Abstract
Objective:
Preoperative core biopsy in breast cancer is becoming the standard of care. The aim of this study was to analyze the various methods of core biopsy with respect to diagnostic accuracy and to examine the management and outcome of those patients with false-negative biopsies.
Methods:
All patients undergoing core biopsy for breast abnormalities over a 5-year period (1999–2003) were reviewed. The accuracy rates for each method of core biopsy, the histologic agreement between the core pathology and subsequent excision pathology, and the length of follow-up for cases of benign disease were studied. Patients whose biopsies were benign but who were subsequently diagnosed with cancer underwent detailed review.
Results:
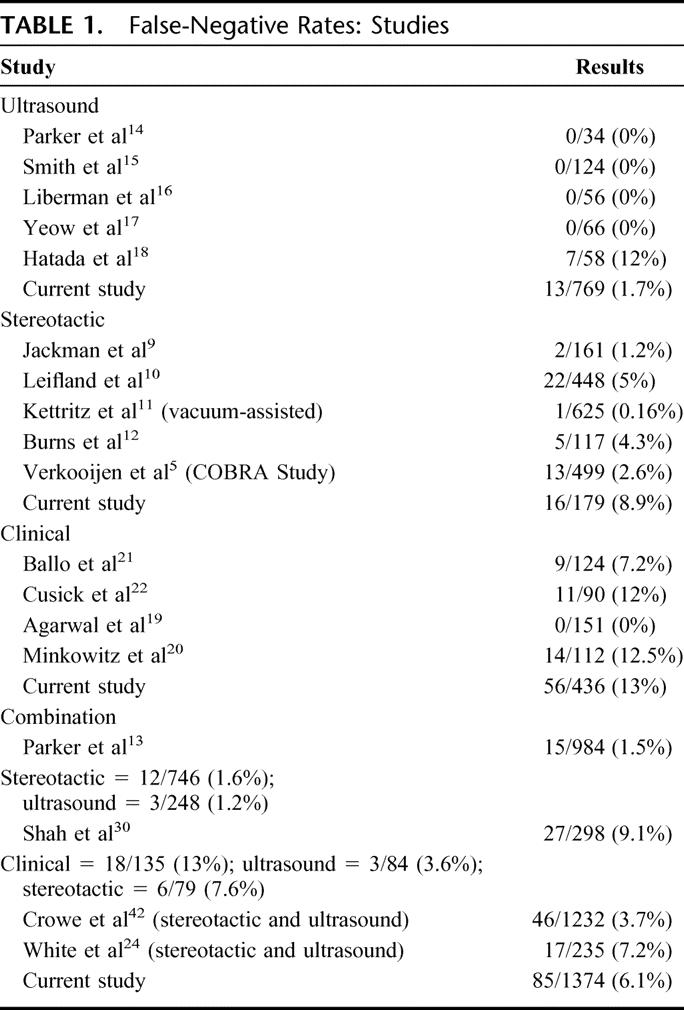
There were 2427 core biopsies performed over the 5-year period, resulting in a final diagnosis of cancer in 1384 patients, benign disease in 954 patients, and atypical disease in 89 patients. Biopsy type consisted of 1279 ultrasound-guided cores, 739 clinically guided cores, and 409 stereotactic-guided cores. The overall false-negative rate was 6.1%, with specific rates for ultrasound-, clinical-, and stereotactic-guided cores of 1.7%, 13%, and 8.9%, respectively. False-negative biopsies occurred in 85 patients, and in 8 of these patients the diagnosis was delayed by greater than 2 months. In all other false-negative cases, “triple assessment” review allowed prompt recognition of discordant biopsy results and further evaluation.
Conclusion:
Ultrasound guidance should be used to perform core biopsies in evaluating all breast abnormalities visible on ultrasound. Adherence to principles of triple assessment following biopsy allows for early recognition of the majority of false-negative cases.
In this study, 2427 core biopsies of the breast were examined, analyzing the diagnostic accuracy of ultrasound, stereotactic, and clinical cores in palpable and nonpalpable lesions. Eighty-five patients (6.1%) with false-negative core biopsies were examined in detail to determine the factors that led to an initial false-negative diagnosis.
