. [DOI] [PubMed] [Google Scholar]
- 36.Melton LJ III. The epidemiology of primary hyperparathyroidism in North America. J Bone Miner Res. 2002;17:N12–N17. [PubMed] [Google Scholar]
- 37.Watson LC, Marx CE. New onset of neuropsychiatric symptoms in the elderly: possible primary hyperparathyroidism. Psychosomatics. 2002;43:413–417. [DOI] [PubMed] [Google Scholar]
- 38.Goyal A, Chumber S, Tandon N, et al. Neuropsychiatric manifestations in patients of primary hyperthyroidism and outcome following surgery. Indian J Med Sci. 2001;55:677–686. [PubMed] [Google Scholar]
- 39.McDonald WM, Salzman C, Schatzberg AF. Depression in the elderly. Psychopharmacol Bull. 2002;36:112–122. [PubMed] [Google Scholar]
- 40.Geffken GR, Ward HE, Staab JP, et al. Psychiatric morbidity in endocrine disorders. Psychoendocrinology. 1998;21:473–489. [DOI] [PubMed] [Google Scholar]
- 41.Spivak B, Radvan M, Ohring R, et al. Primary hyperthyroidism, psychiatric manifestations, diagnosis and management. Psychother Psychosom. 1989;51:38–44. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945;19:87–95. [Google Scholar]
- 43.Wechsler D. Wechsler Adult Intelligence Scale Manual. New York: Psychological Corporation, 1949. [Google Scholar]
- 44.Milner B. Visual recognition and recall after right temporal-lobe excision in man. Neuropsychologia. 1968;6:191–209. [DOI] [PubMed] [Google Scholar]
- 45.Benton AL. The Revised Visual Retention Test. New York: Psychological Corporation, 1963. [Google Scholar]
- 46.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 47.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Indianapolis: Reitan, 1969. [Google Scholar]
- 48.Matarazzo JD. Wechler's Measurement and Appraisal of Adult Intelligence. Baltimore: Williams & Wilkins, 1972:377–427. [Google Scholar]
- 49.Peterson L, Peterson M. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. [DOI] [PubMed] [Google Scholar]
- 50.Reitan RM, Davison LA. Clinical Neuropsychology: Current Status and Applications. New York: John Wiley & Sons, 1974:369. [Google Scholar]
- 51.Cogan MG, Covey CM, Arieff AI, et al. Central nervous system manifestations of hyperparathyroidism. Am J Med. 1978;65:963–970. [DOI] [PubMed] [Google Scholar]
- 52.Raven JC. Guide to the Standard Progressive Matrices. London: Lewis, 1960. [Google Scholar]
- 53.Lezak MD. Neuropsychological Assessment. New York: Oxford University, 1995. [Google Scholar]
- 54.Chiang CY, Andrewest DC, Anderson D, et al. A controlled, prospective study of neuropsychological outcomes post parathyroidectomy in primary hyperparathyroid patients. Clin Endocrinol. 2005;62:99–104. [DOI] [PubMed] [Google Scholar]
- 55.Golden CJ. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago: Stoelting. [Google Scholar]
- 56.Andrwews DC, Bladin P. Post-ictal recognition memory predicts lateralilty of temporal lobe seizure focus: comparison with post-operative data. Neuropsychologia. 1990;28:957–967. [DOI] [PubMed] [Google Scholar]
- 57.Puce A, Andrews DG, Bladin P. Visual recognition memory: neurophysiological evidence for the role of temporal white matter in man. Brain. 1991;114:1647–1666. [DOI] [PubMed] [Google Scholar]
- 58.Asberg M, Montgomery SA, Perris C, et al. Comprehensive Psychopathological Rating Scale. Acta Psychiatr Scand. 1978;271(suppl):5–28. [DOI] [PubMed] [Google Scholar]
- 59.Gupta S, Khandelwal SK, Tandon PN, et al. The development of neuropsychological battery for use of Hindi knowing patients. 1992.