The possibility of “missing” or delaying breast cancer diagnosis as a result of a falsely reassuring benign core biopsy is an outcome that every clinician endeavors to avoid. Although fine needle aspiration cytology is still in use,1–3 many institutions have moved definitively toward core biopsy as a preoperative diagnostic tool in breast disease in an attempt to increase the sensitivity of diagnosis, determine the type and invasive nature of the disease, and prevent unnecessary operations.4–7
The principal modality of biopsy technique in the reported literature is stereotactic-guided cores,5,8–13 with the large majority of studies concentrating on nonpalpable lesions. Ultrasound-guided core biopsy is less well researched (Table 1), 14–18 and the accuracy of clinically guided cores has only occasionally been reported in small scale studies.19–22 In this study, we aimed to determine the efficacy of the 3 different modalities of core biopsy in patients presenting to both symptomatic and screening services, particularly where ultrasound-guided biopsies comprise a significant proportion of the biopsies undertaken. Our secondary aim was to analyze in detail those patients with false-negative biopsies to determine the nature of the clinical, radiologic, or pathologic features that may have contributed to a false-negative result. Finally, we sought to determine whether an appropriate system was in place to ensure that false-negative cases are recognized with the minimum delay.
TABLE 1. False-Negative Rates: Studies
MATERIALS AND METHODS
Study Population
The study population comprised of all women who underwent image-guided or clinically guided core biopsy for evaluation of a breast abnormality at St. Vincent's University Hospital, Dublin, Ireland over a 5-year period from January 1999 to September 2003. Patients were identified from a database of clinical information relating to consecutive patients presenting to the breast symptomatic service and population-based screening service at the hospital. The symptomatic clinic was attended by patients who were referred with breast abnormalities by their primary care physicians. The screening population was derived from those women attending the Irish National Breast Screening Programme (“BreastCheck”), which offers 2-view mammographic screening every 2 years to women 50 to 64 years of age. All patients in the study population underwent clinical examination and radiologic investigation, including mammography. Ultrasound evaluation was performed on the majority of patients.
Image-Guided Biopsy
Image-guided core biopsies were introduced into our clinical practice in September 1999 and gradually replaced clinical core biopsies to become the current standard of care. Ultrasound-guided biopsy was used for the evaluation of sonographically visible lesions and was performed in the supine or decubitus position using a high-resolution 12.5-MHz linear array transducer. Direct visualization of the needle tip pre and post fire was the standard, together with an orthogonal image to ensure that the needle was within the lesion. A 14-gauge automated needle device with a 22-mm throw biopsy gun was used (Tru-Core, Medical Device Technology). The most common indication for stereotactic-guided biopsy was calcifications not seen on ultrasound. Stereotactic-guided core biopsies were performed in the upright position using a digital Siemens Optima machine (Solna, Sweden) and a spring-loaded biopsy device (Tru-Guide, Bard Ltd, Crawley, UK). Fourteen-gauge needles were used for stereotactic biopsies during the study period. A specimen radiograph was performed to ensure the presence of calcifications.
Clinically Guided Core Biopsy
Clinically guided core biopsies were guided by palpation and were preformed using a Pro-Mag biopsy gun (Northbrook, IL) with a 14- or 16-gauge needle.
Pathologic Assessment
Core biopsies were formalin fixed, paraffin embedded, and processed according to standard protocol. Each biopsy was stained with hematoxylin and eosin and examined at a minimum of 2 levels. Biopsies were reported as normal/benign, inadequate, atypical (atypical ductal or lobular hyperplasia, lobular carcinoma in situ, radial scar, papilloma), suspicious but not diagnostic of malignancy, or malignant. Malignant lesions were classified as in situ or invasive carcinoma. Tumor type was specified, and estrogen receptor studies were performed on invasive carcinomas using immunohistochemistry.
Evaluation of Accuracy of Core Biopsy Method
A benign diagnosis on core biopsy followed by a diagnosis of malignant disease was considered a false-negative result. The false-negative rate was obtained by dividing the number of false-negative cases by the number of patients diagnosed with cancer. In patients where atypia was demonstrated on core but a subsequent diagnosis of malignancy was made on excision were considered “underestimates” following multidisciplinary review. A diagnosis of malignancy on core biopsy in patients treated with chemotherapy or endocrine therapy rather than surgery were considered as true positives. In 1 patient a small focus of cancer was discovered on core biopsy but not on excision; the biopsy result was also considered as a true positive biopsy following review.
Triple Assessment
Patients referred to the symptomatic service were assessed in a specialized breast surgical clinic and underwent biopsy on the basis of clinical findings or abnormal mammography. Patients presenting to the National Breast Screening Programme who had abnormal mammograms were also assessed clinically by a consultant surgeon. The clinical, radiologic, and pathologic findings of all patients undergoing core biopsy were reviewed at weekly multidisciplinary breast meetings where recommendations were made to discharge, monitor clinically, further investigate, or refer for diagnostic or therapeutic surgery. Patients from the National Breast Screening Programme who had benign diagnosis were placed on routine recall.
False Negatives
Patients with benign cores and subsequent malignant pathology were reviewed in detail with emphasis on clinical presentation, radiologic findings, indications for further investigations, delay in diagnosis, and final pathologic diagnosis.
Follow-up
In analysis of follow-up, patients with benign cores were divided into those who had excision, those who had a definitive diagnosis of fibroadenoma on core biopsy, and those who were reviewed at subsequent breast clinics or incident round breast screening. Primary care physicians were contacted in cases of early patient discharge from clinic to confirm that malignancy had not been diagnosed in these patients at a later date.
RESULTS
Patients
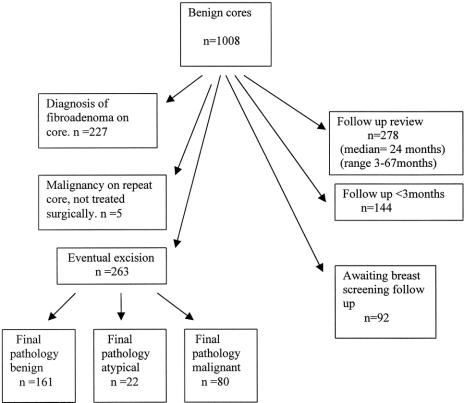
A total of 2427 cores were performed on patients with breast abnormalities: 622 core biopsies on patients from the screening service and 1805 on patients presenting to the symptomatic service. On initial core, 1228 patients were diagnosed with malignant disease, 1008 had benign disease, and 191 patients demonstrated atypical disease. Further to repeat core or excision, 1384 patients had a final diagnosis of malignant disease, 954 patients were diagnosed with benign disease, and 89 patients were diagnosed with atypical disease. The outcome of those with initial benign cores is shown in Figure 1.
FIGURE 1. Analysis of the outcome of those with benign cores: a summary of those who those who had a definite diagnosis of fibroadenoma on core biopsy, those who had an excision biopsy following core biopsy, and those who attended follow-up clinics (or a primary care physician) with no subsequent development of cancer.
Method of Biopsy
The methods of biopsy were ultrasound-guided (n = 1279), stereotactic-guided (n = 409), and clinically guided (n = 739) core biopsy. In those who were subsequently diagnosed with cancer, 769 patients underwent ultrasound-guided core biopsy, 179 patients had stereotactic-guided core biopsy, and 436 patients had clinically guided core biopsy as their initial invasive diagnostic test.
Accuracy of Core
Overall, 85 patients in whom malignancy was subsequently diagnosed had an initial benign core giving a false-negative rate of 6.1% (n = 85 of 1384). The false-negative rate for each biopsy modality was 1.7% (n = 13 of 769) for ultrasound-guided core biopsy, 8.9% (n = 16 of 179) for stereotactic-guided cores, and 13% (n = 56 of 436) for clinically guided core biopsy. In 71 patients, atypia was demonstrated on core and carcinoma was found on excision. These underestimates accounted for 3.4% (n = 26 of 769), 12.8% (n = 23 of 179), and 5.0% (n = 22 of 436) of ultrasound-guided, stereotactic-guided, and clinically guided core biopsy, respectively.
False-Negative Cores
Clinical Features
Eighty-five patients were identified who had an initial benign core biopsy followed by a diagnosis of cancer. The median age was 59 years (range, 37–82 years), with 20 patients less than 50 years. The indication for referral was a breast lump (n = 51), abnormal radiology (n = 22), or both (n = 5). Unusual presentations included blood-filled cysts (n = 2), breast abscess/inflammation (n = 2), skin changes (n = 1), nipple retraction (n = 1), and lymphadenopathy (n = 1). The majority of patients had palpable disease on clinical examination (n = 65).
Radiology
The principal mammographic features of the 85 patients with false-negative biopsies are demonstrated in Table 2, indicating that the majority of these 85 patients had a definite density or mass on mammography. In 17 patients, mammography was normal (n = 6) or demonstrated a lesion that was considered benign (n = 11) (eg, consistent with a cyst). Ultrasound was performed in 13 of the above patients, increasing the level of suspicion in 9 cases. Ten of these 17 cases had suspicious clinical features or a previous history of cancer.