- 60.Brickenkamp R. Test d2-Aufmerksamkeits-Belastungs Test, 8th revised ed. Goettingen: Hogrefe-Verlag GmbH & CoKG, 1994. [Google Scholar]
- 61.Jaeger A, Althoff K. Der WILDE-Intelligenz-Test (WIT), 2nd revised ed. Goettingen: Hogrefe-Verlag GmbH & CoKG, 1994. [Google Scholar]
- 62.Naughton MJ, Shumaker SA. Assessment of health-related quality of life. In: Friedman LM, Furberg CD, DeMets DL, eds. Fundamentals of Clinical Trials. St. Louis: Mosby, 1996:185–203. [Google Scholar]
- 63.Pasieka JL, Parsons LL. A retrospective analysis on the change in symptoms resulting from hyperparathyroidism following surgical intervention: allowing for the validation of a prospective surgical outcome study questionnaire. World Cong Surg. 1995;338–383. [Google Scholar]
- 64.Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery. 2000;128:531–539. [DOI] [PubMed] [Google Scholar]
- 65.Pasieka JL, Parsons LL, Demeure MJ, et al. Patient-based surgical outcome tool demonstrating alleviation of symptoms following parathyroidectomy in patients with primary hyperparathyroidism. World J Surg. 2002;26:942–949. [DOI] [PubMed] [Google Scholar]
- 66.Ware JE, Sherbourne CD. The MOS 35-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 67.Talpos GB, Bobe HG, Kleerekoper M, et al. Randomized trial of parathyroidectomy in mild asymptomatic primary hyperparathyroidism: patient description and effects of the SF-36 health survey. Surgery. 2000;128:1013–1021. [DOI] [PubMed] [Google Scholar]
- 68.Kania C. The health status questionnaire: a three-consortium update. Qual Source. 1996;4:1–7. [Google Scholar]
- 69.NIH Conference. Diagnosis and management of asymptomatic primary hyperparathyroidism: consensus development conference statement. Ann Intern Med. 1991;114:593–597. [DOI] [PubMed] [Google Scholar]
- 70.Harrison BJ, Wheeler MH. Asymptomatic primary hyperparathyroidism. World J Surg. 1991;15:724–729. [DOI] [PubMed] [Google Scholar]
- 71.Preisman RA, Mehnert JH. A plethora of primary hyperparathyroidism. Arch Surg. 1971;103:12–13. [DOI] [PubMed] [Google Scholar]
- 72.Wilhelm S, Lee J, Prinz RA. Major depression due to primary hyperparathyroidism: a frequent and correctable disorder. Am Surgeon. 2004;70:175–180. [PubMed] [Google Scholar]
- 73.Rorie KD. Depression, Lesion Location, and Neuropsychological Test Performance [Unpublished dissertation]. Washington, DC: Howard University, 1997. [Google Scholar]
- 74.Porter JR, Gallagher P, Thompson JM, et al. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–220. [DOI] [PubMed] [Google Scholar]
- 75.Parmelee PA, Katz IR, Lawton MP. Incidence of depression in long-term care settings. J Gerontol. 1992;47:M189–M196. [DOI] [PubMed] [Google Scholar]
- 76.LaRue A. Patterns of performance on the Fuld Object Memory Evaluation in elderly inpatients with depression or dementia. J Clin Exp Neuropsychol. 1989;11:409–422. [DOI] [PubMed] [Google Scholar]
- 77.Rohling M, Scogin F. Automatic and effortful processes in depressed persons. J Gerontol. 1993;48:P87–P95. [DOI] [PubMed] [Google Scholar]
- 78.Feehan M, Knight R, Partridge F. Cognitive complaint and test performance in elderly patients suffering from depression or dementia. Int J Am Geriatric Psychiatry. 1991;6:287–293. [Google Scholar]
- 79.Sternberg DE, Jarvik ME. Memory functions in depression. Arch Gen Psychiatry. 1976;33:219–224. [DOI] [PubMed] [Google Scholar]