TABLE 2. Imaging Features of False-Negative Cores
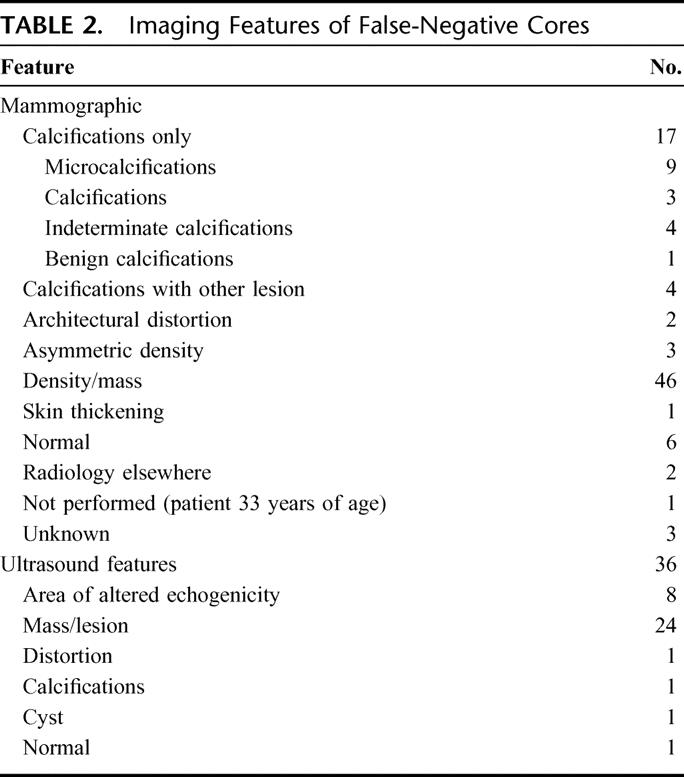
Sixteen of the 85 false-negative cores were performed under stereotactic guidance. Thirteen were performed on calcifications, 1 on calcifications with stromal deformity, and 2 on masses. In 5 cases, the radiologist recorded concern that too few calcifications were captured on core biopsy. Multiple fragments were received in pathology in all but 3 patients.
A smaller subgroup of the 85 patients with false-negative core biopsies had biopsies under ultrasound guidance (n = 13 of 85). These core biopsies were performed on masses (n = 7), hypoechoic areas (n = 2), an area of distortion (n = 1), and an area of malignant calcification (n = 1). Measurements of the lesion were taken in 10 patients, demonstrating a lesion size less than 0.8 cm in 3 patients, and greater than 2 cm in 4 patients.
Pathology-Core Tissue Biopsies
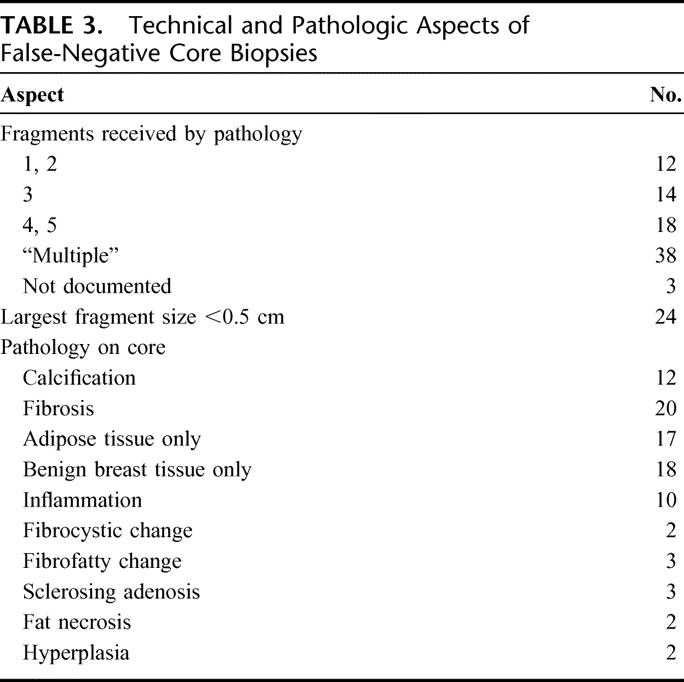
In 26 of the 85 false-negative cases, 3 or less cores or fragments were sent to pathology (Table 3). The histologic findings of the core are detailed in Table 2. Fibrosis was found in 20 of the initial cores and inflammation in 10.
TABLE 3. Technical and Pathologic Aspects of False-Negative Core Biopsies
Cytology
Cytology was performed at or near the time of the initial false-negative core biopsy in 50 of the 85 patients, yielding a positive or suspicious result in 12 patients.
Further Investigation
Further investigation in the 85 patients with false-negative core biopsies was prompted by abnormal mammography and clinical findings (n = 37), abnormal ultrasound and clinical findings (n = 7), and abnormal mammographic findings (n = 28). Clinical findings prompted further investigation in 9 patients, and technical reasons for false-negative core biopsies were recognized in 4 patients. Abnormal cytology was also a factor in prompting further investigation in 12 of the 85 patients.
A repeat core biopsy was performed in 45 of the 85 patients, yielding a diagnosis of cancer in 31 cases. Three sets of core biopsies were performed in 3 patients, without obtaining a diagnosis of cancer.
Delay in Diagnosis
Seventy-one patients had a second core biopsy or decision to proceed to excision biopsy within 1 month of the initial biopsy. Eight patients had a delayed diagnosis or a delay in a decision to proceed with surgery by more than 2 months. In 2 of these patients, it was not possible to determine whether cancers developed after the original biopsy or were “missed cancers” and they were therefore included in the analysis.
Of the 8 patients with delayed diagnosis, 5 had nonsuspicious imaging. Persistent clinical findings despite benign imaging led to repeat core biopsy in 2 patients. A third patient's imaging was consistent with a fibroadenoma; the lesion regressed clinically, and the patient failed to return to follow-up. She was referred 11 months later from a population-based screening program with a diagnosis of cancer at the same site. Two patients had calcifications on imaging that were not considered malignant. One of these patients had a clinically suspicious lump; the other patient had calcifications at the site of her previous excision for breast cancer. In both patients, the calcifications appeared increasingly suspicious 1 year later and repeat biopsy confirmed malignancy. A further patient was referred with abnormal imaging, but repeat imaging and core biopsy were benign. Worsening clinical signs prompted repeat investigations, and cancer was diagnosed 18 months later. In another case, a patient presented with a large blood-filled cyst that failed to heal and 3 sets of biopsies were benign. A clinical decision to excise was made 3 months after the original biopsy, and the pathology was consistent with an intraductal papillary carcinoma. The eighth patient was scheduled for diagnostic excision but persistently failed to attend follow-up clinic and was diagnosed with cancer 26 months later.
Final Pathology
Eighty of the 85 cases with false-negative biopsy had an operative procedure in our hospital. Sixty-three patients had invasive disease (ductal 45, lobular 8, mixed 7, mucinous 1, tubular 1, papillary carcinoma 1), and 13 patients had ductal carcinoma in situ, including 3 with of papillary carcinoma in situ. Four patients had phylloides tumors. Of the 45 patients with invasive ductal carcinomas, 36 had associated ductal carcinoma in situ, including 10 with extensive intraductal component. Of the 78 patients with measurable lesions, 43 patients had tumors <2 cm (T1) and 35 patients had tumors >2 cm (T2). Eleven of the tumors were grade 1, 38 were grade 2, 23 were grade 3, and 8 were of unrecorded grade.
DISCUSSION
In the past decade, the approach to obtaining a diagnosis in breast cancer has undergone major change4,6,7,23–27 and generated considerable controversy.21,28,29 Open excision biopsies have increasingly been replaced by preoperative diagnostic techniques such as fine needle aspiration cytology, and more commonly, core biopsies. In particular, image-guided biopsy has become an established technique. Fears over the accuracy of core biopsy techniques have been assuaged by a large number of studies documenting high sensitivity rates for core biopsies. However, many issues around core biopsy have not been resolved. There is for instance, little information regarding the best modality of biopsy in obtaining a diagnosis of breast cancer. Furthermore, there is still concern that ideal diagnostic protocols may not be in place to prevent some cancers from being missed.30
Stereotactic techniques are widely accepted as the principal alternative to open biopsy for nonpalpable lesions. The only meta-analysis that has been published on image-guided biopsies focuses exclusively on stereotactic techniques. The pooled sensitivity of stereotactic-guided biopsies in Verkooijen's meta-analysis was 97% and varies in other studies from 90% to 99%.5,9–12,24 The sensitivity of stereotactic techniques in our study was relatively low (91%), and this is probably because of its almost exclusive use for evaluation of calcifications. There is evidence that the accuracy of stereotactic biopsies may be lower for calcifications than mass lesions.13,31,32 Targeting of calcifications is difficult, and any patient movement, which is more common on the upright stereotactic table, negates the technique.
This study represents one of the largest studies to date on ultrasound-guided biopsy, particularly in relation to the proportion of breast cancer cases in the study population. Ultrasound-guided biopsies have considerable advantages over stereotactic techniques. Ultrasound-guided techniques are performed in real time, and direct needle visualization with ultrasound allows accuracy of sampling to be assessed. The procedure time is quicker,33 it causes less patient discomfort,33 it does not involve ionizing radiation, and it is less expensive than stereotactic techniques.16 Given that the majority of breast lesions can be visualized by ultrasound, our finding of a false-negative rate as low as 1.7% is important. Other studies on this technique have, in the majority of cases, reported very low false-negative rates often approaching or equalling 0%14–17 (Table 1). In studies of ultrasound and stereotactic techniques, ultrasound sensitivity rates are reported at rates as least as good as stereotactic techniques.13,30,34
Agarwal et al,19 in a study of 151 breast cancer patients undergoing clinically guided core biopsies, suggested that the sensitivity of clinically guided core biopsies is so high as to make image-guided biopsies unnecessary in palpable lesions. Our study, which to our knowledge is the largest study on clinically guided cores, demonstrates that of the 3 modalities, clinically guided cores were the least accurate with a false-negative rate of 13%. Clinically guided biopsy therefore cannot be advocated unless image guidance is not available. Our results are supported by Shah et al,30 who demonstrated a false-negative rate of 12% in clinically-guided cores. Other smaller studies report sensitivity rates between 65% and 95%.20,22,29,35–37 Because clinically guided cores will remain a diagnostic modality in lesions not visible under imaging, it is important to realize its limitations.