- 80.Breslow R, Kocsis J, Belkin B. Memory deficits in depression: evidence utilizing the Wechsler Memory Scale. Percept Mot Skills. 1980;51:541–542. [DOI] [PubMed] [Google Scholar]
- 81.Rush AJ, Weissenburger J, Vinson DB, et al. Neuropsychological dysfunction in unipolar nonpsychotic major depression. J Affective Disord. 1983;5:281–287. [DOI] [PubMed] [Google Scholar]
- 82.King DA, Caine ED, Conwell Y, et al. The neuropsychology of depression in the elderly: a comparative study of normal aging and Alzheimer's disease. J Neuropsychiatr Clin Neurosci. 1991;3:163–168. [DOI] [PubMed] [Google Scholar]
- 83.Clark D, Clayton P, Andreasen N, et al. Comparative Psychiatry. 1985;26:313–325. [DOI] [PubMed]
- 84.Cassens G, Wolfe L, Zola M. The neuropsychology of depressions. J Neuropsychiatr Clin Neurosci. 1990;2:202–213. [DOI] [PubMed] [Google Scholar]
- 85.Mariani G, Gulec S, Rubello D, et al. Preoperative localization and radioguided parathyroid surgery. J Nucl Med. 2003;44:1443–1458. [PubMed] [Google Scholar]
- 86.McBiles M, Lambert AT, Cote MG, et al. Sestamibi parathyroid imaging. Semin Nucl Med. 1995;25:221–234. [DOI] [PubMed] [Google Scholar]
- 87.Lorberboym M, Minski I, Macadziob S, et al. Incremental diagnostic value of preoperative 99mTc-MIBI SPECT in patients with a parathyroid adenoma. J Nucl Med. 2003;44:904–908. [PubMed] [Google Scholar]
- 88.Carter AB, Howanitz PJ. Intraoperative testing for parathyroid hormone: a comprehensive review of the use of the assay and the relevant literature. Arch Pathol Lab Med. 2003;127:1424–1442. [DOI] [PubMed] [Google Scholar]
- 89.Irvin GL, Carneiro DM. Management changes in primary hyperparathyroidism. JAMA. 2000;284:934–936. [DOI] [PubMed] [Google Scholar]
- 90.Hedback G, Tisell L-E, Bengtsson B-A, et al. Premature death in patients operated on for primary hyperparathyroidism. World J Surg. 1990;14:829–836. [DOI] [PubMed] [Google Scholar]
- 91.Hedback G, Oden A, Tisell L-E. The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg. 1991;15:399–407. [DOI] [PubMed] [Google Scholar]
- 92.Hedback G, Oden A. Increased risk of death from primary hyperparathyroidism: an update. Eur J Clin Invest. 1998;28:271–276. [DOI] [PubMed] [Google Scholar]
- 93.Stefenelli T, Mayr H, Bergler-Klein J, et al. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med. 1993;95:197–202. [DOI] [PubMed] [Google Scholar]
- 94.Palmer M, Adami HO, Bergstrom R, et al. Survival and renal function in untreated hypercalcemia.. Lancet. 1987;1:59–62. [DOI] [PubMed] [Google Scholar]
- 95.Chigot J-P, Menegauz F, Achrafi H. Should primary hyperparathyroidism be treated surgically in elderly patients older than 75 years? Surgery. 1995;117:397–401. [DOI] [PubMed] [Google Scholar]
- 96.Flint RS, Harman CR, Carter J, et al. Primary hyperparathyroidism: referral patterns and outcomes of surgery. ANZ J Surg. 2002;72:200–203. [DOI] [PubMed] [Google Scholar]
- 97.Gough IR. Invited commentary. World J Surg. 1998;22:531–536.9597924 [Google Scholar]
- 98.Ettinger WH Jr, Fried LP, Harris T, et al. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;1994:1035–1044. [DOI] [PubMed] [Google Scholar]
- 99.Schneider EL, Guralnik JM. The aging of America: impact on health care costs. JAMA. 1990;263:2335–2340. [PubMed] [Google Scholar]
- 100.Guralnik JM, Ferrucci L, Peiper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]