Other than the biopsy method, the contributing reasons for a false-negative biopsy are likely to be the result of a number of factors and may vary in importance depending on the modality in question. Clinically, only a minority of these patients presented with unusual signs, and most had palpable lesions. Therefore, inability to locate the tumor, small tumor size, or unusual presentation did not appear to be a primary cause of false-negative core biopsies. Nonsuspicious mammography occurred in a number of patients with false-negative biopsies, and a further 17 patients had calcifications on imaging, which may be more difficult to biopsy. In a proportion of ultrasound-guided biopsies, small tumor size may have been a contributing factor. Technical factors appear to be important in determining the accuracy of core biopsies. Studies have shown that a minimum of 4 to 5 cores are necessary to obtain a definitive diagnosis,2,32,36,38,39 and this may need to be increased in the case of calcifications. In 35 of our patients with false-negative biopsies, less than 5 fragments were received in pathology. Benign biopsies with minimal tissue yield should be highlighted as inadequate and referred automatically for repeat biopsy.
In the analysis of the management and outcome of patients with false-negative cores, it was demonstrated that, if a patient's clinical, radiologic, and pathologic results are reviewed after biopsy, in accordance with the principles of triple assessment, few cancer diagnoses are delayed by more than 1 month. In the management of potential breast cancer, the indication to rebiopsy is traditionally governed by suspicion raised by any one of these 3 elements. In our study, radiologic findings were the most frequent reason why patients were further investigated. Conversely, while the majority of false-negative cases demonstrated a definite density or mass on mammography, 17 patients had relatively normal mammography and a benign core. Ultrasound and clinical findings were found to raise the level of suspicion in most of these patients. From a clinical perspective, in 8 of the 85 patients with false-negative biopsies, it was primarily the clinical findings that prompted further investigation; and in a further 7 patients, it was the clinical and ultrasound findings that increased suspicion. Moreover, in 35 of the 85 patients, the tumor size was >2 cm, which is likely to have aided a clinical decision for early reinvestigation. While radiologic findings contributed most to promoting reinvestigation, it is clear, from the analysis of patients with a delayed diagnosis, that clinical suspicion in these patients contributed a considerable role in eliciting the final diagnosis. This emphasizes the need for both multidisciplinary review and clinical follow-up of patients with a benign core biopsy of the breast.
We also demonstrated a benefit in repeating the core as this yielded a definite diagnosis in 69% (n = 31 of 45) of patients in whom this was performed. Furthermore, we found that concomitant cytology contributed as a diagnostic adjunct at or around the time of initial biopsy, yielding suspicious findings in 12 of the 50 patients in whom this was performed. This is supported by other studies that have shown that combining core and fine needle aspiration cytology techniques increases the sensitivity of diagnosis.3,18,29
The core histology was also of interest. Fibrosis was found in a relatively high proportion of biopsies (24%). Revelon et al40 who analyzed more than 2000 biopsies, including excision biopsies, found focal fibrosis in only 2.1% of cases. A finding of fibrosis in a biopsy in the presence of a suspicious mammogram may be an additional risk factor for malignant disease.41
While it is reassuring that most false-negative cases are recognized within a month of the initial biopsy, we recognize that our study is limited by lack of long-term follow-up for all patients with benign biopsies. Despite this, we believe that this study highlights important differences in the efficacy of the various biopsy techniques, in a setting where ultrasound-guided biopsy is the principal biopsy method performed. We have also analysed the characteristics of patients with false-negative biopsies and demonstrated the need for multidisciplinary evaluation and clinical reassessment after every benign biopsy performed.
CONCLUSION
This study has shown that ultrasound-guided biopsy is highly accurate in obtaining a histologic diagnosis in breast cancer. We would recommend that this modality is used in patients who have breast lesions visible on ultrasound. We also have found that radiologic follow-up alone is not enough to prevent a cancer from being missed. Careful adherence to the principles of triple assessment following biopsy and the support of multidisciplinary review is essential to avoid delay in diagnosis of breast cancer in patients with false-negative core biopsies.
Footnotes
Reprints: Arnold D. K. Hill, MCh, FRCSI, Department of Surgery, St. Vincent's University Hospital, Elm Park, Dublin 4, Ireland. E-mail: adkhill@ucd.ie.
REFERENCES
- 1.Farshid G, Rush G. The use of fine needle aspiration cytology and core biopsy in the assessment of highly suspicious mammographic calcifications: analysis of outcome for 182 lesions detected in the setting of population based breast cancer screening program. Cancer. 2003;99:357–364. [DOI] [PubMed] [Google Scholar]
- 2.Dennison G, Anand R, Maker SH, et al. A prospective study of the use of fine needle aspiration cytology and core biopsy in the diagnosis of breast cancer. Breast J. 2003;9:491–493. [DOI] [PubMed] [Google Scholar]
- 3.Westenend PJ, Sever AR, de Volder B, et al. A comparison of aspiration cytology and core needle biopsy in the evaluation of breast lesions. Cancer. 2001;93:146–150. [DOI] [PubMed] [Google Scholar]
- 4.Fuhrman GM, Cederbom GJ, Bolton JS, et al. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with non palpable imaging abnormalities. Ann Surg. 1998;227:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verkooijen HM. Diagnostic accuracy of stereotactic large-core needle biopsy for nonpalpable breast disease: results of a multicenter prospective study with 95% surgical confirmation. Int J Cancer. 2002;99:853–859. [DOI] [PubMed] [Google Scholar]
- 6.Verkooijen HM, Borel Rinkes IH, Peeters PH, et al. Impact of stereotactic large-core needle biopsy on diagnosis and surgical treatment of nonpalpable breast cancer. Eur J Surg Oncol. 2001;27:244–249. [DOI] [PubMed] [Google Scholar]
- 7.March DE, Raslavicus A, Coughlin BF. Use of breast core biopsy in the United States: results of a national survey. AJR Am J Roentgenol. 1997;169:697–701. [DOI] [PubMed] [Google Scholar]
- 8.Verkooijen HM, Peeters PHM, Buskens E, et al. Diagnostic accuracy of large-core needle biopsy for nonpalpable breast disease: a meta-analysis. Br J Can. 2000;82:1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackman JJ, Nowels KW, Rodriguez-Soto J, et al. Stereotactic, automated, large-core needle biopsy of non palpable breast lesions: false negative and histologic underestimation rates after long term follow up. Radiology. 1999;210:799–805. [DOI] [PubMed] [Google Scholar]
- 10.Leifland K, Lagerstedt U, Svane G, et al. Comparison of stereotactic fine needle aspiration cytology and core needle biopsy in 522 non-palpable breast lesions. Acta Radiol. 2003;44:387–391. [DOI] [PubMed] [Google Scholar]
- 11.Kettritz U, Rotter K, Schreer I. Stereotactic vacuum-assisted breast biopsy in 2874 patients. Cancer. 2004;100:245–251. [DOI] [PubMed] [Google Scholar]
- 12.Burns RB, Brown JP, Roe SM, et al. Stereotactic core-needle breast biopsy by surgeons. Ann Surg. 2000;232:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large core breast biopsy: a multi-institutional study. Radiology. 1994;193:359–364. [DOI] [PubMed] [Google Scholar]
- 14.Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507–511. [DOI] [PubMed] [Google Scholar]
- 15.Smith DN, Rosenfield Darling ML, et al. The utility of ultrasonographically guided large-core needle biopsy. J Ultrasound Med. 2001;20:43–49. [DOI] [PubMed] [Google Scholar]
- 16.Liberman L, Feng TL, Dershaw DD. US-guided core breast biopsy: use and cost effectiveness. Radiology. 1998;208:717–723. [DOI] [PubMed] [Google Scholar]
- 17.Yeow KM, Lo YF, Wang CS. Ultrasound-guided core needle biopsy as an initial diagnostic test for palpable breast masses. J Vasc Interv Radiol. 2001;12:1313–1317. [DOI] [PubMed] [Google Scholar]
- 18.Hatada T, Ishii H, Ichii S, et al. Diagnostic value of ultrasound-guided fine needle aspiration biopsy, core needle biopsy, and evaluation of combined use in the diagnosis of breast lesions. J Am Coll Surg. 2000;190:299–303. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal T, Patel B, Ragan P, et al. Core biopsy versus FNAC for palpable breast cancers: is image guidance necessary? Eur J Can. 2003;39:52–56. [DOI] [PubMed] [Google Scholar]
- 20.Minkowitz S, Moskowitz R, Khafif RA, et al. TRU-CUT needle biopsy of the breast: an analysis of its specificity and sensitivity. Cancer. 1986;57:320–323. [DOI] [PubMed] [Google Scholar]
- 21.Ballo MS, Sneige N. Can core needle biopsy replace fine needle aspiration cytology in the diagnosis of palpable breast carcinoma: a comparative study of 124 women. Cancer. 1996;78:773–777. [DOI] [PubMed] [Google Scholar]
- 22.Cusick JD, Dotan J, Jaecks RD, et al. The role of Tru-Cut needle biopsy in the diagnosis of carcinoma of the breast. Gynecol Obstret. 1990;170:407–410. [PubMed] [Google Scholar]
- 23.Nguyen M, McCombs MM, Ghandehari S, et al. An update on core needle biopsy for radiologically detected breast lesions. Cancer. 1996;76:2340–2345. [PubMed] [Google Scholar]
- 24.White RR, Halperin TJ, Olsen JA, et al. Impact of core needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg. 2001;233:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litherland JC, Evans AJ, Wilson AR, et al. The impact of core-biopsy on pre-operative diagnosis rate of screen detected breast cancers. Clin Radiol. 1996;51:562–565. [DOI] [PubMed] [Google Scholar]
- 26.Britton PD, Flower CD, Freeman AH, et al. Changing to core biopsy in an NHS breast screening unit. Clin Radiol. 1997;52:764–767. [DOI] [PubMed] [Google Scholar]
- 27.Crowe JP, Rim A, Patrick RJ, et al. A prospective review of the decline of excisional breast biopsy. Am J Surg. 2002;184:353–355. [DOI] [PubMed] [Google Scholar]
- 28.Kopans DB. Caution on core. Radiology. 1994;193:325–328. [DOI] [PubMed] [Google Scholar]
- 29.Scopa CD, Koukouras D, Spiliotis J, et al. Comparison of fine needle aspiration and Tru-Cut biopsy of palpable mammary lesions. Cancer Detect Prev. 1996;20:620–624. [PubMed] [Google Scholar]
- 30.Shah VI, Raju U, Chitale D, et al. False negative core needle biopsies of the breast. Cancer. 2003;97:1824–1831. [DOI] [PubMed] [Google Scholar]
- 31.Liberman L, Dershaw DD, Glassman JR, et al. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151–157. [DOI] [PubMed] [Google Scholar]
- 32.Liberman L, Dershaw DD, Rosen PP, et al. Stereotactic 14-gauge breast biopsy: how many core biopsy specimens are needed? Radiology. 1994;192:793–795. [DOI] [PubMed] [Google Scholar]
- 33.Mainierio MB, Gareen IF, Bird CE, et al. Preferential use of sonographically guided biopsy to minimise patient discomfort and procedure time in a percutaneous image guided breast biopsy program. J Ultrasound Med. 2002;21:1221–1226. [DOI] [PubMed] [Google Scholar]
- 34.Fajardo LL, Pisano ED, Caudry DJ, et al. Stereotactic and sonographic large core biopsy of nonpalpable breast lesions: results of the Radiological Diagnostic Oncology Group V study. Acad Radiol. 2004;11:293–308. [DOI] [PubMed] [Google Scholar]
- 35.Barreto V, Hamed H, Griffiths AB, et al. Automatic needle biopsy in the diagnosis of early breast cancer. Eur J Surg Oncol. 1991;17:237–239. [PubMed] [Google Scholar]
- 36.Dennison G, Anand R, Makar SH, et al. A prospective study of the use of fine-needle aspiration cytology and core biopsy in the diagnosis of breast cancer. Breast J. 2003;9:491–493. [DOI] [PubMed] [Google Scholar]
- 37.McMahon AJ, Lufty AM, Matthew A, et al. Needle core biopsy of the breast with a spring loaded device. Br J Surg. 1992;79:1042–1045. [DOI] [PubMed] [Google Scholar]
- 38.Fishman JE, Milikowski C, Ramsinghani R, et al. US-guided core needle biopsy of the breast: how many specimens are necessary? Radiology. 2003;226:779–782. [DOI] [PubMed] [Google Scholar]
- 39.Brenner RJ, Fajardo L, Fisher PR, et al. Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol. 1996;166:341–346. [DOI] [PubMed] [Google Scholar]
- 40.Revelon G, Sherman ME, Gateway OMB, et al. Focal fibrosis of the breast: imaging characteristics and histopathologic correlation. Radiology. 2000;216:215–219. [DOI] [PubMed] [Google Scholar]
- 41.Liberman L, Drotman M, Morris EA, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000;89:2538–2546. [DOI] [PubMed] [Google Scholar]
- 42.Crowe JP, Rim A, Patrick RJ, et al. Does core needle biopsy accurately reflect breast pathology? Surgery. 2003;134:23–28. [DOI] [PubMed] [Google Scholar]